Key Points
Question
Is osocimab, a monoclonal antibody against factor XIa, noninferior to enoxaparin for thromboprophylaxis after knee arthroplasty?
Findings
In this phase 2, noninferiority trial that randomized 813 patients undergoing knee arthroplasty, venous thromboembolism (determined by bilateral venography and symptomatic events) at 10 to 13 days postoperatively occurred in 23.7% of patients receiving 0.3 mg/kg, 15.7% receiving 0.6 mg/kg, 16.5% receiving 1.2 mg/kg, and 17.9% receiving 1.8 mg/kg of osocimab postoperatively; 29.9% receiving 0.3 mg/kg and 11.3% receiving 1.8 mg/kg osocimab preoperatively; and 26.3% receiving enoxaparin and 14.5% receiving apixaban. Given postoperatively, 0.6 mg/kg, 1.2 mg/kg, and 1.8 mg/kg doses of osocimab met criteria for noninferiority compared with enoxaparin at the prespecified noninferiority margin of 5%. Preoperative osocimab 1.8 mg/kg met criteria for superiority compared with enoxaparin.
Meaning
Further studies are needed to establish efficacy and safety of osocimab relative to standard therapies for venous thromboprophylaxis.
Abstract
Importance
The efficacy of factor XIa inhibition for thromboprophylaxis is unknown. Osocimab is a long-acting, fully human monoclonal antibody that inhibits factor XIa.
Objective
To compare different doses of osocimab with enoxaparin and apixaban for thromboprophylaxis in patients who have undergone knee arthroplasty.
Design, Setting, and Participants
Randomized, open-label, adjudicator-blinded, phase 2 noninferiority trial with observer blinding for osocimab doses, conducted at 54 hospitals in 13 countries. Adult patients undergoing unilateral knee arthroplasty were randomized from October 2017 through August 2018 and followed up until January 2019.
Interventions
Single intravenous osocimab postoperative doses of 0.3 mg/kg (n = 107), 0.6 mg/kg (n = 65), 1.2 mg/kg (n = 108), or 1.8 mg/kg (n = 106); preoperative doses of 0.3 mg/kg (n = 109) or 1.8 mg/kg (n = 108); or 40 mg of subcutaneous enoxaparin once daily (n = 105) or 2.5 mg of oral apixaban twice daily (n = 105) for at least 10 days or until venography.
Main Outcomes and Measures
The primary outcome was venous thromboembolism incidence between 10 and 13 days postoperatively (assessed by mandatory bilateral venography performed 10 to 13 days after surgery or confirmed symptomatic deep vein thrombosis or pulmonary embolism). A 5% noninferiority margin compared with enoxaparin was chosen. The primary safety outcome of major or clinically relevant nonmajor bleeding was assessed until 10 to 13 days postoperatively.
Results
Of 813 randomized participants (mean [SD] age, 66.5 years [8.2 years]; body mass index, 32.7 [5.7]; and 74.2% women), 600 were included in the per-protocol population used for the primary analysis. The primary outcome occurred in 18 patients (23.7%) receiving 0.3 mg/kg, 8 (15.7%) receiving 0.6 mg/kg, 13 (16.5%) receiving 1.2 mg/kg, and 14 (17.9%) receiving 1.8 mg/kg of osocimab postoperatively; 23 (29.9%) receiving 0.3 mg/kg and 9 (11.3%) receiving 1.8 mg/kg of osocimab preoperatively; 20 (26.3%) receiving enoxaparin; and 12 (14.5%) receiving apixaban. Osocimab given postoperatively met criteria for noninferiority compared with enoxaparin with risk differences (1-sided 95% CIs) of 10.6% (95% CI, –1.2% to ∞) at the 0.6-mg/kg dose; 9.9% (95% CI, –0.9% to ∞) at the 1.2-mg/kg dose, and 8.4% (95% CI, –2.6 to ∞) at the 1.8-mg/kg dose. The preoperative dose of 1.8 mg/kg of osocimab met criteria for superiority compared with enoxaparin with a risk difference of 15.1%; 2-sided 90% CI, 4.9% to 25.2%). Postoperative and preoperative doses of 0.3 mg/kg of osocimab did not meet the prespecified criteria for noninferiority, with risk differences (1-sided 95% CIs) of 2.6% (95% CI, –8.9% to ∞) and –3.6% (95% CI, –15.5% to ∞), respectively. Major or clinically relevant nonmajor bleeding was observed in up to 4.7% of those receiving osocimab, 5.9% receiving enoxaparin, and 2% receiving apixaban.
Conclusions and Relevance
Among patients undergoing knee arthroplasty, postoperative osocimab 0.6 mg/kg, 1.2 mg/kg, and 1.8 mg/kg met criteria for noninferiority compared with enoxaparin, and the preoperative 1.8-mg/kg dose of osocimab met criteria for superiority compared with enoxaparin for the primary outcome of incidence of venous thromboembolism at 10 to 13 days postoperatively. Further studies are needed to establish efficacy and safety of osocimab relative to standard thromboprophylaxis.
Trial Registration
ClinicalTrials.gov Identifier: NCT03276143
This phase 2 clinical noninferiority trial compared 4 doses of postoperative osocimab, a monoclonal antibody against factor XI, vs enoxaparin and apixiban on venous thromboembolism incidence among patients undergoing knee replacement.
Introduction
Patients undergoing knee arthroplasty are at risk of postoperative venous thromboembolism (VTE). To reduce this risk, anticoagulants such as enoxaparin, which inhibits factor Xa and thrombin, or apixaban, which only inhibits factor Xa, are often administered postoperatively. Although effective, these agents are associated with a risk of bleeding, which has prompted ongoing efforts to identify safer anticoagulants.
