This cohort study of adolescents and young adults without cancer identifies opioid prescribing patterns associated with opioid overdose in adolescents and young adults.
Key Points
Question
In adolescents and young adults without cancer, which opioid prescribing patterns are associated with prescription opioid overdose?
Findings
In this claims-based cohort study of 2 752 612 adolescents and young adults without cancer, daily opioid dosage, concurrent benzodiazepine use, and extended-release or long-acting opioid use were associated with increased overdose risk.
Meaning
The findings suggest that when prescribing opioids to adolescents and young adults, practitioners could potentially mitigate overdose risk by using the lowest effective dosage, avoiding concurrent opioid and benzodiazepine use, and relying on short-acting opioids.
Abstract
Importance
Safe opioid prescribing practices are critical to mitigate the risk of prescription opioid overdose in adolescents and young adults. However, studies that examine opioid prescribing patterns associated with prescription opioid overdose have mostly focused on older adults. The generalizability of these studies to adolescents and young adults is unclear.
Objective
To identify opioid prescribing patterns associated with prescription opioid overdose in adolescents and young adults.
Design, Setting, and Participants
This retrospective cohort study assessed privately insured patients aged 12 to 21 years with opioid prescription claims in the IBM MarketScan Commercial Claims and Encounters database between July 1, 2009, and October 1, 2017, and no cancer diagnosis. Data analysis was performed from January 1 to April 30, 2019.
Main Outcomes and Measures
The outcome was a treated opioid overdose as indicated by diagnosis codes. On the basis of days supplied, opioid prescription claims were converted to person-days (the unit of analysis) on which opioid exposure would occur if patients took medications as prescribed. Logistic regression with clustered SEs at the patient level was used to model the occurrence of overdose on a person-day as a function of daily opioid dosage category (<30, 30-59, 60-89, 90-119, or ≥120 morphine milligram equivalents), concurrent benzodiazepine use, and extended-release or long-acting opioid use. Regressions controlled for demographic characteristics, year, opioid use within 180 days, and comorbidities (mental health disorder, substance use disorder, and other chronic condition).
Results
A total of 2 752 612 patients (mean [SD] age at cohort entry, 17.2 [2.5] years; 1 451 918 [52.8%] female) participated in the study. Patients had 4 686 355 opioid prescription claims, corresponding to 21 605 444 person-days. Overdose occurred on 255 person-days among 249 patients (0.01% of the sample). Each increase in daily opioid dosage category was associated with higher overdose risk (adjusted odds ratio [AOR], 1.18; 95% CI, 1.05-1.31). Compared with no use, both concurrent benzodiazepine use (AOR, 1.83; 95% CI, 1.24-2.71) and extended-release or long-acting opioid use (AOR, 2.01; 95% CI, 1.16-3.46) were associated with increased overdose risk.
Conclusions and Relevance
The findings suggest that when prescribing opioids to adolescents and young adults, practitioners could potentially mitigate overdose risk by using the lowest effective daily dosage, avoiding concurrent opioid and benzodiazepine prescribing, and relying on short-acting opioids. Findings are broadly consistent with prior opioid safety studies focused on older adults.
Introduction
Opioids are frequently prescribed to adolescents and young adults.1 In a national study2 of claims from privately insured US adolescents and young adults, 12.6% of patients were prescribed opioids during 2015. Opioid prescribing to adolescents and young adults is associated with an increased risk of misuse, diversion to friends and family members, and long-term opioid use.2,3,4,5,6,7,8 In addition, this prescribing is associated with an increased risk of overdose.9 In 2016, almost 30% of the 2985 opioid-related overdose deaths among US adolescents and young adults involved prescription opioids.10,11 The burden of these deaths and the frequency of opioid prescribing highlight the importance of mitigating overdose risk when prescribing opioids to adolescents and young adults.
To achieve this goal, practitioners could follow the 2016 US Centers for Disease Control and Prevention (CDC) opioid prescribing guideline for adults with chronic pain.12 However, this guideline is largely based on studies12,13,14,15,16,17,18,19,20 of older adults, particularly US veterans. For example, the CDC’s recommendations to use the lowest daily opioid dosage and to avoid concurrent opioid and benzodiazepine use was informed by studies of veterans in which less than 5% of patients were younger than 30 years.13,18 Similarly, the CDC’s recommendation to avoid initiating therapy with extended-release or long-acting opioids was informed by a study of veterans in which the median age of patients was 60 years.19 Because of the underrepresentation of pediatric patients in prior opioid safety studies, the CDC guideline specifically excludes children younger than 18 years.19 Identifying high-risk prescribing patterns specifically in pediatric patients is crucial for ongoing efforts to develop evidence-based state and national pediatric opioid prescribing guidelines.21
In this study, we used a national US private insurance claims database to identify opioid prescribing patterns associated with prescription opioid overdose in a cohort of 2.7 million adolescents and young adults aged 12 to 21 years without cancer. On the basis of prior literature,12,13,14,15,16,17,18,19,20 we focused on the risks associated with daily opioid dosage, concurrent benzodiazepine use, and extended-release or long-acting opioid use.
Methods
Data Source
From January 1 to April 30, 2019, we conducted a retrospective cohort study using the 2009-2017 IBM MarketScan Commercial Claims and Encounters database, a national sample of claims from nonelderly patients across the United States with private employer–sponsored coverage. Data contributors are mostly self-insured large companies.22 Annual sample sizes were 45 million to 50 million from 2009 to 2014 but decreased to 30 million after 2014 because of a loss of data contributors. Despite this loss, the database still represents a substantial portion of the US population. For example, 4 581 278 patients aged 12 to 21 years were contained in the 2016 MarketScan Commercial Claims and Encounters database. On the basis of the 2016 American Community Survey, these patients represented 10.7% of all US patients and 16.2% of all privately insured US patients in this age group.23 We identified prescription claims for opioids and benzodiazepines using a list of national drug codes derived from a search of the IBM MarketScan Red Book (eAppendix 1 in the Supplement). Because data were deidentified, this study was not regulated as human research by the institutional review board of the University of Michigan Medical School, and informed consent was not required.
