Key Points
Question
What is the benefit of bone-targeted radioisotope (RI) use in metastatic castration-resistant prostate cancer, and is there any difference between α-emitting and β-emitting RIs?
Finding
This meta-analysis of individual patient data was based on 6 randomized clinical trials including 2081 patients that compared RI use with no RI use study arms with no overall significant difference. While an α-emitting RI (radium 223) was significantly associated with higher overall survival and higher symptomatic skeletal event–free survival, a β-emitting RI (strontium-89) was not associated with these outcomes.
Meaning
This meta-analysis suggests a benefit of α-emitting RIs but not of β-emitting RIs for overall survival and symptomatic skeletal event–free survival, although caution is necessary for generalizability of these results, given the between-trial heterogeneity.
This meta-analysis of randomized clinical trials assesses overall survival in men with bone metastases from castration-resistant prostate cancer treated with bone-targeted radioisotopes and compares the effects of α-emitting with β-emitting radioisotopes.
Abstract
Importance
Both α-emitting and β-emitting bone-targeted radioisotopes (RIs) have been developed to treat men with metastatic castration-resistant prostate cancer (CRPC). Only 1 phase 3 randomized clinical trial has demonstrated an overall survival (OS) benefit from an α-emitting RI, radium 223 (223Ra), vs standard of care. Yet no head-to-head comparison has been done between α-emitting and β-emitting RIs.
Objective
To assess OS in men with bone metastases from CRPC treated with bone-targeted RIs and to compare the effects of α-emitting RIs with β-emitting RIs.
Data Sources
PubMed, Cochrane Library, ClinicalTrials.gov, and meeting proceedings between January 1993 and June 2013 were reviewed. Key terms included randomized trials, radioisotopes, radiopharmaceuticals, and prostate cancer. Data were collected, checked, and analyzed from February 2017 to October 2018.
Study Selection
Selected trials included patients with prostate cancer, recruited more than 50 patients from January 1993 to June 2013, compared RI use with no RI use (placebo, external radiotherapy, or chemotherapy), and were randomized. Patients were diagnosed with histologically proven prostate cancer and disease progression after both surgical or chemical castration and have evidence of bone metastasis. Nine randomized clinical trials were identified as eligible, but 3 were excluded for insufficient data.
Data Extraction and Synthesis
Individual patient data were requested for each eligible trial, and all data were checked with a standard procedure. The log-rank test stratified by trial was used to estimate hazard ratios (HRs), and a similar fixed-effects (FE) model was used to estimate odds ratios (ORs). The between-trial heterogeneity of treatment effects was evaluated by Cochran test and I2 and was accounted by a random-effects (RE) model.
Main Outcomes and Measures
Overall survival; secondary outcomes were symptomatic skeletal event (SSE)–free survival and adverse events.
Results
Based on 6 randomized clinical trials including 2081 patients, RI use was significantly associated with OS compared with no RI use (HR, 0.86; 95% CI, 0.77-0.95; P = .004) with high heterogeneity (χ25 = 24.46; P < .001; I2 = 80%), but this association disappeared when using an RE model (HR, 0.80; 95% CI, 0.61-1.06; P = .12; τ2 = 0.08). The heterogeneity is explained both by the type of RI and by the inclusion of 2 outlier trials that included 275 patients; the OS benefit was significantly higher with the α-emitting RI 223Ra (HR, 0.70; 95% CI, 0.58-0.83) but not significant with the β-emitting RI strontium-89 (HR, 0.96; 95% CI, 0.84-1.10) (P for interaction = .004). Excluding the outlier trials led to an overall HR of 0.82 (95% CI, 0.73-0.92; P < .001) (between-trial heterogeneity: χ23 = 6.51; P = .09; I2 = 54%) using an FE model and an HR of 0.80 (95% CI, 0.65-0.99; P = .04; τ2 = 0.02) using an RE model. The HR for SSE-free survival was 0.81 (95% CI, 0.69-0.93; P = .004) (between-trial heterogeneity: χ23 = 6.71; P = .08; I2 = 55%) when using an FE model and was 0.76 (95% CI, 0.58-1.01; P = .06; τ2 = 0.04) when using an RE model. There were more hematological toxic effects with RI use compared with no RI use (OR, 1.48; 95% CI, 1.17-1.88; P = .001).
Conclusions and Relevance
In metastatic CRPC, a significant improvement of OS and SSE-free survival was obtained with bone-targeted α-emitting but not β-emitting RIs. Caution is necessary for generalizability of these results, given the between-trial heterogeneity.
Introduction
Prostate cancer currently has the highest incidence and is the second leading cause of cancer death in men in most western countries.1 Although prostate cancer is initially sensitive to androgen deprivation, most deaths result from progression to castration-resistant prostate cancer (CRPC), with metastases spread usually involving the bones, and bone metastases are the main driver of prognosis.2 Docetaxel was the first drug with demonstrated survival advantage in CRPC,3 and since 2010, androgen receptor pathway inhibitors (abiraterone4 and enzalutamide5), a chemotherapy agent (cabazitaxel6), and immunotherapy with sipuleucel-T7 have also been shown to prolong survival. Until recently, many bone-targeting therapies (zoledronic acid,8 denosumab,9 and radioisotopes [RIs]10,11,12) were approved on the basis of pain relief and/or risk reduction of skeletal complications (skeletal-related events or symptomatic skeletal events [SSEs]13,14) without survival benefit. To our knowledge, radium 223 (223Ra), an α-emitting RI, is the only bone-targeted agent with a clearly demonstrated overall survival (OS) benefit in men with CRPC. In the double-blind, phase 3 Alpharadin in Symptomatic Prostate Cancer Patients (ALSYMPCA) randomized clinical trial,15 patients with symptomatic CRPC and bone metastases randomly received 223Ra or a placebo treatment. Radium 223 significantly improved OS with a favorable toxic effect profile.
The main objective of the Meta-analysis of Bone-Targeting Radiopharmaceutical Therapy in Patients With Bone Metastases From Prostate Cancer (MORPHEP) was to evaluate the association of different types of RIs (those emitting α and β radiation) with OS in men with bone metastases from CRPC. Thus, we aim to study whether the effects of α-emitting RIs in this setting is only because of their inherent activity or because of differences in study design and patient selection.
Methods
Protocol and Registration
This meta-analysis was registered on PROSPERO (CRD42016026842). A collaborative group comprising researchers involved in the randomized clinical trials included in the project (MORPHEP Collaborative Group) was established, and the meta-analysis was conducted and is reported on its behalf. We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline for reporting the results of this meta-analysis.
Study Selection
Selected trials must have (1) included patients with prostate cancer; (2) recruited more than 50 patients; (3) compared RI with placebo, external radiotherapy, or chemotherapy; (4) been randomized; and (5) completed recruitment between January 1993 and June 2013. Patients must have been diagnosed with histologically proven prostate cancer and disease progression after both surgical or chemical castration and have evidence of bone metastasis.
