This phase 3 randomized clinical trial assesses whether dasatinib given at 80 mg/m2 is more effective than imatinib mesylate at 300 mg/m2 to improve event-free survival in children with Philadelphia chromosome–positive acute lymphoblastic leukemia.
Key Points
Question
Is dasatinib more effective than imatinib mesylate for childhood Philadelphia chromosome–positive acute lymphoblastic leukemia?
Findings
In this randomized clinical trial of 189 children with Philadelphia chromosome–positive acute lymphoblastic leukemia, the 92 patients treated with dasatinib at 80 mg/m2 per day had significantly higher rates of 4-year event-free survival (71.0% vs 48.9%) and overall survival (88.4% vs 69.2%) and lower relapse rates (19.8% vs 34.4%) than the 97 treated with imatinib mesylate at 300 mg/m2 per day. There were no significant differences in severe toxic effects between the 2 groups.
Meaning
These findings support the use of dasatinib at a dosage of 80 mg/m2 per day in children with Philadelphia chromosome–positive acute lymphoblastic leukemia.
Abstract
Importance
A randomized clinical trial is needed to determine whether the second-generation Abl–tyrosine kinase inhibitor dasatinib is more effective than the first-generation inhibitor imatinib mesylate for childhood Philadelphia chromosome–positive acute lymphoblastic leukemia (ALL).
Objective
To determine whether dasatinib given at a daily dosage of 80 mg/m2 is more effective than imatinib mesylate at a daily dosage of 300 mg/m2 to improve event-free survival of children with Philadelphia chromosome–positive ALL in the context of intensive chemotherapy without prophylactic cranial irradiation.
Design, Setting, and Participants
This open-label, phase 3 randomized clinical trial was conducted at 20 hospitals in China. Enrollment occurred from January 1, 2015, through September 18, 2018, and randomization was stopped on October 4, 2018, when the early stopping criterion of the trial was met. Patients aged 0 to 18 years were recruited. Of the 225 patients with the diagnosis, 35 declined participation and 1 died before treatment, leaving 189 patients available for analysis. Data were analyzed from January 1 through August 4, 2019.
Interventions
Patients were randomized to receive daily dasatinib (n = 92) or imatinib (n = 97) continuously for the entire duration of ALL therapy from the time of diagnosis made during remission induction to the end of continuation therapy.
Main Outcomes and Measures
The primary outcome was event-free survival, analyzed based on intention to treat. The secondary outcomes were relapse, death due to toxic effects, and overall survival.
Results
Among the 189 participants (136 male [72.0%]; median age, 7.8 [interquartile range (IQR), 5.2-11.3] years) and a median follow-up of 26.4 (IQR, 16.3-34.1) months, the 4-year event-free survival and overall survival rates were 71.0% (95% CI, 56.2%-89.6%) and 88.4% (95% CI, 81.3%-96.1%), respectively, in the dasatinib group and 48.9% (95% CI, 32.0%-74.5%; P = .005, log-rank test) and 69.2% (95% CI, 55.6%-86.2%; P = .04, log-rank test), respectively, in the imatinib group. The 4-year cumulative risk of any relapse was 19.8% (95% CI, 4.2%-35.4%) in the dasatinib group and 34.4% (95% CI, 15.6%-53.2%) in the imatinib group (P = .01, Gray test), whereas the 4-year cumulative risk of an isolated central nervous system relapse was 2.7% (95% CI, 0.0%-8.1%) in the dasatinib group and 8.4% (95% CI, 1.2%-15.6%) in the imatinib group (P = .06, Gray test). There were no significant differences in the frequency of severe toxic effects between the 2 treatment groups.
Conclusions and Relevance
Intensive chemotherapy including dasatinib at a dosage of 80 mg/m2 per day yielded superior results in the treatment of Philadelphia chromosome–positive ALL compared with imatinib mesylate at a dosage of 300 mg/m2 per day and provided excellent control of central nervous system leukemia without the use of prophylactic cranial irradiation.
Trial Registration
Chinese Clinical Trial Registry: ChiCTR-IPR-14005706
Introduction
The Philadelphia chromosome occurs in approximately 3% to 4% of cases of childhood acute lymphoblastic leukemia (ALL).1,2 Historically, it was associated with a dismal prognosis, with 5-year event-free survival ranging from 28% to 32%, and was an indication for prophylactic cranial irradiation and allogeneic hematopoietic cell transplant.3,4,5 The addition of the first-generation Abl–tyrosine kinase inhibitor imatinib mesylate has improved the 5-year event-free survival rates to 57%, but virtually all patients received cranial irradiation, and 38% to 100% underwent transplant.6,7,8,9,10
Because relapse and drug resistance were relatively frequent events in patients treated with imatinib,11,12 2 second-generation tyrosine kinase inhibitors, dasatinib and nilotinib hydrochloride, were developed to overcome resistance-inducing ABL1 (OMIM 189980) kinase domain mutations.12 Dasatinib is the more commonly used dual Abl/Src kinase inhibitor13 and can cross the blood-brain barrier to eradicate central nervous system (CNS) leukemia.14 Several nonrandomized clinical trials15,16,17,18 suggested that dasatinib could secure results comparable to those achieved with imatinib despite a lower proportion of patients undergoing allogeneic transplant or cranial irradiation. Because of substantial differences between these trials in the use of historical controls and the proportion of patients treated with transplant and cranial irradiation, the relative efficacy between imatinib and dasatinib remains uncertain. We report herein the early results of the first randomized clinical study, to our knowledge, comparing the efficacy of imatinib and dasatinib in children with Philadelphia chromosome–positive ALL.