Study Population
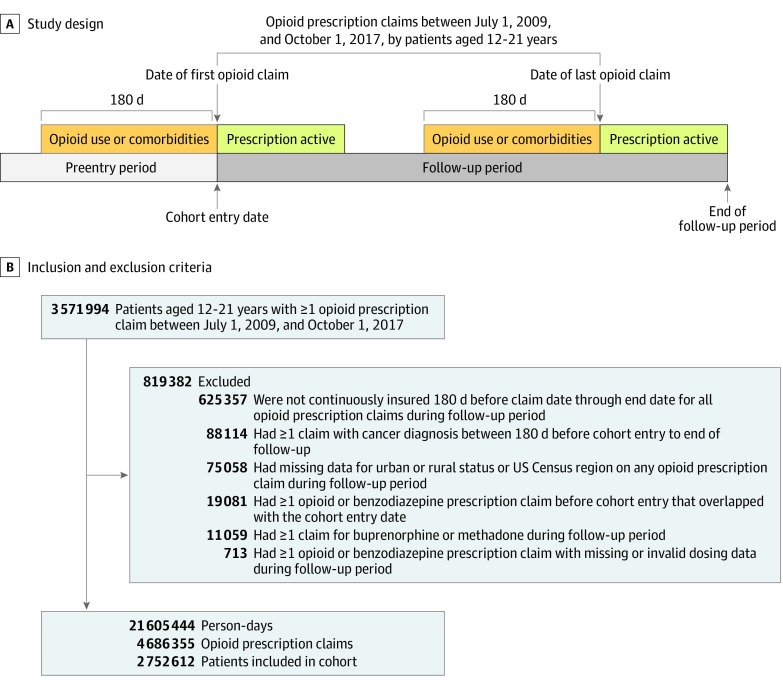
We defined adolescents and young adults as 12 to 21 years of age to capture the population seen by many pediatric practitioners but present results for those aged 12 to 25 and 15 to 24 years in eAppendix 2 in the Supplement. We identified all opioid prescription claims between July 1, 2009, and October 1, 2017, by patients aged 12 to 21 years. We initially included patients with 1 or more such claim. The cohort entry date was the date of the first opioid prescription claim between July 1, 2009, and October 1, 2017. The follow-up period started on the cohort entry date and terminated at the end of the active period (claim date plus days supplied minus one) of the last opioid prescription claim (Figure 1A).
Figure 1. Study Design and Sample Inclusion and Exclusion Criteria.
Study design for a patient with 2 opioid prescription claims. For a patient with only 1 claim, follow-up terminated at the end of the active period of this claim. Recent opioid use was defined based on the presence of an opioid prescription claim during the 180 days to 1 day before the date of the opioid prescription claim from which person-days derived. Comorbidities were defined based on the presence of diagnosis codes on claims that occurred on the date of each opioid prescription claim or during the 180 days before this date.
We excluded patients if they had 1 or more claim with a cancer diagnosis code during the 180 days before cohort entry to the end of follow-up (eAppendix 3 in the Supplement); had 1 or more opioid or benzodiazepine prescription claim before cohort entry that overlapped with the cohort entry date; had 1 or more opioid or benzodiazepine claim during the follow-up period with missing dose data or days supplied greater than 90 days (to account for possible data error); lacked continuous enrollment during the 180 days before the claim date to the end of the active period of any opioid prescription claim during the follow-up period (to ensure sufficient time to observe pre-prescription information); had missing data for US census region or urban or rural residence on any of these claims; or had 1 or more claim for buprenorphine or methadone during the follow-up period (because of inconsistent morphine equivalency24) (Figure 1B).
We only examined associations between prescribing patterns and overdose on days that patients had active opioid prescriptions (person-days). To identify these person-days, we assumed, based on prior literature,13 that patients took medications as prescribed. For example, we converted an opioid prescription claim on January 1 with a 3-day supply to 3 person-days (January 1 to January 3). Person-days were the units of analysis because exposures such as daily opioid dosage vary when there are overlapping opioid prescriptions. Analyses excluded days without active opioid prescriptions because exposures are unclear in the absence of prescriptions.
Outcome
For each person-day, we created an indicator for a treated opioid overdose, defined as a claim that contained an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) or International Statistical Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis code for nonheroin opioid poisoning (eAppendix 4 in the Supplement). Validation studies25,26 in children and adults indicate that ICD-9-CM opioid poisoning codes have high positive predictive value for detecting opioid overdose, albeit low sensitivity. We included the ICD-10-CM opioid poisoning codes used in US federal estimates of opioid overdose deaths.11 Although these codes have not been validated by medical record review to date, conclusions were unchanged when ending the study before the US transitioned to ICD-10-CM (eAppendix 5 in the Supplement).