Search Strategy
Both published and unpublished trials were included in the meta-analysis.16 To identify as many relevant trials as possible, systematic searches of several sources were carried out using electronic database searching for the period of January 1993 to June 2013. Searching included PubMed (eMethods 1 in the Supplement), the Cochrane Library, handsearching, and internet searching of review articles, meeting proceedings, and 1 trials register (ClinicalTrials.gov). The search was updated during the study by the MORPHEP Collaborative Group.
Data Collection Process and Quality Control
Individual patient data (IPD) were requested for each eligible trial, including patient and tumor characteristics, dates of randomization, SSEs and death, treatment arm allocation, details on treatments received, and toxic effects. Follow-up information was updated whenever possible. When IPD were not available, all efforts were done to collect detailed summary data. All data were checked with a standard procedure,17 which follows the recommendations of the Individual Participant Data Meta-analysis Cochrane working group. Internal consistency was checked (eg, chronology of dates, outlier values), and data were compared with trial protocols and published reports. Randomization validity was evaluated by checking patterns of treatment allocation and balance of baseline characteristics between treatment arms. Follow-up of patients was also compared between treatment arms. Data checking on IPD allowed evaluating risk of bias in individual trials.
Outcomes
The primary end point was OS, defined as the time from randomization date until death or last follow-up. Secondary end points were SSE-free survival and toxic effects. Symptomatic skeletal event–free survival was defined as the time from randomization date to the first of symptomatic pathologic bone fractures, spinal cord compression (SCC), or bone metastasis for which external beam radiotherapy (ERT) or surgical intervention had been performed. Toxic effects included grade 3 and higher hematological toxic effects (hemoglobin, white blood cells, and platelets), nausea and/or vomiting, and febrile neutropenia. Other criteria listed in the protocol, such as pain relief or quality of life, were not analyzed because data were not homogeneously measured or not available.
Statistical Analysis
The median follow-up of each trial was estimated using the reverse Kaplan-Meier method,18 and the overall median follow-up was estimated by the weighted (based on sample size) average of individual median follow-ups. Analyses were stratified by trial. Although a 1-step meta-analysis of IPD was initially planned, a 2-step meta-analysis was performed because working directly on the IPD was not possible for all eligible trials. For time-to-event end points (OS and SSE-free survival), the log-rank expected number of events and variance was used to calculate individual and overall hazard ratios (HRs) of treatment effect (RI use vs no RI use arms) with 95% CIs using a fixed-effects (FE) model.19 A similar model was used to estimate the odds ratios (ORs) for the toxic effects analyses after excluding studies with more than 20% missing data. We imputed the value 0.5 if no event occurred in an arm. For evaluation of between-trial heterogeneity, refer to eMethods 2 in the Supplement, including the use of a random-effects (RE) model. The Peto method20 was used to estimate the stratified survival curves comparing the RI use with no RI use arms. From this, the absolute benefits at 1, 2, and more than 2 years with their 95% CIs were estimated. We performed subset analyses to study the association of trial-level characteristics (type of radiation emitted from RI and type of comparison) with treatment effect using a test of heterogeneity between the different groups of trials. We computed residual heterogeneity within groups by subtracting the statistic of the heterogeneity test between groups from the statistic of the overall heterogeneity test.21 Subgroup analyses for efficacy end points according to age, performance status score (0 to 1 vs 2 or greater), serum prostate-specific antigen (PSA) level, alkaline phosphatase (ALP) level, hemoglobin level, and number of bone metastases at baseline (6 or less vs more than 6) were also performed, and we used the pooling of within-trial covariate interaction method22 to estimate the interaction between treatment effect and patient subgroups (eMethods 2 in the Supplement). All analyses were carried out by intention to treat, which means that the patients were analyzed according to the treatment allocated irrespective of whether they had received that treatment or not. Unplanned subset toxic effects analyses were performed for hematological and febrile neutropenia. The P values for testing the treatment effect and the interactions were calculated from the Wald statistic and between-trials heterogeneity test from the Q-Cochran statistic. These test statistics follow a χ2 distribution. All P values were 2-sided, and a P value less than .05 was considered statistically significant. For tests of heterogeneity and interaction, a P value less than .10 was considered significant (eMethods 2 in the Supplement). Analyses were performed using SAS version 9.4 (SAS Institute).
Results
Study Selection and Characteristics
A total of 9 randomized clinical trials comparing RI use with no RI use arms between January 1993 and June 2013 were identified as eligible for the MORPHEP meta-analysis; the PRISMA flow diagram is shown in eFigure 1 in the Supplement. From these 9 eligible trials, IPD or detailed aggregated data were not available for 3 trials11,23,24 including 341 patients—2 because we could not contact the investigators and 1 because of difficulties to recover data. As a result, 6 trials15,25,26,27,28,29 including 2081 patients (minimum, 64 patients; maximum, 921 patients), with 2 large randomized clinical trials (ALSYMPCA trial15 and the Taxane Radioisotope Zoledronic Acid [TRAPEZE] trial29) representing 80% of data, were included (eTable 1 in the Supplement). For the TRAPEZE trial,29 a 2 × 2 randomized trial, all data comparing RI use with no RI use arms (ratio 1:1) were considered, ie, including patients treated or not by zoledronic acid because there is no known interaction between strontium-89 (Sr89) and zoledronic acid. Two trials15,28 used single 223Ra RIs compared with placebo (n = 985). Three trials25,26,29 tested Sr89 combined with either chemotherapy or ERT vs chemotherapy or ERT alone (n = 893). Among them, 1 chemotherapy with Sr89 combination study25 included only responding or stable patients after an induction chemotherapy (doxorubicin, vinblastine, ketoconazole, and estramustine). One trial27 compared Sr89 RI use with ERT (n = 203).
The dose received depended on the type of radioemitter: the Sr89 groups received a single 150-MBq dose, while the 223Ra groups received 50 kBq/kg every 4 weeks for 4 to 6 injections (eTables 1 and 2 in the Supplement). In 2 trials,15,28 ad hoc summary data were available for data checking, efficacy, and toxic effects analyses, and on request, aggregated data were available for the subgroup analyses.
The overall median (range) follow-up was 26.7 (0.4-188.1) months, and the median (interquartile range) age of patients was 70 (64-75) years. The performance status score was more than 2 in less than 18% of patients overall (ranging from less than 10% in the trial by Tu et al25 to approximately 40% in the trials by Oosterhof et al27 and Smeland et al26) (Table 1) (eTables 3 and 4 in the Supplement). The proportion of patients with more than 6 bone metastases ranged from 67.2% to 85.9% (Table 1). The details of trials’ characteristics are reported in eTables 3 and 4 in the Supplement.
