Key Points
Question
What are the risks of intervention, operation, endoscopy, hospitalization, and mortality up to 5 years associated with the 2 most common bariatric surgical procedures?
Findings
In this national, multicenter cohort study comparing outcomes of 33 560 adults undergoing either gastric bypass or sleeve gastrectomy, operations or interventions as well as hospitalization and endoscopy were more commonly associated with gastric bypass. There were no significant differences in mortality between the 2 surgical procedures through 5 years of follow-up.
Meaning
This information on 5-year bariatric surgical procedure outcomes helps to inform procedure-specific decision-making for prospective patients with severe obesity and their physicians.
Abstract
Importance
Additional data comparing longer-term problems associated with various bariatric surgical procedures are needed for shared decision-making.
Objective
To compare the risks of intervention, operation, endoscopy, hospitalization, and mortality up to 5 years after 2 bariatric surgical procedures.
Design, Setting, and Participants
Adults who underwent Roux-en-Y gastric bypass (RYGB) or sleeve gastrectomy (SG) between January 1, 2005, and September 30, 2015, within the National Patient-Centered Clinical Research Network. Data from 33 560 adults at 10 centers within 4 clinical data research networks were included in this cohort study. Information was extracted from electronic health records using a common data model and linked to insurance claims and mortality indices. Analyses were conducted from January 2018 through October 2019.
Exposures
Bariatric surgical procedures.
Main Outcomes and Measures
The primary outcome was time until operation or intervention. Secondary outcomes included endoscopy, hospitalization, and mortality rates.
Results
Of 33 560 adults, 18 056 (54%) underwent RYGB, and 15 504 (46%) underwent SG. The median (interquartile range) follow-up for operation or intervention was 3.4 (1.6-5.0) years for RYGB and 2.2 (0.9-3.6) years for SG. The overall mean (SD) patient age was 45.0 (11.5) years, and the overall mean (SD) patient body mass index was 49.1 (7.9). The cohort was composed predominantly of women (80%) and white individuals (66%), with 26% of Hispanic ethnicity. Operation or intervention was less likely for SG than for RYGB (hazard ratio, 0.72; 95% CI, 0.65-0.79; P < .001). The estimated, adjusted cumulative incidence rates of operation or intervention at 5 years were 8.94% (95% CI, 8.23%-9.65%) for SG and 12.27% (95% CI, 11.49%-13.05%) for RYGB. Hospitalization was less likely for SG than for RYGB (hazard ratio, 0.82; 95% CI, 0.78-0.87; P < .001), and the 5-year adjusted cumulative incidence rates were 32.79% (95% CI, 31.62%-33.94%) for SG and 38.33% (95% CI, 37.17%-39.46%) for RYGB. Endoscopy was less likely for SG than for RYGB (hazard ratio, 0.47; 95% CI, 0.43-0.52; P < .001), and the adjusted cumulative incidence rates at 5 years were 7.80% (95% CI, 7.15%-8.43%) for SG and 15.83% (95% CI, 14.94%-16.71%) for RYGB. There were no differences in all-cause mortality between SG and RYGB.
Conclusions and Relevance
Interventions, operations, and hospitalizations were relatively common after bariatric surgical procedures and were more often associated with RYGB than SG.
Trial Registration
ClinicalTrials.gov identifier: NCT02741674
This large cohort study uses data from the US National Patient-Centered Clinical Research Network to assess for up to 5 years risks of intervention, operation, endoscopy, hospitalization, and mortality associated with Roux-en-Y gastric bypass or sleeve gastrectomy.
Introduction
Current and common bariatric procedures, including Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG), have been studied for short-term safety outcomes, although fewer studies address outcomes at or beyond 5 years of follow-up.1,2 In addition, a lack of standardized reporting of complications or adverse events after bariatric procedures makes it difficult to clearly establish overall and specific rates of postsurgical problems.3 Shared decision-making regarding bariatric surgery for patients with severe obesity has improved with more data on the comparative risks of postsurgical interventions, operations, and hospitalizations. Currently published data on short-term perioperative risks show a gradient of safety, with risk for mortality or adverse outcomes increasing from adjustable gastric banding (AGB) to SG to RYGB and then malabsorptive procedures (ie, biliopancreatic diversion with or without duodenal switch).4,5 However, emerging longer-term data suggest that AGB has a much higher rate of reoperation for reasons including device intolerance and failed weight loss, which have led to a decrease in the number of AGB procedures worldwide.6,7 The 2 most common bariatric operations currently performed are SG and RYGB,8 and they are the main focus of this report comparing data regarding problems occurring within 5 years after the original surgical procedure. Growing evidence shows that bariatric surgery is associated with greater weight loss and more effective initial type 2 diabetes remission than nonsurgical treatments in people with obesity.9,10,11 Thus, there is an urgent need for large and clinically relevant data sets using real-world clinical data to more completely identify the frequency of adverse outcomes and problems associated with bariatric procedures to balance growing information on the potential benefits of weight loss and metabolic improvements.12
The present cohort study uses a large, standardized distributed research network, the National Patient-Centered Clinical Research Network (PCORnet), to more fully inform health decision-making regarding the risks of bariatric surgery by leveraging big data for evidence gathering and potential future evidence generation.13,14 There are several unique aspects to the structure of this study and data set that can more effectively contribute to addressing some of these critical knowledge gaps, which include standardized definitions of the outcomes and completeness of follow-up in one of the largest cohorts of patients undergoing bariatric surgical procedures in the United States. Critical to a complete assessment of associated long-term adverse events is a link from electronic medical records to health insurance claims and state or national death indices, which captures major adverse events even if people do not follow up with their bariatric surgeons. The study is also geographically and demographically diverse, which improves the generalizability of the findings and allows for examination of treatment effect heterogeneity. The specific aim of this study was to compare the associated risk of operation and intervention after bariatric surgical procedures, with a focus on the most common procedures currently performed, SG and RYGB. Overall, data from this cohort will provide real-world evidence on the 5-year risks and benefits associated with the most commonly used bariatric procedures in current clinical practice.
