Key Points
Question
What are the characteristics of necrobiotic xanthogranuloma, and what criteria should establish the diagnosis?
Findings
This multicenter cross-sectional study and systematic review included 235 patients with necrobiotic xanthogranuloma. Informed by the results, 8 board-certified dermatologists participated in a consensus exercise; in the absence of foreign body, infection, or other identifiable cause, both major criteria (clinical and histopathological features) and at least 1 minor criterion (paraproteinemia, plasma-cell dyscrasia, and/or other associated lymphoproliferative disorder, or a periorbital distribution) were proposed to diagnose necrobiotic xanthogranuloma.
Meaning
These findings and criteria have potential to advance clinical research and should be validated by further studies.
This study combines a multicenter cohort study with a systematic review and a consensus-based expert review to evaluate the characteristics of necrobiotic xanthogranuloma and propose diagnostic criteria for this disease.
Abstract
Importance
Necrobiotic xanthogranuloma (NXG) is a non–Langerhans cell histiocytosis classically associated with paraproteinemia attributable to plasma-cell dyscrasias or lymphoproliferative disorders. Despite the morbidity of NXG, the literature is limited to case reports and small studies, and diagnostic criteria are lacking.
Objective
To evaluate the characteristics of NXG and propose diagnostic criteria.
Design, Setting, and Participants
This multicenter cross-sectional study was conducted at tertiary academic referral centers and followed by a systematic review and a consensus exercise. The multicenter cohort included patients with NXG diagnosed at the Brigham and Women’s and Massachusetts General Hospitals (2000-2018), the University of Iowa Hospitals and Clinics (2000-2018), and the University of Pennsylvania Health System (2008-2018). The systematic review was conducted in 2018 and included patients with NXG identified in the Cochrane, Ovid EMBASE, PubMed, and Web of Science databases. The consensus exercise was conducted by 8 board-certified dermatologists to identify diagnostic criteria.
Main Outcomes and Measures
Demographic factors, comorbidities, clinical features, and treatment response.
Results
Of 235 included patients with NXG (34 from the multicenter cohort and 201 from the systematic review results), the mean (SD) age at presentation was 61.6 (14.2) years; 147 (62.6%) were female. Paraproteinemia was detected in 193 patients (82.1%), most often IgG-κ (117 patients [50.0%]). A malignant condition was detected in 59 patients (25.1%), most often multiple myeloma (33 patients [14.0%]). The overall rate of paraproteinemia and/or a malignant condition was 83.8% (197 patients). In the multicenter cohort, evolution of paraproteinemia into multiple myeloma was observed up to 5.7 years (median [range], 2.4 [0.1-5.7] years) after NXG presentation. Cutaneous lesions consisted of papules, plaques, and/or nodules, typically yellow or orange in color (113 of 187 [60.4%]) with a periorbital distribution (130 of 219 [59.3%]). The eye was the leading site of extracutaneous involvement (34 of 235 [14.5%]). In the multicenter cohort, intravenous immunoglobulin had the best treatment response rate (9 of 9 patients [100%]), followed by antimalarial drugs (4 of 5 patients [80%]), intralesional triamcinolone (6 of 8 patients [75%]), surgery (3 of 4 patients [75%]), chemotherapy (8 of 12 patients [67%]), and lenalidomide or thalidomide (5 of 8 patients [63%]). The consensus exercise yielded 2 major criteria, which were (1) clinical and (2) histopathological features consistent with NXG, and 2 minor criteria, consisting of (1) paraproteinemia, plasma-cell dyscrasia, and/or other associated lymphoproliferative disorder and (2) periorbital distribution of cutaneous lesions. In the absence of foreign body, infection, or another identifiable cause, fulfillment of both major and at least 1 minor criterion were proposed to establish the diagnosis of NXG.
Conclusions and Relevance
Necrobiotic xanthogranuloma is a multisystem disorder associated with paraproteinemia and malignant conditions. The proposed diagnostic criteria may advance clinical research and should be validated.
Introduction
Necrobiotic xanthogranuloma (NXG) is a non–Langerhans cell histiocytosis first described by Kossard and Winkelmann1 in 1980. It is typically associated with paraproteinemia attributable to plasma-cell dyscrasias or lymphoproliferative disorders.2 In addition to serving as a skin sign of systemic disease, NXG may result in cosmetic disfigurement. Extracutaneous involvement including the eye, heart, gastrointestinal tract, liver, and lung may result in organ dysfunction and death.1,3,4,5 Clinically, yellow-to-orange papules, plaques, and/or nodules in a periorbital distribution are classic; however, other morphological characteristics and sites have been described.6 Histopathologically, NXG demonstrates palisading granulomas with lymphoplasmacytic infiltrate and zones of necrobiosis. Cholesterol clefts and Touton or large, bizarre foreign body giant cells are other classical features.6 The pathogenesis of NXG remains unknown; however, a role for paraprotein-lipoprotein interaction has been hypothesized.7,8 Evaluation of 12 skin-biopsy specimens by Wood et al9 in 2009 found no evidence to support a monoclonal plasma-cell proliferation within the inflammatory infiltrate.
The literature on NXG consists of case reports and small studies, and diagnostic criteria are currently unavailable. The first objective of this study was to evaluate the demographic features, comorbidities, clinical features, and treatment response of a large NXG cohort. The second objective was to propose diagnostic criteria for NXG.
Methods
Study Design, Setting, and Participants
The institutional review boards of Partners Healthcare, the University of Iowa, and the University of Pennsylvania approved this study. The requirement for informed consent was waived because of the retrospective nature of the study.
For the multicenter cohort, patients were identified from both the electronic health records and the dermatopathology databases at the Brigham and Women's Hospital, Massachusetts General Hospital, the University of Iowa Hospitals and Clinics, and the University of Pennsylvania Health System using International Classification of Diseases, Ninth Revision and Tenth Revision codes (273.2 and D76.3) and a free-text search for the terms necrobiotic xanthogranuloma and NXG. Patients were identified from 2000 through 2018 at the Brigham and Women's Hospital, Massachusetts General Hospital, and the University of Iowa Hospitals and Clinics and from 2008 through 2018 at the University of Pennsylvania Health System, with years selected based on availability of readily searchable electronic health records. The diagnosis was verified by consensus of the principal investigators (K.A.W., M.R., and A.M.) based on clinical and histopathological features. Exclusion criteria were an age younger than 18 years or a lack of histopathological features interpreted by a dermatopathologist as being consistent with NXG.
For the systematic review, records were identified with the support of a medical librarian in the Cochrane, Ovid EMBASE, PubMed, and Web of Science databases on November 20, 2018, using the medical subject headings (MeSH) terms “necrobiotic disorders” AND “histiocytosis, non-Langerhans-cell” OR “necrobiotic xanthogranuloma” in PubMed and a free text search for “necrobiotic xanthogranuloma” OR “NXG” OR “periocular xanthogranuloma” OR “periorbital xanthogranuloma” in all databases. Titles and abstracts were independently reviewed by 2 authors (C.A.N. and C.S.Z.). Full texts of records deemed relevant were independently reviewed by both authors, and a consensus was reached to include or exclude each article. Exclusion criteria were a patient age younger than 18 years, a study with aggregated data, an alternative diagnosis, a patient duplicated in another study, a language other than English, a lack of histopathological features interpreted by a dermatopathologist as being consistent with NXG, or an excluded study design (a conference abstract or a case report with insufficient data).
Data Collection
Using a standardized data collection instrument, we abstracted patient demographics, comorbidities, and clinical features at the time of NXG presentation. For the multicenter cohort, we also abstracted body mass index (calculated as weight in kilograms divided by height in meters squared), timing of paraproteinemia and malignant conditions relative to NXG, and antilipemic therapy, characteristics inconsistently reported in the literature. In addition, we classified treatment response as a complete response, a partial response, no response, or worsening based on the assessment of the patient’s dermatologist. Response rates were calculated as the percentage of patients with a complete or partial response. We did not evaluate response rates in the literature out of a concern for positive outcome bias.
Consensus Exercise
After reviewing the results, 8 board-certified dermatologists (A.M., C.A.N., K.A.W., M.H.N., M.R., R.A.V., R.G.M., and S.I.) participated in a consensus exercise to propose diagnostic criteria for NXG. In the first online survey, participants identified all possible characteristics of NXG that could be considered for inclusion. In the second round, participants ranked the importance of each characteristic on a Likert scale ranging from 1 (not at all important) to 5 (very important), along with a free-response commentary. We used a mean importance ranking of 3.5 of 5 or greater as the numerical cutoff score for inclusion. In the third round, the participants reviewed the results of the previous survey and provided a free-response commentary on how to structure the criteria. In the final round, the participants reviewed draft criteria, indicated the level of agreement with each criterion using a Likert scale ranging from 1 (signaling strongly disagreement) to 5 (strong agreement), along with a free-response commentary, and indicated approval or disapproval. We required a mean agreement ranking of 3.5 of 5 or greater for each criterion and unanimous approval to establish consensus.
Statistical Analysis
Continuous variables were summarized with means and SDs. Categorical variables were reported as proportions and percentages. All statistical analyses were performed in Excel version 14.7.1 (Microsoft).
Results
Patient Characteristics
For the multicenter cohort, the initial search yielded 71 patients at the Brigham and Women's Hospital and Massachusetts General Hospital (combined), 41 patients at the University of Iowa Hospitals and Clinics, and 61 patients at the University of Pennsylvania Health System. Fifteen patients (21%), 6 patients (15%), and 13 patients (21%) from these 3 centers, respectively, were included after review and verification, for a total of 34 patients.