Table 1. Patient Characteristics, Median Follow-up, and Number of Events by Trial and Overall.
| Characteristica | No. (%) | ||||||
|---|---|---|---|---|---|---|---|
| Oosterhof et al27 (n = 203) | Tu et al25 (n = 72) | Smeland et al26 (n = 64) | Nilsson et al28 (n = 64) | TRAPEZE29 (n = 757) | ALSYMPCA15 (n = 921) | Overall (N = 2081) | |
| Age, median (IQR), y | 70.0 (65.0-75.0) | 67.0 (60.2-70.9) | 70.9 (63.8-75.9) | 72.5 (68.0-78.0) | 68.9 (63.9-73.4) | 71.0 (64.0-76.0) | 70.0 (64.0-75.0) |
| <70 | 88 (43.4) | 47 (65.3) | 30 (46.9) | 23 (35.9) | 415 (54.8) | 395 (42.9) | 998 (48.0) |
| ≥70 | 115 (56.6) | 25 (34.7) | 34 (53.1) | 41 (64.1) | 342 (45.2) | 526 (57.1) | 1083 (52.0) |
| Performance status score | |||||||
| 0-1 | 122 (60.1) | 65 (90.3) | 39 (60.9) | 53 (82.8) | 694 (91.7) | 801 (87.0) | 1774 (85.2) |
| ≥2 | 80 (39.4) | 7 (9.7) | 25 (39.1) | 11 (17.2) | 63 (8.3) | 118 (12.8) | 304 (14.6) |
| Missing | 1 (0.5) | 0 | 0 | 0 | 0 | 2 (0.2) | 3 (0.1) |
| Serum PSA level, ng/mL | |||||||
| <143 | 110 (54.2) | 48 (66.7) | 36 (56.2) | 29 (45.3) | 362 (47.8) | 433 (47.0) | 1018 (48.9) |
| ≥143 | 86 (42.4) | 24 (33.3) | 28 (43.8) | 35 (54.7) | 368 (48.6) | 477 (51.8) | 1018 (48.9) |
| Missing | 7 (3.4) | 0 | 0 | 0 | 27 (3.6) | 11 (1.2) | 45 (2.2) |
| Alkaline phosphatase level, U/L | |||||||
| <248.5 | 83 (40.9) | 44 (61.1) | 11 (17.2) | 31 (48.4) | 341 (45.0) | 518 (56.2) | 1028 (49.4) |
| ≥248.5 | 110 (54.2) | 28 (38.9) | 53 (82.8) | 33 (51.6) | 401 (53.0) | 403 (43.8) | 1028 (49.4) |
| Missing | 10 (4.9) | 0 | 0 | 0 | 15 (2.0) | 0 | 25 (1.2) |
| Hemoglobin, g/dL | |||||||
| <12.4 | 102 (50.2) | 22 (30.6) | 26 (40.6) | 24 (37.5) | 360 (47.6) | 498 (54.1) | 1032 (49.6) |
| ≥12.4 | 91 (44.8) | 50 (69.4) | 38 (59.4) | 40 (62.5) | 388 (51.2) | 423 (45.9) | 1030 (49.5) |
| Missing | 10 (4.9) | 0 | 0 | 0 | 9 (1.2) | 0 | 19 (0.9) |
| No. of bone metastasesb | |||||||
| ≤6 | 65 (32.0) | 15 (20.8) | 9 (14.1) | 19 (29.7) | NA | 138 (15.0) | 246 (18.6) |
| >6 | 133 (65.5) | 57 (79.2) | 55 (85.9) | 45 (70.3) | NA | 779 (84.6) | 1069 (80.7) |
| Missing | 5 (2.5) | 0 | 0 | 0 | 0 | 4 (0.4) | 9 (0.7) |
| Duration of follow-up, median (range), mo | 62.7 (1.8-62.7) | 22.3 (1.0-32.3) | NA (2.6-188.1) | 12.1 (0.5-25.6) | 39.2 (0.4-75.1) | 10.0 (0.4-36.6) | 26.7 (0.4-188.1) |
| No. of deaths | 194 (95.6) | 41 (56.9) | 64 (100) | 50 (78.1) | 618 (81.6) | 528 (57.3) | 1495 (71.8) |
| No. of symptomatic skeletal events | NA | NA | 20 (31.3) | 33 (51.6) | 396 (52.3) | 318 (34.5) | 767 (42.5) |
| Spinal cord compression | NA | NA | 4 (6.3) | 5 (7.8) | 58 (7.7) | 23 (2.5) | 90 (5.0) |
| Pathologic bone fracture | NA | NA | 0 | 2 (3.1) | 25 (3.3) | 34 (3.7) | 60 (3.4) |
| Surgical intervention | NA | NA | 0 | 0 | 1 (0.1) | 2 (0.2) | 3 (0.2) |
| External radiotherapyc | NA | NA | 16 (25.0) | 26 (40.6) | 312 (41.2) | 259 (28.1) | 613 (33.9) |
Abbreviations: ALSYMPCA, Alpharadin in Symptomatic Prostate Cancer Patients; IQR, interquartile range; NA, not applicable; PSA, prostate-specific antigen; TRAPEZE, Taxane Radioisotope Zoledronic Acid.
SI conversion factors: To convert PSA to micrograms per liter, multiply by 1; to convert alkaline phosphatase to microkatals per liter, multiply by 0.0167; to convert hemoglobin to grams per liter, multiply by 10.
Continuous characteristics were divided into 2 classes using the median.
For the trial by Nilsson et al28 and the ALSYMPCA trial,15 superscan was considered as higher than 6 bone metastases. For the TRAPEZE trial,29 NA indicates that the number of bone metastases at baseline was not collected or available in this trial.
This category contains both external beam radiotherapy and use of radioisotope for the TRAPEZE trial.29
Risk of Bias Within Studies
All included trials were validated for adequate randomization, blinding, and identical follow-ups between treatment arms. For details, see eTable 5 in the Supplement.