When opioid poisoning claims occurred on consecutive person-days (eg, multiday overdose hospitalization), we assumed that overdose occurred only on the first person-day to avoid double counting overdoses. We considered opioid poisoning claims separated by 2 days or more to represent distinct overdoses based on prior literature.27 Some patients may have experienced fatal overdose before opioid prescriptions ended, resulting in person-days of active opioid prescriptions occurring after death. However, any immortal time bias from including these person-days would be minimal because overdose was rare.28
Exposures
For each person-day, we determined daily opioid dosage in morphine milligram equivalents (MMEs) using the CDC’s conversion factors.29 When multiple opioid prescriptions were active on a person-day, we summed daily dosage across prescriptions. We assigned person-days to 1 of 5 daily dosage categories: less than 30, 30 to 59, 60 to 89, 90 to 119, and 120 or more MMEs. A 30-MME increment represents the difference between prescribing 1 vs 2 pills that contain 5 mg of hydrocodone every 4 hours. We truncated at 120 MMEs because sample sizes above this cutoff were small.
We defined concurrent benzodiazepine use as 1 or more active benzodiazepine prescription on the person-day and extended-release or long-acting opioid use as 1 or more active prescription for these medications on the person-day. We classified opioids as extended release or long acting based on master form (eg, extended-release patch) or opioid type when no short-acting form existed (levorphanol). We classified other opioids as short acting.
Covariates
To account for the time-varying nature of covariates, we defined covariates on the date of opioid prescription claims and then assigned covariate values to person-days derived from the claim (Figure 1A). When multiple prescriptions were active on a person-day, we assigned the covariate values from the earliest prescription. Covariates included age, sex, US census region, urban or rural residence, year indicators, an indicator of recent opioid use (defined as ≥1 opioid prescription claim between 180 days to 1 day before the claim date), and indicators for prior diagnoses of a mental health disorder, substance use disorder, and other chronic condition (defined as ≥1 claim with diagnosis codes for these comorbidities on or during the 180 days before the claim date) (eAppendix 6 in the Supplement). Codes for other chronic conditions were based on the Pediatric Medical Complexity Algorithm, a validated administrative tool to identify children with complex and noncomplex chronic conditions (eAppendix 7 in the Supplement).30
Statistical Analysis
We used descriptive statistics to assess patient characteristics at cohort entry, the prevalence of exposures and covariates among person-days on which overdose did and did not occur, and the proportion of patients with 1 or more overdose. For each exposure, we calculated the unadjusted rate of overdose and 95% CIs.
We used logistic regression to model the occurrence of overdose as a function of daily opioid dosage category (values 1-5, corresponding to the 5 dosage categories), concurrent benzodiazepine use, and extended-release or long-acting opioid use, controlling for covariates. We did not include higher-order terms for age or daily opioid dosage category because they were not significant. Because person-days were not independent, we clustered standard errors at the patient level. We calculated average marginal effects for each exposure and covariate or the absolute difference in the probability of overdose associated with a 1-unit increase when holding other variables at their observed values.31 Because overdose risk may vary based on whether patients are opioid naive, we conducted a planned subgroup analysis of person-days from patients with and without recent opioid use (≥1 opioid prescription claim from 180 days to 1 day before the date of the claim from which person-days were derived). Our model was conceptually similar to a discrete time survival model with a constant hazard. In a sensitivity analysis, we allowed for the risk of overdose to vary over time by additionally controlling for the number of days since each opioid prescription was filled; results were not substantively different (eAppendix 8 in the Supplement).
We examined the width of 95% CIs to assess power. We defined statistical significance as α = .05 and used 2-sided tests. We conducted analyses using SAS, version 9.4 (SAS Institute Inc) and Stata, version 15.1 SE (StataCorp LLC).
Sensitivity Analyses
When patients with concurrent benzodiazepine use present with overdose symptoms, they could be assigned a diagnosis code for opioid poisoning, benzodiazepine poisoning, or both. Consequently, reliance on opioid poisoning codes alone may result in underestimation of overdose risk associated with concurrent benzodiazepine use. To assess this possibility, we repeated analyses when defining the outcome using diagnosis codes for opioid or benzodiazepine poisoning. We also conducted sensitivity analyses regarding exclusion criteria, categorization of daily opioid dosage, statistical modeling strategy, and outcome definition (eAppendix 8 in the Supplement).
Results
Study Population
Among 3 571 994 patients aged 12 to 21 years with 1 or more opioid prescription claim between July 1, 2009, and October 1, 2017, a total of 2 752 612 (77.1%) were included in the sample (Figure 1B). At cohort entry, the mean (SD) age was 17.2 (2.5) years, 1 451 918 patients (52.8%) were female, 2 330 534 (84.7%) lived in an urban area, and 1 136 799 (41.3%) lived in the South compared with 666 772 (24.2%) in the Midwest, 429 866 (15.6%) in the Northeast, and 519 175 (18.9%) in the West. Patients had 4 686 355 opioid prescription claims during the follow-up period, corresponding to 21 605 444 person-days (mean [SD] of 7.8 [19.2] person-days per patient).
Exposures and Covariates by Overdose Status
Overdose occurred on 255 person-days among 249 patients (0.01% of the 2 752 612 patients in the sample). Among person-days on which overdose occurred, the proportion with 120 MMEs or more per day, concurrent benzodiazepine use, and extended-release or long-acting opioid use was higher than among person-days on which overdose did not occur. Among person-days on which overdose occurred, 49.8% were from patients with mental health disorders and 25.5% were from patients with substance use disorders (Table 1).