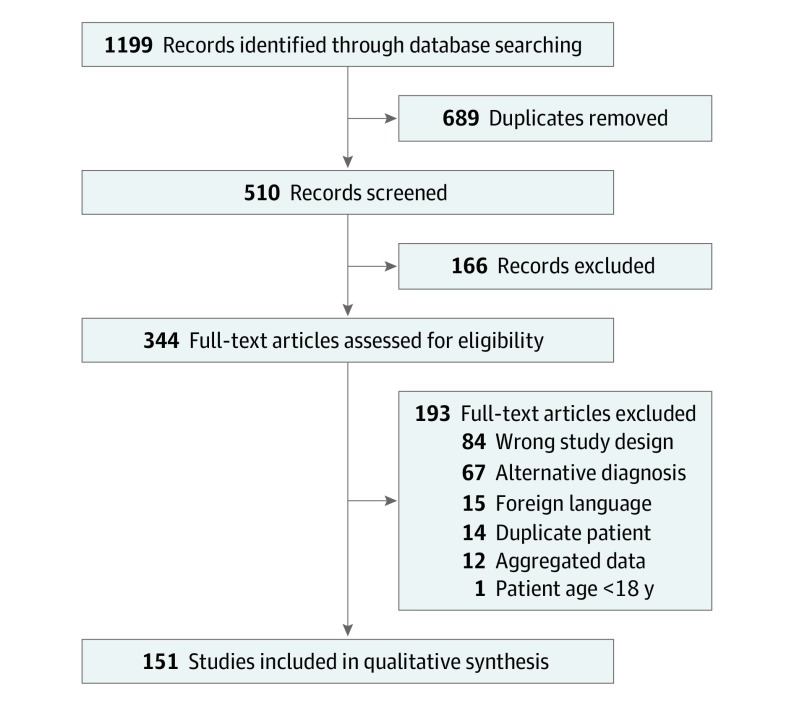
In the systematic review, the initial search yielded 1199 records, 151 of which were included3,4,7,8,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156 (Figure). These reports described 201 patients with NXG, with demographics, comorbidities, and clinical features (detailed in the eTable in the Supplement).
Figure. Flowchart of Selected Studies for the Systematic Review.
Overall, 235 patients with NXG were included in the study. The demographic features and comorbidities of patients with NXG are detailed in Table 1. The mean (SD) age at presentation was 61.6 (14.2) years; 147 patients (62.6%) were female. Most patients were white (78 of 90 for whom these data were available [87%]). Of the 235 patients, 193 (82.1%) had paraproteinemia. The most common subtype was IgG-κ, in 117 of 235 patients (50.0%); however, IgG-λ, IgM, and IgA subtypes were also observed (in 49 patients [20.8%], 5 patients [2.1%], and 2 patients [0.9%], respectively). Fifty-nine of 235 patients (25.1%) had a malignant condition. Multiple myeloma, in 33 patients (14.0%), was the most common type; however, lymphoma, leukemia, and Waldenstrom macroglobulinemia were also observed (in 1 patient [0.4%] each). Solid tumors were rare (2 of 235 [0.9%]) and observed exclusively in patients with coexistent paraproteinemia. The overall rate of paraproteinemia and/or a malignant conditions was 83.8% (affecting 197 patients). In the multicenter cohort, the timing of paraproteinemia relative to NXG was antecedent in 13 of 24 patients (54%), concurrent in 6 of 24 patients (25%), and subsequent in 4 of 24 patients (16.7%). Evolution of paraproteinemia into multiple myeloma was observed up to 5.7 years after NXG presentation (median [range], 2.4 [0.1-5.7] years); in contrast, lymphoma and leukemia preceded NXG presentation (by a median [range] of 5.25 [2.2-6.8] years).
Table 1. Demographics and Comorbidities of Necrobiotic Xanthogranuloma.
| Patient Characteristic | Patients, No./Total No. (%) | ||
|---|---|---|---|
| Multicenter Cohort (n = 34) | Systematic Review (n = 201) | Overall (n = 235) | |
| Age, mean (SD), y | 61.6 (14.1) | 61.9 (14.2) | 61.8 (14.2)a |
| Sex | |||
| Female | 23/34 (68) | 124/201 (61.7) | 147/235 (62.6) |
| Male | 11/34 (32) | 77/201 (38.3) | 88/235 (37.4) |
| Race | |||
| White | 32/33 (97) | 46/57 (81)a | 78/90 (87) |
| Asian | 0 | 11/57 (19) | 11/90 (12) |
| Black or African American | 1/33 (3) | 0 | 1/90 (1) |
| Paraproteinemia | 26/34 (76)b | 167/201 (83.1) | 193/235 (82.1) |
| IgG-κ | 15/34 (44) | 102/201 (50.7) | 117/235 (50.0) |
| IgG-λ | 8/34 (24) | 41/201 (20.4) | 49/235 (20.8) |
| IgM | 1/34 (3) | 4/201 (2.0) | 5/235 (2.1) |
| IgA | 2/201 (1.0) | 2/235 (0.8) | |
| Polyclonal | 2/34 (6) | 6/201 (3.0) | 8/235 (3.4) |
| Unspecified type | 12/201 (6.0) | 12/235 (5.1) | |
| Malignant condition | 8/34 (24)c | 51/201 (25.4) | 59/235 (25.1) |
| Multiple myeloma | 5/34 (15) | 28/201 (13.9) | 33/235 (14.0) |
| Lymphoma | 2/34 (6) | 13/201 (6.5) | 15/235 (6.4) |
| Leukemia | 1/34 (3) | 7/201 (3.5) | 8/235 (3.4) |
| Breast cancer | 0 | 1/201 (0.5) | 1/235 (0.4) |
| Rectal cancer | 0 | 1/201 (0.5) | 1/235 (0.4) |
| Waldenstrom macroglobulinemia | 0 | 1/201 (0.5) | 1/235 (0.4) |
One patient was identified as being of Hispanic or Latino ethnicity.
The timing of paraproteinemia relative to necrobiotic xanthogranuloma was antecedent in 13 of 24 patients (54%), concurrent in 6 of 24 individuals (25%), and subsequent in 4 of 24 patients (17%).
The timing of multiple myeloma relative to necrobiotic xanthogranuloma was subsequent in 5 of 5 patients (100%) at a time interval of up to 5.7 years (median [range], 2.4 [0.1-5.7] years). The timing of lymphoma and leukemia relative to necrobiotic xanthogranuloma was antecedent in 3 of 3 patients (100%).
The clinical features of NXG are detailed in Table 2 and the eTable in the Supplement. Primary morphological characteristics were papules and plaques (163 of 212 patients [76.7%]) and/or nodules (52 of 212 [24.5%]). Ulceration (40 of 211 patients [19.0%]) was the most common secondary feature, followed by telangiectasia (30 of 211 [14.2%]), atrophy (29 of 211 [13.7%]), and induration (15 of 211 [7.1%]). Diverse colors were observed, from yellow or orange (113 of 187 patients [60.4%]) to red or pink (45 of 187 [24.1%]), brown (28 of 187 [14.9%]), and purple (21 of 187 [11.2%]). Pain and pruritus were reported by 27 of 88 patients (30.7%) and 15 of 88 patients (17.0%), respectively. Of 218 patients, 197 (90.4%) presented with multiple cutaneous lesions. The most common distribution was periorbital (130 of 219 [59.3%]); however, lesions were distributed elsewhere on the face (50 of 219 [22.8%]), trunk (116 of 219 [53.0%]), extremities (123 of 219 [56.1%]), and within scars (12 of 219 [5.5%]). The eye was the leading site of extracutaneous involvement. In the multicenter cohort, the rate of eye involvement in patients evaluated by ophthalmology was 27% (4 of 15 patients); however, this denominator was not reliably reported in the literature. Other sites were the brain, gastrointestinal tract, heart, liver, lung, lymphoreticular system, muscle, and parotid gland.
Table 2. Clinical Features of Necrobiotic Xanthogranulomaa.
| Patient Characteristic | Patients, No./Total No. (%) | ||
|---|---|---|---|
| Multicenter Cohort (n = 34) | Systematic Review (n = 201) | Overall (n = 235) | |
| Primary lesionsb | |||
| Papules or plaques | 31/33 (94) | 132/179 (73.7) | 163/212 (76.7) |
| Nodules | 5/33 (15) | 47/179 (26.3) | 52/212 (24.5) |
| Secondary featuresb | |||
| Ulceration | 5/33 (15) | 35/178 (19.7) | 40/211 (19.0) |
| Telangiectasia | 5/33 (15) | 25/178 (14.0) | 30/211 (14.2) |
| Atrophy | 3/33 (9) | 26/178 (14.6) | 29/211 (13.7) |
| Induration | 0/33 (0) | 15/178 (8.4) | 15/211 (7.1) |
| Colorb | |||
| Yellow or orange | 21/33 (64) | 92/154 (59.7) | 113/187 (60.4) |
| Red or pink | 17/33 (51) | 28/154 (18.2) | 45/187 (24.1) |
| Brown | 9/33 (27) | 19/154 (12.3) | 28/187 (14.9) |
| Purple | 5/33 (15) | 16/154 (10.4) | 21/187 (11.3) |
| Symptomsb | |||
| Pain | 5/31 (16) | 22/57 (39) | 27/88 (30.7) |
| Pruritus | 5/31 (16) | 11/57 (19) | 15/88 (17.0) |
| Lesions | |||
| Single | 6/33 (18) | 15/184 (8.2) | 21/217 (9.7) |
| Multiple | 27/33 (82) | 170/185 (92.4) | 197/218 (90.4) |
| Distributionb | |||
| Periorbital | 16/33 (48) | 114/186 (61.3) | 130/219 (59.4) |
| Other face | 9/33 (27) | 41/186 (22.0) | 50/219 (22.8) |
| Trunk | 15/33 (45) | 101/186 (54.3) | 116/219 (53.0) |
| Extremities | 20/33 (61) | 103/186 (55.4) | 123/219 (56.2) |
| Within a scar | 6/33 (18) | 6/186 (3.2) | 12/219 (5.5) |
| Extracutaneous involvementb,c | |||
| Eye | 4/34 (12) | 30/201 (14.9) | 34/235 (14.5) |
| Gastrointestinal tract | 1/34 (3) | 4/201 (2.0) | 5/235 (2.1) |
| Liver | 1/34 (3) | 4/201 (2.0) | 5/235 (2.1) |
| Heart | 0 | 4/201 (2.0) | 4/235 (1.7) |
| Lung | 0 | 3/201 (1.5) | 3/235 (1.3) |
| Lymphoreticular system | 0 | 3/201 (1.5) | 3/235 (1.3) |
| Parotid gland | 0 | 2/201 (1.0) | 2/235 (0.9) |
| Brain | 0 | 1/201 (0.5) | 1/235 (0.4) |
| Muscle | 0 | 1/201 (0.5) | 1/235 (0.4) |
| Abnormal laboratory test results | |||
| BMI, mean (SD)d | 27.0 (5.6) | NA | NA |
| Low high-density lipoprotein cholesterole | 7/17 (41)f | 7/42 (17) | 14/59 (24) |
| Low C4g | 3/4 (75) | 30/50 (60) | 33/54 (61) |
| Cryoglobulinemia | 0 | 9/39 (23) | 9/39 (23) |
| Low 25-hydroxyvitamin D levels | 3/10 (30)h | 1/1 (100)i | 4/11 (36) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not available.
