Key Points
Question
Is consumption of ultraprocessed foods associated with the risk of developing type 2 diabetes (T2D)?
Findings
This observational prospective study of 104 707 participants found that a higher proportion of ultraprocessed foods in the diet was associated with a higher risk of T2D.
Meaning
Ultraprocessed food intake is a modifiable factor that may play a role in T2D etiology. Public health authorities in several countries recently started to recommend privileging unprocessed/minimally processed foods and limiting ultraprocessed food consumption.
This observational study examines whether a higher proportion of ultraprocessed foods in the diet was associated with a higher risk of type 2 diabetes.
Abstract
Importance
Ultraprocessed foods (UPF) are widespread in Western diets. Their consumption has been associated in recent prospective studies with increased risks of all-cause mortality and chronic diseases such as cancer, cardiovascular diseases, hypertension, and dyslipidemia; however, data regarding diabetes are lacking.
Objective
To assess the associations between consumption of UPF and risk of type 2 diabetes (T2D).
Design, Setting, and Participants
In this population-based prospective cohort study, 104 707 participants aged 18 years or older from the French NutriNet-Santé cohort (2009-2019) were included. Dietary intake data were collected using repeated 24-hour dietary records (5.7 per participant on average), designed to register participants' usual consumption for more than 3500 different food items. These were categorized according to their degree of processing by the NOVA classification system.
Main Outcomes and Measures
Associations between UPF consumption and risk of T2D were assessed using cause-specific multivariable Cox proportional hazard models adjusted for known risk factors (sociodemographic, anthropometric, lifestyle, medical history, and nutritional factors).
Results
A total of 104 707 participants (21 800 [20.8%] men and 82 907 [79.2%] women) were included. Mean (SD) baseline age of participants was 42.7 (14.5) years. Absolute T2D rates in the lowest and highest UPF consumers were 113 and 166 per 100 000 person-years, respectively. Consumption of UPF was associated with a higher risk of T2D (multi-adjusted hazard ratio [HR] for an absolute increment of 10 in the percentage of UPF in the diet, 1.15; 95% CI, 1.06-1.25; median follow-up, 6.0 years; 582 252 person-years; 821 incident cases). These results remained statistically significant after adjustment for several markers of the nutritional quality of the diet, for other metabolic comorbidities (HR, 1.13; 95% CI, 1.03-1.23), and for weight change (HR, 1.13; 95% CI, 1.01-1.27). The absolute amount of UPF consumption (grams per day) was consistently associated with T2D risk, even when adjusting for unprocessed or minimally processed food intake (HR for a 100 g/d increase, 1.05; 95% CI, 1.02-1.08).
Conclusions and Relevance
In this large observational prospective study, a higher proportion of UPF in the diet was associated with a higher risk of T2D. Even though these results need to be confirmed in other populations and settings, they provide evidence to support efforts by public health authorities to recommend limiting UPF consumption.
Trial Registration
ClinicalTrials.gov Identifier: NCT03335644
Introduction
Type 2 diabetes mellitus (T2D) is a major public health problem worldwide, affecting 425 million people in 2017, with an estimated projection of 629 million cases by 2045.1 It is therefore urgent to control the disease by intervening on modifiable risk factors, including diet, physical activity, and weight. According to the 2018 Global Burden of Diseases, 34.9% of disability-adjusted life-years (DALYs) of diabetes are attributable to dietary factors,2 such as high intakes of sugar and meat, and low intakes of vegetables, fruits, whole grains, legumes, nuts, and yogurt.3,4
Ultraprocessed foods (UPF) (ie, foods undergoing multiple physical, biological, and/or chemical processes, among which mostly of exclusive industrial use, and generally containing food additives5) are widespread worldwide and especially in Western diets,6,7,8,9,10,11 representing between 25% and 60% of total daily energy.12,13,14,15,16,17,18,19,20,21,22,23,24 During the past decade, scientists’ interest in this topic has increased because several characteristics of these products, beyond a poorer nutritional quality,12,13,14,15,16,17,18,19,20,25 are hypothesized to convey adverse health effects. Indeed, UPF usually go through several physical and chemical processes such as extruding, molding, prefrying, hydrogenation,5 possibly leading to the production of new compounds with potential cardiometabolic disruption properties. They also typically contain food substances of no or rare culinary use (eg, some varieties of refined sugars, hydrogenated oils) and various types of cosmetic additives (eg, emulsifiers, sweeteners, thickening agents, colorants),5 with cardiometabolic effects postulated for some.26,27,28 Finally, UPF often have longer shelf-lives compared with non-UPF, particularly owing to the use of preservatives. Thus, they stay for long periods in their packaging, favoring potential migration of materials in contact with food, such as bisphenol A, associated with increased T2D risk in a recent meta-analysis of observational studies.29
Recently, our research group showed in the NutriNet-Santé cohort that UPF consumption (using the NOVA classification30) was associated with increased risks of cancer,31 mortality,32 depressive symptoms,33 inflammatory bowel syndrome,34 and cardiovascular diseases.35 Other prospective studies also observed associations with mortality risk,36 depression,37 frailty,38 dyslipidemia in children,39 overweight/obesity,40 and hypertension.41 However, no such large-scale prospective study has been conducted regarding T2D. Our objective was to explore the associations between the consumption of UPF and the risk of T2D in a large cohort of French adults.
