Key Points
Question
What is the most effective treatment strategy after a period of first-line induction therapy for patients with metastatic colorectal cancer?
Findings
This systematic review and network meta-analysis of 12 relevant randomized clinical trials comprising 5540 patients with metastatic colorectal cancer who were treated with different strategies found that continuing full cytotoxic chemotherapy until progression without a period of observation or maintenance treatment is not beneficial. Maintenance treatment with a fluoropyrimidine with or without bevacizumab is the preferred maintenance strategy, based on an observed significant improvement in progression-free survival (primary outcome measure) but not in overall survival.
Meaning
Although a maintenance strategy with a fluoropyrimidine with or without bevacizumab is preferred over continuous induction therapy for metastatic colorectal cancer, shared decision-making should include observation as an acceptable option, given the lack of significant overall survival benefits.
Abstract
Importance
In metastatic colorectal cancer, induction combination chemotherapy with a targeted agent is considered the mainstay of treatment. Multiple randomized clinical trials have examined different strategies of continuing cytotoxic therapy until progression compared with a period of either observation or the use of various maintenance agents. However, those randomized clinical trials have shown inconsistent efficacy results that make it challenging to draw any conclusion on which strategy is preferred. Therefore, a network meta-analysis is helpful to compare different agents across randomized clinical trials.
Objective
To evaluate the comparative effectiveness of different treatment strategies for patients with metastatic colorectal cancer.
Evidence Review
MEDLINE, Embase, Scopus, Web of Science, and the Cochrane Central Register of Controlled Trials were searched for randomized clinical trials evaluating different strategies for patients with previously untreated metastatic colorectal cancer. Trials of interest included those including patients with metastatic colorectal cancer who were treated with an initial period of cytotoxic chemotherapy (with or without a biologic) and then switched to one of the following strategies: observation; maintenance with bevacizumab (Bev), fluoropyrimidine (FP), or both (FP + Bev); or continuing the induction regimen until progression. Outcomes of interest included overall survival (OS) and progression-free survival (PFS). The overall effect was pooled using the DerSimonian and Laird random-effects model. Network meta-analysis was conducted using a random-effects consistency model to pool evidence from direct and indirect comparisons. Agents were ranked using surface under the cumulative ranking (SUCRA) probabilities. Higher SUCRA scores correspond to greater efficacy. Initial analysis was performed on December 18, 2018. An updated search was performed in April 2019, and no additional studies were added.
Findings
Twelve trials at low risk of bias (5540 patients; age range, 23-85 years; 64.4 % male) were included. Network meta-analysis showed no benefit of continuing full cytotoxic chemotherapy until progression vs observation in terms of PFS (hazard ratio, 0.71; 95% CI, 0.46-1.09) and OS (hazard ratio, 0.95; 95% CI, 0.85-1.07). Compared with observation, maintenance therapy showed a PFS benefit (hazard ratio, 0.58; 95% CI, 0.43-0.77) but not an OS benefit (hazard ratio, 0.91; 95% CI, 0.83-1.01). All maintenance strategies (FP, FP + Bev, and Bev) showed significant improvement in PFS vs observation. On SUCRA analysis, maintenance treatment (FP or FP + Bev) had the highest likelihood of achieving improved PFS (67.1% for FP, 99.8% for FP + Bev, and 36.5% for Bev) and OS (81.3% for FP, 73.2% for FP + Bev, and 32.6% for Bev).
Conclusions and Relevance
For patients with metastatic colorectal cancer, there is no benefit to continuing the full induction regimen until progression, without a period of either observation or maintenance treatment. A maintenance strategy with a fluoropyrimidine, with or without the addition of bevacizumab, is preferred. However, given the lack of a clear OS benefit, shared decision-making should include observation as an acceptable alternative.
This systematic review and network meta-analysis of randomized clinical trials evaluates the comparative effectiveness of different treatment strategies in metastatic colorectal cancer.