SI conversion factors: To convert 1,25 dihydroxyvitamin D to pmol/L, multiple by 2.6; to convert C3 to g/L, multiply by 0.001; to convert C4 complement to g/L, multiply by 0.001; to convert high-density lipoprotein–cholesterol, multiply by 0.0259.
Further descriptions of all data extracted from the systematic review is in the eTable in the Supplement.
Categories are not mutually exclusive.
Sample sizes cannot be reliably reported because of the heterogeneity of evaluation of extracutaneous sites for involvement with necrobiotic xanthogranuloma. The exception was the rate of eye involvement in patients evaluated by ophthalmology in the multicenter cohort, which was 4 of 15 patients (27%).
Normal limits, 18.5 to 25.
Normal limits, 40 to 50 mg/dL for men and 50 to 59 mg/dL for women.
Four of 7 patients with low and 6 with normal high-density lipoprotein–cholesterol levels (38%) were taking 1 or more medications known to elevate high-density lipoprotein–cholesterol levels.
The normal range for C3 is 10 to 40 mg/dL.
One patient with a low 25-hydroxyvitamin D level had a normal 1,25-dihydroxyvitamin D level (normal range, 18-78 pg/mL).
One patient with a low 25-hydroxyvitamin D level had a high 1,25-dihydroxyvitamin D level (normal range, 18-78 pg/mL).
Patients with NXG in the multicenter cohort had an overweight mean (SD) body mass index (27.0 [5.6]). Overall, 14 of 59 patients (24%) had a low high-density lipoprotein–cholesterol level; however, this rate in the multicenter cohort was higher (7 of 17 patients [41%]), even though 4 of 7 patients (38%) tested were taking 1 or more medications known to elevate high-density lipoprotein cholesterol levels. Thirty-three of 54 patients (61%) and 9 of 39 patients (23%) tested had low complement C4 levels and positive test results for cryoglobulins, respectively. Four of 11 patients (36%) tested had low 25-hydroxyvitamin D; however, 1,25-dihydroxyvitamin D was normal or high in half of these patients (2 of 4 [50%]).
Treatment Response
Treatment response rates in the multicenter cohort are presented in Table 3. Of treatments with response reported in more than 3 patients (10%), intravenous immunoglobulin had the best response rate (9 of 9 patients [100%]), followed by antimalarials (4 of 5 patients [80%]), intralesional triamcinolone (6 of 8 patients [75%]), surgery (3 of 4 patients [75%]), chemotherapy (8 of 12 patients [67%]), and lenalidomide or thalidomide (5 of 8 patients [63%]).
Table 3. Treatment Response for Necrobiotic Xanthogranuloma in the Multicenter Cohort.
| Treatmenta | Response Rate, No./Total No. (%) (n = 34) |
|---|---|
| Intravenous immunoglobulin | 9/9 (100) |
| Antimalarial | 4/5 (80) |
| Intralesional triamcinolone | 6/8 (75) |
| Surgeryb | 3/4 (75) |
| Chemotherapy (alkylating agents, antimetabolites, and/or proteasome inhibitors) | 8/12 (67) |
| Lenalidomide or thalidomide | 5/8 (63) |
Treatments with response reported in less than 10% of patients were antibiotics (topical and systemic), calcineurin inhibitors (topical and intralesional), corticosteroids (topical and systemic), dapsone, electron beam radiation, lasers (carbon dioxide and pulsed dye), nitrogen mustard (topical), pentoxifylline, rituximab, and stem cell transplants.
One of the 4 patients relapsed after a complete response.
Diagnostic Criteria
The proposed diagnostic criteria for NXG are presented in the Box. The response rate for each round of the consensus exercise was 100%. Participants identified 13 characteristics in the first round and ranked their importance in the second round. In the third round, participants expressed preference for proposing major and minor criteria instead of a point-scoring system and requiring the exclusion of other causes, such as a foreign body and infection. Four characteristics that received mean importance rankings of 3.5 of 5 points or greater were drafted into major and minor criteria. In the final round, the major criteria received mean agreement rankings of 4.6 of 5 points and 4.5 of 5 points, respectively; the minor criteria received mean agreement rankings of 4.6 of 5 points and 3.9 of 5 points, respectively; and all participants approved the criteria, establishing consensus.
Box. Diagnostic Criteria for Necrobiotic Xanthogranulomaa.
Major Criteria
Cutaneous papules, plaques, and/or nodules, most often yellow or orange in color.
Histopathological features demonstrating palisading granulomas with lymphoplasmacytic infiltrate and zones of necrobiosis. Characteristic features that are variably present include cholesterol clefts and/or giant cells (Touton or foreign body).
Minor Criteria
Paraproteinemia, most often IgG-κ, plasma-cell dyscrasia, and/or other associated lymphoproliferative disorder.
Periorbital distribution of cutaneous lesions.
Discussion
To our knowledge, this study provides the largest review to date of the demographics, comorbidities, clinical features, and treatment response of NXG. In addition, it presents the outcome of a consensus exercise to propose the first diagnostic criteria for NXG.
The mean age of 61.6 years in the cohort of 235 patients with NXG falls just above the range of 54 to 60 years reported in previous cohorts that included 10 or more patients.5,7,157,158 The female predominance in our cohort (63%) equates to an elevated female-to-male ratio of 1.7. While 1 study reported an elevated female-to-male ratio of 2.7,7 other studies reported an equal sex distribution157,158 or an elevated male-to-female ratio of 1.4.5 The rate of paraproteinemia in this cohort (82.1%) falls within the range of 77% to 84% reported by studies that did not require paraproteinemia as an inclusion criterion,5,158 and the rate of the IgG-κ subtype (50.0%) falls within the previously reported range of 50% to 60%.5,157 The rate of malignant conditions in this cohort (25.1%) is just less than the previously reported range of 26% to 45%, while the rate of multiple myeloma specifically (14%) falls within the previously reported range of 5% to 36%.5,7,157,158 The overall rate of paraproteinemia and/or malignant condition in this cohort was 83.8%. While paraproteinemia in the multicenter cohort most often preceded the presentation of NXG, evolution into multiple myeloma was observed up to 5.7 years afterwards, highlighting the importance of long-term hematologic surveillance.
The primary morphology of papules and plaques (76.7%) and/or nodules (24.5%) in the cohort was consistent with the literature5,7; however, the ulceration rate of 19.0% was less than the rate of 42% reported in a prior study.5 In keeping with the classic description by Kossard and Winkelmann,1 the most frequently observed colors in the cohort were yellow or orange (60.4%). Interestingly, a subset of patients reported pain (30.7%) and/or pruritus (17.0%), symptoms of NXG that have not received attention in prior studies. The most common distribution of cutaneous lesions was periorbital (59.3%), which falls within the previously reported range of 35% to 78%,5,7,157,158 and the eye was the leading site of extracutaneous involvement. Based on the 27% rate of eye involvement in patients evaluated by ophthalmology in this multicenter cohort, we suggest that an ophthalmology referral be considered for all patients with NXG. In addition, these data highlight the potential for multiorgan involvement, including the brain, gastrointestinal tract, heart, liver, lung, lymphoreticular system, muscle, and parotid gland.
Body mass index has been inconsistently reported in the literature; therefore, it is difficult to determine whether the overweight mean body mass index of 27.0 in the multicenter cohort is a reliable feature of NXG. Diagnosis with NXG has been associated with a low high-density lipoprotein–cholesterol phenotype,8 which was found in 24% of patients tested. In the multicenter cohort, 41% of patients tested had low high-density lipoprotein–cholesterol level, even though 38% were taking 1 or more medications known to elevate high-density lipoprotein–cholesterol levels. By comparison, approximately 18% of adults in the United States have low high-density lipoprotein–cholesterol levels.159 Interactions between the paraprotein and lipoproteins are hypothesized to impair macrophage-lipid homeostasis, promote systemic inflammation, and generate immune complexes. Necrobiotic xanthogranuloma has been associated with acquired early complement deficiencies and cryoglobulinemia.7,8 Of the patients tested in our cohort, 61% had low C4 levels, and 23% had cryoglobulinemia. Finally, we identified a pattern of low 25-hydroxyvitamin D with normal or high 1,25-dihydroxyvitamin D. Granuloma-mediated production of 1α-hydroxylase, a well-documented phenomenon in patients with sarcoidosis, may lead to increased synthesis of 1,25-dihydroxyvitamin D and subsequent hypercalcemia.10,160 Given small sample sizes, the associations between these abnormal results of laboratory tests and NXG merit further investigations.
In the multicenter cohort, intravenous immunoglobulin had the best response rate (100%), followed by antimalarial drugs (80%), intralesional triamcinolone (75%), surgery (75%), chemotherapy (67%), and lenalidomide or thalidomide (63%). A diversity of treatments for NXG has been reported; however, the literature consists of case reports, case series, and retrospective studies.161 Prospective, controlled clinical studies are essential to guide practice.