Methods
Trial Design and Oversight
The Chinese Children’s Cancer Group study ALL-2015 (CCCG-ALL-2015) is a prospective, multi-institutional clinical trial involving 20 major hospitals and medical centers. The trial protocol is available in Supplement 1, and the statistical design is described in the eMethods and the eAppendix in Supplement 2. The study was approved by the ethics committee of each participating institution. Written informed consent was obtained from the parents, guardians, or patients, as appropriate. The conduct of the protocol included a central review of minimal residual disease (MRD), periodic internal and on-site monitoring, and external auditing to ensure protocol compliance and appropriate data management. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Eligible patients were children aged 0 to 18 years with a confirmed diagnosis of ALL. All patients received MRD-directed, risk-stratified treatment, modified from the St Jude Children’s Research Hospital Total XV and XVI studies19,20 and the Shanghai Children’s Medical Center ALL-2005 trial.21 CCCG-ALL-2015 consists of 2 open-label, randomized studies. One, which is still under way, tests the efficacy and toxic effects of prolonged pulse therapy with dexamethasone and vincristine sulfate, for which patients with Philadelphia chromosome–positive ALL were not eligible. The other randomized study, which is the subject of this report, compared the efficacy and toxic effects of imatinib vs dasatinib treatment in patients with the t(9;22)(q34:q11.2) translocation detected by conventional cytogenetics, fluorescence in situ hybridization, or BCR (OMIM 151410)–ABL1 fusion identified by reverse transcription polymerase chain reaction analysis. Patients, physicians, and the research staff were not aware of the trial results at any phase of the trial.
Participants and Randomization
All patients were provisionally assigned to the low-risk, the intermediate-risk, or the high-risk group based on their presenting clinical features and immunophenotype; the final risk status was determined by the leukemia molecular subtype and MRD (eTable 1 in Supplement 2). Patients with Philadelphia chromosome–positive ALL were assigned to the intermediate-risk arm and randomly assigned (1:1) to receive imatinib or dasatinib as soon as the diagnosis was made, usually on day 8 of remission induction. Stratified randomization22 was done centrally with an interactive web response system. Randomization was stratified by participating institutions and age at diagnosis (<1, 1-9, and ≥10 years). This was an open-label study.
Treatments
All patients received upfront window therapy with dexamethasone for 4 days followed by remission induction with prednisone acetate, vincristine, daunorubicin hydrochloride, and pegaspargase from days 5 to 28 and cyclophosphamide, cytarabine, and mercaptopurine from days 29 to 35 (eTable 2 in Supplement 2). Patients with Philadelphia chromosome–positive ALL began to receive dasatinib (80 mg/m2 per day) or imatinib mesylate (300 mg/m2 per day) at a median of 8.0 days (interquartile range [IQR], 6.0-12.0) after the initiation of dexamethasone therapy and continuing until the end of therapy. The dosage of dasatinib was based on that used in earlier adult studies,23,24 which was well tolerated in the St Jude Total XVI study of therapy for childhood ALL.25 The dosage of imatinib was chosen from 2 consecutive European studies for their larger experience8,9 than that of the Children’s Oncology Group study, which used a higher dose (340 mg/m2),6,7 and because of the tendency of Asian patients to have higher trough concentrations than white patients while receiving the same dosage.26 All patients with MRD of at least 1% on day 19 of induction therapy received additional early intensification therapy from days 50 to 57. Triple intrathecal therapy was given on days 5, 12, 19, and 29 of induction therapy; additional intrathecal treatments on days 8 and 15 were given to patients with blasts in their cerebrospinal fluid or traumatic lumbar puncture findings at diagnosis.
On completion of induction therapy, high-dose methotrexate, mercaptopurine, and triple intrathecal therapy were given as consolidation treatment (eTable 2 in Supplement 2). Initial continuation treatment consisted of daily mercaptopurine with additional pegaspargase, daunorubicin, vincristine, dexamethasone, and triple intrathecal therapy every 3 weeks, followed by reinduction treatment from weeks 17 to 19 (eTable 2 in Supplement 2). Subsequent continuation treatment consisted of daily mercaptopurine and weekly methotrexate interrupted by 12 pulse therapies with cyclophosphamide, cytarabine, dexamethasone, and vincristine. Intrathecal therapy was given only in the first 5 cycles. Altogether, patients received 19 or 21 doses of triple intrathecal therapy. None of the patients were given prophylactic cranial irradiation. Allogeneic transplant was recommended only for high-risk patients with MRD of at least 1% at the end of remission induction.
Outcome Measures
The primary end point of the trial was event-free survival. The secondary end points were relapse and death due to toxic effects, as well as overall survival. Based on the statistical considerations, randomization of 204 patients would provide an 80% power to detect an approximately 8.5% to 11.0% difference in 5-year event-free survival. Although this number of patients could be accrued within 3 years according to the historical experience at the 20 participating institutions, an interim analysis was planned at 3.5 years to account for slower accrual than anticipated. On October 3, 2018, the results of the interim analysis, which showed significantly improved 3-year event-free survival in the dasatinib group, were provided to the chair of the data and safety monitoring committee, who recommended that randomization be stopped and that imatinib be replaced with dasatinib in all patients still receiving treatment. The trial remains open for new patients, all of whom receive dasatinib.