Introduction
Colorectal cancer is the third most common and lethal cancer in the United States1; around one-fourth of all patients will present with metastatic disease, and approximately half will develop metastases. The mainstay of treatment of advanced metastatic colorectal cancer (mCRC) includes cytotoxic chemotherapy with the addition of a molecularly targeted agent with the aim to prolong survival while preserving or improving quality of life.2 The cytotoxic regimens that are usually used in the first-line setting include a combination of oxaliplatin or irinotecan with a fluoropyrimidine (fluorouracil or capecitabine), in addition to targeted agents (epidermal growth factor receptor [EGFR] inhibitors for patients with RAS (GenBank P01116.1) wild-type mCRC or vascular endothelial growth factor inhibitors).3 These combinations have been associated with a significant improvement in overall survival (OS) among patients with mCRC that is now reaching a median duration surpassing 30 months.3,4,5 The duration of first-line induction chemotherapy after achieving maximum response is a matter of controversy because toxic effects such as neuropathy with oxaliplatin and chronic fatigue with irinotecan can occur after prolonged treatment.3,6 The concept of intermittent treatment with observation or maintenance treatment with “lighter therapy,” instead of continuing induction treatment until progression, has been examined in different randomized clinical trials (RCTs) with oxaliplatin-based or irinotecan-based first-line chemotherapy.7,8,9 However, those RCTs have shown inconsistent efficacy results that make it challenging to draw any conclusion on which strategy or agent is preferred. Therefore, a network meta-analysis is helpful to compare different agents across RCTs. In this systematic review and network meta-analysis, we aim to compare the efficacy of different first-line treatment strategies for patients with mCRC.
Methods
Objective
The present study aimed to compare the efficacy of different treatment strategies (continuous treatment until progression, maintenance chemotherapy or vascular endothelial growth factor inhibitor, or observation) for patients with mCRC after the initial induction chemotherapy period. The reporting of this systematic review follows the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement.10
Eligibility Criteria
Randomized (phase 2 or 3) clinical trials were included if they were published in English. Trials of interest were those comparing continuous induction chemotherapy until progression with an intermittent strategy of observation, or chemotherapy, with or without maintenance therapy, for adult patients with mCRC. Those trials had to include at least 1 of the following strategies: (1) observation; (2) maintenance treatment with a fluoropyrimidine (FP; fluorouracil or capecitabine), bevacizumab (Bev), or both FP + Bev; or continuous induction treatment until progression.
Data Sources and Search Strategies
A comprehensive literature search was performed for full-text articles published in print or online from database inception through April 2019 from electronic databases, MEDLINE, Embase, Scopus, Web of Science, and the Cochrane Central Register of Controlled Trials. The detailed search strategy is as described in the eMethods in the Supplement. The search strategy was designed and conducted by an experienced librarian (D.A.-D.) with input from the study investigators. Two of us (M.B.S. and L.J.M.) identified and reviewed full-text articles that were deemed relevant by screening the list of titles and abstracts. Disagreements between the 2 reviewers were resolved with consensus. Outcomes of interests included OS and progression-free survival (PFS).
Data Extraction
Prespecified data elements were extracted from each trial using a structured data abstraction form, including baseline characteristics, sample size, and interventions used (eTable 1 in the Supplement). Two of us extracted the data from the included trials (M.B.S. and L.J.M.), and disagreements were resolved by referring to a third reviewer (B.F.). Initial analysis was performed on December 18, 2018. An updated search was performed in April 2019, and no additional studies were added.
Risk of Bias and Quality of Evidence
The Cochrane Collaboration’s tool for assessing the risk of bias in the trial was used,11 which includes the following domains: random sequence generation, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting. Two of us independently assessed trial quality (L.J.M. and A.J.L.), and disagreements were resolved by referring to a third reviewer (M.B.S.).The quality of evidence (ie, certainty in the estimates) was evaluated using the GRADE approach (Grading of Recommendations Assessment, Development, and Evaluation).12
Statistical Analysis
We extracted hazard ratios (HRs) from the included trials. Logarithm-transformed HRs were pooled using the DerSimonian and Laird random-effects model. We conducted network meta-analysis based on a random-effects consistency model as described by White et al13 following a multivariate metaregression approach to pool evidence from direct and indirect comparisons. In addition, consistency between indirect and direct comparisons was evaluated. We used the I2 statistic to assess for heterogeneity across the included trials. Two-sided P < .05 was deemed statistically significant. Treatment strategies were ranked using surface under the cumulative ranking (SUCRA) probabilities. Higher SUCRA scores correspond to greater efficacy. Two analyses were planned to compare different maintenance strategies: first, to compare continuous induction chemotherapy vs maintenance vs observation and, second, to compare continuous induction chemotherapy vs FP vs FP + Bev vs Bev vs observation strategies.