The participants in the consensus exercise to propose diagnostic criteria for NXG strongly agreed (mean ranking, 4.6 of 5 points) that cutaneous papules, plaques, and/or nodules should be a major criterion. Given that 60% of NXG lesions in the cohort were yellow or orange in color, this was included with the qualifier most often to indicate a helpful but not mandatory clinical feature. Whether histopathological testing should be required for diagnosis emerged as a key point of discussion. To differentiate NXG from clinical mimics, participants strongly agreed (mean ranking, 4.5 of 5 points) that palisading granulomas with lymphoplasmacytic infiltrate and zones of necrobiosis should be a major criterion. Cholesterol clefts and/or giant cells (Touton or foreign body) were included as characteristic but variably present histopathological features. A second key point of discussion was whether paraproteinemia, most often IgG-κ, plasma-cell dyscrasia, and/or other associated lymphoproliferative disorder should be required for diagnosis. Noting the absence of these associations in 16% of patients with NXG in our cohort, participants strongly agreed (mean ranking, 4.6 of 5 points) that this should be a minor criterion. Participants agreed (mean ranking, 3.9 of 5 points) that periorbital distribution of cutaneous lesions should be a minor criterion. Finally, whether the major criteria should be sufficient to establish the diagnosis of NXG emerged as a third key point of discussion. Participants expressed concern that necrobiosis lipoidica, given shared clinicopathological features, could be misdiagnosed as NXG. Therefore, in the absence of foreign body, infection, or other identifiable cause, both major criteria and at least 1 minor criterion were required for diagnosis. Ongoing research is necessary to validate these criteria so that they may guide clinical research efforts, such as selecting patients for clinical trials.
Limitations
These results should be interpreted in the context of the study design. The retrospective nature of the multicenter cross-sectional study and systematic review may lead to information bias, and the tertiary care setting may limit generalizability to other study populations. A standardized data-collection tool was used to minimize variability; however, data were collected from physician notes and the literature, creating the potential for inconsistent reporting. Moreover, data collected at initial presentation to a tertiary academic referral center may differ from that collected at NXG onset. Response rates are limited by an inability to determine individual response for concurrent treatments. Finally, a potential limitation of the consensus-exercise method was the lack of face-to-face discussion. However, we opted to maintain anonymity to enable participants to formulate and express opinions independently.
Conclusions
This study of 235 patients describes the largest NXG cohort in the published literature, to our knowledge. The results provide insight into the demographics, comorbidities, clinical features, and treatment response of NXG. Eight board-certified dermatologists informed by these results participated in a consensus exercise to propose the first diagnostic criteria for NXG. While these criteria require validation, they represent a step forward in standardizing the diagnosis of this rare disorder for use in clinical research.
eTable. Systematic Review of the Demographics, Comorbidities, and Clinical Features of Necrobiotic Xanthogranuloma
eReferences.
Footnotes
Both major criteria and at least 1 minor criterion are required for diagnosis, applicable only in the absence of foreign body, infection, or other identifiable cause.
References
- 1.Kossard S, Winkelmann RK. Necrobiotic xanthogranuloma. Australas J Dermatol. 1980;21(2):85-88. doi: 10.1111/j.1440-0960.1980.tb00148.x [DOI] [PubMed] [Google Scholar]
- 2.Lipsker D. Monoclonal gammopathy of cutaneous significance: review of a relevant concept. J Eur Acad Dermatol Venereol. 2017;31(1):45-52. doi: 10.1111/jdv.13847 [DOI] [PubMed] [Google Scholar]
- 3.Hunter L, Burry AF. Necrobiotic xanthogranuloma: a systemic disease with paraproteinemia. Pathology. 1985;17(3):533-536. doi: 10.3109/00313028509105517 [DOI] [PubMed] [Google Scholar]
- 4.Winkelmann RK, Litzow MR, Umbert IJ, Lie JT. Giant cell granulomatous pulmonary and myocardial lesions in necrobiotic xanthogranuloma with paraproteinemia. Mayo Clin Proc. 1997;72(11):1028-1033. doi: 10.4065/72.11.1028 [DOI] [PubMed] [Google Scholar]
- 5.Ugurlu S, Bartley GB, Gibson LE. Necrobiotic xanthogranuloma: long-term outcome of ocular and systemic involvement. Am J Ophthalmol. 2000;129(5):651-657. doi: 10.1016/S0002-9394(99)00469-9 [DOI] [PubMed] [Google Scholar]
- 6.Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed Philadelphia: Elsevier Saunders; 2018. [Google Scholar]
- 7.Szalat R, Arnulf B, Karlin L, et al. Pathogenesis and treatment of xanthomatosis associated with monoclonal gammopathy. Blood. 2011;118(14):3777-3784. doi: 10.1182/blood-2011-05-356907 [DOI] [PubMed] [Google Scholar]
- 8.Szalat R, Pirault J, Fermand JP, et al. Physiopathology of necrobiotic xanthogranuloma with monoclonal gammopathy. J Intern Med. 2014;276(3):269-284. doi: 10.1111/joim.12195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wood AJ, Wagner MV, Abbott JJ, Gibson LE. Necrobiotic xanthogranuloma: a review of 17 cases with emphasis on clinical and pathologic correlation. Arch Dermatol. 2009;145(3):279-284. doi: 10.1001/archdermatol.2008.583 [DOI] [PubMed] [Google Scholar]
- 10.Sfeir JG, Zogala RJ, Popii VB. Hypercalcemia in necrobiotic xanthogranuloma: first reported case and insight into treatment. J Bone Miner Res. 2017;32(4):784-787. doi: 10.1002/jbmr.3047 [DOI] [PubMed] [Google Scholar]
- 11.Al-Bermani A, Figueiredo F, Speight EL, Jackson GH, Pandit R. Necrobiotic xanthogranuloma masquerading as posterior scleritis. Eye (Lond). 2009;23(1):239-240. doi: 10.1038/eye.2008.37 [DOI] [PubMed] [Google Scholar]
- 12.Al-Niaimi FA, Dawn G, Cox NH. Necrobiotic xanthogranuloma without paraproteinaemia: marked improvement with psoralen ultraviolet A treatment. Clin Exp Dermatol. 2010;35(3):275-277. doi: 10.1111/j.1365-2230.2009.03447.x [DOI] [PubMed] [Google Scholar]
- 13.AlGain M, Szalat R, Vignon-Pennamen MD, et al. A rare case of disseminated skin and mucosal necrobiotic xanthogranuloma and xanthoma. J Eur Acad Dermatol Venereol. 2017;31(1):e3-e5. doi: 10.1111/jdv.13577 [DOI] [PubMed] [Google Scholar]
- 14.Ali FR, Green R, Chularojanamontri L, Young HS, Motta L. Recurrent posterior scleritis and a ‘sebaceous cyst’: Extrafacial necrobiotic xanthogranuloma. Br J Dermatol. 2012;167:18-19. [Google Scholar]
- 15.Amer R, Pe’er J, Pappo O, Dotan S. Necrobiotic xanthogranuloma associated with choroidal infiltration and syncytial giant cell hepatitis. J Neuroophthalmol. 2005;25(3):189-192. doi: 10.1097/01.wno.0000177299.44845.65 [DOI] [PubMed] [Google Scholar]
- 16.Bain EE III, Meehan SA, Hale EK. Solitary necrobiotic xanthogranuloma of an upper extremity in association with multiple myeloma. J Drugs Dermatol. 2014;13(5):598-600. [PubMed] [Google Scholar]
- 17.Balagula Y, Straus DJ, Pulitzer MP, Lacouture ME. Necrobiotic xanthogranuloma associated with immunoglobulin m paraproteinemia in a patient with Waldenström macroglobulinemia. J Clin Oncol. 2011;29(11):e305-e307. doi: 10.1200/JCO.2010.32.4921 [DOI] [PubMed] [Google Scholar]
- 18.Barzilai A, Trau H, Shpiro D, Yorav S. Necrobiotic xanthogranuloma with paraproteinemia. Cutis. 1996;57(5):320-322. [PubMed] [Google Scholar]
- 19.Betts CM, Pasquinelli G, Costa AM, Fanti PA, Misciali C, Varotti C. Necrobiotic xanthogranuloma without periorbital involvement: an ultrastructural investigation. Ultrastruct Pathol. 2001;25(6):437-444. doi: 10.1080/019131201753343476 [DOI] [PubMed] [Google Scholar]