Methods
Study Setting and Population
The PCORnet Bariatric Surgery study cohort and protocol were previously described.15 This study is a population-based cohort of patients who underwent 3 bariatric surgical procedures: AGB, RYGB, or SG. The comparative results of RYGB and SG are reported and discussed in the present study, and the data on AGB comparative results with SG and RYGB using the same methods are reported in the eAppendix in the Supplement. We identified adults 20 to 79 years of age who underwent a primary (first, nonrevisional) AGB, RYGB, or SG procedure between January 1, 2005, and September 30, 2015, in health systems participating in the Clinical Data Research Networks within PCORnet. The PCORnet uses a common data model to facilitate queries of standardized data. Cohort identification has been previously described, with bariatric procedures identified using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM); Current Procedural Terminology 4; and Healthcare Common Procedure Coding System codes from more than 100 million patient records.15 In the present study, we included only those health systems that linked their electronic health record databases to both insurance claims data and to state or national death indices to support more complete capture of major adverse events. Among sites that contribute bariatric cases to the network, 10 sites met this criterion, and their data are included in this report. Patient-level eligibility criteria are shown in the eFigure in the Supplement. Information was extracted on patient demographics, encounters with physicians and their teams, diagnoses recorded, procedures performed during these encounters, vital signs, and laboratory test results from patients’ electronic health records. The outcomes of interest for this specific analysis were the incidence of operation, intervention, endoscopy, and hospitalization collected after the initial bariatric procedure through September 30, 2015. This study was approved by the Institutional Review Board of Kaiser Permanente Washington Health Research Institute (lead site) and was approved or determined to be exempt from review by participating sites through individual institutional review board review or reliance agreements. Regarding human participation in this study, participating sites provided a waiver or authorization/consent consistent with The Common Rule and the Health Insurance Portability and Accountability Act or deemed the project exempt from institutional review board review under The Common Rule. No one received compensation or was offered any incentive for participating in this study.
Primary Outcome Definitions
A group of content experts consisting of 8 surgeons from both within (A.C., C.L.M., A.T., Marc P. Michalsky, MD, and Thomas Inge, MD, PhD) and outside the study, including leadership from the national bariatric professional society, met to generate evidence-based consensus on the definition of the primary outcome. Operation or intervention, the primary outcome, was identified using ICD-9-CM and Current Procedural Terminology 4 procedure codes and was a composite measure of 2 categories of events: operations and interventions. Operations were defined as any operative procedure on the abdomen, which included subcategories of revision (subsequent bariatric procedures [eg, conversion from SG to RYGB] as well as revisional procedures [eg, gastrectomy]), abdominal wall hernia repairs, and other operations (including internal hernias or paraesophageal/hiatal hernias and other abdominal operative procedures not captured under the prior categories and representing presumed complications). Biliary procedures, including cholecystectomy, were specifically excluded. Interventions were defined as any enteral access procedure (placement of gastrostomy tubes or other feeding devices either percutaneously or through other means of access) or any invasive but nonoperative procedure on the abdomen, such as paracentesis or radiologically guided drainage procedures that do not involve incision.
Secondary Outcome Definitions
Endoscopy was defined as any endoscopic procedure for diagnosis or treatment of the upper gastrointestinal tract. Hospitalization was defined as any inpatient hospitalization following bariatric surgery that was not associated with a delivery, miscarriage, or abortion procedure. All-cause mortality was defined as any death during the study period. Specific codes for cause of death were also collected.
Follow-up Times
Follow-up times were defined as the number of days after the initial surgical procedure until death or an 18-month lapse in health care, defined as 18 consecutive months without evidence of weight, blood pressure, any diagnosis or procedure codes, or any health care encounter. Mortality analyses were not censored for lapse in health care.
Statistical Analysis
For the primary analysis, a Cox proportional hazards model was used to estimate the adjusted hazards ratio (AHR) for the time to operation or to intervention for the comparison of RYGB vs SG. The baseline adjustment variables included body mass index (BMI) calculated as weight in kilograms divided by height in meters squared, demographic variables (age, race/ethnicity), the comorbidities listed in Table 1, and health system. Cox proportional hazards model estimates were also used to estimate the adjusted cumulative percentage of individuals with operation or intervention at 1, 3, and 5 years after surgery. We conducted exploratory analyses for potential heterogeneity in treatment effects across baseline patient characteristics (age <65, ≥65 years; sex; BMI<50, 50-60, or >60; diabetes at the time of surgery; Elixhauser comorbidity index score <0, 0, or >0; gastroesophageal reflux disease [GERD]; and race/ethnicity) by performing an analysis of variance test to determine the statistical significance of an interaction between procedure type and each characteristic of interest in the Cox proportional hazards regression model. Subgroups were chosen based on consensus from study stakeholders, including patients and physicians, and were considered exploratory. As a descriptive analysis, we also report the Kaplan-Meier estimated incidence rates of the first occurrence for each specific component type of operation or intervention and for endoscopy. These estimates were adjusted for censoring but not adjusted for covariates. Secondary exploratory analyses of time to event for all-cause mortality, hospitalization, endoscopy, and revision followed the same approach as the primary analysis. Revision, a component subcategory of operations in the primary outcome of operation or intervention, is of specific clinical interest in the field; thus, revision was addressed as a separate component outcome. All analyses were conducted from January 2018 through October 2019 using R, version 3.4.4 (R Core Team) software. A 2-sided P < .05 was considered statistically significant.
Table 1. Baseline Characteristics.
| Characteristic | Participants by Operation, No. (%) | All Participants (n = 33 560) | ||
|---|---|---|---|---|
| RYGB (n = 18 056) | SG (n = 15 504) | No. (%) | Standard Mean Difference | |
| Age, mean (SD), y | 45.8 (11.4) | 44.1 (11.5) | 45.0 (11.5) | 0.150 |
| Median | 46 | 44 | 45 | |
| Sex | ||||
| Female | 14 481 (80.2) | 12 493 (80.6) | 26 974 (80.4) | 0.010 |
| Male | 3575 (19.8) | 3011 (19.4) | 6586 (19.6) | |
| Race | ||||
| Asian | 186 (1.03) | 227 (1.5) | 413 (1.2) | 0.385 |
| Black | 2246 (12.4) | 3574 (23.1) | 5820 (17.3) | |
| Missing | 1950 (10.8) | 2675 (17.3) | 4625 (13.8) | |
| Multiple | 10 (0.1) | 10 (0.1) | 20 (0.1) | |
| Native | 161 (0.9) | 129 (0.8) | 290 (0.9) | |
| Other | 154 (0.9) | 73 (0.5) | 227 (0.7) | |
| Pacific Islander | 72 (0.4) | 80 (0.5) | 152 (0.4) | |
| White | 13 277 (73.5) | 8736 (56.4) | 22 013 (65.6) | |
| Hispanic ethnicity | ||||
| Missing | 1970 (10.9) | 1124 (7.3) | 3094 (9.2) | 0.284 |
| No | 12 432 (68.9) | 9399 (60.6) | 21 831 (65.1) | |
| Yes | 3654 (20.2) | 4981 (32.1) | 8635 (25.7) | |
| BMI, mean (SD) | 49.7 (8.1) | 48.5 (7.6) | 49.1 (7.9) | 0.154 |
| Median | 48.1 | 46.8 | 47.5 | |
| Weight, mean (SD), kg | 127.9 (25.6) | 123.6 (24.5) | 125.9 (25.2) | 0.175 |
| Median | 123.6 | 118.9 | 121.6 | |
| Procedure year | ||||
| 2005-2009 | 4038 (22.4) | 412 (2.7) | 4450 (13.3) | 0.776 |
| 2010 | 2840 (15.7) | 1085 (7.0) | 3925 (11.7) | |
| 2011 | 3300 (18.3) | 2886 (18.6) | 6186 (18.4) | |
| 2012 | 2573 (14.3) | 2978 (19.2) | 5551 (16.5) | |
| 2013 | 1824 (10.1) | 2651 (17.1) | 4475 (13.3) | |
| 2014 | 1907 (10.6) | 2921 (18.8) | 4828 (14.4) | |
| 2015 | 1574 (8.7) | 2571 (16.6) | 4145 (12.4) | |
| Comorbidity | ||||
| Anxiety | 3891 (21.6) | 3248 (21.0) | 7139 (21.3) | 0.015 |
| Depression | 6361 (35.2) | 4288 (27.7) | 10 649 (31.7) | 0.164 |
| Diabetes | 8018 (44.4) | 4094 (26.4) | 12 112 (36.1) | 0.383 |
| DVT | 122 (0.7) | 56 (0.4) | 178 (0.5) | 0.044 |
| Dyslipidemia | 9782 (54.2) | 6991 (45.1) | 16 773 (50.0) | 0.182 |
| Eating disorder | 3421 (19.0) | 450 (2.9) | 3871 (11.5) | 0.532 |
| GERD | 7925 (43.9) | 4463 (28.8) | 12 388 (36.9) | 0.318 |
| Hypertension | 11 220 (62.1) | 7897 (50.9) | 19 117 (57.0) | 0.227 |
| Kidney disease | 1976 (10.9) | 1168 (7.5) | 3144 (9.4) | 0.118 |
| NAFLD | 4534 (25.1) | 1688 (10.9) | 6222 (18.5) | 0.377 |
| Osteoarthritis | 438 (2.4) | 325 (2.1) | 763 (2.3) | 0.022 |
| PE | 258 (1.4) | 108 (0.7) | 366 (1.1) | 0.071 |
| Sleep apnea | 10 017 (55.5) | 6067 (39.1) | 16 084 (47.9) | 0.332 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DVT, deep vein thrombosis; GERD, gastroesophageal reflux disease; NAFLD, nonalcoholic fatty liver disease; PE, pulmonary embolism; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy.