Tissue factor exposed at the surgical site is a major driver of postoperative VTE.1 Tissue factor initiates coagulation via the extrinsic pathway and triggers thrombin generation. The importance of the intrinsic pathway in the pathogenesis of postoperative venous thrombosis is uncertain, but thrombin can feed back and activate factor XI, thereby amplifying thrombin generation and promoting thrombus formation.2
There is emerging evidence that targeting factor XI, a key component of the intrinsic pathway, attenuates thrombosis with minimal disruption of hemostasis. Patients with vs without congenital factor XI deficiency are at lower risk of VTE but rarely experience spontaneous bleeding, although bleeding can occur with surgery.3,4 Compared with enoxaparin, knock-down of factor XI with an antisense oligonucleotide, starting 35 days before knee arthroplasty, reduced the risk of postoperative VTE without increasing the risk of bleeding.5 It remains unknown, however, whether inhibition of factor XIa has a similar effect.
Osocimab is a fully human monoclonal immunoglobulin G1 antibody that binds adjacent to the active site of factor XIa, and prevents it from activating factor IX.6 After intravenous infusion in healthy volunteers, osocimab has a geometric mean time to maximum plasma concentration of 1 to 4 hours and a half-life of 30 to 44 days,7 thereby enabling single-dose administration for surgical thromboprophylaxis. As proof-of-principle, osocimab was compared with enoxaparin and apixaban in the phase 2 Factor XIa Inhibition for the Prevention of Venous Thromboembolism in Patients Undergoing Total Knee Arthroplasty (FOXTROT) study.
Methods
Study Design and Oversight
This was a phase 2, randomized trial that used an open-label, parallel group, adaptive design, to determine whether osocimab met criteria for noninferiority compared with enoxaparin for thromboprophylaxis after knee arthroplasty. An exploratory comparison of osocimab with apixaban was performed in addition. The full protocol and statistical analysis plan are available in Supplement 1. The institutional review board at each participating center approved the protocol. All patients provided written informed consent. An independent committee, whose members were blinded to treatment assignment, adjudicated all venograms for the presence of thrombosis and all clinically suspected episodes of VTE or bleeding. A separate, independent data and safety monitoring committee reviewed trial outcomes at regular intervals.
Participants
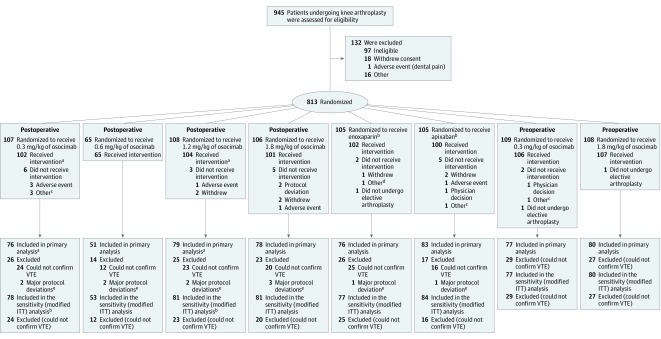
Patients 18 years or older undergoing elective, primary, unilateral, total knee arthroplasty, who were willing to adhere to the study procedures, were eligible for participation (Figure). The main exclusion criteria were active bleeding or a high risk of bleeding; a history of brain, spinal, or ophthalmologic surgery within the 3 months before randomization; sustained uncontrolled hypertension; body weight of more than 135 kg; hemoglobin level of less than 10 g/dL for men and 11 g/dL for women; platelet count of less than 150 × 109/L; activated partial thromboplastin time above the upper limit of normal; calculated creatinine clearance below 60 mL/min; clinically significant liver disease; and a history of prior deep vein thrombosis (DVT). Additional exclusion criteria were epidural analgesia after surgery for postoperative dosing, and spinal anesthesia and epidural analgesia for preoperative dosing. The full list of inclusion and exclusion criteria is provided in the protocol (Supplement 1).
Figure. Enrollment, Randomization, and Populations for Analysis in the FOXTROT Trial of Osocimab to Prevent Venous Thromboembolism (VTE).
aA patient assigned to receive osocimab 1.2 mg/kg postoperatively received 0.3 mg/kg.
bOf these patients, 62 and 43 were randomized as part of the postoperative and preoperative osocimab phases.
cRescheduled or canceled knee arthroplasty.
dReceived commercial enoxaparin instead of study drug.
eDeviations from inclusion or exclusion criteria, use of prohibited concomitant medications impacting on the primary outcome, and significant changes to study drug intake were considered major protocol deviations.
Interventions
The study was conducted in 2 phases. Initially, 4 osocimab doses (0.3, 0.6, 1.2, and 1.8 mg/kg), given postoperatively on the day after surgery, were evaluated. Subsequently, 2 preoperative osocimab doses (0.3 and 1.8 mg/kg) given on the day before surgery were assessed; these preoperative doses were selected once primary efficacy outcome data were available from 75% of patients receiving osocimab postoperatively, as prespecified in the protocol. Each osocimab dose was administered as a single, 60-minute, intravenous infusion in a dose-blinded manner. During both phases, 40 mg of subcutaneous enoxaparin once daily was started either in the evening before surgery or 6 to 8 hours postoperatively (at the investigator’s discretion), and 2.5 mg of oral apixaban twice daily was started 12 to 24 hours postoperatively. Enoxaparin and apixaban were to be continued for at least 10 days or until venography was performed 10 to 13 days postoperatively.
Randomization and Blinding
After enrollment by the investigator, patients were randomized on study day 1 (the day before surgery) using a single-stream and block method, in which 2 lists of random numbers were generated using a standard SAS randomization program. The first list was used to randomize patients in a 5:1:1 ratio to osocimab, enoxaparin, and apixaban with a block size of 14; the second list was used to randomize patients equally between the doses of osocimab with a block size of 15.
