Key Points
Question
What is the maximum plasma concentration of 6 sunscreen active ingredients from 4 commercially available sunscreen products (formulated as lotion, aerosol spray, nonaerosol spray, and pump spray)?
Findings
In this randomized clinical trial with 48 healthy participants, maximum plasma concentrations (geometric mean [coefficient of variation %]) for the active ingredient avobenzone (primary end point) were 7.1 ng/mL (73.9%) for lotion, 3.5 ng/mL (70.9%) for aerosol spray, 3.5 ng/mL (73.0%) for nonaerosol spray, and 3.3 ng/mL (47.8%) for pump spray following a single application of these products on day 1 and multiple applications through day 4.
Meaning
Sunscreen active ingredients are systemically absorbed, which supports the need for additional studies to determine the clinical significance of these findings.
Abstract
Importance
A prior pilot study demonstrated the systemic absorption of 4 sunscreen active ingredients; additional studies are needed to determine the systemic absorption of additional active ingredients and how quickly systemic exposure exceeds 0.5 ng/mL as recommended by the US Food and Drug Administration (FDA).
Objective
To assess the systemic absorption and pharmacokinetics of the 6 active ingredients (avobenzone, oxybenzone, octocrylene, homosalate, octisalate, and octinoxate) in 4 sunscreen products under single- and maximal-use conditions.
Design, Setting, and Participants
Randomized clinical trial at a clinical pharmacology unit (West Bend, Wisconsin) was conducted in 48 healthy participants. The study was conducted between January and February 2019.
Interventions
Participants were randomized to 1 of 4 sunscreen products, formulated as lotion (n = 12), aerosol spray (n = 12), nonaerosol spray (n = 12), and pump spray (n = 12). Sunscreen product was applied at 2 mg/cm2 to 75% of body surface area at 0 hours on day 1 and 4 times on day 2 through day 4 at 2-hour intervals, and 34 blood samples were collected over 21 days from each participant.
Main Outcomes and Measures
The primary outcome was the maximum plasma concentration of avobenzone over days 1 through 21. Secondary outcomes were the maximum plasma concentrations of oxybenzone, octocrylene, homosalate, octisalate, and octinoxate over days 1 through 21.
Results
Among 48 randomized participants (mean [SD] age, 38.7 [13.2] years; 24 women [50%]; 23 white [48%], 23 African American [48%], 1 Asian [2%], and 1 of unknown race/ethnicity [2%]), 44 (92%) completed the trial. Geometric mean maximum plasma concentrations of all 6 active ingredients were greater than 0.5 ng/mL, and this threshold was surpassed on day 1 after a single application for all active ingredients. For avobenzone, the overall maximum plasma concentrations were 7.1 ng/mL (coefficient of variation [CV], 73.9%) for lotion, 3.5 ng/mL (CV, 70.9%) for aerosol spray, 3.5 ng/mL (CV, 73.0%) for nonaerosol spray, and 3.3 ng/mL (CV, 47.8%) for pump spray. For oxybenzone, the concentrations were 258.1 ng/mL (CV, 53.0%) for lotion and 180.1 ng/mL (CV, 57.3%) for aerosol spray. For octocrylene, the concentrations were 7.8 ng/mL (CV, 87.1%) for lotion, 6.6 ng/mL (CV, 78.1%) for aerosol spray, and 6.6 ng/mL (CV, 103.9%) for nonaerosol spray. For homosalate, concentrations were 23.1 ng/mL (CV, 68.0%) for aerosol spray, 17.9 ng/mL (CV, 61.7%) for nonaerosol spray, and 13.9 ng/mL (CV, 70.2%) for pump spray. For octisalate, concentrations were 5.1 ng/mL (CV, 81.6%) for aerosol spray, 5.8 ng/mL (CV, 77.4%) for nonaerosol spray, and 4.6 ng/mL (CV, 97.6%) for pump spray. For octinoxate, concentrations were 7.9 ng/mL (CV, 86.5%) for nonaerosol spray and 5.2 ng/mL (CV, 68.2%) for pump spray. The most common adverse event was rash, which developed in 14 participants.
Results
Among 48 randomized participants (mean [SD] age, 38.7 [13.2] years; 24 women [50%]; 23 white [48%], 23 African American [48%], 1 Asian [2%], and 1 of unknown race/ethnicity [2%]), 44 (92%) completed the trial. Geometric mean maximum plasma concentrations of all 6 active ingredients were greater than 0.5 ng/mL, and this threshold was surpassed on day 1 after a single application for all active ingredients. The overall maximum plasma concentrations for each active ingredient for each product formulation are shown in the table. The most common adverse event was rash, which developed in 14 participants.
| Geometric Mean Maximum Plasma Concentration, Coefficient of Variation (%), ng/mL | ||||
|---|---|---|---|---|
| Lotion | Aerosol Spray | Nonaerosol Spray | Pump Spray | |
| Avobenzone | 7.1 (73.9) | 3.5 (70.9) | 3.5 (73.0) | 3.3 (47.8) |
| Oxybenzone | 258.1 (53.0) | 180.1 (57.3) | Not applicable | Not applicable |
| Octocrylene | 7.8 (87.1) | 6.6 (78.1) | 6.6 (103.9) | Not applicable |
| Homosalate | Not applicable | 23.1 (68.0) | 17.9 (61.7) | 13.9 (70.2) |
| Octisalate | Not applicable | 5.1 (81.6) | 5.8 (77.4) | 4.6 (97.6) |
| Octinoxate | Not applicable | Not applicable | 7.9 (86.5) | 5.2 (68.2) |
Conclusions and Relevance
In this study conducted in a clinical pharmacology unit and examining sunscreen application among healthy participants, all 6 of the tested active ingredients administered in 4 different sunscreen formulations were systemically absorbed and had plasma concentrations that surpassed the FDA threshold for potentially waiving some of the additional safety studies for sunscreens. These findings do not indicate that individuals should refrain from the use of sunscreen.
Trial Registration
ClinicalTrials.gov Identifier: NCT03582215
This randomized clinical trial assesses the systemic absorption and pharmacokinetics of the 6 active ingredients (avobenzone, oxybenzone, octocrylene, homosalate, octisalate, and octinoxate) in lotion, aerosol spray, nonaerosol spray, and pump spray sunscreen products under single- and maximal-use conditions.