Results
There were 33 560 adults at 10 centers in 4 clinical data research networks that met the eligibility criteria for this study (eFigure in the Supplement). Overall, the mean (SD) age was 45.0 (11.5) years, BMI was 49.1 (7.9), and weight was 125.9 (25.2) kg. The cohort was predominantly female (80%) and white (66%), with 17% black participants and 26% of Hispanic ethnicity (Table 1). Among them, 18 056 (54%) underwent RYGB, and 15 504 (46%) underwent SG, with more SG procedures being performed during each consecutive year of the study.15 Overall, 36% had diabetes, 50% had dyslipidemia, 57% had hypertension, 37% had GERD, 48% had sleep apnea, and 32% had depression (Table 1). The SG group was slightly younger (mean [SD]: 44.1 [11.5] years vs 45.8 [11.4] years), with more ethnic and racial diversity and lower rates of diabetes (26.4% vs 44.4%) (Table 1). Median (interquartile range) follow-up for the primary outcome (operation or intervention) was 3.4 (1.6-5.0) years for RYGB and 2.2 (0.9-3.6) years for SG.
Primary Outcome
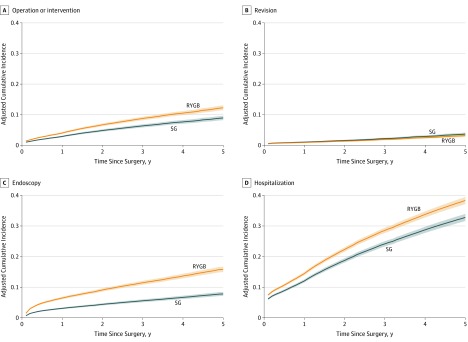
For the primary outcome, the hazard ratios (HRs) comparing RYGB vs SG procedures for time until operation or intervention are given in Table 2. Operation or intervention was less likely for SG than RYGB (HR, 0.72; 95% CI, 0.65-0.79; P < .001). Accordingly, the estimated cumulative probability (95% CI) of operation or intervention was lower for SG (2.89%; 95% CI, 2.65%-3.14%) at 1 year, 6.35% (95% CI, 5.85%-6.85%) at 3 years, and 8.94% (95% CI, 8.23%-9.65%) at 5 years, than for RYGB (4.02%; 95% CI, 3.74%-4.30%) at 1 year, 8.76% (95% CI, 8.22%-9.30%) at 3 years, and 12.27% (95% CI, 11.49%-13.05%) at 5 years (Table 3 and Figure, A). Revision appeared more common after SG than after RYGB (HR, 1.17; 95% CI, 0.98-1.39; P = .09] (Table 2 and Figure, B), but this difference was not significant. The composite primary outcome of operation or intervention was more likely following AGB compared with either RYGB or SG (eAppendix in the Supplement). Kaplan-Meier estimates of the cumulative incidence rates of each of the components of operation or intervention are given in eTable 1 in the Supplement and in the eAppendix in the Supplement for AGB. By 5 years, 13.6% of those who had RYGB and 8.3% of those who had SG underwent an additional operation. For RYGB, 2.8% underwent a revision by 5 years, whereas 4.0% underwent a revision for SG. Rates of intervention, both enteral access and nonoperative procedures, were low (≤1.3%) (eTable 1 in the Supplement).
Table 2. Adjusted Hazard Ratios for Comparison of Different Events for SG vs RYGB.
| Outcome | AHR (95% CI) | P Value |
|---|---|---|
| Operation or intervention excluding endoscopy | 0.715 (0.646-0.793) | <.001 |
| Endoscopy | 0.471 (0.426-0.520) | <.001 |
| Revision | 1.165 (0.976-1.392) | .09 |
| Hospitalization | 0.822 (0.781-0.866) | <.001 |
| Mortality | 0.942 (0.725-1.225) | .66 |
Abbreviations: AHR, adjusted hazard ratio; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy.
Table 3. Estimated Percentages of Patients With Outcome Event at Specified Time.
| Outcome | Estimated % (95% CI) | ||
|---|---|---|---|
| At 1 y | At 3 y | At 5 y | |
| Operation or intervention excluding endoscopy | |||
| RYGB | 4.02 (3.74-4.30) | 8.76 (8.22-9.30) | 12.27 (11.49-13.05) |
| SG | 2.89 (2.65-3.14) | 6.35 (5.85-6.85) | 8.94 (8.23-9.65) |
| Endoscopy | |||
| RYGB | 6.46 (6.08-6.84) | 11.46 (10.83-12.08) | 15.83 (14.94-16.71) |
| SG | 3.10 (2.84-3.36) | 5.57 (5.12-6.02) | 7.80 (7.15-8.43) |
| Revision | |||
| RYGB | 0.90 (0.78-1.02) | 1.88 (1.65-2.12) | 3.16 (2.75-3.56) |
| SG | 1.05 (0.90-1.19) | 2.19 (1.91-2.47) | 3.67 (3.18-4.16) |
| Hospitalization | |||
| RYGB | 14.44 (13.94-14.95) | 28.55 (27.69-29.40) | 38.33 (37.17-39.46) |
| SG | 12.04 (11.55-12.53) | 24.15 (23.29-25.00) | 32.79 (31.62-33.94) |
| Mortality | |||
| RYGB | 0.17 (0.13-0.21) | 0.45 (0.36-0.54) | 0.89 (0.71-1.06) |
| SG | 0.16 (0.12-0.20) | 0.43 (0.33-0.52) | 0.84 (0.65-1.02) |
Abbreviations: RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy.