Statistical Analysis
Data, available in Supplement 3, were analyzed from January 1 through August 4, 2019. Event-free and overall survival curves were estimated using the Kaplan-Meier method and compared using the log-rank test. Event-free survival time was calculated from diagnosis to the first treatment failure, including induction failure owing to death or drug resistance, relapse in any site, death due to any cause, development of a second malignant tumor, and the instigation of off-protocol therapy by decision of the treating physician based on persistent disease, severe toxic effects, or transplant not according to protocol criteria. Overall survival was considered as the time from diagnosis to death due to any cause. When no events occurred, the observation was censored at the time of last patient contact. The cumulative incidence functions of any relapse, isolated CNS relapse, any CNS relapse (isolated plus combined), or death due to toxic effects were estimated according to Kalbfleisch and Prentice27 and compared using the Gray test to account for competing events.28 Competing events for relapse included death in remission, second malignant tumor, treatment abandonment, refusal of protocol treatment (withdrawal consent) by parents, or off-protocol treatment by decision of the treating physician. Competing events for deaths due to toxic effects included relapse and other events (listed above). We calculated 95% CIs with a large-sample normal approximation. The Fisher exact test was applied to comparisons in contingency tables. Single and multivariate regression analyses of event-free survival and cumulative risk of relapse were performed using the Cox and Fine-Gray regression models,29 respectively. All reported P values are 2-sided and were not adjusted for multiple comparisons, with P < .05 indicating significance. Analyses were primarily based on intention to treat, but secondary analyses for as-treated patients were also performed. In the latter, treatment abandonment and parental refusal of protocol treatment were regarded as failures.
Outcome data reported in this article were updated on May 31, 2019. The median follow-up time for the 161 patients who were alive at the time of analysis was 26.4 months (IQR, 16.3-34.1 months; range, 2.1-50.6 months). All statistical analyses were conducted with R statistical software, version 3.4.4 (R Project for Statistical Computing [https://www.r-project.org/]).
Results
Study Population
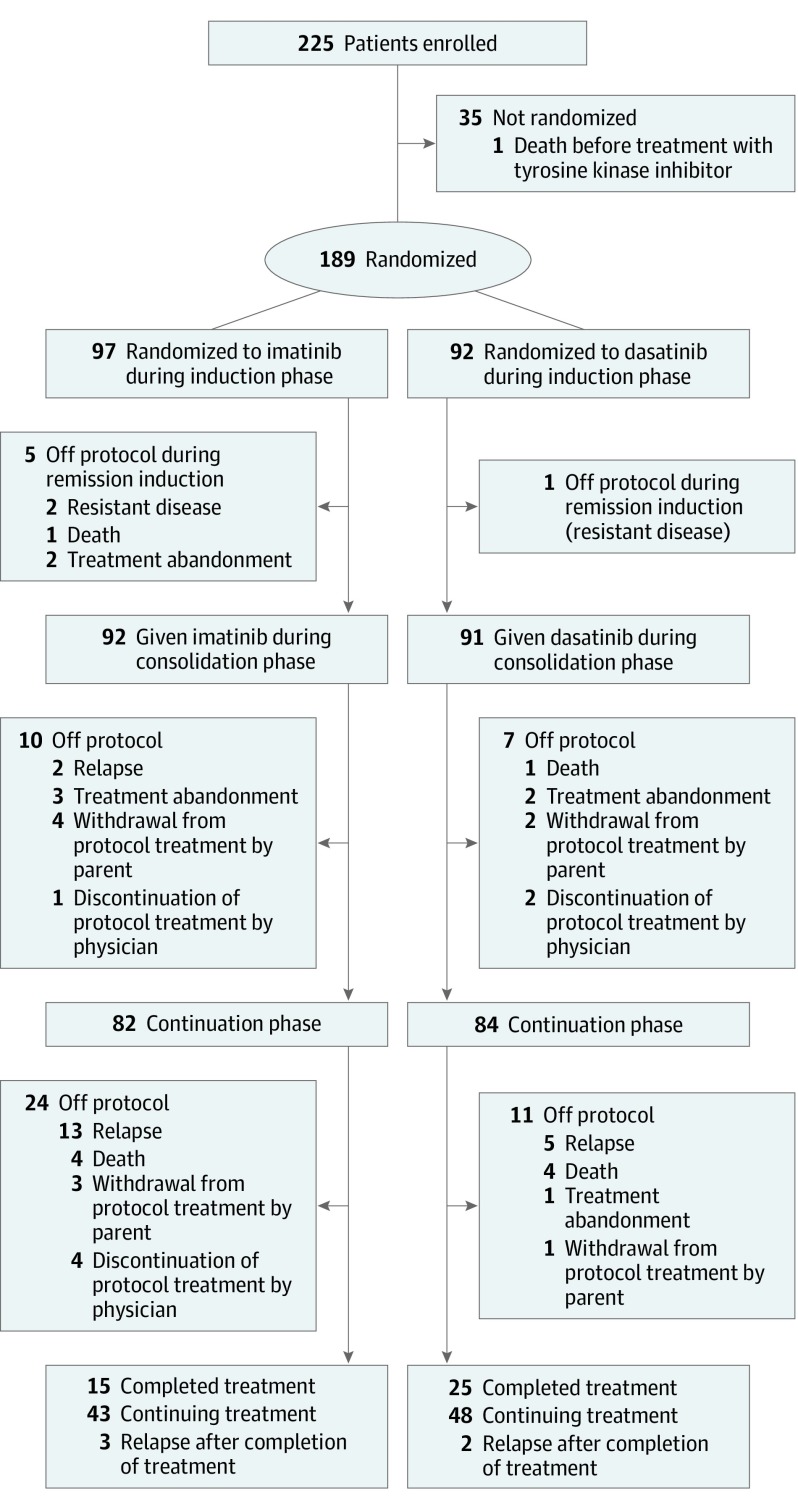
From January 1, 2015, to September 18, 2018, 5525 patients with newly diagnosed ALL were enrolled in the study, of whom 225 (4.1%) had Philadelphia chromosome–positive ALL. Thirty-five patients with this diagnosis declined randomization, and 1 died before treatment (Figure 1). Of the 189 eligible patients (136 male [72.0%] and 53 female [28.0%]; median age, 7. 8 [IQR, 5.2-11.3] years), 92 were randomized to receive dasatinib and 97 to receive imatinib, given daily for the entire duration of ALL therapy. Baseline demographic and disease characteristics were balanced between the 2 groups (eTable 3 in Supplement 2). As expected, p190 was detected more often than p210, and only 4 patients had a T-cell immunophenotype. Based on the MRD level at the end of induction, 184 patients were classified as at intermediate risk and 5 at high risk, of whom 4 (3 receiving imatinib and 1 receiving dasatinib) underwent transplant, and 1 imatinib-treated patient refused transplant. Of these 5 high-risk patients, only 1 imatinib-treated patient was still alive after transplant at 4.0 years from diagnosis.