Introduction
When used with other sun protective measures, sunscreens can prevent skin cancer and protect the skin from sunburn and other UV damage.1 In addition, sunscreens can be used in substantial amounts over the course of a lifetime in primary sunscreen drug products and in sunscreen drug-cosmetic combination products. Consistent with the US Food and Drug Administration (FDA) mission to ensure the safety of drugs, the FDA published a proposed rule in February 20192 that would update regulatory requirements for 16 sunscreen ingredients in the United States. This proposed rule recommends an assessment of the human systemic absorption of sunscreen ingredients with a maximal usage trial as outlined in the FDA guidance on maximal usage trials,3 along with a number of other safety studies. Before publishing a final rule, the FDA will consider public comment on the proposed rule.
To gather initial data on the systemic absorption of sunscreen active ingredients, the FDA conducted a pilot maximal usage trial of 4 sunscreen active ingredients (avobenzone, oxybenzone, octocrylene, and ecamsule) in 4 commercially available sunscreen products, which was previously published.4 The prior study demonstrated that all tested sunscreen active ingredients were absorbed systemically and remained in plasma for at least 3 days after the last application. This current study collected additional information on 1 formulation from the prior study and 3 additional formulations along with 3 active ingredients not evaluated in the previous study (homosalate, octisalate, and octinoxate), the systemic absorption levels after applying sunscreen only once on day 1 and extending follow-up to 21 days, and residual skin levels during the washout phase.
The objective of the current study was to assess the systemic absorption and pharmacokinetics of 6 active ingredients (avobenzone, oxybenzone, octocrylene, homosalate, octisalate, and octinoxate) in 4 sunscreen products.
Methods
Study Setting and Dates
A randomized clinical trial was performed in a clinical pharmacology unit with healthy participants. This study was approved by the clinical site’s local institutional review board (Advarra [https://www.advarra.com]). All participants gave written informed consent. The protocol and statistical analysis plan are available in Supplement 1.
Recruitment
The study was conducted in January and February of 2019. Participants were recruited by standard recruiting for a phase 1 healthy volunteer study. Fitzpatrick skin type (Fitzpatrick Skin Type Questionnaire in Supplement 1) and self-identified race/ethnicity collected in an open-ended format were recorded by clinical staff as a standard component of a clinical trial.5 Study participants remained in the clinic for 7 days and were not exposed to direct sunlight during that time. The study participants had follow-up visits on days 10, 14, and 21. Participants with broken, irritated, or unhealed skin or active sunburn; active autoimmune disease; anemia; or other chronic conditions that could affect blood sample collection were excluded from the study. Additionally, participants using any products containing the listed active ingredients within 7 days of check-in were excluded from enrollment.
Randomization and Interventions
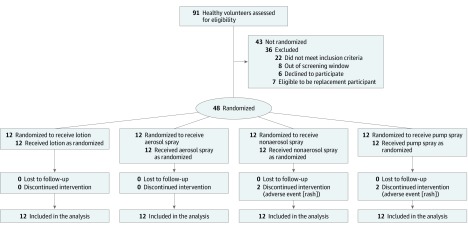
Study participants were randomized by a validated database system to participate in 1 of the 4 treatment groups, which included 4 sunscreen product formulations: lotion, aerosol spray, nonaerosol spray, and pump spray (Figure 1). Randomization was conducted in block sizes of 4 and included equal numbers of women and men in each treatment group. Participants and investigators were not blinded to the randomization due to significant differences between formulations (eTable 1 in Supplement 2). Allocation concealment was not performed. The study product was weighed in advance and applied by clinic staff. Each group had 12 participants with 1 formulation.
Figure 1. Flow of Participants in Study.
Randomization was conducted in block sizes of 4 and included equal numbers of women and men in each treatment group.
Sunscreen product was applied at 2 mg/cm2 to 75% of body surface area (area outside of normal swim wear) during 4 days of the study, with a total of 13 applications. The study product was applied 1 time on day 1 (0 hours) and 4 times per day for the remaining 3 days: 24, 26, 28, and 30 hours on day 2; 48, 50, 52, and 54 hours on day 3; and 72, 74, 76, and 78 hours on day 4 (Pharmacy Manual in Supplement 1). Thirty-four blood samples were collected over 21 days: day 1 at 0, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 9, 10, 12, and 14 hours; day 2 at 23, 28, and 33 hours; day 3 at 47, 52, and 57 hours; day 4 at 71, 73, 74, 76, 78, 81, 82, 84, and 86 hours; day 5 at 95 hours; day 6 at 120 hours; day 7 at 144 hours; day 10 at 216 hours; day 14 at 312 hours; and day 21 at 480 hours after the first sunscreen application.
During days 1 through 7, participants were required per the study protocol to shower each morning after the first pharmacokinetic blood sample collection (and before the first dose of the day), but not at other times during the day. In addition, skin (stratum corneum) samples were collected by tape stripping (6 consecutive strippings)6 of the lower back (around 3.8 cm2) on day 7 and day 14 (Tape Stripping Procedure in Supplement 1). The amounts recovered after tape stripping and plasma concentrations of all 6 active ingredients were measured by a validated liquid chromatographic tandem mass spectrometric method (eMethods 1, 2, 3, 4, and 5 in Supplement 2). Deidentified participant plasma and skin data are available in Supplement 3 (data dictionary for participant data are in the eDictionary in Supplement 2).
Prespecified Outcomes
The prespecified primary outcome was the maximum plasma concentration of avobenzone over days 1 through 21. The secondary outcomes were the maximum plasma concentrations of oxybenzone, octocrylene, homosalate, octisalate, and octinoxate over days 1 through 21.
Exploratory outcomes and analyses included were maximum plasma concentrations following a single application on day 1; maximum plasma concentrations on day 4; area under the plasma concentration vs time curve (AUC) on single (day 1) and multiple (day 4) applications; terminal half-life; and active ingredient concentrations on days 7, 14, and 21 (last application was on day 4). All adverse events were recorded by clinic staff and adjudicated by the principal investigator.
Post Hoc Assessment
Two post hoc assessments were performed. First, the number and percentage of participants with systemic exposure exceeding the 0.5-ng/mL threshold were summarized. The 0.5-ng/mL threshold was selected because the approximated cancer risk associated with plasma concentrations below this threshold would be less than 1 in 100 000 after a single dose.2,7 Second, the amount of active ingredients remaining in the skin on days 7 and 14 was summarized using descriptive statistics.
Statistical Analysis
Because this was an exploratory study to assess general methodology for a sunscreen maximum usage trial, the sample size (N = 48) was determined empirically with reference to the sunscreen guidance recommendation for pilot studies.3 Plasma concentrations below the limit of quantitation were assigned as zero during pharmacokinetic parameter calculation. For all participants who missed 1 or more applications, all samples obtained after the missed application were removed from the analysis. Available data from these participants at earlier time points were included in all analyses, but these participants were not included in descriptive statistics on or following the first missed application. No adjustments for multiplicity were made in the statistical analyses. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary outcomes, exploratory pharmacokinetic parameters, and post hoc assessments should be interpreted as exploratory. Standard noncompartmental pharmacokinetic parameter calculations and statistical analyses were performed in R (version 3.4.3; The R Project for Statistical Computing). AUC and maximum plasma concentrations were separately determined for day 1, day 4, and for the overall study.