Methods
Study Population and Data Collection
The NutriNet-Santé study is an ongoing web-based cohort launched in 2009 in France aiming to study the associations between nutrition and health.42 It was approved by the relevant institutional review boards (Inserm and Cnil). Participants provided electronic informed consent. Adults aged 18 years or older are continuously recruited among the general population and followed using a dedicated web interface (etude-nutrinet-sante.fr). At baseline, participants completed a set of 5 questionnaires related to sociodemographic and lifestyle characteristics43 (eg, sex, date of birth, educational level, smoking status), anthropometry44,45 (eg, height, weight), physical activity (7-day International Physical Activity Questionnaire [IPAQ]),46 health status, and dietary intake. In addition, fasting blood samples were collected for 19 772 participants.
Dietary Data and Food Processing Categorization
Participants were invited to complete a series of 3 nonconsecutive validated web-based 24-hour dietary records at baseline and every 6 months (to vary the season of completion), randomly assigned over a 2-week period (2 weekdays and 1 weekend day).47,48,49 Corresponding nutrient intakes were calculated using the NutriNet-Santé food composition database, containing more than 3500 food items. Mean dietary intake from 24-hour dietary records available during the first 2 years of each participant’s follow-up were averaged and considered as baseline usual dietary intakes in this prospective analysis. The NutriNet-Santé web-based self-administered 24-hour dietary records have been tested and validated against an interview by a trained dietitian,47 and against blood and urinary biomarkers.48,49 Details on dietary and biological data collection is provided in the eMethods in the Supplement.
All food and beverage items of the NutriNet-Santé composition table were categorized into 1 of the 4 NOVA categories (unprocessed/minimally processed foods, culinary ingredients, processed foods, ultraprocessed foods),50 a food classification system based on the extent and purpose of food processing.30,51,52 This study primarily focused on the “ultraprocessed foods” NOVA group. Definitions and examples are presented in the eMethods in the Supplement.
Case Ascertainment
Participants were asked to declare major health events though the yearly health questionnaire, through a specific health check-up questionnaire every 3 months, or at any time through a specific interface on the study website. They were also asked to declare all currently taken medications and treatments via the check-up and yearly questionnaires. In addition, data are linked to medico-administrative databases of the SNIIRAM, providing detailed information about the reimbursement of medication and medical consultations. Details on T2D case ascertainment (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10] code E11) are provided in the eMethods in the Supplement, including Anatomical Therapeutic Chemical (ATC) Classification codes considered for T2D medication. Mortality cases were identified using a linkage to CépiDC, the French national mortality registry.
Statistical Analyses
The flowchart for participants’ selection is presented in the Figure. The final population study included 104 707 individuals. For each participant, the proportion (percentage) of UPF in the total weight of food and/or beverages consumed (grams per day) was calculated. It was determined by making a weight ratio rather than an energy ratio to take into account UPF that do not provide energy (eg, artificially sweetened beverages). Multiple imputation for missing data was performed using the multivariate imputation by chained equations (MICE) method.53 Unadjusted means of the proportion of UPF in the diet were calculated and presented across strata of the population using appropriate unpaired, 2-tailed t tests or analysis of variance for assessing the differences between groups. Regression analysis was performed to assess the association between nutrient and food group intakes, and UPF proportion, adjusted for sex, age, and total energy intake. The distribution of the ultraprocessed variable in the sample is described in eFigure 1 in the Supplement.
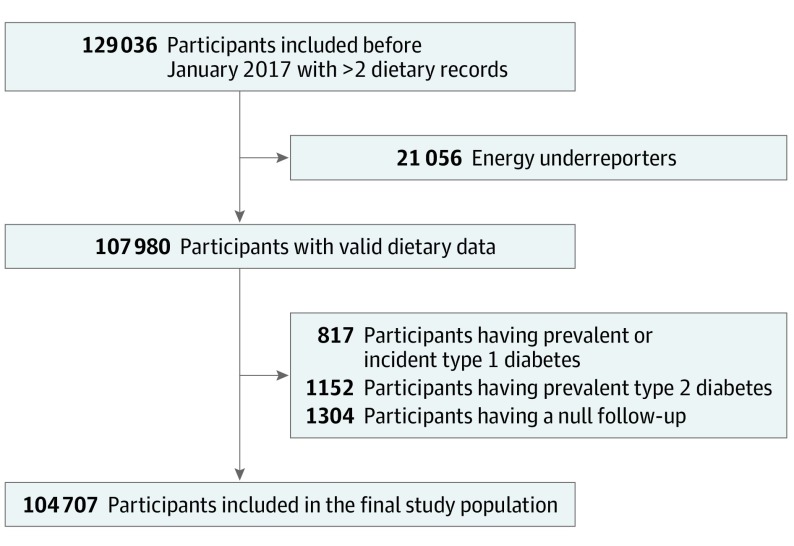
Figure. Flowchart for the Selection of the Study Population, NutriNet-Santé Cohort, 2009 to 2019.