Results
Study Selection
A total of 4741 titles and abstracts were identified by the screening electronic search strategy, of which 72 full-text articles met the eligibility for assessment (eFigure 1 in the Supplement). Fourteen articles describing 12 trials met the inclusion criteria and were included in the qualitative synthesis, while 11 RCTs were included in the network meta-analysis (eFigure 1 in the Supplement).3,7,9,14,15,16,17,18,19,20,21,22,23 CONCEPT (The Combined Oxaliplatin Neurotoxicity Prevention Trial) was not included in the quantitative analysis because it did not provide PFS or OS estimates, which are our end points.3
Study Characteristics
The 12 trials comprised 5540 patients. Seven trials had observation as the control group, with 3 compared with Bev, 2 compared with FP, and 2 compared with FP + Bev (eTable 1 and eFigure 2 in the Supplement). Ten trials used oxaliplatin with either capecitabine (CapeOx) or fluorouracil and leucovorin (FOLFOX) as part of the induction regimen, and 2 used irinotecan-based therapy. The age of the patients in these trials ranged from 23 to 85 years. The length of induction treatment varied between trials, ranging between 12 and 24 weeks (eTable 1 in the Supplement). Nine trials stated whether reinduction with the same chemotherapy regimen used in the induction phase was allowed. The characteristics of the included RCTs are outlined in eTable 1 in the Supplement.
Disruption of Treatment and Loss of Benefit
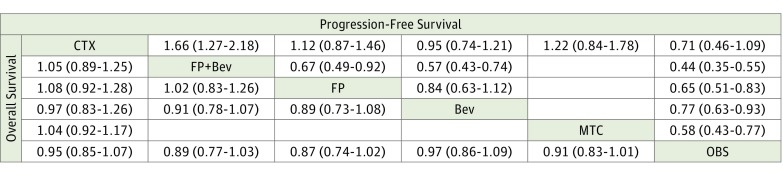
One study examined continuing full induction chemotherapy until progression compared with observation,8 and 3 examined continuing full induction chemotherapy until progression compared with maintenance therapy9,18,23 (eTable 1 and eFigure 2 in the Supplement). In the network meta-analysis, we found no benefit in continuing induction chemotherapy until progression compared with a period of observation in terms of PFS (HR, 0.71; 95% CI, 0.46-1.09) and OS (HR, 0.95; 95% CI, 0.85-1.07) (Figure 1). Similarly, there was no benefit of continuing induction chemotherapy vs maintenance therapy in terms of PFS (HR, 1.22; 95% CI, 0.84-1.78) and OS (HR, 1.04; 95% CI, 0.92-1.17) (Figure 1).
Figure 1. Hazard Ratios for the Pairwise Comparisons of the Network Meta-analysis.
Direct and indirect comparisons should be read from left to right. Hazard ratios for comparisons are in the cell in common between the column-defining and row-defining treatment. For progression-free survival, a hazard ratio of less than 1 favors row-defining treatment. For overall survival, a hazard ratio of less than 1 favors column-defining treatment. Bev indicates bevacizumab; CTX, continuing induction regimen; FP, fluoropyrimidine; MTC, maintenance; and OBS, observation.
Maintenance Therapy vs Observation
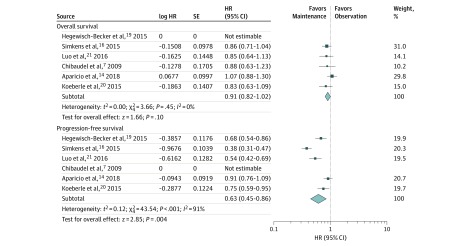
Six trials compared maintenance therapy with observation7,14,15,16,19,20,21,22 (Figure 1; eFigure 2 in the Supplement). The pooled direct comparisons showed a benefit of maintenance therapy vs observation in terms of PFS (HR, 0.58; 95% CI, 0.43-0.77) but not in terms of OS (HR, 0.91; 95% CI, 0.83-1.01) (Figure 2). Similar results are found in the network meta-analysis (Figure 1). On SUCRA analysis, maintenance treatment was ranked highest in terms of PFS (92.2) and OS (85.7) (eTable 2, eFigure 3, and eFigure 4 in the Supplement).