Data are reported with standard descriptive statistics for all demographics (arithmetic means) and pharmacokinetic parameters (geometric mean, coefficient of variation [CV], confidence intervals, minimum, and maximum). 90% CIs were chosen because they are standard in pharmacokinetic studies.8,9 Terminal half-life is reported only for participants with 3 or more concentration values in the terminal portion of the curve and an adjusted coefficient of determination (R2 > 0.70).
Results
A total of 48 participants (24 female, 24 male) were randomized to 4 groups (mean age [SD], 38.7 [13.2] years). Participants in the study were identified as having Fitzpatrick skin type II (n = 9, 19%), III (n = 30, 63%), or IV (n = 9, 19%) (Table 1). Three participants discontinued dosing on day 3 and 1 participant showered immediately after the last dose application on day 4. Samples obtained after these events for these 4 participants were removed from analysis. Four participants had day 14 sampling delayed until day 16 due to inclement weather. Actual sampling time was used in calculating pharmacokinetic parameters for these participants, but nominal time was used for all other analyses. All the predose samples (baseline) and 11%, 13%, 20%, and 21% of other assessments were below the lower limit of quantitation (LLOQ) for lotion, aerosol spray, nonaerosol spray, and pump spray, respectively.
Table 1. Study Participant Demographics and Product Characteristics.
| Demographic | No. (%) | ||||
|---|---|---|---|---|---|
| Population Total (N = 48) | Lotion (n = 12) | Aerosol Spray (n = 12) | Nonaerosol Spray (n = 12) | Pump Spray (n = 12) | |
| Age, mean (SD), y | 38.7 (13.2) | 45.1 (13.8) | 41.4 (13.40 | 39.2 (12.2) | 29.0 (8.5) |
| Sex | |||||
| Male | 24 (50) | 6 (50) | 6 (50) | 6 (50) | 6 (50) |
| Female | 24 (50) | 6 (50) | 6 (50) | 6 (50) | 6 (50) |
| Race | |||||
| Black or African American | 23 (48) | 4 (33) | 5 (42) | 4 (33) | 10 (83) |
| White | 23 (48) | 8 (67) | 7 (58) | 6 (50) | 2 (17) |
| Asian | 1 (2) | 0 | 0 | 1 (8) | 0 |
| Unknown | 1 (2) | 0 | 0 | 1 (8) | 0 |
| Hispanic or Latino ethnicity | 3 (6) | 1 (8) | 0 | 1 (8) | 1 (8) |
| Body mass index, mean (SD)a | 26.0 (2.9) | 26.2 (2.2) | 25.8 (3.7) | 25.8 (2.4) | 26.0 (3.3) |
| Body surface area, mean (SD), m2b | 1.9 (0.2) | 1.9 (0.2) | 1.9 (0.2) | 1.8 (0.2) | 1.9 (0.2) |
| Fitzpatrick skin typec | |||||
| I | 0 | 0 | 0 | 0 | 0 |
| II | 9 (19) | 3 (25) | 2 (17) | 3 (25) | 1 (8) |
| III | 30 (63) | 5 (42) | 10 (83) | 6 (50) | 9 (75) |
| IV | 9 (19) | 4 (33) | 0 | 2 (25) | 2 (17) |
| V | 0 | 0 | 0 | 0 | 0 |
| VI | 0 | 0 | 0 | 0 | 0 |
| Product composition, %d | |||||
| Avobenzone | 3 | 3 | 3 | 3 | |
| Oxybenzone | 4 | 6 | |||
| Octocrylene | 6 | 10 | 10 | ||
| Homosalate | 15 | 10 | 10 | ||
| Octisalate | 5 | 5 | 5 | ||
| Octinoxate | 7.5 | 7.5 | |||
Calculated as weight in kilograms divided by height in meters squared.
Body surface area was calculated using the Mosteller formula: BSA (m2) = (Height [cm] × Weight [kg]/3600)0.5.
Fitzpatrick skin type score: A numerical classification schema used to describe a person’s skin type in terms of response to UV radiation exposure. Participants were provided with a 10-question questionnaire and instructed to select 1 answer for each question. The Fitzpatrick skin type is determined by adding scores for each question, grouped in categories of genetic disposition, reaction to sun exposure, and tanning habits. The scoring scale for this study is as follows: type I: 0-7; type II: 8-16; type III: 17-24; type IV: 25-30; and V or VI: >30.
Maximum allowed on US market is 3% weight/volume (w/v) avobenzone, 6% w/v oxybenzone, 10% w/v octocrylene, 15% w/v homosalate, 5% w/v octisalate, and 7.5% w/v octinoxate.
Exposure Throughout the Study (Days 1-21)
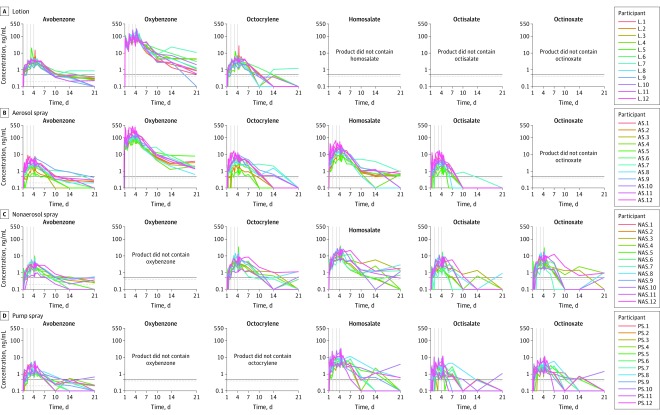
The maximum plasma concentrations of avobenzone were 7.1 ng/mL (CV, 73.9%), 3.5 ng/mL (CV, 70.9%), 3.5 ng/mL (CV, 73.0%), and 3.3 ng/mL (CV, 47.8%) for lotion, aerosol spray, nonaerosol spray, and pump spray, respectively (Table 2). The maximum plasma concentrations of oxybenzone were 258.1 ng/mL (CV, 53.0%) and 180.1 ng/mL (CV, 57.3%) for lotion and aerosol spray, respectively. The maximum plasma concentrations of octocrylene were 7.8 ng/mL (CV, 87.1%), 6.6 ng/mL (CV, 78.1%), and 6.6 ng/mL (CV, 103.9%) for lotion, aerosol spray, and nonaerosol spray, respectively. The maximum plasma concentrations of homosalate were 23.1 ng/mL (CV, 68.0%), 17.9 ng/mL (CV, 61.7%), and 13.9 ng/mL (CV, 70.2%) for aerosol spray, nonaerosol spray, and pump spray, respectively. The maximum plasma concentrations of octisalate were 5.1 ng/mL (CV, 81.6%), 5.8 ng/mL (CV, 77.4%), and 4.6 ng/mL (CV, 97.6%) for aerosol spray, nonaerosol spray, and pump spray, respectively. The maximum plasma concentrations of octinoxate were 7.9 ng/mL (CV, 86.5%) and 5.2 ng/mL (CV, 68.2%) for nonaerosol spray and pump spray, respectively. Pharmacokinetic profiles for individual participants are shown in Figure 2 and geometric mean pharmacokinetic profiles are shown in eFigure 1 in Supplement 2. Complete pharmacokinetic parameters are summarized in Table 2 and eTable 2 in Supplement 2.