Figure 1. Trial Profile.
Imatinib was administered as imatinib mesylate.
There were no significant differences in the initial treatment response based on the detection of MRD on days 19 and 46 of remission induction or the complete remission rate between dasatinib- or imatinib-treated patients (eTable 4 in Supplement 2). Five imatinib-treated patients did not achieve remission because of treatment abandonment (n = 2), refractory disease with 31% and 32% blasts at the end of induction therapy (n = 2), or death due to infection (n = 1). One dasatinib-treated patient did not achieve remission owing to refractory disease (5.7% blasts at the end of induction).
After remission induction, 6 patients (3 receiving dasatinib and 3 receiving imatinib) were taken off protocol due to treatment abandonment. Another 10 patients (3 receiving dasatinib and 7 receiving imatinib) withdrew from protocol treatment for other options such as transplant (7 were still alive for 8.2 to 34.4 months; the remaining 3 had unknown outcomes) (Figure 1). The 18 patients whose parents chose treatment abandonment (n = 8) or withdrew from protocol treatment (n = 10) were censored in the intention-to-treat analysis but were considered to have treatment failure in the as-treated analysis. The treating physicians recommended discontinuation of protocol treatment in favor of other treatment options, including transplant for 7 other patients (2 receiving dasatinib and 5 receiving imatinib), all of whom were considered to have treatment failures in the intention-to-treat and as-treated analyses. At analysis, 4 of these patients were alive, 1 had died of transplant-related toxic effects, and 2 had an unknown status. Among the remaining patients, there were a total of 12 adverse events in the dasatinib group (4 hematologic relapses, 1 isolated CNS relapse, 2 combined hematologic and CNS relapses, and 5 deaths in remission), compared with 22 adverse events in the imatinib group (10 hematologic relapses, 6 isolated CNS relapses, 1 combined hematologic and CNS relapse, 1 ocular relapse, and 4 deaths in remission).
Primary Outcomes
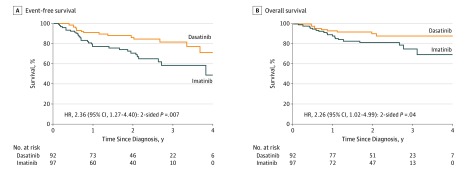
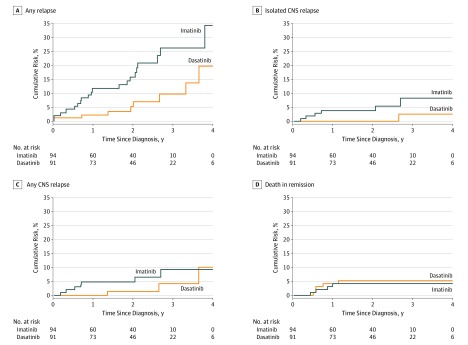
By intention-to-treat analysis, the 4-year event-free survival rate in the dasatinib group (71.0%; 95% CI, 56.2%-89.6%) was significantly better than in the imatinib group (48.9%; 95% CI, 32.0%-74.5%; P = .005, log-rank test) (Figure 2). The 4-year overall survival rate was 88.4% (95% CI, 81.3%-96.1%) in the dasatinib group vs 69.2% (95% CI, 55.6%-86.2%; P = .04, log-rank test) in the imatinib group. The 4-year cumulative risk of any relapse was significantly lower in the dasatinib group (19.8%; 95% CI, 4.2%-35.4%) than in the imatinib group (34.4%; 95% CI, 15.6%-53.2%; P = .01, Gray test) (Figure 3). The 4-year cumulative risk of isolated CNS relapse was also lower in the dasatinib group (2.7%; 95% CI, 0.0%-8.1%) than in the imatinib group (8.4%; 95% CI, 1.2%-15.6%; P = .06, Gray test), whereas the cumulative risk of any CNS relapse (isolated plus combined with hematologic) (10.1% [95% CI, 0.0%-23.3%] vs 9.4% [95% CI, 1.9%-19.9%]; P = .20, Gray test) and the cumulative risk of deaths in remission (5.6% [95% CI, 0.8%-10.4%] vs 4.3% [95% CI, 0.2%-8.4%]; P = .67, Gray test) did not differ significantly between the 2 groups (Figure 3).
Figure 2. Kaplan-Meier Analysis of Survival by Treatment Group.
The hazard ratio (HR) and 95% CI, along with the P value, were obtained from Cox proportional hazards regression modeling. Imatinib was given as imatinib mesylate.
Figure 3. Cumulative Risk of Relapse and Death.
Imatinib was given as imatinib mesylate. CNS indicates central nervous system.
Factors associated with an inferior event-free survival in the intention-to-treat analysis in the imatinib group were being 10 years or older and having a leukocyte cell count of at least 100 ×103/μL at diagnosis, T-cell phenotype, and increased MRD on days 19 and 46. Factors in the dasatinib group were leukocyte cell count of at least 100 ×103/μL at diagnosis, CNS3 status (leukocyte count, ≥5/μL of cerebrospinal fluid, with blasts or cranial palsy), high-risk classification, and increased MRD on days 19 and 46 (eTable 5 in Supplement 2). In the multivariate analysis, treatment with imatinib, being 10 years or older, leukocyte count of at least 100 ×103/μL at diagnosis, and T-cell phenotype were independently associated with a poorer event-free survival, although MRD on day 46 was not significant (Table).