Treatment Efficacy
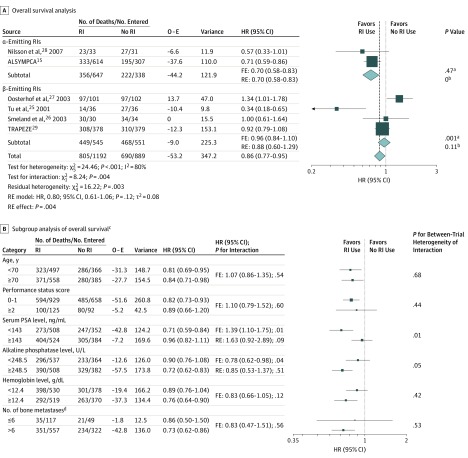
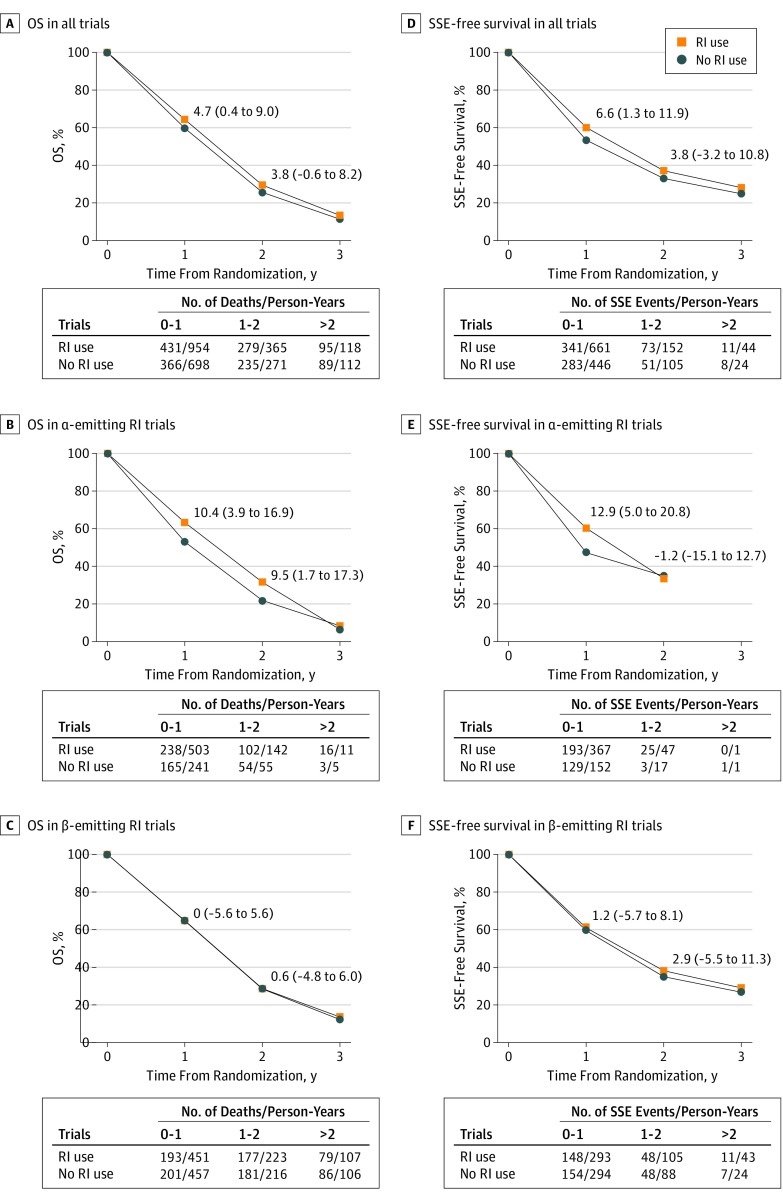
Overall, 1495 deaths (71.8%) were observed. In an FE model, RI use was associated with a significant OS benefit compared with no RI use (HR, 0.86; 95% CI, 0.77-0.95; P = .004), but there was significant (χ25 = 24.46; P < .001) and substantial (I2 = 80%) heterogeneity between trials (Figure 1A). An RE model showed no significant treatment effect (HR, 0.80; 95% CI, 0.61-1.06; P = .12; τ2 = 0.08). The absolute OS difference was 4.7% (95% CI, 0.4-9.0) and 3.8% (95% CI, −0.6 to 8.2) at 1 and 2 years, respectively (Figure 2A). When excluding 2 trials25,27 with 95% CIs that did not overlap that of the overall treatment effect, the heterogeneity remained significant (χ23 = 6.51; P = .09) but decreased to moderate (I2 = 54%) with similar overall treatment effect (FE model: HR, 0.82; 95% CI, 0.73-0.92; P < .001; RE model: HR, 0.80; 95% CI, 0.65-0.99; P = .04; τ2 = 0.02) (eFigure 2 in the Supplement).
Figure 1. Overall Survival and Subgroup Analysis of Trials Comparing Patients Receiving Radioisotopes (RIs) With Patients Receiving No RIs by Type of Radiation.
A, Overall survival in all trials. τ2 values were estimated using the DerSimonian and Laird method.23 Test for heterogeneity: α-emitting RIs: χ21 = 0.53; P = .47; I2 = 0%; β-emitting RIs: χ23 = 15.67; P < .001; I2 = 81%. B, Subgroup analysis of OS. ALSYMPCA indicates Alpharadin in Symptomatic Prostate Cancer Patients; FE, fixed-effects model; HR, hazard ratio; O − E, observed minus expected number of deaths in the experimental arm; PSA, prostate-specific antigen; RE, random-effects model; and RI, radioisotope. To convert PSA to micrograms per liter, multiply by 1; to convert alkaline phosphatase to microkatals per liter, multiply by 0.0167; to convert hemoglobin to grams per liter, multiply by 10.
aP value corresponds to the test for between-trial heterogeneity.
bτ2.
cThe trials by Tu et al25 and Oosterhof et al27 were excluded because they were considered outliers.
dData from the Taxane Radioisotope Zoledronic Acid (TRAPEZE) trial29 were not included in the subgroup analysis of bone metastases at baseline because this information was not available.
Figure 2. Stratified Survival Curves for Overall Survival (OS) and Symptomatic Skeletal Event (SSE)–Free Survivala.
The absolute differences (95% CIs) at 1 and 2 years are given. RI indicates radioisotope.
aThe absolute difference at more than 2 years is not reported because of timing lack of precision.
We observed a significant difference of the overall treatment effect between α-emitting RI and β-emitting RI groups of trials; a significant OS benefit was observed in the α-emitting RI trials (FE model: HR, 0.70; 95% CI, 0.58-0.83; P for heterogeneity = .47; 2 trials; n = 985), but no significant benefit was seen in the β-emitting RI trials (FE model: HR, 0.96; 95% CI, 0.84-1.10; P for heterogeneity = .001; RE model: HR, 0.88; 95% CI, 0.60-1.29; τ2 = 0.11; 4 trials; n = 1096) (χ21 = 8.24; P for interaction = .004) (Figure 1A). The absolute OS benefit of RI use in the α-emitter group was 10.4% (95% CI, 3.9-16.9) at 1 year and 9.5% (95% CI, 1.7-17.3) at 2 years (Figure 2B). There was no significant OS benefit of β-emitting RI use at 1 year (absolute difference, 0.0%; 95% CI, −5.6 to 5.6) and 2 years (absolute difference, 0.6%; 95% CI, −4.8 to 6.0) (Figure 2C). A significant difference was also observed by comparator treatment (eFigure 3 in the Supplement). This subset analysis is similar to the previous one when regrouping the 4 trials25,26,27,29 comparing RI use in combination or not with chemotherapy or ERT. These 2 subset analyses did not explain the overall between-trial heterogeneity of treatment effect, since significant residual heterogeneity remained (type of radiation: χ24 = 16.22; P = .003; type of comparison: χ22 = 9.39; P = .009). When excluding the trials by Tu et al25 and Oosterhof et al,27 the type of RI explained the observed heterogeneity between trials, since the residual heterogeneity was close to 0 with an HR of 0.93 (95% CI, 0.80-1.08) for β-emitting RIs, which remained nonsignificant (eFigure 2 in the Supplement). The difference in type of emitter may be confounded by the timing of the interventions (before vs after Docetaxel era) and the type of control arm.