Table 1. Prevalence of Exposures and Covariates Among Person-Days on Which Overdose Did and Did Not Occur.
| Exposure or Covariate | Overdose, No. (%) | |
|---|---|---|
| Yes (n = 255 Person-Days) | No (n = 21 605 189 Person-Days) | |
| Daily opioid dosage category, MMEs | ||
| <30 | 85 (33.0) | 8 913 266 (41.3) |
| 30-59 | 105 (41.2) | 9 050 867 (41.9) |
| 60-89 | 20 (7.8) | 1 932 856 (9.0) |
| 90-119 | 20 (7.8) | 751 821 (3.5) |
| ≥120 | 25 (9.8) | 956 379 (4.4) |
| Concurrent benzodiazepine use | 32 (12.6) | 673 621 (3.1) |
| Extended-release or long-acting opioid use | 19 (7.5) | 321 770 (1.5) |
| Recent opioid use within 180 d | 136 (53.3) | 7 205 529 (33.4) |
| Age, y | ||
| 12-18 | 119 (46.7) | 12 286 346 (56.9) |
| 19-21 | 136 (54.3) | 9 318 843 (43.1) |
| Female | 139 (54.5) | 11 684 644 (54.1) |
| Urban residence | 225 (88.2) | 18 048 910 (83.5) |
| US census region | ||
| Northeast | 38 (14.9) | 2 948 638 (13.7) |
| Midwest | 75 (29.4) | 5 252 248 (24.3) |
| South | 88 (34.5) | 9 188 697 (42.5) |
| West | 54 (21.2) | 4 215 606 (19.5) |
| Mental health disorder | 127 (49.8) | 3 783 923 (17.5) |
| Substance use disorder | 65 (25.5) | 1 103 681 (5.1) |
| Other chronic condition | 115 (45.1) | 6 374 257 (29.5) |
| Study year | ||
| 2009 | 21 (8.2) | 1 904 777 (8.8) |
| 2010 | 33 (12.9) | 3 261 970 (15.1) |
| 2011 | 46 (18.0) | 3 352 582 (15.5) |
| 2012 | 43 (16.9) | 3 511 450 (16.3) |
| 2013 | 24 (9.4) | 2 628 189 (12.2) |
| 2014 | 34 (13.3) | 2 416 542 (11.2) |
| 2015 | 25 (9.8) | 1 918 711 (8.9) |
| 2016 | 20 (7.8) | 1 722 879 (8.0) |
| 2017 | 9 (3.5) | 888 089 (4.1) |
Abbreviation: MMEs, morphine milligram equivalents.
Unadjusted Overdose Rates by Exposure
The overall unadjusted overdose rate was 1.2 (95% CI, 1.0-1.3) per 100 000 person-days. eAppendix 9 in the Supplement lists unadjusted overdose rates for each daily opioid dosage category. The overdose rate was 1.0 (95% CI, 0.8-1.2) per 100 000 person-days among person-days in the first category (<30 MMEs) and 2.6 (95% CI, 1.7-3.9) per 100 000 person-days among person-days in the last category (≥120 MMEs), 4.8 (95% CI, 3.3-6.7) per 100 000 person-days among person-days with concurrent benzodiazepine use and 1.1 (95% CI, 0.9-1.2) per 100 000 person-days among person-days without concurrent benzodiazepine use, and 5.9 (95% CI, 3.6-9.2) per 100 000 person-days among person-days with extended-release or long-acting opioid use and 1.1 (95% CI, 1.0-1.3) per 100 000 person-days among person-days without extended-release or long-acting opioid use.
Adjusted Results in Overall Sample
Table 2 gives the adjusted odds ratios (AORs) and average marginal effects, expressed as changes per 100 000 person-days. Each increase in daily opioid dosage category was associated with higher overdose risk (AOR, 1.18; 95% CI, 1.05-1.31). Compared with no use, concurrent benzodiazepine use was associated with higher overdose risk (AOR, 1.83; 95% CI, 1.24-2.71), as was extended-release or long-acting opioid use (AOR, 2.01; 95% CI, 1.16-3.46). Covariates associated with higher overdose risk included recent opioid use (AOR, 1.38; 95% CI, 1.02-1.86), mental health disorder diagnosis (AOR, 3.14; 95% CI, 2.40-4.12), and substance use disorder diagnosis (AOR, 3.36; 95% CI, 2.41-4.69) (Figure 2).
Table 2. Adjusted Associations Between Opioid Prescribing Patterns and Opioid Overdose Risk.
| Variable | AOR (95% CI) | Average Marginal Effect (95% CI)a |
|---|---|---|
| Daily opioid dosage categoryb | 1.18 (1.05 to 1.31) | 0.19 (0.06 to 0.32) |
| Concurrent benzodiazepine use | 1.83 (1.24 to 2.71) | 0.71 (0.24 to 1.19) |
| Extended-release or long-acting opioid use | 2.01 (1.16 to 3.46) | 0.82 (0.17 to 1.48) |
| Recent opioid use within 180 d | 1.38 (1.02 to 1.86) | 0.38 (0.02 to 0.74) |
| Age, in single y | 1.02 (0.97 to 1.08) | 0.02 (–0.04 to 0.09) |
| Female | 1.00 (0.77 to 1.29) | 0.00 (–0.30 to 0.30) |
| Urban residence | 1.43 (0.97 to 2.13) | 0.38 (0.01 to 0.74) |
| US census region (vs Northeast) | ||
| Midwest | 1.11 (0.74 to 1.66) | 0.13 (–0.39 to 0.65) |
| South | 0.83 (0.56 to 1.23) | –0.21 (–0.68 to 0.26) |
| West | 0.98 (0.63 to 1.52) | –0.03 (–0.56 to 0.51) |
| Mental health disorder | 3.14 (2.40 to 4.12) | 1.67 (1.17 to 2.17) |
| Substance use disorder | 3.36 (2.41 to 4.69) | 2.29 (1.34 to 3.24) |
| Other chronic condition | 1.21 (0.91 to 1.59) | 0.22 (–0.11 to 0.56) |
| Study year (vs 2009) | ||
| 2010 | 0.88 (0.50 to 1.54) | –0.15 (–0.79 to 0.50) |
| 2011 | 1.18 (0.69 to 2.01) | 0.21 (–0.47 to 0.89) |
| 2012 | 1.01 (0.59 to 1.73) | 0.01 (–0.64 to 0.66) |
| 2013 | 0.72 (0.40 to 1.32) | –0.33 (–0.97 to 0.31) |
| 2014 | 1.12 (0.63 to 1.99) | 0.15 (–0.57 to 0.87) |
| 2015 | 1.03 (0.56 to 1.89) | 0.04 (–0.70 to 0.77) |
| 2016 | 1.00 (0.53 to 1.86) | 0.00 (–0.75 to 0.74) |
| 2017 | 0.90 (0.41 to 1.97) | –0.13 (–1.00 to 0.75) |
Abbreviation: AOR, adjusted odds ratio.