Figure. Cumulative Incidence Rates of Operation or Intervention, Revision, Endoscopy, and Hospitalization.
RYGB indicates Roux-en-Y gastric bypass; SG, sleeve gastrectomy; solid lines, estimated cumulative probabilities; and shading, 95% CIs.
Heterogeneity of Treatment Effects for Primary Outcome
Heterogeneity of treatment effects examines whether there is evidence of a differential effect of bariatric procedures associated with the primary outcome, operation or intervention, across predefined subgroups of interest. There was no evidence of heterogeneity of treatment effects for RYGB vs SG across subgroups for age, sex, diabetes status, or GERD status (Table 4). There was evidence of heterogeneity of treatment effects across other subgroups, namely, those defined by BMI, baseline comorbidity score, or race/ethnicity (Table 4). Lower baseline BMI categories showed more relative benefit of SG over RYGB for the primary outcome (eg, AHR, 0.71; 95% CI, 0.63-0.80 for BMI <50 vs AHR, 0.78; 95% CI, 0.54-1.10 for BMI >60; P = .007). For baseline comorbidity scores, the relative benefit for SG was smaller for the highest comorbidity group (eg, for comorbidity <0: AHR, 0.69; 95% CI, 0.59-0.81 vs for comorbidity >0: AHR, 0.81; 95% CI, 0.67-1.00; P < .001). For race and ethnicity, the relative benefit with SG was slightly greater for those with Hispanic ethnicity (white patients: AHR, 0.69; 95% CI, 0.60-0.79; black patients: AHR, 0.69; 95% CI, 0.56-0.85; Hispanic patients: AHR, 0.63; 95% CI, 0.52-0.76; other patients: AHR, 0.68; 95% CI, 0.37-1.27; P < .001) (Table 4).
Table 4. Heterogeneity of Treatment Effects.
| Evidence of Heterogeneous Treatment Effect of Procedures | Estimated % of Patients (95% CI) | ||
|---|---|---|---|
| At 1 y | At 3 y | At 5 y | |
| Age at Baseline | <65 y | ≥65 y | |
| SG vs RYGB, AHR (95% CI) | 0.711 (0.640-0.790) | 0.783 (0.562-1.089) | |
| RYGB | |||
| <65 y | 3.95 (3.67-4.23) | 8.61 (8.06-9.15) | 12.06 (11.28-12.84) |
| ≥65 y | 5.61 (4.59-6.62) | 12.10 (9.99-14.16) | 16.82 (13.94-19.61) |
| SG | |||
| <65 y | 2.83 (2.58-3.07) | 6.20 (5.70-6.70) | 8.74 (8.01-9.45) |
| ≥65 y | 4.42 (3.18-5.64) | 9.61 (6.98-12.15) | 13.42 (9.81-16.9) |
| Sex | Female | Male | |
| SG vs RYGB, AHR (95% CI) | 0.699 (0.625-0.782) | 0.784 (0.642-0.959) | |
| RYGB | |||
| Female | 4.04 (3.75-4.34) | 8.81 (8.23-9.39) | 12.34 (11.52-13.17) |
| Male | 3.93 (3.46-4.40) | 8.58 (7.60-9.54) | 12.02 (10.66-13.36) |
| SG | |||
| Female | 2.84 (2.58-3.11) | 6.25 (5.70-6.79) | 8.80 (8.02-9.57) |
| Male | 3.10 (2.57-3.62) | 6.79 (5.68-7.89) | 9.56 (8.00-11.09) |
| Baseline BMIa | <50 | 50-60 | >60 |
| SG vs RYGB, AHR (95% CI) | 0.708 (0.632-0.795) | 0.746 (0.610-0.913) | 0.775 (0.543-1.105) |
| RYGB | |||
| <50 | 3.90 (3.60-4.19) | 8.50 (7.92-9.08) | 11.91 (11.08-12.74) |
| 50-60 | 4.29 (3.83-4.74) | 9.32 (8.4-10.24) | 13.04 (11.74-14.32) |
| >60 | 4.78 (3.95-5.6) | 10.36 (8.63-12.05) | 14.45 (12.06-16.76) |
| SG | |||
| <50 | 2.78 (2.51-3.04) | 6.10 (5.56-6.65) | 8.59 (7.82-9.37) |
| 50-60 | 3.22 (2.66-3.77) | 7.05 (5.86-8.22) | 9.90 (8.24-11.53) |
| >60 | 3.72 (2.57-4.86) | 8.12 (5.66-10.52) | 11.39 (7.97-14.67) |
| Diabetes | No | Yes | |
| SG vs RYGB, AHR (95% CI) | 0.678 (0.600-0.765) | 0.796 (0.68-0.931) | |
| RYGB | |||
| No diabetes | 4.11 (3.78-4.45) | 8.96 (8.29-9.62) | 12.54 (11.59-13.47) |
| Diabetes | 3.90 (3.55-4.25) | 8.51 (7.80-9.21) | 11.92 (10.92-12.91) |
| SG | |||
| No diabetes | 2.81 (2.53-3.08) | 6.16 (5.58-6.74) | 8.68 (7.86-9.49) |
| Diabetes | 3.12 (2.68-3.55) | 6.83 (5.90-7.75) | 9.61 (8.31-10.89) |
| Baseline Comorbidity Index Scoreb | <0 | 0 | >0 |
| SG vs RYGB, AHR (95% CI) | 0.689 (0.587-0.809) | 0.702 (0.610-0.807) | 0.813 (0.665-0.995) |
| RYGB | |||
| Comorbidity <0 | 3.81 (3.40-4.23) | 8.33 (7.47-9.18) | 11.68 (10.47-12.87) |
| Comorbidity = 0 | 4.07 (3.72-4.42) | 8.88 (8.17-9.58) | 12.43 (11.43-13.42) |
| Comorbidity >0 | 4.27 (3.73-4.80) | 9.30 (8.18-10.40) | 13.01 (11.46-14.53) |
| SG | |||
| Comorbidity <0 | 2.64 (2.26-3.02) | 5.82 (5.00-6.62) | 8.20 (7.05-9.33) |
| Comorbidity = 0 | 2.87 (2.54-3.21) | 6.32 (5.61-7.02) | 8.90 (7.90-9.88) |
| Comorbidity >0 | 3.49 (2.87-4.10) | 7.63 (6.32-8.93) | 10.72 (8.89-12.51) |
| GERD | No | Yes | |
| SG vs RYGB, AHR (95% CI) | 0.716 (0.633-0.811) | 0.724 (0.622-0.843) | |
| RYGB | |||
| No GERD | 3.84 (3.53-4.15) | 8.38 (7.76-9.00) | 11.75 (10.86-12.63) |
| GERD | 4.30 (3.94-4.66) | 9.36 (8.63-10.08) | 13.09 (12.06-14.11) |
| SG | |||
| No GERD | 2.77 (2.49-3.04) | 6.08 (5.50-6.65) | 8.56 (7.74-9.38) |
| GERD | 3.13 (2.71-3.55) | 6.87 (5.98-7.74) | 9.66 (8.42-10.88) |
| Race/Ethnicityb,c | White, Non-Hispanic | Black, Non-Hispanic | Hispanic |
| SG vs RYGB, AHR (95% CI) | 0.687 (0.599-0.786) | 0.694 (0.564-0.854) | 0.628 (0.523-0.755) |
| RYGB | |||
| White, non-Hispanic | 3.95 (3.61-4.28) | 8.60 (7.92-9.26) | 12.05 (11.10-13.00) |
| Black, non-Hispanic | 4.45 (3.82-5.08) | 9.65 (8.34-10.94) | 13.50 (11.69-15.28) |