The initial protocol for postoperative dosing called for random assignment of 100 patients to each of 3 dose levels of osocimab (0.3, 0.6, and 1.2 mg/kg). After approximately 60 patients in each dose group had received osocimab, the steering committee discontinued enrollment into the 0.6-mg/kg dose group and added the 1.8-mg/kg dose group, as permitted by the study protocol. There were no safety concerns for any of the osocimab doses, and at that point, the rate of the primary efficacy outcome was highest in the 0.6-mg/kg dose group. Adding the 1.8-mg/kg group and maintaining the 0.3-mg/kg group enabled evaluation of a broad range of osocimab doses. As prespecified, when efficacy outcome data were available from 75% of the patients receiving osocimab postoperatively, the steering committee recommended initiation of preoperative dosing using the lowest and highest osocimab doses tested postoperatively.
The study was open-label for treatment assignment and observer-blinded with respect to osocimab doses. An unblinded pharmacist or other investigator-nominated person was responsible for maintaining blinding integrity but was not otherwise involved in the study. Efficacy and safety parameters were centrally adjudicated by an independent committee that was blinded to treatment allocation.
Outcomes
The primary efficacy outcome was the incidence of the composite of asymptomatic DVT (detected by mandatory bilateral venography, performed 10 to 13 days postoperatively), objectively confirmed symptomatic DVT or nonfatal pulmonary embolism (PE), documented fatal PE, or unexplained death, for which PE could not be excluded at 10 to 13 days postoperatively.
The secondary efficacy outcome was the incidence of the composite of asymptomatic DVT detected by venography at 10 to 13 days postoperatively, or objectively confirmed symptomatic DVT or nonfatal PE, fatal PE, or unexplained death for which PE could not be excluded during the 150 days after randomization.
The primary and secondary safety outcomes were the incidence of adjudicated, clinically relevant bleeding, which was the composite of major or clinically relevant nonmajor bleeding from randomization to 10 to 13 days postoperatively and up to study day 150, respectively. Bleeding was defined as major if it was overt and associated with a decrease in hemoglobin of 2 g/dL or more, or necessitated transfusion of 2 or more units of blood with a temporal association within 24 to 48 hours of the bleed; or if the bleeding occurred in a critical area or organ, or contributed to death. Bleeding at the surgical site was defined as major only if it required a second intervention (reoperation, or arthroscopic or endovascular); caused hemodynamic instability; or caused hemarthrosis that delayed mobilization or wound healing and resulted in prolonged hospitalization or deep wound infection. Clinically relevant nonmajor bleeding was defined as overt bleeding that did not meet the criteria for major bleeding but led to hospital admission, a physician-guided medical or surgical treatment for bleeding, hematomas exceeding the usual size, or a change in antithrombotic therapy (details are provided in Supplement 2).
Adverse events included thrombocytopenia and hypersensitivity and infusion-related reactions. Intraoperative blood loss and frequency of transfusion of heterologous blood were evaluated post hoc as exploratory variables.
Activated partial thromboplastin times and prothrombin times were determined before and after osocimab administration. Ratios were calculated by dividing the results determined after study drug administration by those measured at baseline.
Surveillance and Follow-up
Vital signs were assessed before and after osocimab infusion. Patients were seen in the hospital or attended the study center on days 2, 3, and 4, and then on day 6 (±1), between days 12 and 15 (at the time of venography, which corresponded to 10 to 13 days postoperatively), and days 30 (±5), 90 (±7), and 150 (±7). At each of these visits, patients were evaluated for symptoms suggestive of VTE, bleeding, or adverse events. Blood samples were obtained for safety parameters (biochemistry, hematology, and coagulation) and for pharmacokinetic and pharmacodynamic measurements. Urinalysis was also performed.
Statistical Analysis
The primary efficacy analysis tested the hypothesis that osocimab would be noninferior to enoxaparin in the per-protocol population. The per-protocol population included patients who received at least 1 dose of study medication and who had an evaluable venogram at 10 to 13 days postoperatively, or who had an objectively confirmed symptomatic or fatal VTE event, and who did not have protocol deviations that affected the primary efficacy outcome. A sensitivity analysis for efficacy was conducted using a modified intention-to-treat (ITT) approach that included all patients who received at least 1 dose of study medication and who had an evaluable venogram at 10 to 13 days postoperatively, or an who had an objectively confirmed symptomatic or fatal VTE event. Bleeding and adverse events were evaluated for all patients who received at least 1 dose of study medication.
Based on clinical judgment, the protocol prespecified that noninferiority would be demonstrated if the lower limit of the 2-sided 90% CI for the between-group difference in risk was 5% or more. If noninferiority was shown, superiority testing would be performed by demonstrating that the lower limit of the 2-sided 90% CI for the between-group difference in risk was greater than or equal to 0. We calculated CIs based on normal approximation and P values on the asymptotic Wald test. A fixed-sequence procedure was applied to adjust for multiplicity of testing within the osocimab groups, but there was no adjustment for multiplicity for comparisons between the osocimab dose groups and the 2 comparators. Because of the multiple comparisons, results should be considered exploratory.
A sample size of 80 patients per osocimab dose group would provide 97% power to show noninferiority, and 80% power to demonstrate superiority, of osocimab compared with enoxaparin using a 1-sided significance level of .05 and a noninferiority margin of 5% in absolute risk difference. This calculation assumed rates of 10% for the primary efficacy outcome with all doses of osocimab and 25% with enoxaparin.5,8,9 The noninferiority margin of 5% in absolute risk difference was selected to retain at least 90% of the enoxaparin treatment effect, using a placebo rate of 45%.10 Approximately 100 patients per treatment group were to be enrolled assuming that 20% of venograms would be nonevaluable.