A total of 129 036 participants enrolled before January 2017 provided at least 2 valid 24-hour dietary records during their first 2 years of follow-up were eligible for the present study. After exclusion of energy underreporters, participants having prevalent or incident type 1 diabetes, those having prevalent type 2 diabetes, and those with a null follow-up, 104 707 participants were included in the final study population.
Cause-specific Cox models were performed, with a left-truncation to account for delayed entries, and age as timescale, to evaluate the association between the proportion of UPF in the diet (coded as a continuous variable for a 10-point increment) and incidence of T2D. For purposes of analysis, T2D and death during follow-up were handled as competing events. Cause-specific hazard ratios (HRs) and 95% CIs were computed. The Cox model assumptions were verified (eFigures 2 and 3 in the Supplement). Participants contributed person-time until the date of T2D diagnosis or death (competing events), the date of last completed questionnaire, or January 9, 2019, whichever occurred first. The Fine and Gray model for competing events54 was additionally tested in sensitivity analyses.
Models were adjusted for age (time-scale), sex, educational level, body mass index (BMI, calculated as weight in kilograms divided by height in meters squared), physical activity level, smoking status, alcohol intake, number of 24-hour dietary records, energy intake without alcohol, family history of diabetes, and overall nutritional quality of the diet as measured by the validated Food Standard Agency nutrient profiling system dietary index (FSAm-NPS DI) (Model 1). This index, based on the British FSA nutrient profiling system is the one underlying the official French, Belgian, Spanish and German front-of-package food labeling (the Nutri-Score). It was extensively described and validated elsewhere55,56,57 and its computation is detailed in the eMethods in the Supplement. In secondary analyses, Model 2 was further adjusted for several nutrient intakes, whereas Model 3 was adjusted for various food groups associated with T2D risk with consistent evidence.3 Model 4 was based on Model 1 and included further adjustments for baseline dyslipidemia and hypertension and treatments for these conditions, and Model 5 was adjusted for weight change during follow-up. The associations with T2D risk were also tested for the proportion of UPF among specific food groups, the absolute amount of UPF consumption (in grams per day) (adjusted for the absolute amount of unprocessed/minimally processed food), and the proportion of unprocessed/minimally processed foods in the diet. A series of sensitivity analyses was performed to assess the robustness of the findings (eMethods in the Supplement). All tests were 2-sided, and P < .05 was considered statistically significant. For analyses, SAS statistical software (version 9.4; SAS Institute, Inc) was used.
Results
A total of 104 707 participants (21 800 [20.8%] men and 82 907 [79.2%] women) were included. Mean (SD) baseline age of participants was 42.7 (14.5) years. The mean weight contribution of UPF to the diet was 17.3%. Overall, UPF proportions were higher in younger participants, obese individuals, those with lower physical activity levels, and current smokers (Table 1). Higher consumption of UPF was associated with higher FSAm-NPS DI scores (reflecting a poorer nutritional quality of the diet), higher intakes of energy, SFA, sodium and sugar, lower intakes of fiber and alcohol, higher consumptions of sugary drinks and red and processed meats, and lower consumptions of whole grains, yogurt, nuts, and fruits and vegetables (Table 2).
Table 1. Proportion (in Weight) of Ultraprocessed Food in the Diet of 104 707 Participants, NutriNet-Santé Cohort (2009-2019).
| Characteristic | No. (%) | Ultraprocessed Food in the Diet, % | |
|---|---|---|---|
| Mean (SD) | P Valuea | ||
| All | 104 707 (100.0) | 17.29 (9.81) | |
| Age at baseline, y (n = 104 707) | |||
| 18-44 | 59 247 (56.58) | 19.42 (10.65) | <.001 |
| 45-59 | 28 930 (27.62) | 14.77 (8.06) | |
| ≥60 | 16 530 (15.79) | 14.04 (7.20) | |
| Sex (n = 104 707) | |||
| Men | 21 800 (20.82) | 17.58 (9.99) | <.001 |
| Women | 82 907 (79.18) | 17.21 (9.77) | |
| Educational level (n = 98 024) | |||
| <High school degree | 17 952 (17.14) | 17.25 (10.34) | <.001 |
| <2 y after high school | 17 882 (17.08) | 18.96 (10.94) | |
| ≥2 y after high school | 62 190 (59.39) | 16.97 (9.34) | |
| Smoking status (n = 104 633) | |||
| Current | 17 892 (17.09) | 18.95 (11.39) | <.001 |
| Former | 34 217 (32.68) | 16.00 (8.92) | |
| Never | 52 524 (50.16) | 17.56 (9.68) | |
| IPAQ physical activity level (n = 90 146) | |||
| High | 29 382 (28.06) | 16.09 (9.30) | <.001 |
| Moderate | 38 788 (37.04) | 17.06 (9.43) | |
| Low | 21 976 (20.99) | 18.69 (10.37) | |
| Body mass indexb (n = 101 823) | |||
| <25 | 72 357 (69.10) | 17.14 (9.68) | <.001 |
| 25-29.9 | 21 209 (20.25) | 17.02 (9.64) | |
| ≥30 | 8257 (7.88) | 18.86 (11.13) | |
Abbreviation: IPAQ, International Physical Activity Questionnaire.