Figure 2. Meta-analyses of the Included Studies Examining Progression-Free Survival and Overall Survival for Maintenance Treatment vs Observation.
The marker size indicates the relative weight of the study as it contributes to the results of the overall comparison. IV indicates inverse-variance random-effect analysis. Percentages do not total 100 owing to rounding. HR indicates hazard ratio.
Preferred Maintenance Treatment
In the network meta-analysis, we found that any maintenance treatment with Bev or FP as monotherapy or in combination showed significant improvement in PFS vs observation. However, none of those strategies translated into an OS benefit (Figure 1). In addition, when comparing those maintenance agents with each other, the network meta-analysis shows that FP or FP + Bev ranked the highest on SUCRA in terms of PFS (67.1% for FP and 99.8% for FP + Bev) and OS (81.3% for FP and 73.2% for FP + Bev) (eTable 2, eFigure 5, and eFigure 6 in the Supplement).
Risk of Bias
A qualitative assessment was performed by assessing various indicators for each individual study using the Cochrane tool for risk of bias.11 Overall, the trials were deemed to be at low risk for bias, except for detection bias, for which only 2 trials had blinded assessment of outcomes, whereas the others were either not blinded or were unclear (eFigure 7 and eFigure 8 in the Supplement).
Quality of Evidence
The quality of the evidence for the indirect comparisons of maintenance therapy vs observation, continuing full induction chemotherapy vs observation, and maintenance therapy vs continuing full induction chemotherapy was moderate owing to imprecision (eTable 3 in the Supplement).
Discussion
In this systematic review and network meta-analysis of patients with mCRC, we found no benefit in continuing the full induction regimen until progression compared with a period of observation or maintenance therapy in terms of PFS or OS. In addition, we found that maintenance therapy provides a significant advantage over observation in terms of delaying progression but no benefit in terms of OS. Overall, FP as monotherapy or combined with Bev seems to be the preferred maintenance strategy. Single-agent Bev does not appear to be an acceptable option.
The optimal duration of first-line induction chemotherapy in mCRC after initial response or stable disease has been investigated since the era of single-agent FP, with evidence suggesting the feasibility of a “treatment break” and restarting the same treatment on disease progression.24 In this study, we confirm that maintenance therapy and even a cessation of treatment are acceptable options vs continuing the full regimen. A clear advantage for deescalating treatment after achieving a maximum response (typically 3-4 months) with induction therapy includes minimizing toxic effects, maximizing quality of life, and likely improving cost.9,23 These outcomes are clearly shown in the included trials in which patients receiving maintenance therapy have lower rates of toxic effects compared with those receiving the continuous induction regimen9,23 (eTable 4 in the Supplement). For most patients with mCRC, the primary goal of systemic therapy remains palliative.6
The other question is whether biologic agents without a cytotoxic component allow for maintaining a benefit while further reducing toxic effects. Bevacizumab use has resulted in improvement in outcomes when added to first-line induction cytotoxic regimens for patients with mCRC.25,26 In this study, we confirmed that any benefit from Bev is only apparent when combined with FP in the maintenance setting. Furthermore, we found that maintenance treatment with FP is more likely to have a delay in progression benefit when compared with observation or single-agent Bev. In addition, in the maintenance setting, maintenance Bev did not show any superior