- 20.Bhari N, Chiramel MJ, Vedi KK, et al. Necrobiotic xanthogranuloma with multiple myeloma. Clin Exp Dermatol. 2015;40(7):811-814. doi: 10.1111/ced.12620 [DOI] [PubMed] [Google Scholar]
- 21.Bortolani A, Barisoni D, Bontempini L. Necrobiotic xanthogranuloma of the eyelids with paraproteinemia. Eur J Plast Surg. 1999;22(1):36-39. doi: 10.1007/s002380050141 [DOI] [Google Scholar]
- 22.Bullock JD, Bartley GB, Campbell RJ, Yanes B, Connelly PJ, Funkhouser JW. Necrobiotic xanthogranuloma with paraproteinemia: case report and a pathogenetic theory. Trans Am Ophthalmol Soc. 1986;84:342-354. [PMC free article] [PubMed] [Google Scholar]
- 23.Burdick AE, Sanchez J, Elgart GW. Necrobiotic xanthogranuloma associated with a benign monoclonal gammopathy. Cutis. 2003;72(1):47-50. [PubMed] [Google Scholar]
- 24.Butterfield JH, Bartley GB. Atypical periorbital xanthogranulomas associated with systemic benign lymphoepithelial lesions. Ophthalmic Plast Reconstr Surg. 1994;10(4):287-292. doi: 10.1097/00002341-199412000-00014 [DOI] [PubMed] [Google Scholar]
- 25.Carabelli A, Cecca E, Crovetti G, et al. IgG lambda multiple myeloma and diffuse necrobiotic xanthogranuloma association: a case report. Eur J Dermatol. 1997;7(7):519-521. [Google Scholar]
- 26.Chandra S, Finklestein E, Gill D. Necrobiotic xanthogranuloma occurring within linear morphoea. Australas J Dermatol. 2002;43(1):52-54. doi: 10.1046/j.1440-0960.2002.00553.x [DOI] [PubMed] [Google Scholar]
- 27.Chang SE, Lee WS, Lee MW, et al. A case of necrobiotic xanthogranuloma without paraproteinemia presenting as a solitary tumor on the thigh. Int J Dermatol. 2003;42(6):470-472. doi: 10.1046/j.1365-4362.2003.01716_1.x [DOI] [PubMed] [Google Scholar]
- 28.Char DH, LeBoit PE, Ljung BM, Wara W. Radiation therapy for ocular necrobiotic xanthogranuloma. Arch Ophthalmol. 1987;105(2):174-175. doi: 10.1001/archopht.1987.01060020028014 [DOI] [PubMed] [Google Scholar]
- 29.Chave TA, Chowdhury MM, Holt PJ. Recalcitrant necrobiotic xanthogranuloma responding to pulsed high-dose oral dexamethasone plus maintenance therapy with oral prednisolone. Br J Dermatol. 2001;144(1):158-161. doi: 10.1046/j.1365-2133.2001.03967.x [DOI] [PubMed] [Google Scholar]
- 30.Chave TA, Hutchinson PE. Necrobiotic xanthogranuloma with two monoclonal paraproteins and no periorbital involvement at presentation. Clin Exp Dermatol. 2001;26(6):493-496. doi: 10.1046/j.1365-2230.2001.00873.x [DOI] [PubMed] [Google Scholar]
- 31.Chen KY, Leslie W, Mahon B, Venugopal P. A patient with necrobiotic xanthogranuloma presenting with an anterior mediastinal mass, plasma cell dyscrasia, and a lymphoproliferative disorder. Clin Adv Hematol Oncol. 2011;9(9):696-700. [PubMed] [Google Scholar]
- 32.Codère F, Lee RD, Anderson RL. Necrobiotic xanthogranuloma of the eyelid. Arch Ophthalmol. 1983;101(1):60-63. doi: 10.1001/archopht.1983.01040010062009 [DOI] [PubMed] [Google Scholar]
- 33.Cornblath WT, Dotan SA, Trobe JD, Headington JT. Varied clinical spectrum of necrobiotic xanthogranuloma. Ophthalmology. 1992;99(1):103-107. doi: 10.1016/S0161-6420(92)32031-7 [DOI] [PubMed] [Google Scholar]
- 34.Criado PR, Vasconcellos C, Pegas JR, et al. Necrobiotic xanthogranuloma with lambda paraproteinemia: case report of successful treatment with melphalan and prednisone. J Dermatolog Treat. 2002;13(2):87-89. doi: 10.1080/095466302317584458 [DOI] [PubMed] [Google Scholar]
- 35.Criton S, Asokan PU, Pailey S, Kuttappan SS, Rodriguez FP; Remani . Necrobiotic xanthogranuloma with paraproteinaemia. Indian J Dermatol Venereol Leprol. 1996;62(6):383-385. [PubMed] [Google Scholar]
- 36.Dabiri S, Morales A, Ma L, et al. The frequency of dual TCR-PCR clonality in granulomatous disorders. J Cutan Pathol. 2011;38(9):704-709. doi: 10.1111/j.1600-0560.2011.01727.x [DOI] [PubMed] [Google Scholar]
- 37.Davies MJ, Whitehead K, Quagliotto G, Wood D, Patheja RS, Sullivan TJ. Adult orbital and adnexal xanthogranulomatous disease. Asia Pac J Ophthalmol (Phila). 2017;6(5):435-443. [DOI] [PubMed] [Google Scholar]
- 38.DeLuca IJ, Grossman ME. Vulvar necrobiotic xanthogranuloma. J Am Acad Dermatol. 2014;71(6):e247-e248. doi: 10.1016/j.jaad.2014.04.039 [DOI] [PubMed] [Google Scholar]
- 39.Dholaria BR, Cappel M, Roy V. Necrobiotic xanthogranuloma associated with monoclonal gammopathy: successful treatment with lenalidomide and dexamethasone. Ann Hematol. 2016;95(4):671-672. doi: 10.1007/s00277-016-2604-3 [DOI] [PubMed] [Google Scholar]
- 40.Dilnawaz M. An unusual skin presentation of necrobiotic xanthogranuloma. J Coll Physicians Surg Pak. 2010;20(4):274-275. [PubMed] [Google Scholar]
- 41.Dupré A, Viraben R. Necrobiotic xanthogranuloma: a case without paraproteinemia but with transepithelial elimination. J Cutan Pathol. 1988;15(2):116-119. doi: 10.1111/j.1600-0560.1988.tb00530.x [DOI] [PubMed] [Google Scholar]
- 42.Efebera Y, Blanchard E, Allam C, Han A, Lee S, Munshi N. Complete response to thalidomide and dexamethasone in a patient with necrobiotic xanthogranuloma associated with monoclonal gammopathy: a case report and review of the literature. Clin Lymphoma Myeloma Leuk. 2011;11(3):298-302. doi: 10.1016/j.clml.2011.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elner VM, Mintz R, Demirci H, Hassan AS. Local corticosteroid treatment of eyelid and orbital xanthogranuloma. Ophthalmic Plast Reconstr Surg. 2006;22(1):36-40. doi: 10.1097/01.iop.0000192645.24814.e8 [DOI] [PubMed] [Google Scholar]
- 44.Esmaeli N, Ghanadan A, Mansouri P, Ghaedi F. Necrobiotic xanthogranuloma after penicillin injection: A case report. Iranian J Dermatol. 2017;20(1):26-28. [Google Scholar]
- 45.Ferrara G, Palombi N, Lipizzi A, Zalaudek I, Argenziano G. Nonnecrobiotic necrobiotic xanthogranuloma. Am J Dermatopathol. 2007;29(3):306-308. doi: 10.1097/DAD.0b013e3180332b8b [DOI] [PubMed] [Google Scholar]
- 46.Finelli LG, Ratz JL. Plasmapheresis, a treatment modality for necrobiotic xanthogranuloma. J Am Acad Dermatol. 1987;17(2 Pt 2):351-354. doi: 10.1016/S0190-9622(87)70211-4 [DOI] [PubMed] [Google Scholar]
- 47.Fink C, Schneiderbauer R, Hartschuh W, Enk A, Toberer F. Necrobiotic xanthogranuloma associated with chronic lymphocytic leukemia. Int J Dermatol. 2018;57(6):719-720. doi: 10.1111/ijd.13951 [DOI] [PubMed] [Google Scholar]
- 48.Flann S, Wain EM, Halpern S, Andrews V, Whittaker S. Necrobiotic xanthogranuloma with paraproteinaemia. Clin Exp Dermatol. 2006;31(2):248-251. doi: 10.1111/j.1365-2230.2005.02042.x [DOI] [PubMed] [Google Scholar]
- 49.Fortson JS, Schroeter AL. Necrobiotic xanthogranuloma with IgA paraproteinemia and extracutaneous involvement. Am J Dermatopathol. 1990;12(6):579-584. doi: 10.1097/00000372-199012000-00008 [DOI] [PubMed] [Google Scholar]
- 50.Fu T, Zwerner J, Kim J, Tang J. Subcutaneous nodules in an elderly patient. Necrobiotic xanthogranuloma. Arch Dermatol. 2011;147(10):1215-1220. doi: 10.1001/archdermatol.2011.285-a [DOI] [PubMed] [Google Scholar]
- 51.Furner BB, Stevens CS. Diffuse, ulcerating plaques and nodules. Necrobiotic xanthogranuloma (NXG) with paraproteinemia. Arch Dermatol. 1989;125(2):287-288, 290. doi: 10.1001/archderm.1989.01670140139028 [DOI] [PubMed] [Google Scholar]
- 52.Gacto P, Barrera F, Pereyra JJ, Fernández-Ortega P. [Necrobiotic xanthogranuloma: efficacy of surgery in 2 patients] [in Spanish]. Actas Dermosifiliogr. 2009;100(6):499-502. doi: 10.1016/S0001-7310(09)71597-2 [DOI] [PubMed] [Google Scholar]
- 53.Georgiou S, Monastirli A, Kapranos N, Pasmatzi E, Sakkis Th, Tsambaos D. Interferon alpha-2a monotherapy for necrobiotic xanthogranuloma. Acta Derm Venereol. 1999;79(6):484-485. doi: 10.1080/000155599750010030 [DOI] [PubMed] [Google Scholar]
- 54.Gergen N, Biebl K, Berg BC, Suwattee P. Slowly growing yellow nodules: challenge. Necrobiotic xanthogranuloma. Am J Dermatopathol. 2011;33(1):92-93, 103-104. doi: 10.1097/DAD.0b013e3181d435a0 [DOI] [PubMed] [Google Scholar]
- 55.Ghani S, Al Ustwani O, Khalid B, et al. Periorbital necrobiotic xanthogranuloma treated successfully with novel multiple myeloma therapy. Clin Adv Hematol Oncol. 2013;11(10):678-680. [PubMed] [Google Scholar]