Table 2. Pharmacokinetic Parameters of Sunscreen Active Ingredients.
| Geometric Mean (CV%) [Range] {No. of Participants} | ||||
|---|---|---|---|---|
| Lotion (n = 12) | Aerosol Spray (n = 12) | Nonaerosol Spray (n = 12) | Pump Spray (n = 12) | |
| Avobenzone | ||||
| Maximum plasma concentration, ng/mLa | ||||
| Overall | 7.1 (73.9) [2.9-28.0] | 3.5 (70.9) [1.0-9.0] | 3.5 (73.0) [1.0-10.0] | 3.3 (47.8) [1.2-6.2] |
| Day 1 | 1.6 (49.0) [0.7-2.7] | 1.2 (90.6) [0.2-5.2] | 1.0 (65.2) [0.3-1.9] | 0.7 (64.5) [0.3-2] |
| Day 4 | 7.1 (73.9) [2.9-28.0] | 3.5 (70.9) [1.0-9.0] | 3.5 (73.0) [1-10] {n = 10} | 3.1 (40.3) [2.0-6.2] {n = 10} |
| AUC, ng × h/mLa | ||||
| Day 1 | 23.1 (45.3) [10.6-40.6] | 16.5 (85.7) [3.2-48.2] | 11.6 (99.1) [1.2-23.9] | 10.3 (61.5) [3.5-29.9] |
| Day 4 | 93.4 (33.1) [53.3-150.0] | 55.1 (64.4) [16.4-130.2] | 51.6 (45.2) [20.2-92.5] {n = 10} | 42.7 (37.3) [26.5-89.8] {n = 10} |
| Day 7 | ||||
| Concentration, ng/mL | 1.6 (29.4) [1.1-2.4] | 1.1 (56.7) [0.5-3.1] | 1.1 (54.0) [0.6-2.7] {n = 10} | 0.8 (34.2) [0.4-1.4] {n = 10} |
| Skin, ng/cm2 | 927.9 (90.8) [396.2-6556.6] {n = 10} | 1000.1 (89.0) [393.8-5544.2] {n = 11} | 1345.2 (109.6) [333.9-5238.1] {n = 9} | 498.7 (200.3) [52.9-1852.9] {n = 8} |
| Day 14 | ||||
| Concentration, ng/mL | 0.4 (41.9) [0.2-1.0] {n = 11} | 0.1 (473.8) [0-0.9] {n = 11} | 0.2 (332.8) [0-0.6] {n = 10} | 0.1 (465.2) [0-0.6] {n = 10} |
| Skin, ng/cm2 | 39.8 (99.0) [12.7-133.1] {n = 10} | 25.9 (209.1) [6.2-733.5] {n = 11} | 75.8 (378.1) [5.3-854.9] {n = 9} | 48.3 (295.0) [8.9-650.4] {n = 8} |
| Day 21 concentration, ng/mL | 0.1 (393.6) [0-1] | 0.1 (461.5) [0-0.4] {n = 11} | 0.1 (475.8) [0-0.6] {n = 10} | 0 (472.4) [0-0.7] {n = 10} |
| Terminal half-life, h | 112.3 (69.7) [63.1-481.6] {n = 11} | 94.2 (80.1) [29-434.5] {n = 12} | 101.1 (66.6) [50.4-306] {n = 7} | 73.7 (93.1) [29.1-299.2] {n = 6} |
| Oxybenzone | ||||
| Maximum plasma concentration, ng/mLa | ||||
| Overall | 258.1 (53.0) [131.3-498.1] | 180.1 (57.3) [70.1-476.9] | NA | NA |
| Day 1 | 94.2 (44.3) [44.6-177.6] | 85.4 (73.9) [34.8-401.2] | NA | NA |
| Day 4 | 258.1 (53.0) [131.3-498.1] | 180.1 (57.3) [70.1-476.9] | NA | NA |
| AUC, ng × h/mLa | ||||
| Day 1 | 1204 (33.4) [683.2-1901.4] | 1159.6 (51.3) [524.4-3137.6] | NA | NA |
| Day 4 | 3443.7 (34.8) [2053.7-5579.6] | 2757.3 (41.3) [1390.7-5216] | NA | NA |
| Day 7 | ||||
| Concentration, ng/mL | 30.6 (53.3) [13.7-70.0] | 28.3 (42.5) [16.8-62.4] | NA | NA |
| Skin, ng/cm2 | 358.5 (194.7) [40.7-5815.2] {n = 10} | 698.5 (155.0) [114.2-6734.8] {n = 11} | NA | NA |
| Day 14 | ||||
| Concentration, ng/mL | 5.1 (92.3) [1.6-32.5] {n = 11} | 3.8 (68.2) [1.3-9.0] {n = 11} | NA | NA |
| Skin, ng/cm2 | 18.2 (195) [3.9-341.3] {n = 10} | 29.1 (238.2) [7.0-979.6] {n = 11} | NA | NA |
| Day 21 concentration, ng/mL | 1.3 (517.6) [0-14.4] | 2.0 (73.0) [0.6-8.0] {n = 11} | NA | NA |
| Terminal half-life, h | 78.5 (40.6) [41.8-135.3] | 79.2 (49.3) [27.4-206.7] | NA | NA |
| Octocrylene | ||||
| Maximum plasma concentration, ng/mLa | ||||
| Overall | 7.8 (87.1) [2.6-38.7] | 6.6 (78.1) [1.4-16.2] | 6.6 (103.9) [1.7-34.4] | NA |
| Day 1 | 1.5 (67.8) [0.5-3.6] | 1.3 (438.0) [0-12.3] | 1.4 (73.9) [0.4-3.1] | NA |
| Day 4 | 7.8 (87.1) [2.6-38.7] | 6.6 (78.1) [1.4-16.2] | 6.6 (103.9) [1.7-34.4] {n = 10} | NA |
| AUC, ng × h/mLa | ||||
| Day 1 | 20 (62.9) [6.4-38.1] | 14 (1772.3) [0-94.2] | 16.9 (66.8) [6.5-40.4] | NA |
| Day 4 | 94.7 (48.6) [41.4-210.0] | 89.4 (67.4) [23.2-178.2] | 89.5 (60) [33.2-177.0] {n = 10} | NA |
| Day 7 | ||||
| Concentration, ng/mL | 1.9 (44.2) [1.0-2.9] | 2.1 (63.8) [0.6-4.9] | 2.3 (92.9) [0.6-8.6] {n = 10} | NA |
| Skin, ng/cm2 | 2090.7 (99.6) [730.6-16 676.6] {n = 10} | 3463.1 (75.0) [1403.9-19 841.8] {n = 11} | 4488.8 (120.4) [1072.3-20 606.2] {n = 9} | NA |
| Day 14 | ||||