| Hispanic | 4.05 (3.54-4.56) | 8.81 (7.75-9.87) | 12.35 (10.87-13.81) |
| Other, non-Hispanic | 4.07 (2.66-5.46) | 8.85 (5.84-11.76) | 12.40 (8.24-16.38) |
| SG | |||
| White, non-Hispanic | 3.05 (2.69-3.40) | 6.67 (5.93-7.41) | 9.40 (8.35-10.43) |
| Black, non-Hispanic | 2.96 (2.48-3.44) | 6.48 (5.46-7.48) | 9.13 (7.71-10.53) |
| Hispanic | 2.68 (2.28-3.08) | 5.89 (5.04-6.73) | 8.30 (7.11-9.49) |
| Other, non-Hispanic | 2.65 (1.31-3.97) | 5.81 (2.90-8.63) | 8.20 (4.12-12.11) |
Abbreviations: AHR, adjusted hazard ratio; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); GERD, gastroesophageal reflux disease; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy.
P < .01 for heterogeneity of treatment effects.
P < .001 for heterogeneity of treatment effects.
With an AHR of 0.682 (95% CI, 0.367-1.266) for other, non-Hispanic.
Secondary Outcomes
Endoscopy
Endoscopy for any reason (diagnostic or therapeutic) was less likely for SG vs RYGB (HR, 0.47; 95% CI, 0.43-0.52; P < .001) (Table 2 and Figure, C). The corresponding cumulative rates of endoscopy for SG were 3.10% (95% CI, 2.84%-3.36%) at 1 year, 5.57% (95% CI, 5.12%-6.02%) at 3 years, and 7.80% (95% CI, 7.15%-8.43%) at 5 years, and for RYGB they were 6.46% (95% CI, 6.08%-6.84%) at 1 year, 11.46% (95% CI, 10.83%-12.08%) at 3 years, and 15.83% (95% CI, 14.94%-16.71%) at 5 years (Table 3).
Hospitalization
Hospitalization was less likely after SG than after RYGB (HR, 0.82; 95% CI, 0.78-0.87; P < .001) (Table 2). The estimated, adjusted cumulative incidence rates of hospitalization for SG were 12.04% (95% CI, 11.55%-12.53%) at 1 year, 24.15% (95% CI, 23.29%-25.00%) at 3 years, and 32.79% (95% CI, 31.62%-33.94%) at 5 years, and for RYGB the rates were 14.44% (95% CI, 13.94%-14.95%) at 1 year, 28.55% (95% CI, 27.69%-29.40%) at 3 years, and 38.33% (95% CI, 37.17%-39.46%) at 5 years (Table 3 and Figure, D).
Mortality
There were no significant differences in the HR for time to total all-cause mortality between surgical procedures (SG vs RYGB: HR, 0.94; 95% CI, 0.73-1.23; P = .66) (Table 2). In addition, the estimated, adjusted cumulative risk of all-cause mortality at 5 years was less than 1% for both surgical procedures (Table 3). The 2 most common specified causes of death were cardiovascular disease and malignant neoplasm/cancer (eTable 2 in the Supplement). Substance or alcohol use, unintentional injury, and self-harming behaviors were among the 15 most frequent specified causes of death recorded in this cohort (eTable 2 in the Supplement).
Discussion
As the literature about the improvements in weight, metabolic, and other health outcomes following bariatric surgery has grown, so too has the demand for information on the later rates of operations, interventions, hospitalizations, and mortality to support informed decision-making. In the present cohort study of 33 560 individuals undergoing the 2 most common bariatric surgical procedures in 10 health systems, we showed that the 5-year risk for operation or intervention is greater for RYGB when compared with SG. Only 1 component of operation or intervention, the revision of a bariatric procedure, appeared more common with SG than with RYGB, although this difference was not statistically significant. There was also evidence of heterogeneous treatment effects for the primary outcome for procedure comparisons across several subgroups, including BMI, baseline comorbidity score, and race/ethnicity, such that the benefit of SG over RYGB for the primary outcome was (1) greater with lower BMI, (2) greater for patients with lower comorbidity, and (3) slightly greater for those with Hispanic ethnicity. In addition, endoscopy procedures and hospitalizations were more common among patients who underwent RYGB than among those who underwent SG, and the estimated rates of hospitalization at 5 years for SG and RYGB were 32.8% and 38.3%, respectively. There were no significant differences in total all-cause mortality between procedures, 5-year mortality rates were low, and the most common causes of death were cardiovascular and cancer.
Prior studies have shown that people undergoing bariatric surgery experience a reduction in all-cause, long-term mortality compared with people receiving usual care.16,17,18 In those studies, the reduction in mortality was not observed until 5 or more years after surgery. In the study by Arterburn et al,18 at follow-up of 5 and 10 years, all-cause mortality was 6.4% and 13.8% for patients who underwent bariatric surgery compared with 10.4% and 23.9% for matched nonsurgical controls (HR, 0.45 and 0.47), respectively. Of note, mortality studies of bariatric surgery have been carried out mostly in patients undergoing RYGB and not SG. The present study included up to 5 years’ follow-up and showed both low and similar mortality rates associated with SG and RYGB. Additional years of follow-up for this cohort will be required to address whether any mortality differences between surgical procedures will emerge.