outcome to FP in the pairwise meta-analysis. We found that maintenance strategies with FP alone or combined with Bev were ranked the highest in attaining PFS and OS benefit. Because the added value of Bev is unclear in the maintenance setting, the choice for adding it to FP (FP vs FP + Bev) will depend on many factors, including patient preferences, risk of toxic effects, and added cost. The cost-effectiveness of capecitabine and Bev maintenance was studied in 2 different trials from Europe and the United States.27,28 Recently, a US study used the outcomes from the CAIRO3 (Maintenance Treatment Versus Observation After Induction in Advanced Colorectal Carcinoma) trial to determine the association of capecitabine and Bev drug prices with cost-effectiveness.28 The combination was not found to be cost-effective from Medicare payer perspective, with an estimated 93% decrease in drug prices needed for the combination to reach cost-effectiveness. Despite the limitations of the cost-effectiveness analysis, such information would further assist with the shared decision-making with the patient when determining the best maintenance strategy, especially when out-of-pocket treatment expenses appear to constitute more than 60% of the income for many patients with cancer.29
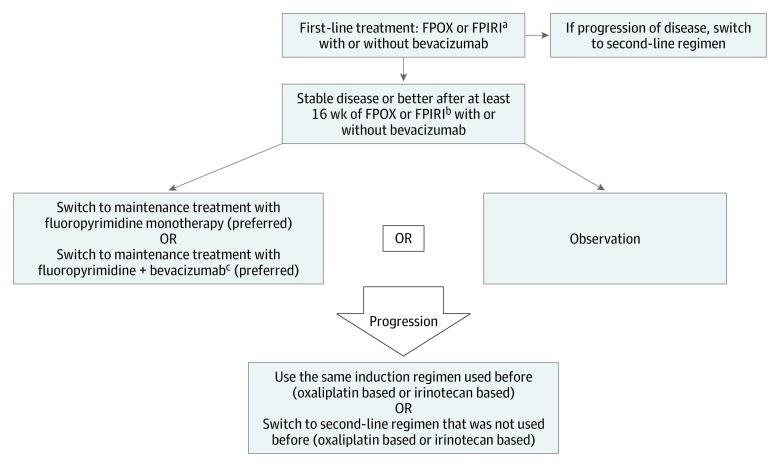
In our study, FP as monotherapy or combined with Bev ranked highest on SUCRA in the probability of achieving a survival benefit. However, the pairwise comparison in the meta-analysis did not show this improvement to be statistically significant for each of them when compared with observation. With the understanding that OS was not the primary outcome measure in the studies included in our analysis, our findings support observation to continuously be considered as a valid option for some patients. In Figure 3, we suggest an algorithm for maintenance strategies for patients with mCRC.
Figure 3. Flow Diagram Showing Suggested Algorithm for Maintenance Strategies in Metastatic Colorectal Cancer.
FPIRI indicates fluoropyrimidine with irinotecan; FPOX, fluoropyrimidine with oxaliplatin.
aThe same recommendations could be considered for FOLFOXIRI (fluorouracil, leucovorin, oxaliplatin, and irinotecan) with less supporting evidence.
bMost of the maintenance trials used oxalipatin-based regimens. The fluoropyrimidine used can be either capecitabine or fluorouracil.
cCan consider adding bevacizumab to the maintenance treatment with fluoropyrimidine if bevacizumab was added to the induction period.
We focused on PFS and OS as the coprimary end points in our analysis because there is clear consensus on the definition of such end points in mCRC clinical trials. The end points used in some of the included trials, such as tumor control duration (PRODIGE9)14 and time to failure of strategy (AIO),19 are controversial and lack validation in RCTs examining maintenance strategies, especially given the relatively low percentage of patients who are exposed to reinduction treatment.19,21
Other biologics in clinical use for mCRC have been examined in smaller studies. Maintenance strategies with EGFR-directed therapies have been investigated, with conflicting results.30,31 The phase 2 MACRO-2 trial showed noninferiority of cetuximab as maintenance treatment after induction therapy with FOLFOX and cetuximab when compared with continuous therapy.30 In contrast, maintenance with single-agent panitumumab appeared to be inferior to fluorouracil plus panitumumab after induction in the phase 2 VALENTINO trial.31 The latter study did not separate the contributing effects from adding panitumumab to fluorouracil as a single agent and, as such, is inconclusive. Future trials should focus on identifying the subset of patients who are most likely to benefit from more aggressive treatments or maintenance strategies vs observation alone.