- 56.Ghiasi N, Alavi A, Coutts PM, Ghazarian D, Sibbald RG. Necrobiotic xanthogranuloma as an unusual cause of refractive chronic bilateral leg ulceration. Int J Low Extrem Wounds. 2012;11(4):293-295. doi: 10.1177/1534734612465434 [DOI] [PubMed] [Google Scholar]
- 57.Girisha BS, Holla AP, Fernandes M, Noronha TM. Necrobiotic xanthogranuloma. J Cutan Aesthet Surg. 2012;5(1):43-45. doi: 10.4103/0974-2077.94341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goede JS, Misselwitz B, Taverna C, et al. Necrobiotic xanthogranuloma successfully treated with autologous stem cell transplantation. Ann Hematol. 2007;86(4):303-306. doi: 10.1007/s00277-006-0231-0 [DOI] [PubMed] [Google Scholar]
- 59.Gonzales JA, Haemel A, Gross AJ, Acharya NR. Management of uveitis and scleritis in necrobiotic xanthogranuloma. J Ocul Pharmacol Ther. 2017;33(4):325-333. doi: 10.1089/jop.2016.0135 [DOI] [PubMed] [Google Scholar]
- 60.Goyal A, O’Leary D, Vercellotti G, Miller D, McGlave P. Intravenous immunoglobulin for treatment of necrobiotic xanthogranuloma. Dermatol Ther. 2019;32(1):e12744. doi: 10.1111/dth.12744 [DOI] [PubMed] [Google Scholar]
- 61.Gün D, Demirçay Z, Demirkesen C. Necrobiotic xanthogranuloma in a burn scar. Int J Dermatol. 2004;43(4):293-295. doi: 10.1111/j.1365-4632.2004.01858.x [DOI] [PubMed] [Google Scholar]
- 62.Hallermann C, Tittelbach J, Norgauer J, Ziemer M. Successful treatment of necrobiotic xanthogranuloma with intravenous immunoglobulin. Arch Dermatol. 2010;146(9):957-960. doi: 10.1001/archdermatol.2010.236 [DOI] [PubMed] [Google Scholar]
- 63.Hamilton HK, Dover JS, Arndt KA. Successful treatment of disfiguring hemosiderin-containing hyperpigmentation with the Q-switched 650-nm wavelength laser. JAMA Dermatol. 2014;150(11):1221-1222. doi: 10.1001/jamadermatol.2014.1838 [DOI] [PubMed] [Google Scholar]
- 64.Hashemi P, Rashidi A, Chapas AM, Balfour EM. Necrobiotic xanthogranuloma of the extremities with paraproteinemia and without periorbital involvement at presentation. Cutis. 2012;89(1):41-44. [PubMed] [Google Scholar]
- 65.Hauser C, Schifferli J, Saurat JH. Complement consumption in a patient with necrobiotic xanthogranuloma and paraproteinemia. J Am Acad Dermatol. 1991;24(5 pt 2):908-911. doi: 10.1016/0190-9622(91)70145-R [DOI] [PubMed] [Google Scholar]
- 66.Herd TJ, Fischer R, Christensen LC, Rajpara A. Irregular yellow-brown plaques on the trunk and thighs. Cutis. 2018;101(1):12-15, 15. [PubMed] [Google Scholar]
- 67.Holden CA, Winkelmann RK, Wilson Jones E. Necrobiotic xanthogranuloma: a report of four cases. Br J Dermatol. 1986;114(2):241-250. doi: 10.1111/j.1365-2133.1986.tb02804.x [DOI] [PubMed] [Google Scholar]
- 68.Inthasotti S, Wanitphakdeedecha R, Manonukul J. A 7-year history of necrobiotic xanthogranuloma following asymptomatic multiple myeloma: a case report. Dermatol Res Pract. 2011;2011:927852. doi: 10.1155/2011/927852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ito Y, Nishimura K, Yamanaka K, et al. Necrobiotic xanthogranuloma with paraproteinemia; an atypical case. J Dtsch Dermatol Ges. 2008;6(1):40-43. [DOI] [PubMed] [Google Scholar]
- 70.Jeziorska M, Hassan A, Mackness MI, et al. Clinical, biochemical, and immunohistochemical features of necrobiotic xanthogranulomatosis. J Clin Pathol. 2003;56(1):64-68. doi: 10.1136/jcp.56.1.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnston KA, Grimwood RE, Meffert JJ, Deering KC. Necrobiotic xanthogranuloma with paraproteinemia: an evolving presentation. Cutis. 1997;59(6):333-336. [PubMed] [Google Scholar]
- 72.Kadakia S, Nadkarni N, Sonavane S, Ghate S. Spectacular skin nodules: cutaneous necrobiotic xanthogranuloma without paraproteinemia. Indian J Dermatol. 2012;57(5):396-398. doi: 10.4103/0019-5154.100499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kawakami Y, Yamamoto T. Letter: Necrobiotic xanthogranuloma of extremities in an elderly patient successfully treated with low-dose prednisolone. Dermatol Online J. 2011;17(6):13. [PubMed] [Google Scholar]
- 74.Keorochana N, Klanarongran K, Satayasoontorn K, Chaiamnuay S. Necrobiotic xanthogranuloma scleritis in a case of granulomatosis with polyangiitis (Wegener’s granulomatosis). Int Med Case Rep J. 2017;10:323-328. doi: 10.2147/IMCRJ.S145943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khan IJ, Azam NA, Sullivan SC, Habboush HW, Christian A. Necrobiotic xanthogranuloma successfully treated with a combination of dexamethasone and oral cyclophosphamide. Can J Ophthalmol. 2009;44(3):335-336. doi: 10.3129/i09-021 [DOI] [PubMed] [Google Scholar]
- 76.Khan M, Ahmed I, Carr R. An unusual subcutaneous lump. Clin Exp Dermatol. 2012;37(7):808-810. doi: 10.1111/j.1365-2230.2011.04280.x [DOI] [PubMed] [Google Scholar]
- 77.Koch D, Lucas G, Durrington P, Lear JT. Ulcerating necrobiotic xanthogranulomata and paraproteinaemia treated with thalidomide. Br J Dermatol. 2008;159:16-17. [Google Scholar]
- 78.Koch PS, Goerdt S, Géraud C. Erythematous papules, plaques, and nodular lesions on the trunk and within preexisting scars. JAMA Dermatol. 2013;149(9):1103-1104. doi: 10.1001/jamadermatol.2013.186 [DOI] [PubMed] [Google Scholar]
- 79.Kossard S, Chow E, Wilkinson B, Killingsworth M. Lipid and giant cell poor necrobiotic xanthogranuloma. J Cutan Pathol. 2000;27(7):374-378. doi: 10.1034/j.1600-0560.2000.027007374.x [DOI] [PubMed] [Google Scholar]
- 80.Kovalyshyn I, Busam KJ, Marghoob AA. Orange-yellow diffuse cutaneous eruption in an 82-year-old woman. Arch Dermatol. 2009;145(10):1183-1188. doi: 10.1001/archdermatol.2009.213-a [DOI] [PubMed] [Google Scholar]
- 81.Kunikata N, Kikuchi K, Hashimoto A, Tagami H. Necrobiotic xanthogranuloma of the nose without paraproteinemia. J Dermatol. 2006;33(11):809-812. doi: 10.1111/j.1346-8138.2006.00184.x [DOI] [PubMed] [Google Scholar]
- 82.Lam K, Brownstein S, Jordan DR, van der Jagt R, Jastrzebski A, Dionne MA. Bilateral necrobiotic xanthogranuloma of the eyelids followed by a diagnosis of multiple myeloma 20 years later. Ophthalmic Plast Reconstr Surg. 2013;29(5):e119-e120. doi: 10.1097/IOP.0b013e318279fdef [DOI] [PubMed] [Google Scholar]
- 83.Langlois S, Brochot P, Reguiai Z, et al. Necrobiotic xanthogranuloma with multiple myeloma. Case report and pathogenic hypotheses. Joint Bone Spine. 2006;73(1):120-122. doi: 10.1016/j.jbspin.2005.03.008 [DOI] [PubMed] [Google Scholar]
- 84.Le AV, Fenske NA. An unusual granulomatous disease in the elderly. Arch Dermatol. 1997;133(10):1303-1304, 1306-1307. doi: 10.1001/archderm.1997.03890460129018 [DOI] [PubMed] [Google Scholar]
- 85.Lebey PB, Determer I, Bazex J, el Sayed F, Marguery MC. Periorbital papules and nodules. Necrobiotic xanthogranuloma. Arch Dermatol. 1997;133(1):99-102, 102. doi: 10.1001/archderm.133.1.99 [DOI] [PubMed] [Google Scholar]
- 86.Lee HJ, Kim JM, Kim GW, et al. Necrobiotic xanthogranuloma treated with a combination of oral methylprednisolone and cyclosporin. J Dermatol. 2017;44(10):1190-1191. doi: 10.1111/1346-8138.13648 [DOI] [PubMed] [Google Scholar]
- 87.Li S, Chen AJ, Fang S, Li H. Successful treatment of necrobiotic xanthogranuloma with total glucosides of paeony. Dermatol Ther. 2014;27(5):304-306. doi: 10.1111/dth.12133 [DOI] [PubMed] [Google Scholar]
- 88.Liszewski W, Wisniewski JD, Safah H, Boh EE. Treatment of refractory necrobiotic xanthogranulomas with extracorporeal photopheresis and intravenous immunoglobulin. Dermatol Ther. 2014;27(5):268-271. doi: 10.1111/dth.12135 [DOI] [PubMed] [Google Scholar]
- 89.Luck J, Layton A, Noble BA. Necrobiotic xanthogranuloma with orbital involvement. J R Soc Med. 1992;85(6):357-358. [PMC free article] [PubMed] [Google Scholar]
- 90.Lukács J, Goetze S, Elsner P. Periocular necrobiotic xanthogranuloma successfully treated with intravenous immunoglobulin. Acta Derm Venereol. 2017;97(6):754-755. doi: 10.2340/00015555-2626 [DOI] [PubMed] [Google Scholar]