Average marginal effect is the absolute difference in probability of overdose associated with a 1-unit increase in the variable, holding other variables at their observed values (expressed as changes in the overdose date per 100 000 person-days). For categorical variables, such as US census region, average marginal effect represents the difference in probability of overdose relative to the reference category.
Daily opioid dosage category was represented by a variable with values of 1 to 5, with 1 corresponding to less than 30, 2 corresponding to 30 to 59, 3 corresponding to 60 to 89, 4 corresponding to 90 to 119, and 5 corresponding to 120 or more morphine milligram equivalents per day. The AOR refers to a 1-unit increase in daily opioid dosage category.
Figure 2. Adjusted Odds Ratios (AORs) of Opioid Overdose for Exposures and Selected Covariates.
Daily opioid dosage category was represented by a variable with values of 1 to 5, with 1 corresponding to less than 30, 2 corresponding to 30 to 59, 3 corresponding to 60 to 89, 4 corresponding to 90 to 119, and 5 corresponding to 120 or more morphine milligram equivalents per day. The AOR refers to a 1-unit increase in daily opioid dosage category. The AOR for age in years refers to a 1-year increase in patient age. Boxes represent the point estimate. Horizontal lines represent 95% CIs.
Subgroup Analysis
Among 14 399 779 person-days from patients without recent opioid use within 180 days, conclusions were similar to the main analysis. Among 7 205 665 person-days from patients with recent opioid use, neither daily opioid dosage category (AOR, 1.12; 95% CI, 0.98-1.27) nor extended-release or long-acting opioid use (AOR, 1.55; 95% CI, 0.80-3.01) was associated with overdose, but concurrent benzodiazepine use was still associated with overdose (AOR, 1.59; 95% CI, 1.00-2.52) (Table 3).
Table 3. Subgroup Analysis by Recent Opioid Use Statusa.
| Variable | AOR (95% CI) | |
|---|---|---|
| Recent Opioid Use (n = 7 205 665 Person-Days) | No Recent Opioid Use (n = 14 399 779 Person-Days) | |
| Daily opioid dosage category | 1.12 (0.98-1.27) | 1.26 (1.06-1.50) |
| Concurrent benzodiazepine use | 1.59 (1.00-2.52) | 2.86 (1.52-5.40) |
| Extended-release or long-acting opioid use | 1.55 (0.80-3.01) | 4.31 (1.92-9.67) |
| Age, in single y | 1.01 (0.94-1.09) | 1.02 (0.95-1.10) |
| Female | 0.94 (0.66-1.34) | 1.08 (0.76-1.54) |
| Urban residence | 1.28 (0.76-2.16) | 1.68 (0.92-3.06) |
| US census region (vs Northeast) | ||
| Midwest | 0.66 (0.39-1.11) | 2.20 (1.12-4.32) |
| South | 0.47 (0.28-0.78) | 1.78 (0.93-3.42) |
| West | 0.67 (0.38-1.16) | 1.77 (0.86-3.64) |
| Mental health disorder | 3.34 (2.31-4.82) | 2.85 (1.93-4.21) |
| Substance use disorder | 2.18 (1.40-3.37) | 6.79 (4.40-10.47) |
| Other chronic condition | 1.06 (0.74-1.52) | 1.40 (0.94-2.08) |
| Study year (vs 2009) | ||
| 2010 | 0.90 (0.39-2.08) | 0.88 (0.41-1.88) |
| 2011 | 1.70 (0.79-3.69) | 0.74 (0.34-1.62) |
| 2012 | 0.96 (0.43-2.18) | 1.08 (0.53-2.20) |
| 2013 | 1.08 (0.46-2.50) | 0.44 (0.18-1.10) |
| 2014 | 1.57 (0.69-3.57) | 0.78 (0.35-1.72) |
| 2015 | 1.44 (0.60-3.45) | 0.74 (0.32-1.71) |
| 2016 | 1.22 (0.48-3.13) | 0.82 (0.36-1.90) |
| 2017 | 0.29 (0.04-2.30) | 1.15 (0.46-2.87) |
Abbreviation: AOR, adjusted odds ratio.
Recent opioid use was defined based on the presence of an opioid prescription claim in the 180 days to 1 day before the date of the opioid prescription claim from which person-days derived.
Sensitivity Analyses
When including benzodiazepine poisoning codes, the point estimate for the association between overdose and concurrent benzodiazepine use increased (AOR, 3.42; 95% CI, 2.52-4.63) compared with the main analysis (AOR, 1.83; 95% CI, 1.24-2.70) (eAppendix 10 in the Supplement). Conclusions were unchanged in other sensitivity analyses regarding exclusion criteria, categorization of daily opioid dosage, statistical modeling strategy, and outcome definition (eAppendix 8 in the Supplement).