Planned subgroup analyses (Figure 1B) excluding the trials by Tu et al25 and Oosterhof et al27 showed a significant interaction between treatment effect and serum PSA level (HR, 1.39; 95% CI, 1.10-1.75; P for interaction = .01) and between treatment effect and ALP level (HR, 0.78; 95% CI, 0.62-0.98; P for interaction = .04) but with heterogeneity of trial interactions. When an RE model was used, only the interaction between treatment and serum PSA level remained statistically significant at 10% (HR of interaction, 1.63; 95% CI, 0.92-2.89; P = .09) (eMethods 2 in the Supplement); patients with a lower serum PSA level (less than 143 ng/mL [to convert to micrograms per liter, multiply by 1]) (FE model: HR, 0.71; 95% CI, 0.59-0.84) had a better benefit of RI use compared with patients with a higher serum PSA level (FE model: HR, 0.96; 95% CI, 0.82-1.11) (eFigure 4 in the Supplement). After excluding the trial by Nilsson et al28 because of between-trial heterogeneity within a class (eFigure 4 in the Supplement), this interaction remained statistically significant (HR of interaction, 1.29; 95% CI, 1.02-1.64; P = .03) with no between-trial heterogeneity and homogeneous overall treatment effect in each class. No significant interaction between treatment effect and other patients’ characteristics was observed (Figure 1B), including the unplanned analysis of ALP level with the cutoff of 120 U/L (data not shown; to convert to microkatals per liter, multiply by 0.0167).
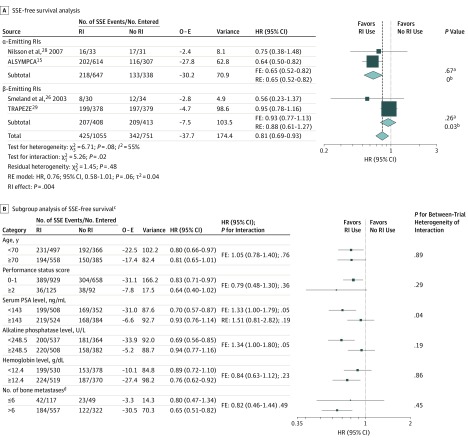
For SSE-free survival, data were available from 4 trials15,26,28,29 including 1806 patients, with 767 SSEs (90 SCCs, 61 pathologic bone fractures, 3 surgical interventions, and 613 ERTs). No information was collected for the Tu et al.25 The trial by Oosterhof et al27 was also excluded from this analysis since SSEs were not collected systematically. In the trial by Smeland et al,26 only SCC and ERT data were collected. In contrast with the trial by Nilsson et al28 and the ALSYMPCA trial,15 the TRAPEZE trial29 did not distinguish between ERT and the reuse of RI, and thus these men (111 of 314 [35.4%]) were considered in only 1 category. Compared with no RI use, RI use had a significant benefit on SSE-free survival (FE model: HR, 0.81; 95% CI, 0.69-0.93; P = .004) (between-trial heterogeneity: χ23 = 6.71; P = .08; I2 = 55%) (Figure 3A), which disappeared with an RE model (HR, 0.76; 95% CI, 0.58-1.01; P = .06; τ2 = 0.04). The absolute SSE-free survival difference was 6.6% (95% CI, 1.3-11.9) at 1 year and 3.8% (95% CI, −3.2 to 10.8) at 2 years (Figure 2D).
Figure 3. Symptomatic Skeletal Event (SSE)–Free Survival and Subgroup Analyses of Trials Comparing Patients Receiving Radioisotopes (RIs) With Patients Receiving No RIs by Type of Radiation.
A, Symptomatic skeletal event–free survival excluding 2 trials.25,27 τ2 values were estimated using the DerSimonian and Laird method.23 Test for heterogeneity: α-emitting RIs: χ21 = 0.18; P = .67; I2 = 0%; β-emitting RIs: χ21 = 1.28; P = .26; I2 = 22%. B, Subgroup analysis of SSE-free survival. ALSYMPCA indicates Alpharadin in Symptomatic Prostate Cancer Patients; FE, fixed-effects model; HR, hazard ratio; O − E, observed minus expected number of SSEs in the experimental arm; PSA, prostate-specific antigen; RE, random-effects model; and RI, radioisotope. To convert PSA to micrograms per liter, multiply by 1; to convert alkaline phosphatase to microkatals per liter, multiply by 0.0167; to convert hemoglobin to grams per liter, multiply by 10.
aP value corresponds to the test for between-trial heterogeneity.
bτ2.
cThe trials by Tu et al25 and Oosterhof et al27 were excluded because no information was available for the former and data were not reliable for the latter.
dData from the Taxane Radioisotope Zoledronic Acid (TRAPEZE) trial29 were not included in the subgroup analysis of bone metastases at baseline because this information was not requested in this trial.
The interaction between emitter type and treatment effect on SSE-free survival was significant (χ21 = 5.26; P = .02) with no significant residual heterogeneity (χ22 = 1.45; P = .48) (Figure 3A). The risk reduction of SSE was restricted to the α-emitting RI trials (FE model: HR, 0.65; 95% CI, 0.52-0.82; P for heterogeneity = .67; 2 trials; n = 985). In terms of SSE-free rates, the differences were 12.9% (95% CI, 5.0-20.8;) at 1 year and −1.2% (95% CI, −15.1 to 12.7) at 2 years (Figure 2E). Use of β-emitting RIs was not significantly associated with SSE-free survival (FE model: HR, 0.93; 95% CI, 0.77-1.13; P for heterogeneity = .26; 2 trials; n = 821), with absolute differences of 1.2% (95% CI, −5.7 to 8.1) at 1 year and 2.9% (95% CI, −5.5 to 11.3) at 2 years (Figure 2F). A significant interaction between the type of comparison and the treatment effect was observed (χ22 = 6.54; P = .04) (eFigure 5 in the Supplement).
Planned subgroup analyses for SSE-free survival (Figure 3B) showed a significant interaction between treatment effect and serum PSA level (HR of interaction, 1.33; 95% CI, 1.00-1.79; P = .05), which disappeared after taking into account significant heterogeneity (χ23 = 8.12; P = .04) by an RE model (HR, 1.51; 95% CI, 0.81-2.82; P = .19), and a significant interaction between treatment effect and ALP level (HR of interaction, 1.34; 95% CI, 1.00-1.80; P = .05), with no between-trial heterogeneity of interaction (χ23 = 4.80; P = .19); patients with a lower ALP level (HR, 0.69; 95% CI, 0.56-0.85) had a better benefit when using RIs compared with patients with a higher ALP level (HR, 0.94; 95% CI, 0.77-1.16) (eFigure 6A in the Supplement). This interaction remained significant (χ21 = 2.99; P = .08), with homogeneous treatment effect in each class after excluding the trials by Smeland et al26 and Nilsson et al,28 2 trials with large interaction effects (eFigure 6B in the Supplement). No significant interaction between treatment effect and patients’ other characteristics was observed (Figure 3B), including the unplanned analysis of ALP level using the cutoff of 120 U/L (data not shown). As suggested by a reviewer, we performed an unplanned sensitivity analysis including only patients not treated with zoledronic acid from the TRAPEZE trial29 in the meta-analysis (eMethods 3 in the Supplement).