| Concentration, ng/mL | 0 (669.5) [0-1.2] {n = 11} | 0 (1645.7) [0-2] {n = 11} | 0.1 (1254.0) [0-1.1] {n = 9} | NA |
| Skin, ng/cm2 | 123.3 (74.6) [50-327] {n = 10} | 111.1 (187.1) [35.1-3239.2] {n = 11} | 358.1 (338.9) [39.2-3144.1] {n = 9} | NA |
| Day 21 concentration, ng/mL | 0 (262.2) [0-1.4] | 0 (0) [0-0] {n = 11} | 0 (1253.9) [0-1.2] {n = 10} | NA |
| Terminal half-life, h | 49.5 (59.1) [23.7-121.8] {n = 10} | 48.4 (36.7) [28.9-70.9] {n = 8} | 79.1 (40.1) [41.2-118.8] {n = 8} | NA |
| Homosalate | ||||
| Maximum plasma concentration, ng/mLa | ||||
| Overall | NA | 23.1 (68.0) [8.7-67.7] | 17.9 (61.7) [7.4-39] | 13.9 (70.2) [3.7-35.8] |
| Day 1 | NA | 7.6 (108.7) [1.5-44.9] | 4.6 (48.7) [2-8.8] | 3.8 (110.0) [0.9-23.1] |
| Day 4 | NA | 23.1 (68.0) [8.7-67.7] | 17.9 (61.7) [7.4-39] {n = 10} | 14.3 (60.7) [6.7-35.8] {n = 10} |
| AUC, ng × h/mLa | ||||
| Day 1 | NA | 104 (87.1) [24-424.9] | 62.3 (42.4) [32.5-111.6] | 47.6 (79.5) [14.6-130.7] |
| Day 4 | NA | 350.8 (52.1) [140.6-726.7] | 267.4 (46.8) [142.3-457.3] {n = 10} | 199.0 (52.9) [101.9-452.9] {n = 10} |
| Day 7 | ||||
| Concentration, ng/mL | NA | 5.2 (41.4) [2.7-10.3] | 4.7 (103.8) [1.4-21.7] {n = 10} | 3.5 (114.1) [0.5-11.5] {n = 10} |
| Skin, ng/cm2 | NA | 4517 (102.2) [1180.3-32 776.8] {n = 10} | 2814.9 (178.7) [361.9-15 029.4] {n = 9} | 2165.7 (164.1) [401.9-7248] {n = 6} |
| Day 14 | ||||
| Concentration, ng/mL | NA | 0.4 (637.8) [0-3.8] {n = 11} | 0.7 (410.2) [0-5.8] {n = 10} | 0.2 (2388.7) [0-2.3] {n = 10} |
| Skin, ng/cm2 | NA | 190.2 (202.9) [72.8-5218.6] {n = 10} | 436.9 (253.6) [56.5-5988.6] {n = 9} | 181.8 (157.1) [31.3-839.6] {n = 6} |
| Day 21 concentration, ng/mL | NA | 0.2 (723) [0-1] {n = 11} | 0.3 (1654.1) [0-2.9] {n = 10} | 0 (1552.1) [0-3.9] {n = 10} |
| Terminal half-life, h | NA | 67.9 (62.2) [24.4-155.6] {n = 11} | 78.4 (61.6) [49-162.4] {n = 4} | 46.9 (67.5) [18-82.4] {n = 6} |
| Octisalate | ||||
| Maximum plasma concentration, ng/mLa | ||||
| Overall | NA | 5.1 (81.6) [2-17.3] | 5.8 (77.4) [2.3-16.7] | 4.6 (97.6) [0.9-12.2] |
| Day 1 | NA | 1.3 (441.4) [0-12.9] | 1.4 (51.6) [0.5-2.3] | 0.7 (974.7) [0-10.6] |
| Day 4 | NA | 5.1 (81.6) [2-17.3] | 5.8 (77.4) [2.3-16.7] {n = 10} | 4.6 (85.1) [1.5-12.2] {n = 10} |
| AUC, ng × h/mLa | ||||
| Day 1 | NA | 12.0 (1529.4) [0-94.6] | 14.7 (89.6) [2.7-28 | 4.5 (9367.4) [0-75.7] |
| Day 4 | NA | 67.9 (55.5) [30.1-172.6] | 76.9 (62.8) [32.5-137.5 {n = 10} | 54.0 (83.3) [13.8-134.3] {n = 10} |
| Day 7 | ||||
| Concentration, ng/mL | NA | 0.4 (444.3) [0-1.4] | 0.6 (1247.5) [0-5.9] {n = 10} | 0.5 (1011.8) [0-4.5] {n = 10} |
| Skin, ng/cm2 | NA | 1265.4 (107.3) [277.4-9311.2] {n = 10} | 1128.3 (204.5) [125.4-6746.4] {n = 9} | 907.8 (168.7) [193.6-2767.7] {n = 6} |
| Day 14 | ||||
| Concentration, ng/mL | NA | 0 (163.6) [0-0.4] {n = 10} | 0 (630.3) [0-1.4] {n = 10} | 0 (557.2) [0-0.5] {n = 10} |
| Skin, ng/cm2 | NA | 63.9 (174.6) [17.9-1194.1] {n = 10} | 195.7 (250.4) [28.9-2591.1] {n = 9} | 82.3 (85.6) [32.3-263.4] {n = 6} |
| Day 21 concentration, ng/mL | NA | 0 (0) [0-0] {n = 11} | 0 (260.8) [0-0.9] {n = 10} | 0 (286.2) [0-1.1] {n = 10} |
| Terminal half-life, h | NA | 37.4 (61.2) [19.3-122.5] {n = 11} | 27.3 (37.2) [16.9-41] {n = 5} | 77.1 (213.8) [22.9-323.6] {n = 5} |
| Octinoxate | ||||
| Maximum plasma concentration, ng/mLa | ||||
| Overall | NA | NA | 7.9 (86.5) [2.6-30.6] | 5.2 (68.2) [1.5-11.8] |
| Day 1 | NA | NA | 2.0 (96.0) [0.6-5] | 1.1 (326.2) [0-4.1] |
| Day 4 | NA | NA | 7.9 (86.5) [2.6-30.6] {n = 10} | 6.1 (53.8) [3.2-11.8] {n = 10} |
| AUC, ng × h/mLa | ||||
| Day 1 | NA | NA | 21.6 (116.4) [2.7-57.7] | 9.9 (1435.1) [0-53.3] |
| Day 4 | NA | NA | 103.4 (56.2) [45.1-213.5] {n = 10} | 79.5 (50.1) [40.4-144.7] {n = 10} |
| Day 7 | ||||
| Concentration, ng/mL | NA | NA | 1.3 (571.9) [0-12.5] {n = 10} | 1.0 (453.9) [0-5.8] {n = 10} |