A previously reported meta-analysis of 8 trials in 2014 examined continuous vs intermittent chemotherapy strategies for patients with mCRC.32 The results from that study found that intermittent strategies do not result in a clinically significant reduction in OS. In contrast, our study included a more powerful method (network meta-analysis). In addition, our analysis included only RCTs that used combination induction chemotherapy that is currently considered the standard of care for patients with mCRC. We also included only vascular endothelial growth factor inhibitors in our meta-analysis and excluded EGFR-directed therapies, given that the study of EGFR inhibitors is complicated by an expanded exclusionary mutational landscape as well as associations with sidedness in the colon (right colon vs left colon).33,34 Therefore, it would be challenging to interpret the results of such trials if they had been included in our analysis because they did not account for those stratification factors during the randomization process.17 Finally, our study expands on the previously reported meta-analysis by including additional landmark quality trials and providing simultaneous assessment of the relative efficacy of the different maintenance regimens (FP vs FP + Bev vs Bev).
Limitations
There are several limitations to our study as related to the network analysis and the individual trials. For example, PFS should be interpreted with caution in clinical trials when outcome assessors are not blinded (detection bias). The only 2 trials that clearly stated blinding of the outcome assessors were CAIRO3 and CONCEPT.3,15,17 In addition, our analysis was performed with study-level data rather than individual patient data, which would limit the power of our analysis. Despite these limitations, we believe that our study allows for the most comprehensive and up-to-date analysis of maintenance strategies in mCRC.
Conclusions
For patients with mCRC who achieve at least stable disease after a specified period of induction regimen (3-4 months), a maintenance strategy with at least FP with or without the addition of Bev is preferred. However, given the lack of a clear OS benefit, observation remains an acceptable alternative. Optimal maintenance strategies should be dependent on factors such as patient preference, cost of care, and potential toxic effects.
eFigure 1. Screening and Selection Process
eFigure 2. Evidence Network
eFigure 3. Ranking Probabilities of the Different Comparisons for Overall Survival
eFigure 4. Ranking Probabilities of the Different Comparisons for Progression-Free Survival
eFigure 5. Ranking Probabilities of the Different Comparisons for Overall Survival
eFigure 6. Ranking Probabilities of the Different Comparisons for Progression-Free Survival
eFigure 7. Risk of Bias Graph
eFigure 8. Risk of Bias Graph
eTable 1. Baseline Characteristics
eTable 2. Ranking Probabilities of Different Maintenance Strategies
eTable 3. Quality of Evidence Table (GRADE)
eTable 4. Adverse Events
eMethods. Search Strategy
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):-. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Oliveira J; ESMO Guidelines Working Group . Advanced colorectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(suppl 4):61-63. doi: 10.1093/annonc/mdp130 [DOI] [PubMed] [Google Scholar]
- 3.Hochster HS, Grothey A, Hart L, et al. Improved time to treatment failure with an intermittent oxaliplatin strategy: results of CONcePT. Ann Oncol. 2014;25(6):1172-1178. doi: 10.1093/annonc/mdu107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogel A, Hofheinz RD, Kubicka S, Arnold D. Treatment decisions in metastatic colorectal cancer—beyond first and second line combination therapies. Cancer Treat Rev. 2017;59:54-60. doi: 10.1016/j.ctrv.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 5.Yoshino T, Arnold D, Taniguchi H, et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol. 2018;29(1):44-70. doi: 10.1093/annonc/mdx738 [DOI] [PubMed] [Google Scholar]