No statistical hypothesis was defined for the comparison with apixaban because this was considered exploratory; only 2-sided 90% CIs were calculated. The analyses of secondary efficacy outcomes, bleeding and adverse events, and comparisons between osocimab doses were also performed descriptively. Given that these evaluations were considered exploratory, no correction was made for type I error.
Missing data were expected owing to the complexity of venography. The readability of venograms is independent of the results of the read; therefore, estimation of treatment effect only in patients with evaluable venograms is valid. A prespecified, descriptive analysis of the primary outcome in all patients who received at least 1 dose of study medication was performed after multiple imputation of missing data using a logistic regression model with baseline characteristics and treatment groups as covariates.
The statistical evaluation was performed using SAS release 9.4 (SAS Institute Inc).
Results
Participants
Of 945 patients assessed for eligibility, 813 patients at 54 centers in 13 countries underwent randomization according to the protocol from October 2017 through April 2018 for postoperative osocimab dosing and from April 2018 through August 2018 for preoperative dosing. Randomization to enoxaparin and apixaban was continuous throughout these time frames. Follow-up was completed in January 2019. Of the 813 patients randomized, 600 (73.8%) comprised the per-protocol population (Figure); baseline characteristics were similar across the study groups (Table 1). None of the 11 patients excluded from the per-protocol population owing to protocol deviation had VTE. Overall, evaluable venograms were obtained from 741 of 787 patients (94.2%) who received study medication. Baseline characteristics were similar among patients with and without valid venography (eTable 1 in Supplement 2).
Table 1. Demographic and Baseline Characteristics of Participants in the Per-Protocol Populationa.
| No. (%) of Patients | ||||||||
|---|---|---|---|---|---|---|---|---|
| Postoperative Osocimab, mg/kg | Preoperative Osocimab, mg/kg | Enoxaparin (n = 76) |
Apixaban (n = 83) |
|||||
| 0.3 (n = 76) |
0.6 (n = 51) |
1.2 (n = 79) |
1.8 (n = 78) |
0.3 (n = 77) |
1.8 (n = 80) |
|||
| Age, mean (SD), y | 65.6 (7.7) | 66.1 (9.9) | 66.1 (8.4) | 68.0 (7.4) | 66.2 (8.2) | 67.8 (7.3) | 67.0 (8.8) | 64.9 (8.4) |
| Women | 53 (69.7) | 35 (68.6) | 59 (74.7) | 51 (65.4) | 58 (75.3) | 60 (75.0) | 55 (72.4) | 65 (78.3) |
| Men | 23 (30.3) | 16 (31.4) | 20 (25.3) | 27 (34.6) | 19 (24.7) | 20 (25.0) | 21 (27.6) | 18 (21.7) |
| Race, No.b | 75 | 51 | 77 | 77 | 75 | 78 | 74 | 82 |
| White | 73 (97.3) | 48 (94.1) | 75 (97.4) | 77 (100) | 75 (100) | 77 (98.7) | 72 (97.3) | 77 (93.9) |
| Black | 2 (2.7) | 3 (5.9) | 1 (1.3) | 0 | 0 | 0 | 2 (2.7) | 5 (6.1) |
| Asian | 0 | 0 | 1 (1.3) | 0 | 0 | 0 | 0 | 0 |
| Native Hawaiianc | 0 | 0 | 0 | 0 | 0 | 1 (1.3) | 0 | 0 |
| Weight, mean (SD), kg | 88.1 (16.9) | 85.0 (13.5) | 88.6 (17.3) | 84.6 (14.1) | 86.5 (18.7) | 88.9 (16.0) | 87.9 (17.9) | 87.2 (16.7) |
| BMI, mean (SD) | 32.5 (5.5) | 31.2 (5.1) | 33.4 (5.6) | 31.3 (5.3) | 32.2 (6.3) | 33.8 (5.9) | 32.4 (5.5) | 32.6 (5.8) |
| Creatinine clearance, mean (SD), mL/min/1.73 m2 | 88.5 (24.8) | 85.8 (23.3) | 85.0 (24.0) | 82.4 (22.0) | 81.5 (18.4) | 84.1 (21.5) | 82.4 (17.8) | 86.8 (17.8) |
| Duration of surgery, median (IQR), min | 90 (75-110) | 85 (70-105) | 85 (75-106) | 80 (70-100) | 80 (70-105) | 85 (72.5-102) | 85 (65-109) | 85 (75-105) |
| Type of anesthesia | ||||||||
| Spinald | 63 (82.9) | 41 (80.4) | 63 (79.7) | 66 (84.6) | 0 | 1 (1.2) | 33 (43.4) | 37 (44.6) |
| General | 13 (17.1) | 7 (13.7) | 12 (15.2) | 12 (15.4) | 77 (100) | 79 (98.8) | 43 (56.6) | 43 (51.8) |
| Other | 0 | 3 (5.9) | 4 (5.1) | 0 | 0 | 0 | 0 | 3 (3.6) |
| Tourniquet applied | 55 (72.4) | 33 (64.7) | 50 (63.3) | 39 (50.0) | 51 (66.2) | 51 (63.8) | 47 (61.8) | 56 (67.5) |
| Duration of tourniquet use, median (IQR), min | 65 (44-90) | 65 (50-89) | 76 (62.5-95) | 70 (52-85) | 79 (69-95) | 75 (61-85) | 74 (55-95) | 69 (50-99) |
| aPTT at baseline, mean (SD), s | 29.6 (2.2) | 30.6 (6.6) | 29.3 (2.9) | 29.6 (2.9) | 32.4 (22.6) | 29.4 (2.3) | 29.5 (2.2) | 29.8 (2.7) |
| Duration of comparator treatment, median (range), d | 13 (10-16) | 12 (4-16) | ||||||
Abbreviations: aPTT, activated partial thromboplastin time; BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; IQR, interquartile range.