| Skin, ng/cm2 | NA | NA | 2373.6 (149.7) [493.2-12 200.5] {n = 9} | 1675.2 (132.7) [470-5856.9] {n = 7} |
| Day 14 | ||||
| Concentration, ng/mL | NA | NA | 0 (1653.6) [0-2.3] {n = 10} | 0 (961.9) [0-0.8] {n = 10} |
| Skin, ng/cm2 | NA | NA | 284.0 (353.3) [22.2-2977.6] {n = 9} | 151.3 (410.9) [24-1809.8] {n = 7} |
| Day 21 concentration, ng/mL | NA | NA | 0 (1399.2) [0-1] {n = 10} | 0 (332.7) [0-1.5] {n = 10} |
| Terminal half-life, h | NA | NA | 50.6 (74.4) [25.8-109.5] {n = 5} | 157.4 (229.8) [31.7-671] {n = 5} |
Abbreviations: AUC, area under the plasma concentration vs time curve; CV, coefficient of variation; NA, not applicable.
Maximum plasma concentration is the maximum ingredient concentration observed over the study duration. Maximum plasma concentration on day 1 (single application) was the maximum concentration over the interval of 0 to 23 hours and on day 4 was the maximum concentration over the interval of 73 to 95 hours. AUC on day 1 (single application) was the area under the curve over the interval 0 to 23 hours and on day 4 was the area under the curve from 73 to 95 hours.
Figure 2. Pharmacokinetic Profiles of Sunscreen Active Ingredients by Product on Multiple Applications.
Concentration profiles for each participant are shown for the study duration. A single application was applied at time 0 on day 1. The gray vertical lines indicate the 6-hour window (eg, at 0, 2, 4, and 6 hours) of sunscreen application on days 2, 3, and 4. The black horizontal line denotes 0.5 ng/mL. The lower limit of quantitation (LLOQ) was 0.2 ng/mL for avobenzone and 0.4 ng/mL for oxybenzone, octocrylene, homosalate, octisalate, and octinoxate (horizontal gray line). All samples below the LLOQ were set to 0.1 ng/mL for plotting individual profiles in this figure.
Single-Application Exposure (Day 1)
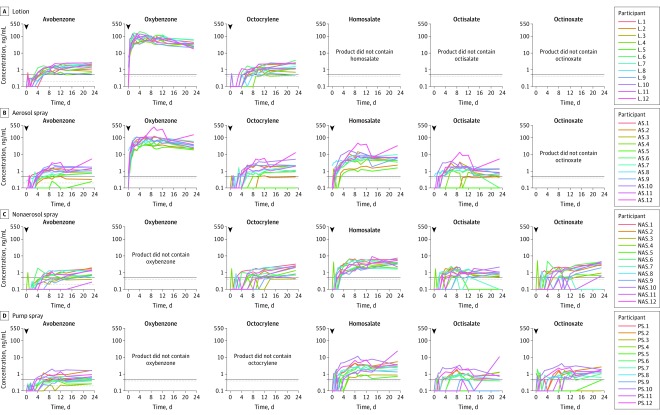
The systemic exposures, as measured by geometric mean maximum plasma concentrations, of all the tested active ingredients were higher than 0.5 ng/mL after a single application (individual participant pharmacokinetic profiles are shown in Figure 3 and geometric mean pharmacokinetic profiles in eFigure 2 in Supplement 2). For example, the maximum plasma concentrations of avobenzone were 1.6 ng/mL (CV, 49.0%), 1.2 ng/mL (CV, 90.6%), 1.0 ng/mL (CV, 65.2%), and 0.7 ng/mL (CV, 64.5%) for lotion, aerosol spray, nonaerosol spray, and pump spray, respectively, and the maximum plasma concentrations of oxybenzone were 94.2 ng/mL (CV, 44.3%) and 85.4 ng/mL (CV, 73.9%) for lotion and aerosol spray, respectively. Three participants had samples below the LLOQ for 1 or more active ingredients on day 1. All octisalate samples for 2 participants administered pump spray were below the LLOQ on day 1; 1 of these participants also had octinoxate samples below the LLOQ on day 1. A participant administered aerosol spray had octisalate and octocrylene samples below the LLOQ on day 1. These individuals were included in all summary pharmacokinetic parameter summaries.
Figure 3. Pharmacokinetic Profiles of Sunscreen Active Ingredients by Product on Single Application.
Concentration profiles for each participant are shown for day 1. The arrows indicate sunscreen application at 0 hours. The black horizontal line denotes 0.5 ng/mL. The lower limits of quantitation (LLOQ) was 0.2 ng/mL for avobenzone and 0.4 ng/mL for oxybenzone, octocrylene, homosalate, octisalate, and octinoxate (horizontal gray line). All samples below the LLOQ were set to 0.1 ng/mL for plotting individual profiles in this figure.
Additional Exploratory Assessments
The other outcomes, including maximum plasma concentrations on day 4 and AUC on single and multiple applications, are summarized in Table 2 and eTable 2 in Supplement 2. All the active ingredients in all tested products had long terminal half-lives (mean range, 27.3-157.4 hours) (Table 2).