Discussion
In this study of 2.7 million privately insured US adolescents and young adults without cancer, daily opioid dosage, concurrent benzodiazepine use, and extended-release or long-acting opioid use were associated with higher prescription opioid overdose risk. These findings suggest that practitioners who treat adolescents and young adults could potentially mitigate opioid overdose risk by using the lowest effective daily dosage, avoiding concurrent opioid and benzodiazepine prescribing, and relying on short-acting opioids.
Overall, approximately 1 in 10 000 adolescents and young adults overdosed while they had active opioid prescriptions. In a prior study32 of privately insured adolescents aged 11 to 17 years with a median of 1.75 years of follow-up after their first opioid prescription, 6 of 10 000 had an overdose event during the follow-up period; these events included overdoses attributable to the initial opioid exposure and overdoses attributable to other sources. A more comparable estimate comes from a national study33 of nonelderly adults prescribed opioids after surgical procedures. In this study,33 the overdose rate within 30 days of surgery was also 1 in 10 000, suggesting that adolescents and young adults prescribed opioids have a similar immediate overdose risk compared with older populations.
In adjusted analyses, each increase in daily opioid dosage category was associated with an 18% higher odds of overdose. In subgroup analyses, the association between daily opioid dosage and overdose risk persisted in the absence of recent opioid use within 180 days but not in its presence. This finding, combined with the demonstrated dose-response association between daily dosage and overdose risk, suggests that the safest approach may be to initiate opioid therapy using the lowest potentially effective daily dosage and to titrate upward carefully in adolescents and young adults. Our findings contrast with the aforementioned study32 of privately insured adolescents aged 11 to 17 years, which did not find an association between the daily opioid dosage of initial opioid prescriptions and subsequent overdose at any point after these prescriptions. This discrepancy may be because daily dosage is a more useful indicator of overdose risk when examining the immediate response to opioid exposure.
Concurrent benzodiazepine use occurred on 3.1% of days on which opioid prescriptions were active despite the well-known ability of benzodiazepines to potentiate opioid-related respiratory depression.12 In adjusted analyses, concurrent benzodiazepine use was associated with 1.8-fold higher odds of overdose compared with no use. Findings highlight the importance of avoiding concurrent opioid and benzodiazepine prescribing when possible as well as the importance of identifying opportunities to reduce unnecessary use of benzodiazepines in adolescents and young adults. For example, benzodiazepines have questionable efficacy for pediatric anxiety.34
Extended-release or long-acting opioid use occurred on 1.5% of days on which opioid prescriptions were active. In adjusted analyses, extended-release or long-acting opioid use was associated with 2.0-fold higher odds of overdose compared with no use. This association was adjusted for daily opioid dosage, which is typically high when extended-release or long-acting opioids are used.19 Findings suggest that these agents’ duration of action may account for their association with overdose. In subgroup analyses, the association between extended-release or long-acting opioid use and overdose persisted in the absence of recent opioid use within 180 days but not in its presence. Results suggest that short-acting opioids may be preferred for opioid-naive adolescents and young adults.
Half of overdoses occurred among patients with recent diagnoses of mental health disorders and one-quarter among patients with substance use disorders. These disorders were associated with a 3.1- to 3.3-fold higher odds of overdose, suggesting that they are equally important risk factors for overdose as opioid prescribing patterns, if not more so. Results suggest that a careful assessment for mental health and substance use disorders should be conducted before prescribing opioids to adolescents and young adults. When these common risk factors are present and opioids are required, practitioners should consider instituting heightened measures to mitigate overdose risk, such as close follow-up and naloxone coprescribing.12
Strengths and Limitations
This study has several strengths, including the use of a large national data set and a sample size of more than 21 million person-days of opioid exposure. Furthermore, many prior opioid safety studies13,16,35 have focused on a single exposure, such as daily opioid dosage. In contrast, this study’s estimates reflect the independent association between overdose and each exposure after accounting for the other 2 exposures.
This study also has several limitations. First, we did not examine harms of prescription opioid exposure other than overdose.3 Other more common harms of this exposure include increased risk of opioid misuse, long-term opioid use, and heroin use.5,6,36,37,38 Second, even though regressions controlled for key demographic characteristics and comorbidities, confounding by unmeasured factors is possible. For example, our database lacked data on race/ethnicity and any information on prescribers. Randomized clinical trials are infeasible because of the rareness of overdose. However, findings are consistent with prior studies13,14,15,16,17,18,19,20 and are biologically plausible. Third, patients may not have taken opioids as prescribed. For example, some adolescents and young adults misuse opioids prescribed to them, whereas others may not take the entire prescription.3,39 Estimates should be interpreted as associations between overdose risk and the use patterns practitioners intended.13 Fourth, subgroup analyses may have been underpowered because of limited sample size. Fifth, opioid poisoning codes have high positive predictive value but low sensitivity for detecting overdose.26,27 In addition, overdose may occur when opioid prescriptions are not active or may not result in claims (eg, overdose death at home). Consequently, this study likely failed to capture all overdoses, potentially resulting in underestimation of overdose risk associated with prescribing patterns. Sixth, misuse of opioids belonging to others is the most common type of opioid misuse among adolescents and young adults, but this phenomenon was beyond the scope of our analysis.3 Seventh, we may have underestimated the prevalence of mental health and substance use disorders because we assessed these comorbidities based on diagnosis codes that may have less than perfect sensitivity and that occurred during a relatively short time frame. Eighth, we could not differentiate between intentional or unintentional overdose.40
Conclusions
To prevent prescription opioid overdose in adolescents and young adults, it is crucial to identify evidence-based strategies to mitigate overdose risk when prescribing opioids to this population. Findings from this study suggest that such strategies may include using the lowest effective daily dosage, avoiding concurrent opioid and benzodiazepine prescribing, and relying on short-acting opioids. Furthermore, findings suggest that practitioners should carefully assess adolescents and young adults for mental health and substance use disorders before prescribing opioids and should consider implementing heightened measures to mitigate overdose risk when these disorders are present. Future studies should evaluate whether this study’s findings generalize to other populations, including adolescents and young adults with public insurance coverage.