SI conversion factor: to convert creatinine clearance from mL/min/1.73 m2 to mL/s/m2, multiply by 0.0167.
The per-protocol population included all patients who received at least 1 dose of study medication who could be evaluated for the primary efficacy outcome and did not have an important deviation from the protocol with an impact on the primary efficacy variable.
Excluding those for whom race was not reported.
Or other Pacific Islander.
Spinal anesthesia was an exclusion criterion for preoperative administration of osocimab; however, 1 patient receiving osocimab 1.8 mg/kg preoperatively accidentally underwent spinal anesthesia without complications.
Efficacy
Among patients who received osocimab postoperatively, the primary outcome occurred in of 18 participants (23.7%) who received 0.3 mg/kg; 8 (15.7%) who received 0.6 mg/kg; 13 (16.5%) who received 1.2 mg/kg; and 14 (17.9%) who received 1.8 mg/kg of osocimab postoperatively. Among patients who received osocimab preoperatively, the primary outcome occurred in 23 (29.9%) in the 0.3-mg/kg group and 9 (11.3%) in the 1.8-mg/kg group. The primary outcome occurred in 20 (26.3%) of those receiving enoxaparin and 12 (14.5%) receiving apixaban (Table 2). One-sided significance-level analysis showed that postoperative osocimab met criteria for noninferiority compared with enoxaparin with risk differences of 10.6% (95% CI, –1.2% to ∞) in the 0.6-mg/kg group, 9.9% (95% CI, –0.9% to ∞) in the 1.2-mg/kg group, and 8.4% (95% CI, –2.6% to ∞) in the 1.8-mg/kg group. Two-sided 90% CI analysis showed that preoperative osocimab at the 1.8-mg/kg dose level met criteria for superiority compared with enoxaparin (risk difference, 15.1% [90% CI, 4.9% to 25.2%]; P = .007). Administration of 0.3 mg/kg of osocimab did not meet the prespecified criteria for noninferiority compared with enoxaparin: postoperative risk difference, 2.6% (1-sided 95% CI, –8.9% to ∞) and preoperative risk difference, –3.6% (1-sided 95% CIs, –15.5% to ∞) (Table 2). No patient experienced pulmonary embolism during the period between randomization and completion of venography at 10 to 13 days postoperatively, and no deaths occurred during the study. Results of the prespecified sensitivity analysis of the primary outcome in the modified ITT population, the sensitivity analysis of the primary outcome after multiple imputation of missing data and secondary efficacy outcome data are shown in eTables 2, 3, and 4, respectively, in Supplement 2.
Table 2. Rates of Venous Thromboembolism (Per-Protocol Analysis)a.
| Postoperative Osocimab, mg/kg | Preoperative Osocimab, mg/kg | Enoxaparin (n = 76) |
Apixaban (n = 83) |
|||||
|---|---|---|---|---|---|---|---|---|
| 0.3 (n = 76) |
0.6 (n = 51) |
1.2 (n = 79) |
1.8 (n = 78) |
0.3 (n = 77) |
1.8 (n = 80) |
|||
| Primary VTE Outcome, No. (%) [90% CI]b | ||||||||
| 18 (23.7) [15.9 to 33.1] |
8 (15.7) [8.1 to 26.5] |
13 (16.5) [10.0 to 24.9] |
14 (17.9) [11.2 to 26.6] |
23 (29.9) [21.4 to 39.6] |
9 (11.3) [6.0 to 18.8] |
20 (26.3) [18.2 to 35.9] |
12 (14.5) [8.6 to 22.4] |
|
| Noninferiority of Osocimab vs Enoxaparin, % (1-Sided 95% CI) | ||||||||
| Risk difference | 2.6 (–8.9 to ∞) |
10.6 (–1.2 to ∞) |
9.9 (–0.9 to ∞) |
8.4 (–2.6 to ∞) |
–3.6 (–15.5 to ∞) |
15.1 (4.9 to ∞) |
||
| P value | .14 | .01 | .01 | .02 | .42 | <.001 | ||
| Superiority of Osocimab vs Enoxaparin, % (2-Sided 90% CI)c | ||||||||
| Risk difference | 10.6 (–1.2 to 22.4) |
9.9 (–0.9 to 20.6) |
8.4 (–2.6 to 19.3) |
15.1 (4.9 to 25.2) |
||||
| P value | .07 | .07 | .10 | .007 | ||||
| Exploratory Comparison of Osocimab vs Apixaban, % (90% CI)d | ||||||||
| Risk difference | –9.2 (–19.5 to 1.0) |
–1.2 (–11.7 to 9.3) |
–2.0 (–11.3 to 7.4) |
–3.5 (–13.1 to 6.1) |
–15.4 (–26.1 to –4.7) |
3.2 (–5.4 to 11.8) |
||
| DVT Components of the Primary Outcome, No. (%)b | ||||||||
| Asymptomatic | 18 (23.7) | 7 (13.7) | 13 (16.5) | 14 (17.9) | 22 (28.6) | 9 (11.3) | 20 (26.3) | 11 (13.3) |
| Symptomatic | 1 (1.3) | 1 (2.0) | 1 (1.3) | 2 (2.6) | 1 (1.3) | 0 | 1 (1.3) | 1 (1.2) |
| Proximale | 2 (2.6) | 3 (5.9) | 3 (3.8) | 3 (3.8) | 5 (6.5) | 2 (2.5) | 3 (3.9) | 2 (2.4) |
| Distale | 18 (23.7) | 8 (15.7) | 13 (16.5) | 13 (16.7) | 24 (31.2) | 9 (11.3) | 19 (25.0) | 12 (14.5) |
Abbreviations: DVT, deep vein thrombosis; VTE, venous thromboembolism.