P values were obtained with unpaired 2-tailed t tests (comparison between 2 categories) or analysis of variance (comparison between 3 categories).
Calculated as weight in kilograms divided by height in meters squared.
Table 2. Associations Between the Proportion (in Weight) of Ultraprocessed Food in the Diet and Nutritional Factors (Nutrients and Food Group Intakes) of 104 707 Participations in the NutriNet-Santé Cohort (2009-2019).
| Nutritional Factor | Mean (SD) | Proportion of Ultraprocessed Food in the Diet | |
|---|---|---|---|
| Change in Nutritional Factor, β (SE)a | P Valueb | ||
| FSAm-NPS dietary index | 6.59 (2.46) | 0.62 (0.01) | <.001 |
| Energy intake without alcohol, Kcal/d | 1847.14 (450.86) | 29.95 (1.36) | <.001 |
| Alcohol intake, g/1000 kcal/d | 3.91 (5.53) | −0.50 (0.02) | <.001 |
| Sodium intake, mg/1000 kcal/d | 1479.10 (369.21) | 6.97 (1.20) | <.001 |
| Saturated fatty acids, g/1000 kcal/d | 17.78 (4.02) | 0.31 (0.01) | <.001 |
| Fiber, g/1000 kcal/d | 10.72 (3.57) | –0.78 (0.01) | <.001 |
| Sugar, g/1000 kcal/d | 50.39 (13.45) | 1.32 (0.04) | <.001 |
| Whole grains, g/1000 kcal/d | 18.96 (24.36) | –3.14 (0.08) | <.001 |
| Yogurt, g/1000 kcal/d | 33.68 (42.75) | –2.58 (0.14) | <.001 |
| Sugary drinks, g/1000 kcal/d | 24.94 (53.64) | 25.22 (0.15) | <.001 |
| Red and processed meat, g/1000 kcal/d | 40.01 (27.34) | 1.68 (0.09) | <.001 |
| Nuts, g/1000 kcal/d | 2.52 (5.40) | –0.49 (0.02) | <.001 |
| Fruits and vegetables, g/1000 kcal/d | 228.93 (129.04) | –37.54 (0.37) | <.001 |
Abbreviation: FSAm-NPS DI, Food Standards Agency nutrient profiling system dietary index, described in eMethods in the Supplement.
Change for an absolute increase of 0.1 in the proportion (in weight) of ultraprocessed food in the diet.
P values were obtained from linear regression models adjusted for sex, age, and energy intake.
The contribution of main food groups to the ultraprocessed category, along with the proportions of the other NOVA categories are presented in the eResults in the Supplement.
During follow-up (582 252 person-years; median follow-up time, 6.0 years; interquartile range [IQR], 2.8-8.4 years), 821 incident T2D cases were detected. Absolute incidence rates for T2D in the whole population were 132 per 100 000 person-years; age and sex corrected absolute rates were 113, 125, 143 and 166 per 100 000 person-years in the first quarter (lowest consumers), second, third, and fourth quarter (highest consumers) of the proportion of UPF intake in the diet, respectively.
Ultraprocessed food intake was associated with an increased T2D risk (Model 1: HR for a 10-point increment in the percentage of UPF in the diet, 1.15; 95% CI, 1.06-1.25; P = .001). The associations remained significant after further adjustments for Model 2, 3, 4, and 5 covariates (Table 3). The findings also remained robust throughout all sensitivity models (eTable 1 in the Supplement). Although HRs were in the same direction, this association was significant in women only, but statistical power was reduced for men (eTable 1 in the Supplement). The absolute amount of UPF consumption in grams per day was consistently associated with T2D risk, even when adjusting for the absolute amount (in g/d) of unprocessed/minimally processed food intake (HR for a 100 g/d increase in UPF consumption, 1.05; 95% CI, 1.02-1.08; P = .003).
Table 3. Associations Between the Proportion (in Weight) of UPF in the Diet and Risk of Type 2 Diabetes From Cause-Specific Multiadjusted Cox Proportional Hazard Models in 104 707 Patients in the NutriNet-Santé Cohort (2009-2019)a.
| Variable | Absolute Increment of 10% of UPF in the Diet, HR (95% CI) | P Value |
|---|---|---|
| No. of cases/total | 821/104 707 | |
| Model 1 | 1.15 (1.06-1.25) | .001 |
| Model 2 | 1.19 (1.09-1.30) | <.001 |
| Model 3 | 1.14 (1.04-1.25) | .005 |
| Model 4 | 1.13 (1.03-1.23) | .006 |
| Model 5b | 1.13 (1.01-1.27) | .04 |
Abbreviations: UPF, ultraprocessed foods; FSAm-NPS DI, Food Standards Agency nutrient profiling system dietary index, described in eMethods in the Supplement.