- 91.Macfarlane AW, Verbov JL. Necrobiotic xanthogranuloma with paraproteinaemia. Br J Dermatol. 1985;113(3):339-343. doi: 10.1111/j.1365-2133.1985.tb02087.x [DOI] [PubMed] [Google Scholar]
- 92.McGregor JM, Miller J, Smith NP, Hay RJ. Necrobiotic xanthogranuloma without periorbital lesions. J Am Acad Dermatol. 1993;29(3):466-469. doi: 10.1016/0190-9622(93)70212-C [DOI] [PubMed] [Google Scholar]
- 93.Machado S, Alves R, Lima M, Leal I, Massa A. Cutaneous necrobiotic xanthogranuloma (NXG)—successfully treated with low dose chlorambucil. Eur J Dermatol. 2001;11(5):458-462. [PubMed] [Google Scholar]
- 94.Mahendran P, Wee J, Chong H, Natkunarajah J. Necrobiotic xanthogranuloma treated with lenalidomide. Clin Exp Dermatol. 2018;43(3):345-347. doi: 10.1111/ced.13293 [DOI] [PubMed] [Google Scholar]
- 95.Martínez Fernández M, Rodríguez Prieto MA, Ruiz González I, Sánchez Sambucety P, Delgado Vicente S. Necrobiotic xanthogranuloma associated with myeloma. J Eur Acad Dermatol Venereol. 2004;18(3):328-331. doi: 10.1111/j.1468-3083.2004.00906.x [DOI] [PubMed] [Google Scholar]
- 96.Mathias M, Permi HS, Shetty P, Jayaprakash Shetty K, Girish BS. Necrobiotic xanthogranuloma presenting as a clinical variant without paraproteinemia. Nitte University Journal of Health Science. 2011;1(1-3):72-74. [Google Scholar]
- 97.Matsuura F, Yamashita S, Hirano K, et al. Activation of monocytes in vivo causes intracellular accumulation of lipoprotein-derived lipids and marked hypocholesterolemia—a possible pathogenesis of necrobiotic xanthogranuloma. Atherosclerosis. 1999;142(2):355-365. doi: 10.1016/S0021-9150(98)00260-3 [DOI] [PubMed] [Google Scholar]
- 98.Meyer S, Szeimies RM, Landthaler M, Hohenleutner S. Cyclophosphamide-dexamethasone pulsed therapy for treatment of recalcitrant necrobiotic xanthogranuloma with paraproteinemia and ocular involvement. Br J Dermatol. 2005;153(2):443-445. doi: 10.1111/j.1365-2133.2005.06737.x [DOI] [PubMed] [Google Scholar]
- 99.Minocha R, Lee S, Choi JYJ, Mann S. Necrobiotic xanthogranuloma: a report of two fascinating cases. Hong Kong J Dermat Venereol. 2017;25(2):84-89. [Google Scholar]
- 100.Miszkiel KA, Sohaib SAA, Rose GE, Cree IA, Moseley IF. Radiological and clinicopathological features of orbital xanthogranuloma. Br J Ophthalmol. 2000;84(3):251-258. doi: 10.1136/bjo.84.3.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mohsenin A, Sinard J, Huang JJ. Necrobiotic xanthogranuloma and chronic lymphocytic leukemia of the conjunctiva masquerading as scleritis and uveitis. Clin Ophthalmol. 2012;6:2045-2047. doi: 10.2147/OPTH.S35743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Muscardin LM, Mastroianni A, Chistolini A, Pulsoni A. Necrobiotic xanthogranuloma without periorbital lesions and without paraproteinaemia. J Eur Acad Dermatol Venereol. 2003;17(2):233-235. doi: 10.1046/j.1468-3083.2003.00577_6.x [DOI] [PubMed] [Google Scholar]
- 103.Naghashpour M, Setoodeh R, Moscinski L, et al. Nonnecrobiotic necrobiotic xanthogranuloma as an initial manifestation of paraproteinemia and small lymphocytic lymphoma in a patient with Sjögren syndrome. Am J Dermatopathol. 2011;33(8):855-857. doi: 10.1097/DAD.0b013e3182051fce [DOI] [PubMed] [Google Scholar]
- 104.Nestle FO, Hofbauer G, Burg G. Necrobiotic xanthogranuloma with monoclonal gammopathy of the IgG lambda type. Dermatology. 1999;198(4):434-435. [PubMed] [Google Scholar]
- 105.Nishimura M, Takano-Nishimura Y, Yano I, Hayashi N, Toshitani S. Necrobiotic xanthogranuloma in a human T-lymphotropic virus type 1 carrier. J Am Acad Dermatol. 1992;27(5 pt 2):886-889. doi: 10.1016/0190-9622(92)70274-J [DOI] [PubMed] [Google Scholar]
- 106.Nockowski P, Woźniak Z, Reich A, Maj J. Xanthoma-like skin changes in an elderly woman with a normal lipid profile. Acta Dermatovenerol Croat. 2017;25(2):167-169. [PubMed] [Google Scholar]
- 107.Novak PM, Robbins TO, Winkelmann RK. Necrobiotic xanthogranuloma with myocardial lesions and nodular transformation of the liver. Hum Pathol. 1992;23(2):195-196. doi: 10.1016/0046-8177(92)90244-W [DOI] [PubMed] [Google Scholar]
- 108.Oestreicher J, Dookeran R, Nijhawan N, Kolin A. Necrobiotic xanthogranuloma with predominant periorbital involvement. Ophthalmic Plast Reconstr Surg. 2010;26(6):473-475. doi: 10.1097/IOP.0b013e3181d92955 [DOI] [PubMed] [Google Scholar]
- 109.Olson RM, Harrison AR, Maltry A, Mokhtarzadeh A. Periorbital necrobiotic xanthogranuloma successfully treated with intravenous immunoglobulin. Case Rep Ophthalmol. 2018;9(1):70-75. doi: 10.1159/000485913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Omarjee L, Janin A, Etienne G, et al. Necrobiotic xanthogranuloma: a paraneoplastic skin lesion of haematological malignancies? Eur J Dermatol. 2018;28(3):384-386. doi: 10.1684/ejd.2018.3256 [DOI] [PubMed] [Google Scholar]
- 111.Oumeish OY, Oumeish I, Tarawneh M, Salman T, Sharaiha A. Necrobiotic xanthogranuloma associated with paraproteinemia and non-Hodgkin’s lymphoma developing into chronic lymphocytic leukemia: the first case reported in the literature and review of the literature. Int J Dermatol. 2006;45(3):306-310. doi: 10.1111/j.1365-4632.2006.02575.x [DOI] [PubMed] [Google Scholar]
- 112.Pedrosa AF, Ferreira O, Calistru A, et al. Necrobiotic xanthogranuloma with giant cell hepatitis, successfully treated with intravenous immunoglobulins. Dermatol Ther. 2015;28(2):68-70. doi: 10.1111/dth.12211 [DOI] [PubMed] [Google Scholar]
- 113.Peyman A, Walsh N, Green P, Dorey MW, Seamone C, Pasternak S. Necrobiotic xanthogranuloma associated with necrotizing scleritis. Am J Dermatopathol. 2012;34(6):644-647. doi: 10.1097/DAD.0b013e318234e73c [DOI] [PubMed] [Google Scholar]
- 114.Plotnick H, Taniguchi Y, Hashimoto K, Negendank W, Tranchida L. Periorbital necrobiotic xanthogranuloma and stage I multiple myeloma: ultrastructure and response to pulsed dexamethasone documented by magnetic resonance imaging. J Am Acad Dermatol. 1991;25(2 pt 2):373-377. doi: 10.1016/0190-9622(91)70208-J [DOI] [PubMed] [Google Scholar]
- 115.Randell PL, Heenan PJ. Necrobiotic xanthogranuloma with paraproteinaemia. Australas J Dermatol. 1999;40(2):114-115. doi: 10.1046/j.1440-0960.1999.00334.x [DOI] [PubMed] [Google Scholar]
- 116.Rashid MM, Chowdhury T, Sultana A, Begum R, Islam S, Ahmed I. Necrobiotic xanthogranuloma—a case report. J Pak Assoc Dermatol. 2009;19(3):175-177. [Google Scholar]
- 117.Rayner SA, Duncombe AS, Keefe M, Theaker J, Manners RM. Necrobiotic xanthogranuloma occurring in an eyelid scar. Orbit. 2008;27(3):191-194. doi: 10.1080/01676830701804057 [DOI] [PubMed] [Google Scholar]
- 118.Reddy VC, Salomão DR, Garrity JA, Baratz KH, Patel SV. Periorbital and ocular necrobiotic xanthogranuloma leading to perforation. Arch Ophthalmol. 2010;128(11):1493-1494. doi: 10.1001/archophthalmol.2010.254 [DOI] [PubMed] [Google Scholar]
- 119.Reeder CB, Connolly SM, Winkelmann RK. The evolution of Hodgkin’s disease and necrobiotic xanthogranuloma syndrome. Mayo Clin Proc. 1991;66(12):1222-1224. doi: 10.1016/S0025-6196(12)62473-2 [DOI] [PubMed] [Google Scholar]
- 120.Rose GE, Patel BC, Garner A, Wright JE. Orbital xanthogranuloma in adults. Br J Ophthalmol. 1991;75(11):680-684. doi: 10.1136/bjo.75.11.680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rose A, Robinson M, Kamino H, Latkowski JA. Necrobiotic xanthogranuloma. Dermatol Online J. 2012;18(12):30. [PubMed] [Google Scholar]
- 122.Rubinstein A, Wolf DJ, Granstein RD. Successful treatment of necrobiotic xanthogranuloma with intravenous immunoglobulin. J Cutan Med Surg. 2013;17(5):347-350. doi: 10.2310/7750.2013.13012 [DOI] [PubMed] [Google Scholar]
- 123.Russo GG. Necrobiotic xanthogranuloma with scleroderma. Cutis. 2002;70(6):311-316. [PubMed] [Google Scholar]
- 124.Ryan E, Warren LJ, Szabo F. Necrobiotic xanthogranuloma: response to chlorambucil. Australas J Dermatol. 2012;53(2):e23-e25. doi: 10.1111/j.1440-0960.2010.00710.x [DOI] [PubMed] [Google Scholar]