Table. Multivariate Cox Proportional Hazards Regression Analysis of Event-Free Survival.
| Category | No. (%) of Patients (n = 183)a | HR (95% CI) | P Value |
|---|---|---|---|
| Tyrosine kinase inhibitor | |||
| Dasatinib | 91 (49.7) | 1 [Reference] | .005 |
| Imatinib mesylate | 92 (50.3) | 2.78 (1.37-5.64) | |
| Age, y | |||
| 1-9 | 123 (67.2) | 1 [Reference] | .04 |
| ≥10 | 60 (32.8) | 2.02 (1.03-3.98) | |
| Leukocyte cell count at diagnosis, ×103/μL | |||
| <100 | 105 (57.4) | 1 [Reference] | <.001 |
| ≥100 | 78 (42.6) | 4.15 (2.02-8.53) | |
| CNS status | |||
| CNS1 | 164 (89.6) | 1 [Reference] | NA |
| CNS2/traumatic lumbar puncture | 13 (7.1) | 0.30 (0.07-1.23) | .09 |
| CNS3 | 6 (3.3) | 1.19 (0.34-4.21) | .78 |
| Immunophenotype | |||
| B | 179 (97.8) | 1 [Reference] | <.001 |
| T | 4 (2.2) | 20.71 (4.44-96.59) | |
| Final risk | |||
| Intermediate | 178 (97.3) | 1 [Reference] | .09 |
| High | 5 (2.7) | 3.14 (0.83-11.84) | |
| Minimal residual disease (MRD) level | |||
| Day 19 | |||
| <5% | 156 (85.2) | 1 [Reference] | .57 |
| ≥5% | 27 (14.8) | 0.75 (0.27-2.05) | |
| Day 46 | |||
| <0.01% | 144 (78.7) | 1 [Reference] | .11 |
| ≥0.01% | 39 (21.3) | 2.07 (0.84-5.10) |
Abbreviations: CNS, central nervous system; HR hazard ratio; NA, not applicable.
The single infant in the imatinib group and 5 patients without MRD results at day 46 were excluded.
Secondary Outcomes
The as-treated analysis showed no significant differences in patient characteristics (eTable 6 in Supplement 2) and the induction treatment response based on MRD level (eTable 7 in Supplement 2). When treatment abandonment and refusal of protocol treatment were included as adverse events, the dasatinib-treated patients had a superior 4-year event-free survival (66.5% [95% CI, 52.4%-84.4%] vs 39.8% [95% CI, 25.4%-62.2%]; P < .001, log-rank test) (eFigure 1 in Supplement 2) as well as a lower 4-year cumulative risk of any relapse (20.3% [95% CI, 4.6%-36.0%] vs 33.8% [95% CI, 15.2%-52.4%]; P = .04, Gray test) and isolated CNS relapse (2.7% [95% CI, 0.0%-8.0%] vs 8.6% [95% CI, 1.2%-16.0%]; P = .06, Gray test) compared with the imatinib-treated patients (eFigure 2 in Supplement 2). Similarly, treatment with imatinib, leukocyte count of at least 100 ×103/μL at diagnosis, T-cell phenotype, and increased MRD level on day 46 were independent adverse prognostic features in a multivariate analysis (eTable 8 in Supplement 2).
Adverse Events
The frequencies of commonly reported serious toxic effects did not differ significantly between the 2 treatment arms in the as-treated analysis (eTable 9 in Supplement 2). Infections followed by pancreatitis were the most common adverse effects, with 5 fatal infections occurring in each treatment arm. No deaths could be attributed directly to dasatinib or imatinib treatment.
Discussion
In this first randomized clinical phase 3 trial of dasatinib and imatinib in childhood Philadelphia chromosome–positive ALL, enrollment was stopped after an interim analysis demonstrated that dasatinib yields a superior outcome compared with imatinib. In the intention-to-treat analysis, in which 7 patients were considered to have treatment failure based on their physician’s decision to discontinue the protocol-specified treatment, the 4-year event-free survival rate for the 92 dasatinib-treated patients was 71.0%, significantly better than the 48.9% for the 97 imatinib-treated patients. In the as-treated analysis, we conservatively included as treatment failures not only these 7 patients but also the other 18 whose parents decided to abandon treatment or withdrew protocol treatment for other treatment options (chiefly transplant). With this approach, the difference in 4-year event-free survival rates were even more striking (66.5% vs 39.8%).
This result stands in contrast to those of 2 recent pediatric phase 2 trials of dasatinib given at a dosage of 60 mg/m2 per day.17,18 The event-free survival rates of these 2 trials did not differ from those of their historical controls treated with imatinib. Of the 60 patients treated in the Children’s Oncology Group study,17 in which 19 patients received transplants and 4 received cranial irradiation for CNS3 status, the 4- and 5-year event-free survival rates were approximately 62% and 60%, respectively. In the international study,18 which enrolled 106 patients (14.2% of whom received transplants and 5% of whom received cranial irradiation for CNS3 status), the 3-year event-free survival rate was 66%. By contrast, despite total omission of prophylactic cranial irradiation with transplant limited to a single patient in the present study, the 4-year event-free survival rate in 92 dasatinib-treated patients was 71.0%. Moreover, isolated CNS relapse occurred in only 1 of 92 dasatinib-treated patients in this study but in 4 of 60 and 4 of 106 patients in the other 2 studies.17,18 Thus, our results appear more favorable than those of the other 2 studies using a lower dosage of dasatinib, although longer follow-up is needed to assess whether they represent significant improvement over the Children’s Oncology Group study. Nonetheless, we expect that the event-free survival of our dasatinib-treated patients will not only remain superior to that of the imatinib-treated patients, but the gap will be widened with longer follow-up based on a previous observation30 that more effective treatment would reduce off-therapy relapse in children with ALL, including those with Philadelphia chromosome–positive ALL. Indeed, of the 40 patients who had completed ALL therapy in this study, thus far, 3 of 15 imatinib-treated patients as opposed to 2 of 25 dasatinib-treated patients had relapsed after the cessation of treatment, pointing to more enduring effects of the latter regimen (Figure 1).