Median follow-up times 6.0 years, 582 252 person-years. Model 1 was a cause-specific Cox proportional hazard model adjusted for age (timescale), sex, educational level (<high school degree/<2 years after high school/≥2 years after high school), baseline body mass index (BMI, continuous, calculated as weight in kilograms divided by height in meters squared), physical activity level (high/moderate/low), smoking status (never/former/current), alcohol intake (g/d, continuous), number of 24-hour dietary records (continuous), energy intake (kcal/d, continuous), FSAm-NPS DI score (continuous), and family history of T2D (yes/no). Model 2 = Model 1 unadjusted for FSAm-NPS DI but adjusted instead for saturated fatty acid intake, sodium intake, sugar intake, dietary fiber intake (continuous variables). Model 3 was Model 1 unadjusted for FSAm-NPS DI but adjusted instead for intakes of red and processed meat, sugary drinks, fruits and vegetables, whole grains, nuts, and yogurt (continuous variables). Model 4 was Model 1 plus baseline prevalent dyslipidemia and hypertension (yes/no), and treatments for these conditions (yes/no). Model 5 was Model 1 plus percentage of weight change (weight in the last anthropometric questionnaire minus weight in the baseline questionnaire divided by weight in the baseline questionnaire multiplied by 100) among participants having available repeated anthropometric data. Overall, there were 340 competing cases of deaths detected during follow-up. Cause-specific hazard ratios for death in the 5 models were respectively: HR, 1.13; 95% CI, 1.00-1.28; P = .049; HR, 1.09; 95% CI, 0.97-1.24; P = .15; HR, 1.08; 95% CI, 0.94 to 1.23), P = .26, 1.13 (1.00 to 1.27), P = .056 and 1.03 (0.93 to 1.15), P = .50. Multiple imputation for missing data was performed using the MICE method53 by fully conditional specification (20 imputed data sets) (62 to 97 additional T2D cases by imputed data set) for the following covariates: BMI, smoking status, educational level (≤5% of missing data), and physical activity (15% of missing data). Results were combined across imputation based on Rubin’s combination rules58,59 using the SAS PROC MIANALYZE procedure.60
Number of cases, 461 of 79 752.
The proportions of UPF in beverages, sugary products, fats/sauces, and dairy products were more specifically associated with increased T2D risk (eTable 2 in the Supplement).
The proportion of unprocessed/minimally processed foods in the diet was inversely associated with T2D risk (HR for a 10-point increment, 0.91; 95% CI, 0.84-0.98; P = .01; [Model 1 covariates]).
Discussion
In this large cohort, UPF consumption was associated with increased T2D risk. To our knowledge, although UPF consumption was previously found to be associated with increased risks of cancer,31 cardiovascular diseases,35 mortality,32,36 depressive symptoms,33,37 and metabolic disorders (obesity,40 hypertension,41 and dyslipidemia39), no prior prospective epidemiological study had evaluated their association with T2D risk.
Several mechanistic hypotheses can be postulated to explain these findings. Overall, UPF usually have a lower nutritional quality35 because they are on average richer in sodium, energy, fat, sugar, and poorer in fiber12,13,14,15,16,17,18,19,20,25 and often exhibit a higher glycemic index.61 Several of these factors are associated with T2D with different levels of consensus.3 Many food groups, mostly ultraprocessed (eg, processed meat, and sugary sweetened beverages) are recognized T2D risk factors.3 Sugary sweetened beverages may impact metabolic health by several mechanistic pathways that are still currently debated.62 Consistently, in a 1-month randomized clinical trial,63 an ultraprocessed diet vs an unprocessed one led to an increased energy intake, which was highly correlated with weight gain. Of note, energy balance and overweight are both associated with T2D risk.3 However, this could not have entirely explained the associations observed because our models were adjusted for BMI and weight change. Moreover, high consumers of UPF in our population had lower consumptions of whole grains, fruits, and vegetables, which are recommended in the prevention of T2D, consistent with our finding of lower T2D risk in higher consumers of minimally/unprocessed foods. However, our analyses showed that the UPF-T2D risk association was not entirely explained by the simultaneous lower consumption of unprocessed/minimally processed foods. Moreover, the UPF-T2D risk association was adjusted for overall diet quality and energy intake, and remained significant in our models after further adjustment for a wide range of dietary factors. Thus, these factors did not fully explain the observed associations.