The incidence of morbidity and complications after the various bariatric surgical procedures varies and depends on the type of study, specific bariatric procedure performed, definition of the complication applied, and patient characteristics.19,20,21 In 11 published randomized clinical trials including all surgical procedure types with 794 total patients that have compared bariatric surgery to nonsurgical treatment, rates of adverse events were higher among surgical patients, with follow-up to 5 years for 2 of the studies and up to 2 to 3 years for the other studies,11,22,23,24 yet the total sample size was not large enough to compare the safety outcomes between surgical procedures. Observational cohort studies of short-term and longer-term safety and problems that occur after bariatric surgery mostly measure rates of reoperation, reintervention, and hospitalization.25,26 Cohort studies at 1 to 5 years’ follow-up show rates of reoperation following bariatric surgery ranging from 5% to 22% and are higher after RYGB compared with SG.27,28,29 A recent study evaluating 5-year outcomes for 35 273 patients who underwent bariatric surgery in an integrated health care system in California and Washington showed a lower risk of any intervention in the SG cohort (21%) compared with those undergoing RYGB (28%) (AHR, 0.78; 95% CI, 0.74-0.84; P < .0001), which was primarily attributed to higher rates of endoscopic interventional procedures in the RYGB cohort.29 Specifically, rates of reoperation were also slightly higher in the cohort that underwent RYGB vs SG, with a risk difference of only 1% (17.8% for RYGB vs 16.8% for SG). Two recent randomized clinical trials comparing SG vs RYGB at the 5-year follow-up showed similar rates of reoperation and reintervention in small but well-studied cohorts.30,31 In the SM-BOSS study,30 107 patients were randomized to SG and 110 to RYGB, and rates of reoperation/reintervention did not significantly differ between the 2 surgical groups (16% for SG vs 22% for RYGB). If the operation or intervention and endoscopy rates in the present study are combined for each procedure at 5 years, those rates are 16% for SG and 28% for RYGB and are thus very similar to the rates observed in the SM-BOSS study of all reoperations and reinterventions, including endoscopy. In the SLEEVEPASS study31 in which reoperation rates included only those for GERD and for internal or incisional/abdominal wall hernia, the 5-year reoperation rates were 10% for SG vs 18% for RYGB, again very similar to the operation or intervention (without endoscopy) rates observed in this study (9% for SG and 12% for RYGB).32 These comparisons highlight that the rates of problems occurring after bariatric surgery differ depending on the definitions used; thus, there is an urgent need to standardize reporting3 to enable more accurate comparisons between studies.
There are a few other studies examining RYGB with follow-up at 10 years, and their reported rates of reoperation ranged from 2% to 60%.27,28,33 Among 2 studies of SG with at least 10 years’ follow-up, rates of reoperation were 32% and 36%.33 For SG—now the most commonly performed surgical procedure—a recent meta-analysis of longer-term (7-year) outcomes estimated a surgical revision/conversion rate of 20% owing to either weight re-gain or GERD.34 In the present study, the only single component of operation or intervention that was slightly greater after SG compared with RYGB was revision/conversion of the SG procedure, the reason(s) for which could not be determined. Longer-term observation of these revision/conversion rates will be important data for comparative effectiveness between surgical procedures. The present data were gathered from clinical care in the real world, yet the results are comparable to controlled studies and therefore lend additional support to the findings of these other types of studies that operation and intervention occur less commonly after SG than after RYGB for up to 5 years.
The present study also sought to identify heterogeneity in the primary outcome, operation or intervention, across clinical subgroups. We found no evidence of heterogeneity of treatment effects for RYGB vs SG across subgroups for age, sex, diabetes status, or GERD status. Gastroesophageal reflux disease was of interest because of the concern for potential future development of incident or worsening GERD and symptomatic hiatal hernia after SG.35 Nevertheless, we did not observe any differences by procedure type based on GERD status, at least up to the present follow-up time. The benefit of SG over RYGB for the primary outcome was higher among patients with lower baseline BMI (eg, <50) and in those with lower comorbidity burden. These findings suggest that people with higher BMI or more comorbid disease do better with SG than with RYGB, but people with lower BMI or less comorbid diseases do even better with SG than RYGB. This finding is counterintuitive to that of clinical practice because SG is often described as the preferred procedure for patients with higher BMI and comorbid burden. Our study was not designed to provide mechanistic explanations for these findings, and we also viewed these analyses as exploratory in nature; thus, they warrant further confirmation in future studies. The benefit of SG over RYGB for the primary outcome was only slightly greater for those with Hispanic ethnicity; therefore, the clinical importance of this finding is unclear. Other studies have found differences in weight and metabolic bariatric surgical outcomes based on racial/ethnic backgrounds,36,37 but less has been published about potential differences with respect to complications and adverse outcomes.
Understanding the reasons for higher rates of endoscopy after RYGB vs after SG observed in the present study will be important to monitor for longer-term follow-up and is a potential target for further study. The estimated cumulative hospitalization rates at 5 years in the present study were rather high, approximately 30% and 40% for SG and RYGB, respectively. The reasons for these hospitalizations were not specified; they may reflect admissions to manage complications but may also reflect care for chronic conditions that may not have been addressed previously because of excess weight (eg, joint replacement or hernia repair). Nevertheless, these global rates of rehospitalization should be factored into patient decision-making processes regarding bariatric surgery because they may contribute to patient dissatisfaction if expectations are not appropriately set. In addition, there are the consequences of increased health care use and costs after surgery. In another large study, rates of rehospitalization 1 to 3 years after bariatric surgery were more than twice the rates in the prior year and were associated with or directly attributable to the bariatric procedure.38 In addition, when patients who undergo RYGB surgery are compared with controls, the former experience more adverse clinical outcomes but have overall decreased comorbid health conditions associated with obesity.39 These observations highlight the importance of increasing information on both sides of the risk-vs-benefit equation. However, a larger proportion of the bariatric surgery literature addresses the weight, health, and other improvements that can occur during postoperative follow-up. The present report contributes additional data to findings about the potential risks, with more information about the probability of hospitalization in the 5 years after surgery, and enables comparisons between surgical procedures.
An earlier publication from the same national cohort used in the present study also addresses 30-day safety outcomes, detailed benefits, and comparative effectiveness of weight loss following bariatric surgery.40 In that study of more than 65 000 patients, adults undergoing RYGB lost more weight than those undergoing SG or AGB at 1, 3, and 5 years; however, RYGB had the highest 30-day rate of major adverse events (5.0% for RYGB, 2.6% for SG, and 2.9% for AGB). One-year mean total weight losses were 31.2% (95% CI, 31.1%-31.3%) for RYGB, 25.2% (95% CI, 25.1%-25.4%) for SG, and 13.7% (95% CI, 13.3%-14.0%) for AGB. Five-year mean total weight losses were 25.5% (95% CI, 25.1%-25.9%) for RYGB, 18.8% (95% CI, 18.0%-19.6%) for SG, and 11.7% (95% CI, 10.2%-13.1%) for AGB. The weight loss among patients with diabetes, those with BMI less than 50, those aged 65 years or older, African American individuals, and Hispanic individuals was slightly less than among patients without those characteristics.40
Limitations and Strengths
The limitations of this study included that it was nonrandomized and that it risked residual unmeasured confounding, including confounding by indication, which may have persisted despite covariate adjustment. In addition, comorbid health conditions and the score calculations were performed using ICD-9-CM diagnosis codes, which may have underestimated comorbidity prevalence rates and therefore disease severity. We could not consider correlations of outcomes by surgeon, and data linkages between electronic health records and claims were not validated; thus, we do not know whether all sites had complete capture of claims data. In addition, we did not consider the reason for the operation or intervention event; therefore, the data may include major abdominal interventions that could be performed for a reason unrelated to bariatric surgery (eg, trauma, plastic surgery). However, there is no reason to believe that nonbariatric abdominal surgery would be differentially performed across bariatric procedure populations; thus, risk of bias in comparisons is low. The SG procedure was originally introduced as the first part of a 2-stage procedure. In the present study, the planned second stage procedure would be considered an operation or intervention outcome. Despite this, the rates of operation or intervention were lower for SG than for RYGB. In addition, because time trends in procedure evolution showed a later increased use of SG in the present cohort, overall follow-up time was less in this group, limiting the SG sample size at 5 years. Future studies should confirm these findings in SG populations with longer-term follow-up for later complications, such as symptomatic hiatal hernia and reflux.41 In addition to the primary analysis of the present study, we also examined several secondary analyses without multiple testing adjustment; thus, these results should be considered hypothesis generating.