We attribute the improved disease control achieved in the dasatinib group to the use of a higher drug dose (80 mg/m2) than typically specified by other protocols.12,15,16,17,18 This modification may overcome the relative drug resistance of some patients by increasing systemic drug exposure and perhaps eradicates leukemia in the CNS by reaching a therapeutic level in the cerebrospinal fluid.14 Indeed, of our 92 dasatinib-treated patients, only 1 developed an isolated CNS relapse and only 2 had combined hematologic and CNS relapses. This explanation is further supported by observations on the 10 patients treated with a similar backbone regimen and dasatinib at a dosage of 80 mg/m2 per day in the St Jude Total XVI study.20 None of these patients received prophylactic cranial irradiation, and only 1 underwent transplant. With a median follow-up of 3.5 (range, 0.8-9.5) years, 1 patient failed induction, 1 died of hematologic relapse after transplant, and 1 died of multiple-organ failure in remission; none of the patients developed CNS relapse. The 5-year event-free survival rate was 70.0% (95% CI, 32.4%-100%); 5-year overall survival, 75.0% (95% CI, 38.2%-100%) (eFigure 3 in Supplement 2).
There were no differences in toxic effects between dasatinib- and imatinib-treated patients. Both drugs were well tolerated, and no deaths could be directly attributed to either agent alone. However, approximately 5% of the patients in each arm died of fatal infections, which accounted for 23% and 42% of the treatment failures in the imatinib and dasatinib groups, respectively. Similarly, the rate of fatal infection in the international study of dasatinib at a dosage of 60 mg/m2 per day was 5.5% (5 of 91 patients).18 Moreover, approximately 7% of our patients in each treatment arm had disseminated fungal infections. These findings suggest that the intensity of chemotherapy should be reduced in future studies or that prophylactic antimicrobial treatment could be instituted.31,32
Although we found that dasatinib treatment at a dosage of 80 mg/m2 per day allows the omission of prophylactic cranial irradiation, our results raise an important question about the role of allogeneic transplant in the treatment. In our trial, 1 dasatinib- and 3 imatinib-treated patients received transplants for MRD of at least 1% at the end of induction, but only 1 remained alive in remission at 4.0 years from diagnosis. Although a leukocyte count of at least 100 ×103/μL and being 10 years or older were independent risk factors (Table), the prevalence of these features was too high to make them useful indicators for transplant. Whether comprehensive genetic studies could be used to identify factors (eg, mutations of IKZF1 and ABL1 genes) to improve risk stratification33 or whether the addition of other active nonchemotherapeutic agents (eg, blinatumomab) or the use of more potent tyrosine kinase inhibitors (eg, ponatinib) could eliminate the need for transplant in this disease12 requires further research.
Limitations
This study has several limitations. Until 2010, most patients with ALL in China, especially those from less developed areas, abandon treatment due to financial reasons.34,35 With improved economic conditions and the development of the New Rural Cooperative Medical Scheme, which covers most expenses related to the treatment of ALL, we undertook the CCCG-ALL-2015 study to advance the quality of therapy for patients with access to treatment. Nonetheless, 3.1% of the overall patients, including 8 of 189 with Philadelphia chromosome–positive disease (4.2%) in this study, still abandoned treatment, mainly because of economic constraints, the perception of incurability, severe adverse effects, and concern over late complications.35 This response is a culturally embraced attitude that must be overcome before greater therapeutic success can be achieved. In this regard, interventions with financial support by charitable organizations can be helpful, as can assistance from community support groups, parental education, and psychosocial guidance. In addition, although the group results were not available to any study investigators before the interim analysis, we cannot totally exclude physician bias as a factor affecting the outcome of this trial. Thus, future studies of dasatinib in children and adults are warranted to confirm our results.
Conclusions
This study found that dasatinib at a dosage of 80 mg/m2 per day was more effective than imatinib mesylate at a dosage of 300 mg/m2 per day in the treatment of children with Philadelphia chromosome–positive ALL. Dasatinib therapy provided excellent control of CNS leukemia without the use of prophylactic cranial irradiation.