Caution is needed in interpreting biological mechanisms underlying these associations because, so far, potentially involved compounds and modes of action are diverse and evidence is still limited. Beyond nutritional values, UPF are often characterized by the presence of food additives. Most of them are likely to be neutral for long-term health and some may even be beneficial (eg, antioxidants), but recent concerns emerged mainly from in vitro/in vivo models for several compounds commonly used in thousands of foods. For instance, carrageenan, a thickening and stabilizing agent, used in more than 5500 products in France and pertaining to the top-20 used additives in France, might contribute to the development of diabetes by impairing glucose tolerance, increasing insulin resistance, and inhibiting insulin signaling in human HepG2 cells.64 However, as for most additives, human data on long-term health impacts are still lacking and potential cocktail effects remain largely unknown. These aspects will soon be investigated through a large-scale multidisciplinary research program, notably based on the NutriNet-Santé cohort,65 combining epidemiological and experimental data. Moreover, 2 meta-analyses observed associations between T2D risk and the intakes of nonnutritive sweeteners and artificially sweetened beverages.28,66
In addition, UPF may be contaminated by the migration of contact materials, especially because they often stay in their packaging for long periods owing to extended expiration dates. In particular, the exposure to bisphenol-A (BPA), a hydrophobic substance of very high concern,66 having endocrine disrupting properties, as well as high BPA serum concentrations, have been associated with increased T2D risk in recent meta-analyses.29,67 Of note, BPA was forbidden for use in food packaging in 2015 in France, after the dietary data collection period in this study. However, BPA is being replaced by other components such as bisphenol-S, also presenting endocrine disruption properties, and suspected to be about 250 times more absorbed orally than BPA.68 A recent study conducted in the United States showed that UPF consumption was associated with increased exposure to phthalates,69 endocrine-disrupting chemicals70 used in industrial plastic packaging.
Furthermore, UPF that went through processes such as high-temperature heating and extruding may contain neoformed contaminants. For instance, acrylamide71 and acrolein72 metabolites were associated with insulin resistance, and urinary biomarkers of polycyclic aromatic hydrocarbons were positively associated with diabetes.73 Acrylamide is found mainly in fried potatoes, biscuits, cakes, bread, or coffee, but is not specific to industrial processes. On the other hand, high levels of furans were observed in industrial breakfast cereals, canned food, and coffee. Even though furans might be detected in cooked/baked home-made food (toasted bread for instance) especially in foods rich in carbohydrates, it is likely that industrial processes lead to substantially higher levels of furans.74 Hepatotoxic and genotoxic properties for this substance were suspected by the European Food Safety Authority.75 Finally, industrial partial oil hydrogenation may lead to the creation of transunsaturated fatty acids in products containing hydrogenated oils. Although still debated, trans fats were linked to increased risks of heart disease76 and T2D.77 Additional research is needed to understand the biological mechanisms underlying the present observations.
Limitations
This study has some limitations. Even though we used a multisource case ascertainment approach, exhaustiveness could not be guaranteed. About 20% of T2D cases are estimated to be underdiagnosed in France.78 This probably resulted in a loss of statistical power. Only 1304 participants had a null follow-up and were excluded. Some of them may have had T2D that was not detected thereafter. However, this should represent a small number of missed cases (<20 cases considering T2D incidence in France78) and, owing to the prospective design, the resulting potential misclassification bias was most likely nondifferential and rather resulted in an underestimation of the associations. Second, causation could not be established from this single observational study and residual confounding cannot be entirely ruled out. Nevertheless, several mechanistic hypotheses support the biological plausibility of these findings, and the results remained unchanged after a series of sensitivity analyses adjusting for many lifestyle and dietary confounders. These findings are in line with previous observational studies showing associations between UPF and cardiometabolic outcomes.32,35,36,39,40,41 To our knowledge, only 1 short-term randomized clinical trial published so far showed a strong effect of an ultraprocessed diet on weight gain and energy intake.63 This kind of trial would not be ethically or logistically feasible to investigate longer-term associations with hard health end points (eg, cancer, cardiovascular diseases, T2D), but provides useful insights into potential mechanisms underlying associations observed in long-term epidemiological cohorts. Third, misclassification bias in the NOVA cannot be ruled out; however, this would have led to a nondifferential measurement error (in cases and noncases), and potentially biasing toward the null hypothesis. Fourth, the ultraprocessed category covers diverse products; this exploratory approach was not designed to focus on a specific food category or to isolate a particular process/additive. However, it allowed us to explore overall exposure to UPF and to observe associations with T2D resulting from cumulative intakes and potential cocktail effects of their ingredients. It would be interesting to explore the associations with chronic diseases of substituting UPF by less processed foods. Classic substitution models, initially designed for macronutrients, are less straightforward for complex food groups,79 but adapted statistical methods should be developed to tackle these challenges. Finally, compared with the general French population, participants to this study were younger, more often women, with higher educational levels80 and healthier dietary habits.81 This might have underestimated the associations owing to a narrower range of UPF intake. Furthermore, T2D incidence was lower (186 cases per 100 000 person-years in our sample after standardization vs 289 per 100 000 in the French population78), thereby limiting statistical power, especially for some stratified analyses (eg, in men).