- 125.Saeki H, Tomita M, Kai H, et al. Necrobiotic xanthogranuloma with paraproteinemia successfully treated with melphalan, prednisolone and skin graft. J Dermatol. 2007;34(11):795-797. doi: 10.1111/j.1346-8138.2007.00387.x [DOI] [PubMed] [Google Scholar]
- 126.Sagiv O, Thakar SD, Morrell G, Tetzlaff MT, Esmaeli B. Rituximab monotherapy is effective in treating orbital necrobiotic xanthogranuloma. Ophthalmic Plast Reconstr Surg. 2018;34(1):e24-e27. doi: 10.1097/IOP.0000000000000988 [DOI] [PubMed] [Google Scholar]
- 127.Santosaputri E, Ellis EJ, Nagiah S, Chrispal A, Thomas A. A multisystem granulomatous disease: necrobiotic xanthogranuloma with hepatic involvement. Med J Aust. 2014;200(8):490-493. doi: 10.5694/mja13.11303 [DOI] [PubMed] [Google Scholar]
- 128.Schaudig U, Al-Samir K. Upper and lower eyelid reconstruction for severe disfiguring necrobiotic xanthogranuloma. Orbit. 2004;23(1):65-76. doi: 10.1076/orbi.23.1.65.28989 [DOI] [PubMed] [Google Scholar]
- 129.Scupham RK, Fretzin DF. Necrobiotic xanthogranuloma with paraproteinemia. Arch Pathol Lab Med. 1989;113(12):1389-1391. [PubMed] [Google Scholar]
- 130.Seastrom S, Bookout A, Hogan DJ. Necrobiotic xanthogranuloma without a monoclonal gammopathy. Cutis. 2014;94(6):293-296. [PubMed] [Google Scholar]
- 131.Shah KC, Poonnoose SI, George R, Jacob M, Rajshekhar V. Necrobiotic xanthogranuloma with cutaneous and cerebral manifestations. case report and review of the literature. J Neurosurg. 2004;100(6):1111-1114. doi: 10.3171/jns.2004.100.6.1111 [DOI] [PubMed] [Google Scholar]
- 132.Silapunt S, Chon SY. Generalized necrobiotic xanthogranuloma successfully treated with lenalidomide. J Drugs Dermatol. 2010;9(3):273-276. [PubMed] [Google Scholar]
- 133.Singh K, Rajan KD, Eberhart C. Orbital necrobiotic xanthogranuloma associated with systemic IgG4 disease. Ocul Immunol Inflamm. 2010;18(5):373-378. doi: 10.3109/09273948.2010.490629 [DOI] [PubMed] [Google Scholar]
- 134.Smith HG, Sargent LA, Lundgrin DB. Necrobiotic xanthogranuloma of the chest wall. Dermatol Online J. 2006;12(1):12. [PubMed] [Google Scholar]
- 135.Stork J, Kodetová D, Vosmík F, Krejca M. Necrobiotic xanthogranuloma presenting as a solitary tumor. Am J Dermatopathol. 2000;22(5):453-456. doi: 10.1097/00000372-200010000-00013 [DOI] [PubMed] [Google Scholar]
- 136.Sutton L, Sutton S, Sutton M. Treatment of necrobiotic xanthogranuloma with 2-chlorodeoxyadenosine. Skinmed. 2013;11(2):121-123. [PubMed] [Google Scholar]
- 137.Torabian SZ, Fazel N, Knuttle R. Necrobiotic xanthogranuloma treated with chlorambucil. Dermatol Online J. 2006;12(5):11. [PubMed] [Google Scholar]
- 138.Tucker NA, Discepola MJ, Blanco G, Burnier MN Jr. Necrobiotic xanthogranuloma without dermatologic involvement. Can J Ophthalmol. 1997;32(6):396-399. [PubMed] [Google Scholar]
- 139.Umbert I, Winkelmann RK. Necrobiotic xanthogranuloma with cardiac involvement. Br J Dermatol. 1995;133(3):438-443. doi: 10.1111/j.1365-2133.1995.tb02674.x [DOI] [PubMed] [Google Scholar]
- 140.Valentine EA, Friedman HD, Zamkoff KW, Streeten BW. Necrobiotic xanthogranuloma with IgA multiple myeloma: a case report and literature review. Am J Hematol. 1990;35(4):283-285. doi: 10.1002/ajh.2830350414 [DOI] [PubMed] [Google Scholar]
- 141.Venencie PY, Puissant A, Verola O, et al. Necrobiotic xanthogranuloma with myeloma: a case report. Cancer. 1987;59(3):588-592. doi: [DOI] [PubMed] [Google Scholar]
- 142.Venencie PY, Le Bras P, Toan ND, Tchernia G, Delfraissy JF. Recombinant interferon alfa-2b treatment of necrobiotic xanthogranuloma with paraproteinemia. J Am Acad Dermatol. 1995;32(4):666-667. doi: 10.1016/0190-9622(95)90370-4 [DOI] [PubMed] [Google Scholar]
- 143.Vieira V, Del Pozo J, Martínez W, Veiga-Barreiro JA, Fonseca E. Necrobiotic xanthogranuloma associated with lymphoplasmacytic lymphoma. Palliative treatment with carbon dioxide laser. Eur J Dermatol. 2005;15(3):182-185. [PubMed] [Google Scholar]
- 144.Vignon M, Placais L, Malphettes M, et al. Non-cirrhotic portal hypertension in necrobiotic xanthogranuloma associated with monoclonal gammopathy. J Eur Acad Dermatol Venereol. 2017;31(9):e403-e405. doi: 10.1111/jdv.14213 [DOI] [PubMed] [Google Scholar]
- 145.Vu K, Gupta R, Frater J, Atkinson J, Ranganathan P. A 55-year-old man with periorbital and inguinal masses, pericarditis, and pleuritis. Arthritis Care Res (Hoboken). 2017;69(5):730-736. doi: 10.1002/acr.22843 [DOI] [PubMed] [Google Scholar]
- 146.Wee SA, Shupack JL. Necrobiotic xanthogranuloma. Dermatol Online J. 2005;11(4):24. [PubMed] [Google Scholar]
- 147.Wei YH, Cheng JJ, Wu YH, et al. Necrobiotic xanthogranuloma: response to dapsone. Dermatol Ther. 2015;28(1):7-9. doi: 10.1111/dth.12179 [DOI] [PubMed] [Google Scholar]
- 148.Wells J, Gillespie R, Zardawi I. Case of recalcitrant necrobiotic xanthogranuloma. Australas J Dermatol. 2004;45(4):213-215. doi: 10.1111/j.1440-0960.2004.00099.x [DOI] [PubMed] [Google Scholar]
- 149.Wolz M, Peters MS. Necrobiotic xanthogranuloma of the extremities. J Drugs Dermatol. 2012;11(1):122-123. [PubMed] [Google Scholar]
- 150.Wruhs M, Feldmann R, Sawetz I, Breier F, Steiner A. Necrobiotic xanthogranuloma in a patient with multiple myeloma. Case Rep Dermatol. 2016;8(3):350-353. doi: 10.1159/000452826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yang CY, Chung WH, Rosaline CYH, Kuo TT, Yang CH. Necrobiotic xanthogranuloma with paraproteinemia without periorbital involvement - a case report. Zhonghua Pifuke Yixue Zazhi. 2010;28(3):125-129. doi: 10.1016/S1027-8117(10)60027-4 [DOI] [Google Scholar]
- 152.Yasukawa K, Kato N, Hamasaka A, Hata H. Necrobiotic xanthogranuloma: isolated skeletal muscle involvement and unusual changes. J Am Acad Dermatol. 2005;52(4):729-731. doi: 10.1016/j.jaad.2004.12.016 [DOI] [PubMed] [Google Scholar]
- 153.Yoon SY, Park HJ, Lee JY, Cho BK. Necrobiotic xanthogranuloma with multiple myeloma and no periorbital involvement. Ann Dermatol. 2007;19(1):22-24. doi: 10.5021/ad.2007.19.1.22 [DOI] [Google Scholar]
- 154.Zainal A, Razif MY, Makhashen M, Swaminathan M, Mazita A. Necrobiotic xanthogranuloma of the parotid gland. J Laryngol Otol. 2010;124(5):569-571. doi: 10.1017/S0022215109991563 [DOI] [PubMed] [Google Scholar]
- 155.Ziemer M, Wedding U, Sander CS, Elsner P. Necrobiotic xanthogranuloma-rapid progression under treatment with melphalan. Eur J Dermatol. 2005;15(5):363-365. [PubMed] [Google Scholar]
- 156.Ziemer M, Norgauer J, Simon JC, Koehler MJ. An unusual histologic variant of necrobiotic xanthogranuloma. Am J Dermatopathol. 2012;34(2):e22-e26. doi: 10.1097/DAD.0b013e3182222aa8 [DOI] [PubMed] [Google Scholar]
- 157.Higgins LS, Go RS, Dingli D, et al. Clinical features and treatment outcomes of patients with necrobiotic xanthogranuloma associated with monoclonal gammopathies. Clin Lymphoma Myeloma Leuk. 2016;16(8):447-452. doi: 10.1016/j.clml.2016.04.009 [DOI] [PubMed] [Google Scholar]
- 158.Hilal T, DiCaudo DJ, Connolly SM, Reeder CB. Necrobiotic xanthogranuloma: a 30-year single-center experience. Ann Hematol. 2018;97(8):1471-1479. doi: 10.1007/s00277-018-3301-1 [DOI] [PubMed] [Google Scholar]
- 159.Carroll MD. Frayar CD, Nguyen DT Total and high-density lipoprotein cholesterol in adults: United States, 2015–2016; NCHS data brief, No. 290, October 2017. https://www.cdc.gov/nchs/data/databriefs/db290.pdf. Published 2017. Accessed November 19, 2019. [PubMed]
- 160.Sodhi A, Aldrich T. Vitamin D supplementation: not so simple in sarcoidosis. Am J Med Sci. 2016;352(3):252-257. doi: 10.1016/j.amjms.2016.05.027 [DOI] [PubMed] [Google Scholar]
- 161.Miguel D, Lukacs J, Illing T, Elsner P. Treatment of necrobiotic xanthogranuloma—a systematic review. J Eur Acad Dermatol Venereol. 2017;31(2):221-235. doi: 10.1111/jdv.13786 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Systematic Review of the Demographics, Comorbidities, and Clinical Features of Necrobiotic Xanthogranuloma
eReferences.