Adverse Events
The rate of severe hematological toxic effects increased with the use of RI (20.5%) compared with no RI use (15.9%) (OR, 1.48; 95% CI, 1.17-1.88; P = .001) (Table 2) (eTable 6 and eFigure 7A in the Supplement). Hematological toxic effects between the α-emitting RI group (OR, 1.77; 95% CI, 1.24-2.54) and the β-emitting RI group (OR, 1.29; 95% CI, 0.94-1.77) (eFigure 7B in the Supplement) was not significantly different (P for interaction = .19) (unplanned analysis). The rate of nausea and/or vomiting was not significantly different between the RI use and no RI use arms (OR, 1.27; 95% CI, 0.74-2.17; P = .39) (eFigure 8 in the Supplement), nor was the rate of febrile neutropenia (OR, 1.00; 95% CI, 0.59-1.68; P = .99) (eFigure 9A in the Supplement). The unplanned analysis of febrile neutropenia rates according to the type of radiation emitted revealed no significant difference between the RI effect in the α-emitter group (OR, 0.60; 95% CI, 0.06-6.36) and in the β-emitter group (OR, 1.02; 95% CI, 0.60-1.75) (P for interaction = .67), with only 2 events observed in the α-emitting RI group (eFigure 9B in the Supplement).
Table 2. Toxic Effects Analyses, Including Hematological Toxic Effects, Nausea and/or Vomiting, and Febrile Neutropeniaa.
| Toxic Effect | No. of Trials | No. of Patients | Proportion of Patients With Toxic Effects Receiving RI, % | Proportion of Patients With Toxic Effects Receiving No RI, No./Total No. (%) | Odds Ratio (95% CI) | P Value | I2, % | P for Heterogeneity |
|---|---|---|---|---|---|---|---|---|
| Hematologicalb | 6 | 2029 | 20.5 | 138/867 (15.9) | 1.48 (1.17-1.88) | .001 | 0 | .67 |
| Nausea and/or vomitingc | 4 | 1061 | 5.9 | 26/527 (4.9) | 1.27 (0.74-2.17) | .39 | 0 | .44 |
| Nausea and/or vomiting (sensitivity analysis)d | 3 | 989 | 5.4 | 20/491 (4.1) | 1.51 (0.84-2.71) | .17 | 0 | .81 |
| Febrile neutropeniae | 5 | 1878 | 3.7 | 29/787 (3.7) | 1.00 (0.59-1.68) | .99 | 0 | .87 |
See eTable 6 in the Supplement for a detailed description of selected toxic effects and eFigure 7 in the Supplement for the forest plots.
All trials were used for hematological toxic effects analysis.
The analysis of nausea and/or vomiting was limited to 4 trials because 2 trials (Nilsson et al28 and the ALSYMPCA trial29) had a large amount of missing data (>20%).
Sensitivity analysis excluded the trial by Tu et al,25 as nausea and/or vomiting was extracted from the publication, which reported grade of 2 or greater. Here, we focused on toxic effects of grade 3 or greater.
The trial by Oosterhof et al27 was excluded because these data were not collected.
Discussion
Bone is the main target of prostate cancer dissemination and a source of major morbidity and mortality.30,31 The present meta-analysis based on IPD from randomized clinical trials shows that RI does not improve OS in men with CRPC and bone metastases. However, an OS benefit was observed with the 223Ra α-emitting RI, while no significant benefit was observed with the Sr89 β-emitting RI. In the subgroup analyses, men with the lowest serum PSA values appeared to benefit significantly more from bone-targeted RI therapy compared with those with the highest serum PSA values. No significant interaction was found between treatment effect and age, performance status score, ALP level, hemoglobin level, and the number of bone metastases at baseline. Furthermore, similar results were observed for SSE-free survival except when excluding patients treated with zoledronic acid in the TRAPEZE trial,29 where we observed a significant overall benefit of RI use both in the FE and RE models (eMethods 3 in the Supplement). An overall risk reduction of SSE-free survival was more important in patients with low ALP levels compared with patients with high ALP levels. Hematological toxic effects were more frequently observed in patients treated by RI compared with those treated without RI, with no significant differences according to the type of radiation.
In the recently reported interim analysis of the Eastern Cooperative Oncology Group (ERA 223) study32 including 401 and 405 patients in the 223Ra with abiraterone acetate and placebo with abiraterone acetate arms, respectively, concurrent treatment with 223Ra and abiraterone acetate did not improve SSE-free survival (HR, 1.12; 95% CI, 0.92-1.37) and OS (HR, 1.21; 95% CI, 0.95-1.51). These results differ from the ALSYMPCA trial.15 One of the explanations of the failure of 223Ra when it is associated with abiraterone is probably owing to the biological activities of both drugs. In fact, we know that abiraterone acetate promotes the osteoblastic activity in the bone and that 223Ra is the most active in the bone remodeling sites. Likely, this makes the 223Ra harmful by targeting not only metastatic bones but also disease-free bones. As this trial was not included in our study because the recruitment was more recent, an unplanned post hoc analysis including ERA 223 trial32 data based on summary data was carried out for OS but not for SSE-free survival, since death was considered as an event in the ERA 223 trial, contrary to our meta-analysis (eMethods 4 in the Supplement).
Furthermore, the use of the β-emitting RI Sr89 was not significantly associated with OS both when used alone and in combination, which is consistent with earlier reported data.10,29 Only 1 study using a β-emitting RI, the trial by Tu et al,25 reported OS and SSE-free survival improvements. In this trial, Sr89 was used as a maintenance strategy in men selected for having a chemosensitive cancer. No improvement in time to SSE was observed with β-emitting RIs. Most likely, physical and biological properties explain the superiority of α-emitting RIs over β-emitting RIs in men with CRPC.33
Strengths and Limitations
The main strength of this meta-analysis is the use of IPD, which allowed detailed checking of each trial that was subsequently reanalyzed and validated by the trialists. The intention-to-treat principle was respected for all analyses, and subgroup analyses have been performed through the estimation of interaction terms.