Conclusions
These results suggest an association between UPF consumption and T2D risk. They need to be confirmed in large prospective cohorts in other settings, and underlying mechanisms need to be explored in ad hoc epidemiological and experimental studies. Beyond nutritional factors, nonnutritional dimensions of the diet may play a role in these associations, such as some additives, neoformed contaminants, and contact materials. Even if a causal link between UPF and chronic diseases cannot be established so far, the accumulation of consistent data leads public health authorities in several countries such as France82 or Brazil83 to recommend privileging the consumption of unprocessed/minimally processed foods, and limiting the consumption of UPF in the name of the precautionary principle.
eMethods.
eFigure 1. Distribution of the main exposure (proportion of ultra-processed food in the diet) in the study sample (N=104,707), NutriNet-Santé, France
eFigure 2. Cox model proportional risk assumption testing (Schoenfeld residuals)
eFigure 3. Spline plot for the linearity assumption of the association between the proportion of ultra-processed food in the diet and the risk of Type-2 Diabetes using Restricted cubic spline (RCS) SAS Macro® developed by Desquilbet and Mariotti
eResults.
eReferences.
References
- 1.International Diabetes Federation International Diabetes Federation - Facts & figures - Atlas 8th Edition 2017. https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html. Accessed May 15, 2019.
- 2.Dicker D, Nguyen G, Abate D, et al. ; GBD 2017 Mortality Collaborators . Global, regional, and national age-sex-specific mortality and life expectancy, 1950-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1684-1735. doi: 10.1016/S0140-6736(18)31891-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forouhi NG, Misra A, Mohan V, Taylor R, Yancy W. Dietary and nutritional approaches for prevention and management of type 2 diabetes. BMJ. 2018;361:k2234. doi: 10.1136/bmj.k2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwingshackl L, Hoffmann G, Lampousi A-M, et al. . Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. 2017;32(5):363-375. doi: 10.1007/s10654-017-0246-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monteiro CA, Cannon G, Levy RB, et al. . Ultra-processed foods: what they are and how to identify them. Public Health Nutr. 2019;22(5):936-941. doi: 10.1017/S1368980018003762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monteiro CA, Moubarac JC, Cannon G, Ng SW, Popkin B. Ultra-processed products are becoming dominant in the global food system. Obes Rev. 2013;14(suppl 2):21-28. doi: 10.1111/obr.12107 [DOI] [PubMed] [Google Scholar]
- 7.Moodie R, Stuckler D, Monteiro C, et al. ; Lancet NCD Action Group . Profits and pandemics: prevention of harmful effects of tobacco, alcohol, and ultra-processed food and drink industries. Lancet. 2013;381(9867):670-679. doi: 10.1016/S0140-6736(12)62089-3 [DOI] [PubMed] [Google Scholar]
- 8.Moubarac JC, Batal M, Martins AP, et al. . Processed and ultra-processed food products: consumption trends in Canada from 1938 to 2011. Can J Diet Pract Res. 2014;75(1):15-21. doi: 10.3148/75.1.2014.15 [DOI] [PubMed] [Google Scholar]
- 9.Martins AP, Levy RB, Claro RM, Moubarac JC, Monteiro CA. Increased contribution of ultra-processed food products in the Brazilian diet (1987-2009). RevSaude Publica. 2013;47:656-665. doi: 10.1590/S0034-8910.2013047004968 [DOI] [PubMed] [Google Scholar]
- 10.Juul F, Hemmingsson E. Trends in consumption of ultra-processed foods and obesity in Sweden between 1960 and 2010. Public Health Nutr. 2015;18(17):3096-3107. doi: 10.1017/S1368980015000506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.PAHO Ultra-Processed Food and Drink Products in Latin America: Trends, Impact on Obesity, Policy Implications.; 2015. http://iris.paho.org/xmlui/bitstream/handle/123456789/7699/9789275118641_eng.pdf?sequence=5&isAllowed=y&ua=1. Accessed May 15, 2019.