Trial Protocol
eMethods. Trial Design and Oversight and Statistical Methods
eTable 1. Criteria for Classification of Risk Groups
eTable 2. Remission Induction, Consolidation, and Continuation/Reinduction Therapy
eTable 3. Baseline Demographic and Disease Characteristics of the Patients
eTable 4. Comparison of Minimal Residual Disease Levels on Day 19 and Day 46 of Remission Induction Between Patient’s Intent-to-Treat With Dasatinib or Imatinib
eTable 5. Univariate Cox Proportional Hazards Rate Regression Analysis of Event-Free Survival Within Each Treatment Arm
eTable 6. Baseline Demographic and Disease Characteristics of As-Treated Patients
eTable 7. Comparison of Minimal Residual Disease Levels on Day 19 and Day 46 of Remission Induction Between Patients As-Treated With Dasatinib or Imatinib
eTable 8. Multivariable Cox Proportional Hazards Regression Analysis of Event-Free Survival Among As-Treated Patients With Treatment Abandonment and Refusal of Protocol Treatment Considered as Adverse Events
eTable 9. Comparison of Toxicity Between Patients As-Treated With Dasatinib or Imatinib
eFigure 1. Survival by As-Treated Groups
eFigure 2. Cumulative Risk by As-Treated Groups
eFigure 3. Event-Free and Overall Survival of Patients Treated in St Jude Total XVI Study
eAppendix. Run-time Printout Log
Data Sets
Data Sharing Statement
References
- 1.Schlieben S, Borkhardt A, Reinisch I, et al. Incidence and clinical outcome of children with BCR/ABL-positive acute lymphoblastic leukemia (ALL): a prospective RT-PCR study based on 673 patients enrolled in the German pediatric multicenter therapy trials ALL-BFM-90 and CoALL-05-92. Leukemia. 1996;10(6):957-963. [PubMed] [Google Scholar]
- 2.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354(2):166-178. doi: 10.1056/NEJMra052603 [DOI] [PubMed] [Google Scholar]
- 3.Aricò M, Valsecchi MG, Camitta B, et al. Outcome of treatment in children with Philadelphia chromosome–positive acute lymphoblastic leukemia. N Engl J Med. 2000;342(14):998-1006. doi: 10.1056/NEJM200004063421402 [DOI] [PubMed] [Google Scholar]
- 4.Aricò M, Schrappe M, Hunger SP, et al. Clinical outcome of children with newly diagnosed Philadelphia chromosome–positive acute lymphoblastic leukemia treated between 1995 and 2005. J Clin Oncol. 2010;28(31):4755-4761. doi: 10.1200/JCO.2010.30.1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao YJ, Guo Y, Hu SY, et al. Philadelphia chromosome-positive acute lymphoblastic leukemia in China: a retrospective study from the Chinese Childhood Cancer Group. Leuk Lymphoma. 2016;57(11):2696-2698. doi: 10.3109/10428194.2016.1157872 [DOI] [PubMed] [Google Scholar]
- 6.Schultz KR, Bowman WP, Aledo A, et al. Improved early event-free survival with imatinib in Philadelphia chromosome–positive acute lymphoblastic leukemia: a Children’s Oncology Group study. J Clin Oncol. 2009;27(31):5175-5181. doi: 10.1200/JCO.2008.21.2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultz KR, Carroll A, Heerema NA, et al. ; Children’s Oncology Group . Long-term follow-up of imatinib in pediatric Philadelphia chromosome–positive acute lymphoblastic leukemia: Children’s Oncology Group study AALL0031. Leukemia. 2014;28(7):1467-1471. doi: 10.1038/leu.2014.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biondi A, Schrappe M, De Lorenzo P, et al. Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome–positive acute lymphoblastic leukaemia (EsPhALL): a randomised, open-label, intergroup study. Lancet Oncol. 2012;13(9):936-945. doi: 10.1016/S1470-2045(12)70377-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biondi A, Gandemer V, De Lorenzo P, et al. Imatinib treatment of paediatric Philadelphia chromosome–positive acute lymphoblastic leukaemia (EsPhALL2010): a prospective, intergroup, open-label, single-arm clinical trial. Lancet Haematol. 2018;5(12):e641-e652. doi: 10.1016/S2352-3026(18)30173-X [DOI] [PubMed] [Google Scholar]
- 10.Manabe A, Kawasaki H, Shimada H, et al. Imatinib use immediately before stem cell transplantation in children with Philadelphia chromosome–positive acute lymphoblastic leukemia: results from Japanese Pediatric Leukemia/Lymphoma Study Group (JPLSG) Study Ph(+) ALL04. Cancer Med. 2015;4(5):682-689. doi: 10.1002/cam4.383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piccaluga PP, Paolini S, Martinelli G. Tyrosine kinase inhibitors for the treatment of Philadelphia chromosome–positive adult acute lymphoblastic leukemia. Cancer. 2007;110(6):1178-1186. doi: 10.1002/cncr.22881 [DOI] [PubMed] [Google Scholar]
- 12.Short NJ, Kantarjian H, Pui CH, Goldstone A, Jabbour E. SOHO state of the art update and next questions: Philadelphia chromosome–positive acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk. 2018;18(7):439-446. doi: 10.1016/j.clml.2018.05.015 [DOI] [PubMed] [Google Scholar]
- 13.O’Hare T, Walters DK, Stoffregen EP, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65(11):4500-4505. doi: 10.1158/0008-5472.CAN-05-0259 [DOI] [PubMed] [Google Scholar]
- 14.Porkka K, Koskenvesa P, Lundán T, et al. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome–positive leukemia. Blood. 2008;112(4):1005-1012. doi: 10.1182/blood-2008-02-140665 [DOI] [PubMed] [Google Scholar]
- 15.Ravandi F, O’Brien SM, Cortes JE, et al. Long-term follow-up of a phase 2 study of chemotherapy plus dasatinib for the initial treatment of patients with Philadelphia chromosome–positive acute lymphoblastic leukemia. Cancer. 2015;121(23):4158-4164. doi: 10.1002/cncr.29646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravandi F, Othus M, O’Brien SM, et al. US Intergroup Study of chemotherapy plus dasatinib and allogeneic stem cell transplant in Philadelphia chromosome positive ALL. Blood Adv. 2016;1(3):250-259. doi: 10.1182/bloodadvances.2016001495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slayton WB, Schultz KR, Kairalla JA, et al. Dasatinib plus intensive chemotherapy in children, adolescents, and young adults with Philadelphia chromosome–positive acute lymphoblastic leukemia: results of Children’s Oncology Group Trial AALL0622. J Clin Oncol. 2018;36(22):2306-2314. doi: 10.1200/JCO.2017.76.7228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunger SP, Saha V, Devidas M, et al. CA180-372: an international collaborative phase 2 trial of dasatinib and chemotherapy in pediatric patients with newly diagnosed Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ ALL). Blood. 2017;130(suppl 1):98. doi:10.1182/blood.V130.Suppl_1.98.9828705853 [Google Scholar]