The primary outcome was assessed in the per-protocol population, which comprised all patients who received at least 1 dose of study medication who could be evaluated for the primary efficacy outcome and did not have an important deviation from the protocol with an impact on the primary efficacy variable.
The primary outcome was the incidence of a composite of asymptomatic and symptomatic VTE up to 10 to 13 days postoperatively, which corresponded to study days 12 to 15. None of the patients had pulmonary embolism, and there were no deaths.
A hierarchical scheme was used in which testing for superiority of osocimab vs enoxaparin was permitted if noninferiority was achieved.
No statistical hypothesis was defined for the exploratory comparison with apixaban; only confidence intervals were calculated.
Proximal and distal DVT that occurred between randomization and up to 10 to 13 days postoperatively, which corresponded to study days 12 to 15. A patient may have been included in both categories if they had both proximal and distal DVT.
Bleeding
Major or clinically relevant nonmajor bleeding for up to 10 to 13 days postoperatively was observed in 2% of patients receiving 0.3 mg/kg, 1% receiving 1.2 mg/kg, and 3% receiving 1.8 mg/kg of osocimab; in 1.9% receiving 0.3 mg/kg and 4.7% receiving 1.8 mg/kg of osocimab preoperatively; and in 5.9% of those receiving enoxaparin and 2% of those receiving apixaban (Table 3). None of the patients receiving 0.6 mg/kg of osocimab postoperatively experienced major or clinically relevant nonmajor bleeding (Table 3).
Table 3. Bleeding and Adverse Eventsa.
| Outcome | No. (%) of Patients | |||||||
|---|---|---|---|---|---|---|---|---|
| Postoperative Osocimab, mg/kg | Preoperative Osocimab, mg/kg | Enoxaparin (n = 102) | Apixaban (n = 100) | |||||
| 0.3 (n = 102) | 0.6 (n = 65) | 1.2 (n = 104) | 1.8 (n = 101) | 0.3 (n = 106) | 1.8 (n = 107) | |||
| Major or nonmajor bleeding, No. (%) [90% CI]b | 2 (2.0) [0.3 to 6] |
0 [0.0 to 4.5] | 1 (1.0) [0.0 to 4.5] |
3 (3.0) [0.8 to 7.5] |
2 (1.9) [0.3 to 5.8] |
5 (4.7) [1.8 to 9.5] |
6 (5.9) [2.6 to 11.3] |
2 (2.0) [0.4 to 6.2] |
| Risk difference | ||||||||
| vs Enoxaparin, % (90% CI) | 3.9 (–0.5 to 8.4) |
5.9 (2.1 to 9.7) |
4.9 (0.8 to 9.1) |
2.9 (–1.8 to 7.6) |
4.0 (–0.4 to 8.4) |
1.3 (–3.8 to 6.3) |
||
| vs Apixaban, % (90% CI) | 0.0 (–3.2 to 3.3) |
2.0 (–0.3 to 4.3) |
1.0 (–1.8 to 3.8) |
–1.0 (–4.6 to 2.6) |
0.1 (–3.1 to 3.3) |
–2.6 (–6.7 to 1.4) |
||
| Bleeding | ||||||||
| Major | 0 | 0 | 0 | 0 | 0 | 1 (0.9) | 0 | 0 |
| Clinically relevant nonmajor | 2 (2.0) | 0 | 1 (1.0) | 3 (3.0) | 2 (1.9) | 4 (3.7) | 6 (5.9) | 2 (2.0) |
| Intraoperative blood loss, median (IQR), mLc | 200 (100 to 300) | 200 (100 to 300) | 150 (70 to 250) | 200 (100 to 300) | 120 (50 to 260) | 130 (50 to 300) | 150 (75 to 300) | 150 (80 to 300) |
| Receipt of blood transfusiond | 16 (15.7) | 12 (18.5) | 12 (11.5) | 7 (6.9) | 15 (14.2) | 17 (15.9) | 24 (23.5) | 19 (19.0) |
| Adverse eventse | ||||||||
| Any | 71 (69.6) | 46 (70.8) | 72 (69.2) | 65 (64.4) | 74 (69.8) | 86 (80.4) | 75 (73.5) | 63 (63.0) |
| Serious | 3 (2.9) | 1 (1.5) | 3 (2.9) | 2 (2.0) | 3 (2.8) | 6 (5.6) | 6 (5.9) | 1 (1.0) |
| Any resulting in permanent discontinuation of study drug | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1.0) | 0 |
| Platelets <lower limit of normal | 2 (2.0) | 6 (9.2) | 4 (3.8) | 6 (5.9) | 8 (7.5) | 10 (9.3) | 6 (5.9) | 2 (2.0) |
| Hypersensitivity and infusion-related reactionsf | 7 (6.9) | 3 (4.6) | 7 (6.7) | 3 (3.0) | 3 (2.8) | 5 (4.7) | 2 (2.0) | 3 (3.0) |
Abbreviations: IQR, interquartile range.
Major or clinically relevant nonmajor bleeding and adverse events were assessed in all patients who received at least 1 dose of study medication.
Major or clinically relevant nonmajor bleeding up to 10 to 13 days postoperatively, which corresponded to study days 12 to 15. No statistical hypothesis testing was defined; only confidence intervals were calculated. Major and clinically relevant nonmajor bleeding occurred before administration of study drug in 1 patient in the postoperative 0.3-mg/kg osocimab group, 1 patient in the postoperative 1.2-mg/kg osocimab group, and in 2 patients in the enoxaparin group (post hoc analysis).
Intraoperative blood loss as reported by the investigator.
Intraoperative blood loss and blood transfusions occurring after the first administration of study drug for patients receiving preoperative osocimab only.