Post Hoc Assessments
For all of the active ingredients, after single application, most participants had maximum plasma concentrations that reached or exceeded the 0.5-ng/mL threshold, with 75% or more of participants reaching that threshold within 23 hours for avobenzone (83%, n = 40/48), 2 hours for oxybenzone (100%, n = 24/24), 8 hours for octocrylene (75%, n = 27/36), 3 hours for homosalate (86%, n = 31/36), 6 hours for octisalate (75%, n = 27/36), and 8 hours for octinoxate (75%, n = 18/24) (eTable 3 in Supplement 2). Observations at 23 hours remained above 0.5 ng/mL in more than 75% of participants for all active ingredients (avobenzone: 85% [n = 41/48]; oxybenzone: 100% [n = 24/24]; octocrylene: 92% [n = 33/36]; homosalate: 100% [n = 36/36]; octisalate: 86% [n = 31/36]; and octinoxate: 92% [n = 22/24]). In addition, many participants had concentrations above the 0.5-ng/mL threshold until day 7 for avobenzone (95%; n = 42/44), octisalate (75%; n = 24/32), and octinoxate (90%; n = 18/20); day 10 for octocrylene (67%; n = 22/33); and day 21 for homosalate (55%; n = 17/31) and oxybenzone (96%; n = 22/23) (Figure 3 and Table 2).
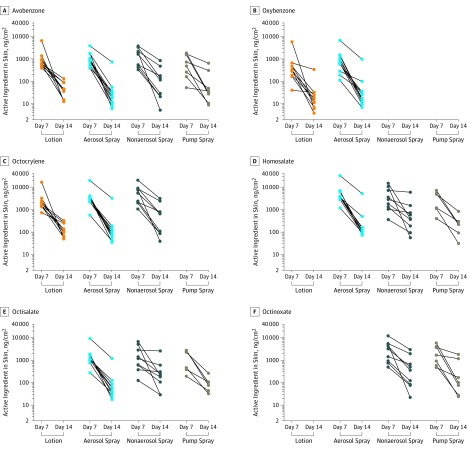
All active ingredients were detectable in skin following tape stripping, with greater amounts detectable at day 7 compared with day 14 (Table 2 and Figure 4). The skin amounts for avobenzone were 927.9 ng/cm2 (CV, 90.8%), 1000.1 ng/cm2 (CV, 89.0%), 1345.2 ng/cm2 (CV, 109.6%), and 498.7 ng/cm2 (CV, 200.3%) on day 7 and 39.8 ng/cm2 (CV, 99.0%), 25.9 ng/cm2 (CV, 209.1%), 75.8 ng/cm2 (CV, 378.1%), and 48.3 ng/cm2 (CV, 295.0%) on day 14 for lotion, aerosol spray, nonaerosol spray, and pump spray, respectively. The skin amounts of other active ingredients are summarized in Table 2 and Figure 4.
Figure 4. Residual Sunscreen Active Ingredient Amount in Skin.
Amount of each analyte in skin detected on day 7 and day 14 for each participant and product. Observations for the same participant are connected by a solid black line. Amounts are summarized by product (along x-axis) and normalized to surface area of tape strips (ng/cm2). There were 10, 10, and 10 paired avobenzone, oxybenzone, and octocrylene observations, respectively, for lotion; there were 11, 10, 10, 11, and 11 paired avobenzone, oxybenzone, octocrylene, homosalate, and octisalate observations, respectively, for aerosol spray; there were 9, 9, 9, 9, and 9 paired avobenzone, octocrylene, homosalate, octisalate, and octinoxate observations, respectively, for nonaerosol spray; and there were 8, 6, 6, and 7 paired avobenzone, homosalate, octisalate, and octinoxate observations, respectively, for pump spray.
Adverse Events
No serious drug-related adverse events were reported. Rash was reported in 14 participants (eTable 4 in Supplement 2).
Discussion
This randomized clinical trial demonstrated systemic exposure of 6 commonly used sunscreen active ingredients when participants were administered 1 application of 4 different sunscreen formulations on day 1 and 4 applications on days 2, 3, and 4. All 6 sunscreen active ingredients tested resulted in exposure above 0.5 ng/mL, and this threshold was reached after 1 application to 75% of body surface area on day 1. This reinforces the need for additional research to determine the effect of systemic exposure of sunscreen ingredients.
In the prior study of 24 healthy volunteers, systemic absorption of sunscreen active ingredients was demonstrated.4 This follow-up study expanded the sample size, tested additional sunscreen active ingredients and formulations, and confirmed the finding that sunscreen active ingredients are systemically absorbed. This included 6 of the 12 active ingredients in the sunscreen over-the-counter monograph for which the FDA has requested additional data to make a determination as to whether these ingredients are generally recognized as safe and effective (GRASE).2
The systemic exposures of avobenzone, oxybenzone, and octocrylene under controlled maximal usage conditions were previously reported.4 To our knowledge, the systemic exposure data of other active ingredients (homosalate, octisalate, and octinoxate) have not been reported in the literature; however, the concentrations in human breast milk were reported for octinoxate along with other sunscreen active ingredients including oxybenzone.10 Furthermore, studies in the literature have raised questions about the potential for oxybenzone and homosalate to affect endocrine activity.11 In addition, multiple active ingredients lack nonclinical safety assessment data, including systemic carcinogenicity, developmental, and reproductive studies to determine the clinical significance of systemic exposure of sunscreen active ingredients.
The FDA’s proposed rule2 considered that most of the currently used sunscreen active ingredients were not classified as GRASE due to lack of sufficient supporting safety data and encouraged interested parties to collect additional safety data of these ingredients to make a final GRASE determination. The rule also discussed the safety data that the FDA recommended to support a determination of whether certain active ingredients are GRASE, including the need for human absorption data. The main purpose of the maximal usage trial is to help the FDA quantify the anticipated level of risk for humans, using an exposure margin calculation. The FDA determines exposure margins taking the animal no-observed-effect levels from toxicology studies and the level of ingredient exposure from the human maximal usage trial. In some cases, results of the maximal usage trial will also help inform the extent of animal testing as outlined in the FDA’s proposed sunscreen rule.2 Results of the maximal usage trial alone are not used to determine the need for animal studies, nor the extent of animal testing.