- 19.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360(26):2730-2741. doi: 10.1056/NEJMoa0900386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pui CH, Campana D, Sandlund JT, et al. Treatment of childhood acute lymphoblastic leukemia without cranial irradiation. Ann Hematol. 2011;90(suppl 1):S61-S63. [Google Scholar]
- 21.Liu Y, Chen J, Tang J, Ni S, Xue H, Pan C. Cost of childhood acute lymphoblastic leukemia care in Shanghai, China. Pediatr Blood Cancer. 2009;53(4):557-562. doi: 10.1002/pbc.22127 [DOI] [PubMed] [Google Scholar]
- 22.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27(7-8):365-375. doi: 10.1016/0021-9681(74)90015-0 [DOI] [PubMed] [Google Scholar]
- 23.Ottmann O, Dombret H, Martinelli G, et al. Dasatinib induces rapid hematologic and cytogenetic responses in adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia with resistance or intolerance to imatinib: interim results of a phase 2 study. Blood. 2007;110(7):2309-2315. doi: 10.1182/blood-2007-02-073528 [DOI] [PubMed] [Google Scholar]
- 24.Kantarjian H, Cortes J, Kim DW, et al. Phase 3 study of dasatinib 140 mg once daily versus 70 mg twice daily in patients with chronic myeloid leukemia in accelerated phase resistant or intolerant to imatinib: 15-month median follow-up. Blood. 2009;113(25):6322-6329. doi: 10.1182/blood-2008-11-186817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeha S, Coustan-Smith E, Pei D, et al. Impact of tyrosine kinase inhibitors on minimal residual disease and outcome in childhood Philadelphia chromosome–positive acute lymphoblastic leukemia. Cancer. 2014;120(10):1514-1519. doi: 10.1002/cncr.28598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh O, Chan JY, Lin K, Heng CC, Chowbay B. SLC22A1-ABCB1 haplotype profiles predict imatinib pharmacokinetics in Asian patients with chronic myeloid leukemia. PLoS One. 2012;7(12):e51771. doi: 10.1371/journal.pone.0051771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY: Wiley; 2002:1-439. doi: 10.1002/9781118032985 [DOI] [Google Scholar]
- 28.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141-1154. doi: 10.1214/aos/1176350951 [DOI] [Google Scholar]
- 29.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 30.Pui CH, Pei D, Campana D, et al. A revised definition for cure of childhood acute lymphoblastic leukemia. Leukemia. 2014;28(12):2336-2343. doi: 10.1038/leu.2014.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeh TC, Liu HC, Hou JY, et al. Severe infections in children with acute leukemia undergoing intensive chemotherapy can successfully be prevented by ciprofloxacin, voriconazole, or micafungin prophylaxis. Cancer. 2014;120(8):1255-1262. doi: 10.1002/cncr.28524 [DOI] [PubMed] [Google Scholar]
- 32.Wolf J, Tang L, Flynn PM, et al. Levofloxacin prophylaxis during induction therapy for pediatric acute lymphoblastic leukemia. Clin Infect Dis. 2017;65(11):1790-1798. doi: 10.1093/cid/cix644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aoe M, Ishida H, Matsubara T, et al. Simultaneous detection of ABL1 mutation and IKZF1 deletion in Philadelphia chromosome–positive acute lymphoblastic leukemia using a customized target enrichment system panel. Int J Lab Hematol. 2018;40(4):427-436. doi: 10.1111/ijlh.12805 [DOI] [PubMed] [Google Scholar]
- 34.Pui CH, Tang JY, Yang JJ, Chen SJ, Chen Z. International collaboration to save children with acute lymphoblastic leukemia. J Glob Oncol. 2019;5:1-2. doi: 10.1200/JGO.19.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai J, Yu J, Zhu X, et al. ; Chinese Children’s Cancer Group Childhood Acute Lymphoblastic Leukaemia (ALL) 2015 Study Group (CCCG-ALL-2015) . Treatment abandonment in childhood acute lymphoblastic leukaemia in China: a retrospective cohort study of the Chinese Children’s Cancer Group. Arch Dis Child. 2019;104(6):522-529. doi: 10.1136/archdischild-2018-316181 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods. Trial Design and Oversight and Statistical Methods
eTable 1. Criteria for Classification of Risk Groups
eTable 2. Remission Induction, Consolidation, and Continuation/Reinduction Therapy
eTable 3. Baseline Demographic and Disease Characteristics of the Patients
eTable 4. Comparison of Minimal Residual Disease Levels on Day 19 and Day 46 of Remission Induction Between Patient’s Intent-to-Treat With Dasatinib or Imatinib
eTable 5. Univariate Cox Proportional Hazards Rate Regression Analysis of Event-Free Survival Within Each Treatment Arm
eTable 6. Baseline Demographic and Disease Characteristics of As-Treated Patients
eTable 7. Comparison of Minimal Residual Disease Levels on Day 19 and Day 46 of Remission Induction Between Patients As-Treated With Dasatinib or Imatinib
eTable 8. Multivariable Cox Proportional Hazards Regression Analysis of Event-Free Survival Among As-Treated Patients With Treatment Abandonment and Refusal of Protocol Treatment Considered as Adverse Events
eTable 9. Comparison of Toxicity Between Patients As-Treated With Dasatinib or Imatinib
eFigure 1. Survival by As-Treated Groups
eFigure 2. Cumulative Risk by As-Treated Groups
eFigure 3. Event-Free and Overall Survival of Patients Treated in St Jude Total XVI Study
eAppendix. Run-time Printout Log
Data Sets
Data Sharing Statement