eAppendix 1. Methodology for Identifying Prescription Claims for Opioids and Benzodiazepines
eAppendix 2. Results When Using Different Age Ranges
eAppendix 3. Diagnosis Codes Used to Define Cancer
eAppendix 4. Diagnosis Codes Used to Define Opioid Overdose
eAppendix 5. Results When Ending Study Period Before ICD-10-CM Transition
eAppendix 6. Diagnosis Codes for Mental Health Conditions and Substance Use Disorders
eAppendix 7. Diagnosis Codes for Other Chronic Conditions (Pediatric Medical Complexity Algorithm)
eAppendix 8. Sensitivity Analyses Regarding Controlling for Time, Exclusion Criteria, Categorization of Daily Opioid Dosage, Statistical Modeling Strategy, and Outcome Definition
eAppendix 9. Unadjusted Overdose Rate by Daily Opioid Dosage Category
eAppendix 10. Sensitivity Analysis Including Benzodiazepine Poisoning Codes
eReferences
References
- 1.McCabe SE, Wilens TE, Boyd CJ, Chua KP, Voepel-Lewis T, Schepis TS. Age-specific risk of substance use disorders associated with controlled medication use and misuse subtypes in the United States. Addict Behav. 2019;90:285-293. doi: 10.1016/j.addbeh.2018.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schroeder AR, Dehghan M, Newman TB, Bentley JP, Park KT. Association of opioid prescriptions from dental clinicians for US adolescents and young adults with subsequent opioid use and abuse. JAMA Intern Med. 2019;179(2):145-152. doi: 10.1001/jamainternmed.2018.5419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Substance Abuse and Mental Health Services Administration Key Substance Use and Mental Health Indicators in the United States: Results From the 2017 National Survey on Drug Use and Health. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2018. [Google Scholar]
- 4.Harbaugh CM, Lee JS, Chua KP, et al. Association between long-term opioid use in family members and persistent opioid use after surgery among adolescents and young adults. JAMA Surg. 2019;154(4):e185838. doi: 10.1001/jamasurg.2018.5838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harbaugh CM, Lee JS, Hu HM, et al. Persistent opioid use among pediatric patients after surgery. Pediatrics. 2018;141(1):e20172439. doi: 10.1542/peds.2017-2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harbaugh CM, Nalliah RP, Hu HM, Englesbe MJ, Waljee JF, Brummett CM. Persistent opioid use after wisdom tooth extraction. JAMA. 2018;320(5):504-506. doi: 10.1001/jama.2018.9023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCabe SE, West BT, Boyd CJ. Medical use, medical misuse, and nonmedical use of prescription opioids: results from a longitudinal study. Pain. 2013;154(5):708-713. doi: 10.1016/j.pain.2013.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinn PD, Hur K, Chang Z, et al. Incident and long-term opioid therapy among patients with psychiatric conditions and medications: a national study of commercial health care claims. Pain. 2017;158(1):140-148. doi: 10.1097/j.pain.0000000000000730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;360:j5790. doi: 10.1136/bmj.j5790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomes T, Tadrous M, Mamdani MM, Paterson JM, Juurlink DN. The burden of opioid-related mortality in the United States. JAMA Netw Open. 2018;1(2):e180217. doi: 10.1001/jamanetworkopen.2018.0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Center for Health Statistics Health, United States, 2017: With Special Feature on Mortality. Hyattsville, MD: National Center for Health Statistics; 2018. [PubMed] [Google Scholar]
- 12.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49. doi: 10.15585/mmwr.rr6501e1 [DOI] [PubMed] [Google Scholar]
- 13.Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321. doi: 10.1001/jama.2011.370 [DOI] [PubMed] [Google Scholar]
- 14.Gwira Baumblatt JA, Wiedeman C, Dunn JR, Schaffner W, Paulozzi LJ, Jones TF. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med. 2014;174(5):796-801. doi: 10.1001/jamainternmed.2013.12711 [DOI] [PubMed] [Google Scholar]
- 15.Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):686-691. doi: 10.1001/archinternmed.2011.117 [DOI] [PubMed] [Google Scholar]
- 16.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92. doi: 10.7326/0003-4819-152-2-201001190-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dasgupta N, Funk MJ, Proescholdbell S, Hirsch A, Ribisl KM, Marshall S. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med. 2016;17(1):85-98. [DOI] [PubMed] [Google Scholar]
- 18.Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. doi: 10.1136/bmj.h2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller M, Barber CW, Leatherman S, et al. Prescription opioid duration of action and the risk of unintentional overdose among patients receiving opioid therapy. JAMA Intern Med. 2015;175(4):608-615. doi: 10.1001/jamainternmed.2014.8071 [DOI] [PubMed] [Google Scholar]
- 20.Bohnert AS, Logan JE, Ganoczy D, Dowell D. A detailed exploration into the association of prescribed opioid dosage and overdose deaths among patients with chronic pain. Med Care. 2016;54(5):435-441. doi: 10.1097/MLR.0000000000000505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pennsylvania Department of Health Safe Prescribing of Opioids in Pediatric and Adolescent Populations Harrisburg: Pennsylvania Department of Health; 2017. https://www.dos.pa.gov/ProfessionalLicensing/BoardsCommissions/Documents/PA%20Guidelines%20-Pediatric%20and%20Adolescent%20Populations.pdf. Accessed April 1, 2019.