- 6.Zhou M, Fu L, Zhang J. Who will benefit more from maintenance therapy of metastatic colorectal cancer? Oncotarget. 2017;9(15):12479-12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chibaudel B, Maindrault-Goebel F, Lledo G, et al. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? the GERCOR OPTIMOX2 study. J Clin Oncol. 2009;27(34):5727-5733. doi: 10.1200/JCO.2009.23.4344 [DOI] [PubMed] [Google Scholar]
- 8.Labianca R, Sobrero A, Isa L, et al. ; Italian Group for the Study of Gastrointestinal Cancer-GISCAD . Intermittent versus continuous chemotherapy in advanced colorectal cancer: a randomised “GISCAD” trial. Ann Oncol. 2011;22(5):1236-1242. doi: 10.1093/annonc/mdq580 [DOI] [PubMed] [Google Scholar]
- 9.Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal cancer—a GERCOR study. J Clin Oncol. 2006;24(3):394-400. doi: 10.1200/JCO.2005.03.0106 [DOI] [PubMed] [Google Scholar]
- 10.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. doi: 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brignardello-Petersen R, Bonner A, Alexander PE, et al. ; GRADE Working Group . Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol. 2018;93:36-44. doi: 10.1016/j.jclinepi.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 13.White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods. 2012;3(2):111-125. doi: 10.1002/jrsm.1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aparicio T, Ghiringhelli F, Boige V, et al. ; PRODIGE 9 Investigators . Bevacizumab maintenance versus no maintenance during chemotherapy-free intervals in metastatic colorectal cancer: a randomized phase III trial (PRODIGE 9). J Clin Oncol. 2018;36(7):674-681. doi: 10.1200/JCO.2017.75.2931 [DOI] [PubMed] [Google Scholar]
- 15.Goey KKH, Elias SG, van Tinteren H, et al. Maintenance treatment with capecitabine and bevacizumab versus observation in metastatic colorectal cancer: updated results and molecular subgroup analyses of the phase 3 CAIRO3 study. Ann Oncol. 2017;28(9):2128-2134. doi: 10.1093/annonc/mdx322 [DOI] [PubMed] [Google Scholar]
- 16.Simkens LH, van Tinteren H, May A, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet. 2015;385(9980):1843-1852. doi: 10.1016/S0140-6736(14)62004-3 [DOI] [PubMed] [Google Scholar]
- 17.Adams RA, Meade AM, Seymour MT, et al. ; MRC COIN Trial Investigators . Intermittent versus continuous oxaliplatin and fluoropyrimidine combination chemotherapy for first-line treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet Oncol. 2011;12(7):642-653. doi: 10.1016/S1470-2045(11)70102-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Díaz-Rubio E, Gómez-España A, Massutí B, et al. ; Spanish Cooperative Group for the Treatment of Digestive Tumors . First-line XELOX plus bevacizumab followed by XELOX plus bevacizumab or single-agent bevacizumab as maintenance therapy in patients with metastatic colorectal cancer: the phase III MACRO TTD Study. Oncologist. 2012;17(1):15-25. doi: 10.1634/theoncologist.2011-0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hegewisch-Becker S, Graeven U, Lerchenmüller CA, et al. Maintenance strategies after first-line oxaliplatin plus fluoropyrimidine plus bevacizumab for patients with metastatic colorectal cancer (AIO 0207): a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2015;16(13):1355-1369. doi: 10.1016/S1470-2045(15)00042-X [DOI] [PubMed] [Google Scholar]
- 20.Koeberle D, Betticher DC, von Moos R, et al. Bevacizumab continuation versus no continuation after first-line chemotherapy plus bevacizumab in patients with metastatic colorectal cancer: a randomized phase III non-inferiority trial (SAKK 41/06). Ann Oncol. 2015;26(4):709-714. doi: 10.1093/annonc/mdv011 [DOI] [PubMed] [Google Scholar]
- 21.Luo HY, Li YH, Wang W, et al. Single-agent capecitabine as maintenance therapy after induction of XELOX (or FOLFOX) in first-line treatment of metastatic colorectal cancer: randomized clinical trial of efficacy and safety. Ann Oncol. 2016;27(6):1074-1081. doi: 10.1093/annonc/mdw101 [DOI] [PubMed] [Google Scholar]