This meta-analysis has limitations. First, data from 3 randomized clinical trials could not be retrieved, and therefore, this meta-analysis was limited to 6 randomized clinical trials, with 2 major trials (the ALSYMPCA and TRAPEZE trials15,29) providing more than 80% of data. This first limit has no effect on the main findings (eMethods 5 in the Supplement). When using an RE approach, used in particular in case of between-trial heterogeneity, the relative weight of the ALSYMPCA and TRAPEZE trials compared with an FE approach was 21.8% and 22.5% instead of 30.9% and 44.8%, respectively. Second, substantial and moderate heterogeneity was observed between trials for OS and SSE-free survival analyses, respectively, leading to a nonsignificant effect when using an RE model. For OS, this between-trial heterogeneity is mainly explained by 2 outliers (with I2 decreasing from 80% to 54%) and by the type of emitted radiations. The 2 outliers correspond to (1) the large treatment effect reported in the trial by Tu et al,25 which may be explained by the selected population—men with a cancer response or stabilization after induction chemotherapy—and (2) the significant negative effect of Sr89 compared with pain treatment with local field radiotherapy reported in the trial by Oosterhof et al,27 which was difficult to explain by the authors. One hypothesis is that Sr89 was given to patients with more impaired global health. When excluding these 2 trials, a similar and significant treatment effect was observed both for the FE and RE models. For SSE-free survival, for which the analysis is limited to 4 trials only, since the 2 trials had no available information, the moderate trial heterogeneity is related to the type of emitted radiations. The methods of DerSimonian and Laird that we used for the estimation of the between-trial variability with the RE model are criticized by some authors.34 Its estimation by a restricted maximum likelihood method gives similar results in this meta-analysis except for the main analysis (τ2 value: restricted maximum likelihood, 0.14; DerSimonian and Laird, 0.08). The residual heterogeneity may be explained by patient characteristics. The trials’ accrual period, which ranged from 1993 to 2013, might have added heterogeneity in the results. The subset analysis by type of comparison is limited owing to the number of trials and is reduced to the subset analysis by type of emitted radiation when excluding the 2 outliers. A further limitation concerns the quality of data collected for the SSE-free survival analysis and the lack of power for the subset analyses of toxic effects.
Conclusions
This meta-analysis supports the role of α-emitting RIs (223Ra) as a treatment for men with CRPC and bone metastases both for OS and the prevention of SSEs but not of β-emitting RIs (Sr89). The action of α-emitting RIs may be explained by their physical properties irrespective of the study design. However, these results must be interpreted with caution because of the observed between-trial heterogeneity. Furthermore, dedicated studies are needed to identify biomarkers of response to 223Ra and to define the best and safest combinations.
eMethods 1. Trial search strategy from PubMed.
eMethods 2. Statistical methods.
eMethods 3. Unplanned sensitivity analysis.
eMethods 4. Unplanned post hoc analysis.
eMethods 5. Unplanned sensitivity analysis.
eTable 1. Description of the 6 randomized clinical trials included for the meta-analysis.
eTable 2. Distribution of the potential confounding factors in the eligible trials.
eTable 3. Patient characteristics, median follow-up, and number of events by trial and overall.
eTable 4. Patient characteristics, median follow-up, and number of events by trial and by arm.
eTable 5. Risk of bias summary: authors’ judgments about each risk of bias item for each included study.
eTable 6. Description of selected toxic effects of grade ≥3 per trial and per arm.
eFigure 1. Flow diagram of studies inclusion and exclusion.
eFigure 2. Overall survival for trials comparing radioisotopes (RIs) with no RIs according to the type of radiation emitted from RIs after excluding the MDA 1996 and EORTC trials.
eFigure 3. Forest plot for subset analysis of overall survival according to the type of comparison.
eFigure 4. Forest plots for serum prostate-specific antigen subgroup analysis for overall survival.
eFigure 5. Forest plot for subset analysis according to the type of comparison for symptomatic skeletal event (SSE)–free survival.
eFigure 6. Forest plots of alkaline phosphatase level subgroup analysis for symptomatic skeletal event (SSE)–free survival.
eFigure 7. Hematological toxic effects analysis.
eFigure 8. Nausea and/or vomiting analysis.
eFigure 9. Febrile neutropenia toxic effects analysis.
eReferences.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356-387. doi: 10.1016/j.ejca.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 2.Fizazi K, Massard C, Smith M, et al. Bone-related parameters are the main prognostic factors for overall survival in men with bone metastases from castration-resistant prostate cancer. Eur Urol. 2015;68(1):42-50. doi: 10.1016/j.eururo.2014.10.001 [DOI] [PubMed] [Google Scholar]
- 3.Tannock IF, de Wit R, Berry WR, et al. ; TAX 327 Investigators . Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502-1512. doi: 10.1056/NEJMoa040720 [DOI] [PubMed] [Google Scholar]
- 4.de Bono JS, Logothetis CJ, Molina A, et al. ; COU-AA-301 Investigators . Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995-2005. doi: 10.1056/NEJMoa1014618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scher HI, Fizazi K, Saad F, et al. ; AFFIRM Investigators . Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187-1197. doi: 10.1056/NEJMoa1207506 [DOI] [PubMed] [Google Scholar]
- 6.de Bono JS, Oudard S, Ozguroglu M, et al. ; TROPIC Investigators . Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147-1154. doi: 10.1016/S0140-6736(10)61389-X [DOI] [PubMed] [Google Scholar]
- 7.Kantoff PW, Higano CS, Shore ND, et al. ; IMPACT Study Investigators . Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411-422. doi: 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- 8.Saad F, Gleason DM, Murray R, et al. ; Zoledronic Acid Prostate Cancer Study Group . A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94(19):1458-1468. doi: 10.1093/jnci/94.19.1458 [DOI] [PubMed] [Google Scholar]
- 9.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813-822. doi: 10.1016/S0140-6736(10)62344-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewington VJ, McEwan AJ, Ackery DM, et al. A prospective, randomised double-blind crossover study to examine the efficacy of strontium-89 in pain palliation in patients with advanced prostate cancer metastatic to bone. Eur J Cancer. 1991;27(8):954-958. doi: 10.1016/0277-5379(91)90257-E [DOI] [PubMed] [Google Scholar]
- 11.Serafini AN, Houston SJ, Resche I, et al. Palliation of pain associated with metastatic bone cancer using samarium-153 lexidronam: a double-blind placebo-controlled clinical trial. J Clin Oncol. 1998;16(4):1574-1581. doi: 10.1200/JCO.1998.16.4.1574 [DOI] [PubMed] [Google Scholar]