- 22.IBM Watson Health. IBM MarketScan research databases for life sciences researchers, 2018. https://www.ibm.com/downloads/cas/0NKLE57Y. Accessed November 11, 2019.
- 23.US Census Bureau 2016 American Community Survey Summary File. 2018. https://www.census.gov/programs-surveys/acs/data/summary-file.html. Accessed April 30, 2018.
- 24.Lawlor PG, Turner KS, Hanson J, Bruera ED. Dose ratio between morphine and methadone in patients with cancer pain: a retrospective study. Cancer. 1998;82(6):1167-1173. doi: [DOI] [PubMed] [Google Scholar]
- 25.Chung CP, Callahan ST, Cooper WO, et al. Development of an algorithm to identify serious opioid toxicity in children. BMC Res Notes. 2015;8:293. doi: 10.1186/s13104-015-1185-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rowe C, Vittinghoff E, Santos GM, Behar E, Turner C, Coffin PO. Performance measures of diagnostic codes for detecting opioid overdose in the emergency department. Acad Emerg Med. 2017;24(4):475-483. doi: 10.1111/acem.13121 [DOI] [PubMed] [Google Scholar]
- 27.Larochelle MR, Liebschutz JM, Zhang F, Ross-Degnan D, Wharam JF. Opioid prescribing after nonfatal overdose and association with repeated overdose. Ann Intern Med. 2016;165(5):376-377. doi: 10.7326/L16-0168 [DOI] [PubMed] [Google Scholar]
- 28.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167(4):492-499. doi: 10.1093/aje/kwm324 [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention Data resources: analyzing prescription data and morphine milligram equivalents (MMEs). 2018. https://www.cdc.gov/drugoverdose/resources/data.html. Accessed April 1, 2019.
- 30.Simon TD, Cawthon ML, Stanford S, et al. ; Center of Excellence on Quality of Care Measures for Children with Complex Needs (COE4CCN) Medical Complexity Working Group . Pediatric Medical Complexity Algorithm: a new method to stratify children by medical complexity. Pediatrics. 2014;133(6):e1647-e1654. doi: 10.1542/peds.2013-3875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norton EC, Dowd BE, Maciejewski ML. Marginal effects: quantifying the effect of changes in risk factors in logistic regression models. JAMA. 2019;321(13):1304-1305. doi: 10.1001/jama.2019.1954 [DOI] [PubMed] [Google Scholar]
- 32.Groenewald CB, Zhou C, Palermo TM, Van Cleve WC Associations between opioid prescribing patterns and overdose among privately insured adolescents. Pediatrics. 2019;144(5):e20184070. doi: 10.1542/peds.2018-4070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ladha KS, Gagne JJ, Patorno E, et al. Opioid overdose after surgical discharge. JAMA. 2018;320(5):502-504. doi: 10.1001/jama.2018.6933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, Whiteside SPH, Sim L, et al. Comparative effectiveness and safety of cognitive behavioral therapy and pharmacotherapy for childhood anxiety disorders: a systematic review and meta-analysis. JAMA Pediatr. 2017;171(11):1049-1056. doi: 10.1001/jamapediatrics.2017.3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung CP, Callahan ST, Cooper WO, et al. Outpatient opioid prescriptions for children and opioid-related adverse events. Pediatrics. 2018;142(2):e20172156. doi: 10.1542/peds.2017-2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bennett KG, Harbaugh CM, Hu HM, et al. Persistent opioid use among children, adolescents, and young adults after common cleft operations. J Craniofac Surg. 2018;29(7):1697-1701. doi: 10.1097/SCS.0000000000004762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miech R, Johnston L, O’Malley PM, Keyes KM, Heard K. Prescription opioids in adolescence and future opioid misuse. Pediatrics. 2015;136(5):e1169-e1177. doi: 10.1542/peds.2015-1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cerda M, Santaella J, Marshall BD, Kim JH, Martins SS Nonmedical prescription opioid use in childhood and early adolescence predicts transitions to heroin use in young adulthood: a national study. J Pediatr. 2015;167(3):605-612, e601-602. [DOI] [PMC free article] [PubMed]
- 39.Monitto CL, Hsu A, Gao S, et al. Opioid prescribing for the treatment of acute pain in children on hospital discharge. Anesth Analg. 2017;125(6):2113-2122. doi: 10.1213/ANE.0000000000002586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bohnert ASB, Ilgen MA. Understanding links among opioid use, overdose, and suicide. N Engl J Med. 2019;380(1):71-79. doi: 10.1056/NEJMra1802148 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Methodology for Identifying Prescription Claims for Opioids and Benzodiazepines
eAppendix 2. Results When Using Different Age Ranges
eAppendix 3. Diagnosis Codes Used to Define Cancer
eAppendix 4. Diagnosis Codes Used to Define Opioid Overdose
eAppendix 5. Results When Ending Study Period Before ICD-10-CM Transition
eAppendix 6. Diagnosis Codes for Mental Health Conditions and Substance Use Disorders
eAppendix 7. Diagnosis Codes for Other Chronic Conditions (Pediatric Medical Complexity Algorithm)
eAppendix 8. Sensitivity Analyses Regarding Controlling for Time, Exclusion Criteria, Categorization of Daily Opioid Dosage, Statistical Modeling Strategy, and Outcome Definition
eAppendix 9. Unadjusted Overdose Rate by Daily Opioid Dosage Category
eAppendix 10. Sensitivity Analysis Including Benzodiazepine Poisoning Codes
eReferences