A major strength of this study was that it was one of the largest bariatric surgery cohorts ever studied, examining in depth the problems that occurred after surgical procedures, including a large number of SG cases, which is currently the most common bariatric operation. In addition, there was more ethnic and racial diversity in this cohort than is typical in bariatric surgery studies, suggesting that the results may be more generalizable. This study also adds to the knowledge on the frequency and types of operations, interventions, and hospitalizations associated with 2 different bariatric surgical procedures. This knowledge will inform decision-making about and between the 2 most commonly used bariatric surgical procedures. In addition, this project is the first of 2 demonstration projects from the Clinical Data Research Networks within PCORnet. Therefore, the present study has established a platform within which clinical trials in bariatric surgery could be facilitated in the future by and within this large data set.14,15
Conclusions
This study showed that problems, including interventions, operations, and hospitalizations, were relatively common after bariatric surgical procedures and were more often associated with RYGB than with SG. The collection of data from midterm to longer-term events was feasible and could be carried out effectively in large data sets linked to insurance claims and mortality data. This information, balanced with the weight loss40 and health outcome results from this study, will help inform procedure-specific decision-making for prospective patients and physicians.
eFigure. Flow Diagram for Identification of the Cohort
eTable 1. Kaplan-Meier Estimated Cumulative Incidence of Operations, Interventions, and Endoscopy
eTable 2. Causes of Death
eAppendix. Results for Adjustable Gastric Band (AGB)
References
- 1.Courcoulas AP, Yanovski SZ, Bonds D, et al. . Long-term outcomes of bariatric surgery: a National Institutes of Health symposium. JAMA Surg. 2014;149(12):1323-1329. doi: 10.1001/jamasurg.2014.2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfe BM, Belle SH. Long-term risks and benefits of bariatric surgery: a research challenge. JAMA. 2014;312(17):1792-1793. doi: 10.1001/jama.2014.12966 [DOI] [PubMed] [Google Scholar]
- 3.Brethauer SA, Kim J, el Chaar M, et al. . Standardized outcomes reporting in metabolic and bariatric surgery. Surg Obes Relat Dis. 2015;11(3):489-506. doi: 10.1016/j.soard.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 4.Khorgami Z, Andalib A, Aminian A, Kroh MD, Schauer PR, Brethauer SA. Predictors of readmission after laparoscopic gastric bypass and sleeve gastrectomy: a comparative analysis of ACS-NSQIP database. Surg Endosc. 2016;30(6):2342-2350. doi: 10.1007/s00464-015-4477-2 [DOI] [PubMed] [Google Scholar]
- 5.Hutter MM, Schirmer BD, Jones DB, et al. . First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg. 2011;254(3):410-420. doi: 10.1097/SLA.0b013e31822c9dac [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Brien PE, MacDonald L, Anderson M, Brennan L, Brown WA. Long-term outcomes after bariatric surgery: fifteen-year follow-up of adjustable gastric banding and a systematic review of the bariatric surgical literature. Ann Surg. 2013;257(1):87-94. doi: 10.1097/SLA.0b013e31827b6c02 [DOI] [PubMed] [Google Scholar]
- 7.Courcoulas AP, King WC, Belle SH, et al. . Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018;153(5):427-434. doi: 10.1001/jamasurg.2017.5025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.English WJ, DeMaria EJ, Brethauer SA, Mattar SG, Rosenthal RJ, Morton JM. American Society for Metabolic and Bariatric Surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surg Obes Relat Dis. 2018;14(3):259-263. doi: 10.1016/j.soard.2017.12.013 [DOI] [PubMed] [Google Scholar]
- 9.Buchwald H, Estok R, Fahrbach K, et al. . Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248-256. doi: 10.1016/j.amjmed.2008.09.041 [DOI] [PubMed] [Google Scholar]
- 10.Brethauer SA, Aminian A, Romero-Talamás H, et al. . Can diabetes be surgically cured? long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg. 2013;258(4):628-636. doi: 10.1097/SLA.0b013e3182a5034b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schauer PR, Bhatt DL, Kirwan JP, et al. ; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376(7):641-651. doi: 10.1056/NEJMoa1600869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arterburn DE, Courcoulas AP. Bariatric surgery for obesity and metabolic conditions in adults. BMJ. 2014;349:g3961. doi: 10.1136/bmj.g3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frieden TR. Evidence for health decision making—beyond randomized, controlled trials. N Engl J Med. 2017;377(5):465-475. doi: 10.1056/NEJMra1614394 [DOI] [PubMed] [Google Scholar]
- 14.Angus DC. Fusing randomized trials with big data: the key to self-learning health care systems? JAMA. 2015;314(8):767-768. doi: 10.1001/jama.2015.7762 [DOI] [PubMed] [Google Scholar]
- 15.Toh S, Rasmussen-Torvik LJ, Harmata EE, et al. ; PCORnet Bariatric Surgery Collaborative . The National Patient-Centered Clinical Research Network (PCORnet) bariatric study cohort: rationale, methods, and baseline characteristics. JMIR Res Protoc. 2017;6(12):e222. doi: 10.2196/resprot.8323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams TD, Gress RE, Smith SC, et al. . Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753-761. doi: 10.1056/NEJMoa066603 [DOI] [PubMed] [Google Scholar]
- 17.Maciejewski ML, Livingston EH, Smith VA, et al. . Survival among high-risk patients after bariatric surgery. JAMA. 2011;305(23):2419-2426. doi: 10.1001/jama.2011.817 [DOI] [PubMed] [Google Scholar]
- 18.Arterburn DE, Olsen MK, Smith VA, et al. . Association between bariatric surgery and long-term survival. JAMA. 2015;313(1):62-70. doi: 10.1001/jama.2014.16968 [DOI] [PubMed] [Google Scholar]