While the prior study was designed to represent maximal usage (ie, application at least every 2 hours), the current study was designed to provide additional data on the systemic exposure of the active ingredients following single application; residual skin amounts during the wash-out phase; plasma concentrations up to 17 days after the last application; and the systemic exposure to additional commonly used sunscreen ingredients, including octinoxate, homosalate, and octisalate. The systemic exposure of all the tested ingredients in all products exceeded 0.5 ng/mL on single application and remained above the threshold until 23 hours after application. The systemic exposures of all tested ingredients remained above 0.5 ng/mL in more than 50% of participants up to 7 days for avobenzone, octisalate, and octinoxate; 10 days for octocrylene; and 21 days for homosalate and oxybenzone. The continued presence of sunscreen active ingredients in skin at days 7 and 14, the long terminal half-life typically exceeding 48 hours, and ingredients remaining detectable through day 21 suggest absorption through skin is the rate-limiting step. However, confirmation of this hypothesis would require administration of the sunscreen active ingredients intravenously to determine their elimination rate constants.
Limitations
This study has several limitations. First, a change in study design from an indoor setting to outdoor setting (with exposure to heat and sunlight) would better represent real-life sunscreen application. However, the data would have likely been more variable because of the need to control for heat, humidity, wind, and cloud cover. Therefore, the study was designed to collect data in a standardized manner to design subsequent studies. Second, this study was not designed to assess the absorption difference by formulation type and Fitzpatrick skin types. Third, given the different amounts of sunscreen ingredients between the products, the data from tape stripping should be viewed in a qualitative manner rather than quantitative across products.
Conclusions
In this study conducted in a clinical pharmacology unit and examining sunscreen application among healthy participants, all 6 of the tested active ingredients administered in 4 different sunscreen formulations were systemically absorbed and had plasma concentrations that surpassed the FDA threshold for potentially waiving some of the additional safety studies for sunscreens. These findings do not indicate that individuals should refrain from the use of sunscreen.
Trial Protocol
eTable 1. Active and Inactive ingredients
eTable 2. Complete Pharmacokinetic Parameters of Sunscreen Active Ingredients
eTable 3. Percentage of Study Participants With Samples Exceeding 0.5 ng/mL at Select Time Points on Day 1 by Product and Ingredient
eTable 4. Number and Percentage of Adverse Events by Treatment Group
eFigure 1. Pharmacokinetic Profiles of Sunscreen Active Ingredients Upon Multiple Applications
eFigure 2. Pharmacokinetic Profiles of Sunscreen Active Ingredients Upon Single Application
eMethods 1. Bioanalytical Method Conditions for Avobenzone and oxybenzone in Plasma
eMethods 2. Bioanalytical Method Conditions for Octocrylene in Plasma
eMethods 3. Bioanalytical Method Conditions for Homosalate, Octisalate, and Octinoxate in Plasma
eMethods 4. Bioanalytical Method Conditions for Avobenzone, Oxybenzone, and Octocrylene in Skin Samples
eMethods 5. Bioanalytical Method Conditions for Homosalate, Octisalate and Octinoxate in Skin Samples
eDictionary. Data Dictionary for Participant Data Listings – Plasma and Skin data
Deidentified Participant Data
Data Sharing Statement
References
- 1.Nash JF. Human safety and efficacy of ultraviolet filters and sunscreen products. Dermatol Clin. 2006;24(1):35-51. doi: 10.1016/j.det.2005.09.006 [DOI] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration Sunscreen Drug Products for Over-The-Counter Human Use; Proposed Rule. Federal Register. https://www.govinfo.gov/content/pkg/FR-2019-02-26/pdf/2019-03019.pdf. Published February 26, 2019. Accessed April 1, 2019.
- 3.US Food and Drug Administration Maximal usage trials for topical active ingredients being considered for inclusion in an over-the-counter monograph: study elements and considerations. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM608356.pdf. Published May 2019. Accessed November 29, 2019.
- 4.Matta MK, Zusterzeel R, Pilli NR, et al. . Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321(21):2082-2091. doi: 10.1001/jama.2019.5586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Food and Drug Administration Collection of race and ethnicity data in clinical trials. https://www.fda.gov/downloads/regulatoryinformation/guidances/ucm126396.pdf. Published October 2016. Accessed February 5, 2019.
- 6.Raj N, Voegeli R, Rawlings AV, et al. . Variation in stratum corneum protein content as a function of anatomical site and ethnic group. Int J Cosmet Sci. 2016;38(3):224-231. doi: 10.1111/ics.12274 [DOI] [PubMed] [Google Scholar]
- 7.US Food and Drug Administration Food additives: threshold of regulation of substances used in food-contract articles: final rule. Federal Register. https://www.govinfo.gov/content/pkg/FR-1995-07-17/pdf/95-17435.pdf. Published July 17, 1995. Accessed April 1, 2019. [Google Scholar]
- 8.US Food and Drug Administration Statistical approaches to establishing bioequivalence. https://www.fda.gov/downloads/drugs/guidances/ucm070244.pdf. Published February 2001. Accessed April 9, 2019.
- 9.US Food and Drug Administration Bioavailability studies submitted in NDAs or INDs—general considerations. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM631943.pdf. Published February 2019. Accessed April 9, 2019.
- 10.Schlumpf M, Kypke K, Wittassek M, et al. . Exposure patterns of UV filters, fragrances, parabens, phthalates, organochlor pesticides, PBDEs, and PCBs in human milk: correlation of UV filters with use of cosmetics. Chemosphere. 2010;81(10):1171-1183. doi: 10.1016/j.chemosphere.2010.09.079 [DOI] [PubMed] [Google Scholar]
- 11.Krause M, Klit A, Blomberg Jensen M, et al. . Sunscreens: are they beneficial for health? an overview of endocrine disrupting properties of UV-filters. Int J Androl. 2012;35(3):424-436. doi: 10.1111/j.1365-2605.2012.01280.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Active and Inactive ingredients
eTable 2. Complete Pharmacokinetic Parameters of Sunscreen Active Ingredients
eTable 3. Percentage of Study Participants With Samples Exceeding 0.5 ng/mL at Select Time Points on Day 1 by Product and Ingredient
eTable 4. Number and Percentage of Adverse Events by Treatment Group
eFigure 1. Pharmacokinetic Profiles of Sunscreen Active Ingredients Upon Multiple Applications
eFigure 2. Pharmacokinetic Profiles of Sunscreen Active Ingredients Upon Single Application
eMethods 1. Bioanalytical Method Conditions for Avobenzone and oxybenzone in Plasma
eMethods 2. Bioanalytical Method Conditions for Octocrylene in Plasma
eMethods 3. Bioanalytical Method Conditions for Homosalate, Octisalate, and Octinoxate in Plasma
eMethods 4. Bioanalytical Method Conditions for Avobenzone, Oxybenzone, and Octocrylene in Skin Samples
eMethods 5. Bioanalytical Method Conditions for Homosalate, Octisalate and Octinoxate in Skin Samples
eDictionary. Data Dictionary for Participant Data Listings – Plasma and Skin data
Deidentified Participant Data
Data Sharing Statement