- 22.Noepel-Duennebacke S, Arnold D, Hertel J, et al. Impact of the localization of the primary tumor and RAS/BRAF mutational status on maintenance strategies after first-line oxaliplatin, fluoropyrimidine, and bevacizumab in metastatic colorectal cancer: results from the AIO 0207 trial. Clin Colorectal Cancer. 2018;17(4):e733-e739. doi: 10.1016/j.clcc.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 23.Yalcin S, Uslu R, Dane F, et al. Bevacizumab + capecitabine as maintenance therapy after initial bevacizumab + XELOX treatment in previously untreated patients with metastatic colorectal cancer: phase III “Stop and Go” study results—a Turkish Oncology Group Trial. Oncology. 2013;85(6):328-335. doi: 10.1159/000355914 [DOI] [PubMed] [Google Scholar]
- 24.Maughan TS, James RD, Kerr DJ, et al. ; Medical Research Council Colorectal Cancer Group . Comparison of intermittent and continuous palliative chemotherapy for advanced colorectal cancer: a multicentre randomised trial. Lancet. 2003;361(9356):457-464. doi: 10.1016/S0140-6736(03)12461-0 [DOI] [PubMed] [Google Scholar]
- 25.Fuchs CS, Marshall J, Barrueco J. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: updated results from the BICC-C study. J Clin Oncol. 2008;26(4):689-690. doi: 10.1200/JCO.2007.15.5390 [DOI] [PubMed] [Google Scholar]
- 26.Hurwitz HI, Tebbutt NC, Kabbinavar F, et al. Efficacy and safety of bevacizumab in metastatic colorectal cancer: pooled analysis from seven randomized controlled trials. Oncologist. 2013;18(9):1004-1012. doi: 10.1634/theoncologist.2013-0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franken MD, van Rooijen EM, May AM, et al. Cost-effectiveness of capecitabine and bevacizumab maintenance treatment after first-line induction treatment in metastatic colorectal cancer. Eur J Cancer. 2017;75:204-212. doi: 10.1016/j.ejca.2017.01.019 [DOI] [PubMed] [Google Scholar]
- 28.Sherman SK, Lange JJ, Dahdaleh FS, et al. Cost-effectiveness of maintenance capecitabine and bevacizumab for metastatic colorectal cancer. JAMA Oncol. 2019;5(2):236-242. doi: 10.1001/jamaoncol.2018.5070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narang AK, Nicholas LH. Out-of-pocket spending and financial burden among Medicare beneficiaries with cancer. JAMA Oncol. 2017;3(6):757-765. doi: 10.1001/jamaoncol.2016.4865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aranda E, García-Alfonso P, Benavides M, et al. ; Spanish Cooperative Group for the Treatment of Digestive Tumours (TTD) . First-line mFOLFOX plus cetuximab followed by mFOLFOX plus cetuximab or single-agent cetuximab as maintenance therapy in patients with metastatic colorectal cancer: phase II randomised MACRO2 TTD study. Eur J Cancer. 2018;101:263-272. doi: 10.1016/j.ejca.2018.06.024 [DOI] [PubMed] [Google Scholar]
- 31.Pietrantonio F, Morano F, Corallo S, et al. First-line FOLFOX plus panitumumab (Pan) followed by 5FU/LV plus Pan or single-agent Pan as maintenance therapy in patients with RAS wild-type metastatic colorectal cancer (mCRC): the VALENTINO study. J Clin Oncol. 2018;36(15_suppl):3505. doi: 10.1200/JCO.2018.36.15_suppl.3505 [DOI] [Google Scholar]
- 32.Berry SR, Cosby R, Asmis T, Chan K, Hammad N, Krzyzanowska MK; Cancer Care Ontario’s Gastrointestinal Disease Site Group . Continuous versus intermittent chemotherapy strategies in metastatic colorectal cancer: a systematic review and meta-analysis. Ann Oncol. 2015;26(3):477-485. doi: 10.1093/annonc/mdu272 [DOI] [PubMed] [Google Scholar]
- 33.Tejpar S, Stintzing S, Ciardiello F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. 2017;3(2):194-201. doi: 10.1001/jamaoncol.2016.3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Venook AP, Niedzwiecki D, Innocenti F, et al. Impact of primary (1°) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol. 2016;34(15_suppl):3504. doi: 10.1200/JCO.2016.34.15_suppl.3504 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Screening and Selection Process
eFigure 2. Evidence Network
eFigure 3. Ranking Probabilities of the Different Comparisons for Overall Survival
eFigure 4. Ranking Probabilities of the Different Comparisons for Progression-Free Survival
eFigure 5. Ranking Probabilities of the Different Comparisons for Overall Survival
eFigure 6. Ranking Probabilities of the Different Comparisons for Progression-Free Survival
eFigure 7. Risk of Bias Graph
eFigure 8. Risk of Bias Graph
eTable 1. Baseline Characteristics
eTable 2. Ranking Probabilities of Different Maintenance Strategies
eTable 3. Quality of Evidence Table (GRADE)
eTable 4. Adverse Events
eMethods. Search Strategy