From randomization until 10 to 13 days postoperatively, which corresponded to study days 12 to 15.
Clinical descriptions of infusion-related reactions are provided in Supplement 2.
All major and nonmajor clinically relevant bleeding events until 10 to 13 days postoperatively consisted of bleeding linked to surgical site. No patient had intracranial bleeding or bleeding into another critical site. Bleeding events occurring during follow-up are described in eTable 5 in Supplement 2.
Other Outcomes
The rates of adverse events are shown in Table 3. Of the 585 patients taking any dose of osocimab, 414 (70.8%) experienced an adverse event and 18 (3.1%) a serious adverse event. Seventy-five of 102 patients (73.5%) taking enoxaparin experienced an adverse event and 6 (5.9%), a serious adverse event. Sixty-three of 100 patients (63%) taking apixaban experienced an adverse event and 1 (1%), a serious adverse event. Thrombocytopenia occurred in 36 patients (6.2%) receiving osocimab; 6 (5.9%), enoxaparin; and 2 (2%), apixaban. Hypersensitivity or infusion-related reactions that were not necessarily study drug–related occurred in 28 patients (4.8%) receiving osocimab; 2 (2%), enoxaparin; and 3 (3%), apixaban. Clinical descriptions of the infusion-related reactions are provided in eTable 6 in Supplement 2. There were no clinically relevant changes in laboratory test results or vital signs. Osocimab increased activated partial thromboplastin time ratios measured within 2 hours of its infusion but not prothrombin time ratios (eFigure 1 in Supplement 2).
Discussion
In this phase 2 noninferiority randomized clinical trial involving patients undergoing knee arthroplasty, postoperative osocimab at doses of 0.6 mg/kg, 1.2 mg/kg, and 1.8 mg/kg were noninferior to enoxaparin, and the preoperative dose of 1.8 mg/kg was superior to enoxaparin with regard to the primary outcome of incidence of VTE at 10 to 13 days postoperatively.
To our knowledge, this is the first study to assess the utility of factor XIa inhibition for preoperative and postoperative thromboprophylaxis after knee arthroplasty. Tissue factor exposed during surgery can trigger thrombin generation and thrombus formation.11 The observation that 3 dosage levels of osocimab administered postoperatively (0.6, 1.2, and 1.8 mg/kg) met criteria for noninferiority compared with enoxaparin supports the concept that feedback activation of factor XI by thrombin is critical for thrombus formation.12 Although additional studies are needed to establish efficacy and safety of osocimab relative to standard therapies for venous thromboprophylaxis, these findings suggest that upstream inhibition of factor XIa prevents thrombosis to a similar extent as downstream inhibition at the level of factor Xa or thrombin (eFigure 2 in Supplement 2).
The findings of this study add to those with the factor XI antisense oligonucleotide.5 There are important differences between osocimab and the antisense oligonucleotide. First, on a mechanistic level, osocimab inhibits factor XIa, whereas the antisense oligonucleotide blocks hepatic synthesis of factor XI, thereby reducing the amount available for activation. Second, osocimab rapidly inhibits factor XIa activity as evidenced by the dose-dependent increase in the activated partial thromboplastin time ratios within 2 hours after its infusion,7 whereas it takes several weeks for the antisense oligonucleotide to lower factor XI to therapeutic levels.13 The rapid onset of action of osocimab enabled investigation into the relative importance of postoperative and preoperative factor XIa inhibition, demonstrating that factor XIa inhibition with osocimab is effective even when initiated after surgery.
A key aim of this phase 2 study with osocimab was to identify potential doses to carry forward in future clinical trials. Rates of VTE with enoxaparin and apixaban in this study were similar to those reported in previous trials.5,14 The per-protocol population was selected for analysis of the primary outcome to avoid diluting the treatment effect by including patients without valid venograms, because it was expected that venographically detected asymptomatic VTE would be the main outcome event. Nevertheless, sensitivity analysis in the modified ITT population yielded similar results.
In this study, the 3 highest doses of osocimab were noninferior to enoxaparin for the primary outcome. Postoperative administration of osocimab was associated with rates of clinically relevant bleeding ranging from 1% with the 0.3 mg/kg dose to 4.7% with the 1.8 mg/kg dose with all but 1 bleed in the clinically relevant nonmajor category. The highest dose of osocimab (1.8 mg/kg) may have been associated with more bleeding when given preoperatively than when given postoperatively, whereas the lowest dose (0.3 mg/kg) was not. There were no clinically relevant bleeding events after study-drug administration with the 0.6 or 1.2 mg/kg doses of osocimab, whereas there were such events with enoxaparin and apixaban. Therefore, osocimab doses between 0.6 and 1.2 mg/kg appear to be the most promising to carry forward into future trials, although there were fewer patients in the 0.6-mg/kg dose group than in the other dose groups.
The rate of serious adverse events with osocimab was similar to that with enoxaparin and apixaban, and none of the infusion-related reactions with osocimab necessitated its interruption or discontinuation.
Limitations
This study has several limitations. First, the strength of the conclusions is limited by the sample size. Nevertheless, evaluating a 6-fold range of osocimab doses and assessing postoperative and preoperative administration enabled the identification of doses suitable for further investigation. Second, the study was open-label. However, investigators were blinded to osocimab doses, and all suspected efficacy and safety outcomes were adjudicated by an independent committee whose members were unaware of treatment assignment. Nevertheless, the lack of blinding to treatment allocation may have influenced decisions regarding the need for surgical interventions to manage bleeding. Third, only patients undergoing general anesthesia were included in the preoperative evaluation of osocimab, so the safety of preoperative dosing in patients undergoing spinal anesthesia remains unknown. Fourth, although the noninferiority margin of 5% was chosen based on clinical judgement, it is more stringent than the 14% used in a previous study.5 Fifth, additional studies are needed to establish the efficacy and safety of osocimab relative to standard therapies for venous thromboprophylaxis.