- 19.Encinosa WE, Bernard DM, Du D, Steiner CA. Recent improvements in bariatric surgery outcomes. Med Care. 2009;47(5):531-535. doi: 10.1097/MLR.0b013e31819434c6 [DOI] [PubMed] [Google Scholar]
- 20.Birkmeyer NJ, Dimick JB, Share D, et al. ; Michigan Bariatric Surgery Collaborative . Hospital complication rates with bariatric surgery in Michigan. JAMA. 2010;304(4):435-442. doi: 10.1001/jama.2010.1034 [DOI] [PubMed] [Google Scholar]
- 21.Finks JF, Kole KL, Yenumula PR, et al. ; Michigan Bariatric Surgery Collaborative from the Center for Healthcare Outcomes and Policy . Predicting risk for serious complications with bariatric surgery: results from the Michigan Bariatric Surgery Collaborative. Ann Surg. 2011;254(4):633-640. doi: 10.1097/SLA.0b013e318230058c [DOI] [PubMed] [Google Scholar]
- 22.Ikramuddin S, Korner J, Lee WJ, et al. . Durability of addition of Roux-en-Y gastric bypass to lifestyle intervention and medical management in achieving primary treatment goals for uncontrolled type 2 diabetes in mild to moderate obesity: a randomized control trial. Diabetes Care. 2016;39(9):1510-1518. doi: 10.2337/dc15-2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mingrone G, Panunzi S, De Gaetano A, et al. . Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386(9997):964-973. doi: 10.1016/S0140-6736(15)00075-6 [DOI] [PubMed] [Google Scholar]
- 24.Courcoulas AP, Belle SH, Neiberg RH, et al. . Three-year outcomes of bariatric surgery vs lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg. 2015;150(10):931-940. doi: 10.1001/jamasurg.2015.1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sudan R, Maciejewski ML, Wilk AR, Nguyen NT, Ponce J, Morton JM. Comparative effectiveness of primary bariatric operations in the United States. Surg Obes Relat Dis. 2017;13(5):826-834. doi: 10.1016/j.soard.2017.01.021 [DOI] [PubMed] [Google Scholar]
- 26.Daigle CR, Brethauer SA, Tu C, et al. . Which postoperative complications matter most after bariatric surgery? prioritizing quality improvement efforts to improve national outcomes. Surg Obes Relat Dis. 2018;14(5):652-657. doi: 10.1016/j.soard.2018.01.008 [DOI] [PubMed] [Google Scholar]
- 27.Mehaffey JH, LaPar DJ, Clement KC, et al. . 10-Year outcomes after Roux-en-Y gastric bypass. Ann Surg. 2016;264(1):121-126. doi: 10.1097/SLA.0000000000001544 [DOI] [PubMed] [Google Scholar]
- 28.Obeid NR, Malick W, Concors SJ, Fielding GA, Kurian MS, Ren-Fielding CJ. Long-term outcomes after Roux-en-Y gastric bypass: 10- to 13-year data. Surg Obes Relat Dis. 2016;12(1):11-20. doi: 10.1016/j.soard.2015.04.011 [DOI] [PubMed] [Google Scholar]
- 29.Li RA, Liu L, Arterburn D, et al. . Five-year longitudinal cohort study of reinterventions after sleeve gastrectomy and Roux-en-Y gastric bypass [published online June 7, 2019]. Ann Surg. doi: 10.1097/SLA.0000000000003401 [DOI] [PubMed] [Google Scholar]
- 30.Peterli R, Wölnerhanssen BK, Peters T, et al. . Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319(3):255-265. doi: 10.1001/jama.2017.20897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salminen P, Helmiö M, Ovaska J, et al. . Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA. 2018;319(3):241-254. doi: 10.1001/jama.2017.20313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arterburn D, Gupta A. Comparing the outcomes of sleeve gastrectomy and Roux-en-Y gastric bypass for severe obesity. JAMA. 2018;319(3):235-237. doi: 10.1001/jama.2017.20449 [DOI] [PubMed] [Google Scholar]
- 33.O’Brien PE, Hindle A, Brennan L, et al. . Long-term outcomes after bariatric surgery: a systematic review and meta-analysis of weight loss at 10 or more years for all bariatric procedures and a single-centre review of 20-year outcomes after adjustable gastric banding. Obes Surg. 2019;29(1):3-14. doi: 10.1007/s11695-018-3525-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clapp B, Wynn M, Martyn C, Foster C, O'Dell M, Tyroch A. Long term (7 or more years) outcomes of the sleeve gastrectomy: a meta-analysis. Surg Obes Relat Dis. 2018;14(6):741-747. doi: 10.1016/j.soard.2018.02.027 [DOI] [PubMed] [Google Scholar]
- 35.Patti MG, Schlottmann F. Gastroesophageal reflux after sleeve gastrectomy. JAMA Surg. 2018;153(12):1147-1148. doi: 10.1001/jamasurg.2018.2437 [DOI] [PubMed] [Google Scholar]
- 36.Coleman KJ, Brookey J. Gender and racial/ethnic background predict weight loss after Roux-en-Y gastric bypass independent of health and lifestyle behaviors. Obes Surg. 2014;24(10):1729-1736. doi: 10.1007/s11695-014-1268-0 [DOI] [PubMed] [Google Scholar]
- 37.Coleman KJ, Huang YC, Koebnick C, et al. . Metabolic syndrome is less likely to resolve in Hispanics and non-Hispanic blacks after bariatric surgery. Ann Surg. 2014;259(2):279-285. doi: 10.1097/SLA.0000000000000258 [DOI] [PubMed] [Google Scholar]
- 38.Zingmond DS, McGory ML, Ko CY. Hospitalization before and after gastric bypass surgery. JAMA. 2005;294(15):1918-1924. doi: 10.1001/jama.294.15.1918 [DOI] [PubMed] [Google Scholar]
- 39.Bolen SD, Chang HY, Weiner JP, et al. . Clinical outcomes after bariatric surgery: a five-year matched cohort analysis in seven US states. Obes Surg. 2012;22(5):749-763. doi: 10.1007/s11695-012-0595-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arterburn D, Wellman R, Emiliano A, et al. ; PCORnet Bariatric Study Collaborative . Comparative effectiveness and safety of bariatric procedures for weight loss: a PCORnet cohort study. Ann Intern Med. 2018;169(11):741-750. doi: 10.7326/M17-2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casillas RA, Um SS, Zelada Getty JL, Sachs S, Kim BB. Revision of primary sleeve gastrectomy to Roux-en-Y gastric bypass: indications and outcomes from a high-volume center. Surg Obes Relat Dis. 2016;12(10):1817-1825. doi: 10.1016/j.soard.2016.09.038 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Flow Diagram for Identification of the Cohort
eTable 1. Kaplan-Meier Estimated Cumulative Incidence of Operations, Interventions, and Endoscopy
eTable 2. Causes of Death
eAppendix. Results for Adjustable Gastric Band (AGB)