- 12.Fizazi K, Beuzeboc P, Lumbroso J, et al. Phase II trial of consolidation docetaxel and samarium-153 in patients with bone metastases from castration-resistant prostate cancer. J Clin Oncol. 2009;27(15):2429-2435. doi: 10.1200/JCO.2008.18.9811 [DOI] [PubMed] [Google Scholar]
- 13.Smith MR, Coleman RE, Klotz L, et al. Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: comparison of skeletal-related events and symptomatic skeletal events. Ann Oncol. 2015;26(2):368-374. doi: 10.1093/annonc/mdu519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillessen S, Attard G, Beer TM, et al. Management of patients with advanced prostate cancer: the report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur Urol. 2018;73(2):178-211. doi: 10.1016/j.eururo.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 15.Parker C, Nilsson S, Heinrich D, et al. ; ALSYMPCA Investigators . Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223. doi: 10.1056/NEJMoa1213755 [DOI] [PubMed] [Google Scholar]
- 16.Pignon JP, Hill C. Meta-analyses of randomised clinical trials in oncology. Lancet Oncol. 2001;2(8):475-482. doi: 10.1016/S1470-2045(01)00453-3 [DOI] [PubMed] [Google Scholar]
- 17.Stewart LA, Clarke MJ; Cochrane Working Group . Practical methodology of meta-analyses (overviews) using updated individual patient data. Stat Med. 1995;14(19):2057-2079. doi: 10.1002/sim.4780141902 [DOI] [PubMed] [Google Scholar]
- 18.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343-346. doi: 10.1016/0197-2456(96)00075-X [DOI] [PubMed] [Google Scholar]
- 19.Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27(5):335-371. doi: 10.1016/S0033-0620(85)80003-7 [DOI] [PubMed] [Google Scholar]
- 20.Lueza B, Rotolo F, Bonastre J, Pignon J-P, Michiels S. Bias and precision of methods for estimating the difference in restricted mean survival time from an individual patient data meta-analysis. BMC Med Res Methodol. 2016;16:37. doi: 10.1186/s12874-016-0137-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Non-small Cell Lung Cancer Collaborative Group Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ. 1995;311(7010):899-909. doi: 10.1136/bmj.311.7010.899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher DJ, Copas AJ, Tierney JF, Parmar MKB. A critical review of methods for the assessment of patient-level interactions in individual participant data meta-analysis of randomized trials, and guidance for practitioners. J Clin Epidemiol. 2011;64(9):949-967. doi: 10.1016/j.jclinepi.2010.11.016 [DOI] [PubMed] [Google Scholar]
- 23.Sartor O, Reid RH, Hoskin PJ, et al. ; Quadramet 424Sm10/11 Study Group . Samarium-153-Lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology. 2004;63(5):940-945. doi: 10.1016/j.urology.2004.01.034 [DOI] [PubMed] [Google Scholar]
- 24.Han SH, de Klerk JMH, Tan S, et al. The PLACORHEN study: a double-blind, placebo-controlled, randomized radionuclide study with (186)Re-etidronate in hormone-resistant prostate cancer patients with painful bone metastases: Placebo Controlled Rhenium Study. J Nucl Med. 2002;43(9):1150-1156. [PubMed] [Google Scholar]
- 25.Tu SM, Millikan RE, Mengistu B, et al. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet. 2001;357(9253):336-341. doi: 10.1016/S0140-6736(00)03639-4 [DOI] [PubMed] [Google Scholar]
- 26.Smeland S, Erikstein B, Aas M, Skovlund E, Hess SL, Fosså SD. Role of strontium-89 as adjuvant to palliative external beam radiotherapy is questionable: results of a double-blind randomized study. Int J Radiat Oncol Biol Phys. 2003;56(5):1397-1404. doi: 10.1016/S0360-3016(03)00274-8 [DOI] [PubMed] [Google Scholar]
- 27.Oosterhof GON, Roberts JT, de Reijke TM, et al. Strontium(89) chloride versus palliative local field radiotherapy in patients with hormonal escaped prostate cancer: a phase III study of the European Organisation for Research and Treatment of Cancer, Genitourinary Group. Eur Urol. 2003;44(5):519-526. doi: 10.1016/S0302-2838(03)00364-6 [DOI] [PubMed] [Google Scholar]
- 28.Nilsson S, Franzén L, Parker C, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007;8(7):587-594. doi: 10.1016/S1470-2045(07)70147-X [DOI] [PubMed] [Google Scholar]
- 29.James ND, Pirrie SJ, Pope AM, et al. Clinical outcomes and survival following treatment of metastatic castrate-refractory prostate cancer with docetaxel alone or with strontium-89, zoledronic acid, or both: the TRAPEZE randomized clinical trial. JAMA Oncol. 2016;2(4):493-499. doi: 10.1001/jamaoncol.2015.5570 [DOI] [PubMed] [Google Scholar]
- 30.Gartrell BA, Coleman R, Efstathiou E, et al. Metastatic prostate cancer and the bone: significance and therapeutic options. Eur Urol. 2015;68(5):850-858. doi: 10.1016/j.eururo.2015.06.039 [DOI] [PubMed] [Google Scholar]
- 31.Gartrell BA, Galsky MD. Clinical decision making in castration-resistant prostate cancer according to baseline prostate-specific antigen: are we measuring disease burden or disease biology? Eur Urol. 2015;67(2):231-232. doi: 10.1016/j.eururo.2014.08.069 [DOI] [PubMed] [Google Scholar]
- 32.Smith M, Parker C, Saad F, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):408-419. doi: 10.1016/S1470-2045(18)30860-X [DOI] [PubMed] [Google Scholar]
- 33.Bruland ØS, Nilsson S, Fisher DR, Larsen RH. High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin Cancer Res. 2006;12(20, pt 2):6250s-6257s. doi: 10.1158/1078-0432.CCR-06-0841 [DOI] [PubMed] [Google Scholar]
- 34.Langan D, Higgins JPT, Jackson D, et al. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res Synth Methods. 2019;10(1):83-98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Trial search strategy from PubMed.
eMethods 2. Statistical methods.
eMethods 3. Unplanned sensitivity analysis.
eMethods 4. Unplanned post hoc analysis.
eMethods 5. Unplanned sensitivity analysis.
eTable 1. Description of the 6 randomized clinical trials included for the meta-analysis.
eTable 2. Distribution of the potential confounding factors in the eligible trials.
eTable 3. Patient characteristics, median follow-up, and number of events by trial and overall.
eTable 4. Patient characteristics, median follow-up, and number of events by trial and by arm.
eTable 5. Risk of bias summary: authors’ judgments about each risk of bias item for each included study.
eTable 6. Description of selected toxic effects of grade ≥3 per trial and per arm.
eFigure 1. Flow diagram of studies inclusion and exclusion.
eFigure 2. Overall survival for trials comparing radioisotopes (RIs) with no RIs according to the type of radiation emitted from RIs after excluding the MDA 1996 and EORTC trials.
eFigure 3. Forest plot for subset analysis of overall survival according to the type of comparison.
eFigure 4. Forest plots for serum prostate-specific antigen subgroup analysis for overall survival.
eFigure 5. Forest plot for subset analysis according to the type of comparison for symptomatic skeletal event (SSE)–free survival.
eFigure 6. Forest plots of alkaline phosphatase level subgroup analysis for symptomatic skeletal event (SSE)–free survival.
eFigure 7. Hematological toxic effects analysis.
eFigure 8. Nausea and/or vomiting analysis.
eFigure 9. Febrile neutropenia toxic effects analysis.
eReferences.