Conclusions
Among patients undergoing knee arthroplasty, postoperative 0.6 mg/kg, 1.2 mg/kg, and 1.8 mg/kg doses of osocimab met criteria for noninferiority compared with enoxaparin, and the preoperative 1.8-mg/kg dose of osocimab met criteria for superiority compared with enoxaparin for the primary outcome of incidence of venous thromboembolism at 10 to 13 days postoperatively. Further studies are needed to establish efficacy and safety of osocimab relative to standard thromboprophylaxis.
Trial Protocol
eAppendix.
eTable 1. Baseline characteristics by validity of venography at 10 to 13 days postoperatively in all patients who received at least one dose of study medication
eTable 2. Sensitivity analysis of the primary efficacy outcome in the modified intention-to-treat population
eTable 3. Sensitivity analysis of the primary efficacy outcome after multiple imputation of non-valid information
eTable 4. Secondary efficacy outcomes in the per-protocol population and in the modified intention-to-treat population
eTable 5. Bleeding and adverse events until study day 150 (±7)
eTable 6. Clinical description of infusion-related reactions
eFigure 1. Activated partial thromboplastin time and prothrombin time ratios measured within two hours after administration of osocimab
eFigure 2. Effect of osocimab, enoxaparin, and apixaban on the coagulation system
Data Sharing Statement
References
- 1.Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451(7181):914-918. doi: 10.1038/nature06797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gailani D, Broze GJ Jr. Factor XI activation in a revised model of blood coagulation. Science. 1991;253(5022):909-912. doi: 10.1126/science.1652157 [DOI] [PubMed] [Google Scholar]
- 3.Duga S, Salomon O. Congenital factor XI deficiency: an update. Semin Thromb Hemost. 2013;39(6):621-631. doi: 10.1055/s-0033-1353420 [DOI] [PubMed] [Google Scholar]
- 4.Salomon O, Steinberg DM, Zucker M, Varon D, Zivelin A, Seligsohn U. Patients with severe factor XI deficiency have a reduced incidence of deep-vein thrombosis. Thromb Haemost. 2011;105(2):269-273. doi: 10.1160/TH10-05-0307 [DOI] [PubMed] [Google Scholar]
- 5.Büller HR, Bethune C, Bhanot S, et al. ; FXI-ASO TKA Investigators . Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med. 2015;372(3):232-240. doi: 10.1056/NEJMoa1405760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaefer M, Buchmueller A, Dittmer F, Straßburger J, Wilmen A. Allosteric inhibition as a new mode of action for BAY 1213790, a neutralizing antibody targeting the activated form of coagulation factor XI [published online October 24, 2019]. J Mol Biol. doi: 10.1016/j.jmb.2019.09.008 [DOI] [PubMed] [Google Scholar]
- 7.Thomas D, Thelen K, Kraff S, et al. BAY 1213790, a fully human IgG1 antibody targeting coagulation factor XIa: First evaluation of safety, pharmacodynamics, and pharmacokinetics. Res Pract Thromb Haemost. 2019;3(2):242-253. doi: 10.1002/rth2.12186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P; ADVANCE-2 investigators . Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet. 2010;375(9717):807-815. doi: 10.1016/S0140-6736(09)62125-5 [DOI] [PubMed] [Google Scholar]
- 9.Lassen MR, Ageno W, Borris LC, et al. RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358(26):2776-2786. doi: 10.1056/NEJMoa076016 [DOI] [PubMed] [Google Scholar]
- 10.Fuji T, Fujita S, Tachibana S, Kawai Y. A dose-ranging study evaluating the oral factor Xa inhibitor edoxaban for the prevention of venous thromboembolism in patients undergoing total knee arthroplasty. J Thromb Haemost. 2010;8(11):2458-2468. doi: 10.1111/j.1538-7836.2010.04021.x [DOI] [PubMed] [Google Scholar]
- 11.Johnson GJ, Leis LA, Bach RR. Tissue factor activity of blood mononuclear cells is increased after total knee arthroplasty. Thromb Haemost. 2009;102(4):728-734. doi: 10.1160/th09-04-0261 [DOI] [PubMed] [Google Scholar]
- 12.von dem Borne PA, Meijers JC, Bouma BN. Feedback activation of factor XI by thrombin in plasma results in additional formation of thrombin that protects fibrin clots from fibrinolysis. Blood. 1995;86(8):3035-3042. doi: 10.1182/blood.V86.8.3035.3035 [DOI] [PubMed] [Google Scholar]
- 13.Younis HS, Crosby J, Huh JI, et al. Antisense inhibition of coagulation factor XI prolongs APTT without increased bleeding risk in cynomolgus monkeys. Blood. 2012;119(10):2401-2408. doi: 10.1182/blood-2011-10-387134 [DOI] [PubMed] [Google Scholar]
- 14.Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361(6):594-604. doi: 10.1056/NEJMoa0810773 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix.
eTable 1. Baseline characteristics by validity of venography at 10 to 13 days postoperatively in all patients who received at least one dose of study medication
eTable 2. Sensitivity analysis of the primary efficacy outcome in the modified intention-to-treat population
eTable 3. Sensitivity analysis of the primary efficacy outcome after multiple imputation of non-valid information
eTable 4. Secondary efficacy outcomes in the per-protocol population and in the modified intention-to-treat population
eTable 5. Bleeding and adverse events until study day 150 (±7)
eTable 6. Clinical description of infusion-related reactions
eFigure 1. Activated partial thromboplastin time and prothrombin time ratios measured within two hours after administration of osocimab
eFigure 2. Effect of osocimab, enoxaparin, and apixaban on the coagulation system
Data Sharing Statement