- 12.Luiten CM, Steenhuis IH, Eyles H, Ni Mhurchu C, Waterlander WE. Ultra-processed foods have the worst nutrient profile, yet they are the most available packaged products in a sample of New Zealand supermarkets—CORRIGENDUM. Public Health Nutr. 2016;19(3):539. doi: 10.1017/S1368980015002840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams J, White M. Characterisation of UK diets according to degree of food processing and associations with socio-demographics and obesity: cross-sectional analysis of UK National Diet and Nutrition Survey (2008-12). Int J Behav Nutr Phys Act. 2015;12:160. doi: 10.1186/s12966-015-0317-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cediel G, Reyes M, da Costa Louzada ML, et al. . Ultra-processed foods and added sugars in the Chilean diet (2010). Public Health Nutr. 2017;21(1):1-9. doi: 10.1017/S1368980017001161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa Louzada ML, Martins AP, Canella DS, et al. . Ultra-processed foods and the nutritional dietary profile in Brazil. Rev Saude Publica. 2015;49:38. doi: 10.1590/S0034-8910.2015049006132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martínez Steele E, Baraldi LG, Louzada ML, Moubarac JC, Mozaffarian D, Monteiro CA. Ultra-processed foods and added sugars in the US diet: evidence from a nationally representative cross-sectional study. BMJ Open. 2016;6(3):e009892. doi: 10.1136/bmjopen-2015-009892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moubarac JC, Batal M, Louzada ML, Martinez Steele E, Monteiro CA. Consumption of ultra-processed foods predicts diet quality in Canada. Appetite. 2017;108:512-520. doi: 10.1016/j.appet.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 18.Moubarac JC, Martins AP, Claro RM, Levy RB, Cannon G, Monteiro CA; Evidence from Canada . Consumption of ultra-processed foods and likely impact on human health. Public Health Nutr. 2013;16(12):2240-2248. doi: 10.1017/S1368980012005009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poti JM, Mendez MA, Ng SW, Popkin BM. Is the degree of food processing and convenience linked with the nutritional quality of foods purchased by US households? Am J Clin Nutr. 2015;101(6):1251-1262. doi: 10.3945/ajcn.114.100925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slimani N, Deharveng G, Southgate DA, et al. . Contribution of highly industrially processed foods to the nutrient intakes and patterns of middle-aged populations in the European Prospective Investigation into Cancer and Nutrition study. Eur J Clin Nutr. 2009;63(suppl 4):S206-S225. doi: 10.1038/ejcn.2009.82 [DOI] [PubMed] [Google Scholar]
- 21.Marrón-Ponce JA, Sánchez-Pimienta TG, Louzada MLDC, Batis C. Energy contribution of NOVA food groups and sociodemographic determinants of ultra-processed food consumption in the Mexican population. Public Health Nutr. 2018;21(1):87-93. doi: 10.1017/S1368980017002129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baraldi LG, Martinez Steele E, Canella DS, Monteiro CA. Consumption of ultra-processed foods and associated sociodemographic factors in the USA between 2007 and 2012: evidence from a nationally representative cross-sectional study. BMJ Open. 2018;8(3):e020574. doi: 10.1136/bmjopen-2017-020574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rauber F, da Costa Louzada ML, Steele EM, Millett C, Monteiro CA, Levy RB. Ultra-processed food consumption and chronic non-communicable diseases-related dietary nutrient profile in the UK (2008-2014). Nutrients. 2018;10(5):587. doi: 10.3390/nu10050587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vandevijvere S, De Ridder K, Fiolet T, Bel S, Tafforeau J. Consumption of ultra-processed food products and diet quality among children, adolescents and adults in Belgium. Eur J Nutr. 2018;(December). doi: 10.1007/s00394-018-1870-3 [DOI] [PubMed] [Google Scholar]
- 25.Louzada ML, Martins AP, Canella DS, et al. . Impact of ultra-processed foods on micronutrient content in the Brazilian diet. Rev Saude Publica. 2015;49:45. doi: 10.1590/S0034-8910.2015049006211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhattacharyya S, O-Sullivan I, Katyal S, Unterman T, Tobacman JK. Exposure to the common food additive carrageenan leads to glucose intolerance, insulin resistance and inhibition of insulin signalling in HepG2 cells and C57BL/6J mice. Diabetologia. 2012;55(1):194-203. doi: 10.1007/s00125-011-2333-z [DOI] [PubMed] [Google Scholar]
- 27.Roca-Saavedra P, Mendez-Vilabrille V, Miranda JM, et al. . Food additives, contaminants and other minor components: effects on human gut microbiota-a review. J Physiol Biochem. 2018;74(1):69-83. doi: 10.1007/s13105-017-0564-2 [DOI] [PubMed] [Google Scholar]
- 28.Azad MB, Abou-Setta AM, Chauhan BF, et al. . Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ. 2017;189(28):E929-E939. doi: 10.1503/cmaj.161390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang S, Lim JE, Choi Y, Jee SH. Bisphenol A exposure and type 2 diabetes mellitus risk: a meta-analysis. BMC Endocr Disord. 2018;18(1):81. doi: 10.1186/s12902-018-0310-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monteiro CA, Cannon G, Levy RB, et al. . NOVA: the star shines bright. World Nutr. 2016;7(1-3):28-38. [Google Scholar]
- 31.Fiolet T, Srour B, Sellem L, et al. . Consumption of ultra-processed foods and cancer risk: results from NutriNet-Santé prospective cohort. BMJ. 2018;360:k322. doi: 10.1136/bmj.k322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnabel L, Kesse-Guyot E, Allès B, et al. . Association between ultraprocessed food consumption and risk of mortality among middle-aged adults in France. JAMA Intern Med. 2019;179(4):490-498. doi: 10.1001/jamainternmed.2018.7289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adjibade M, Julia C, Allès B, et al. . Prospective association between ultra-processed food consumption and incident depressive symptoms in the French NutriNet-Santé cohort. BMC Med. 2019;17(1):78. doi: 10.1186/s12916-019-1312-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schnabel L, Buscail C, Sabate J-M, et al. . Association between ultra-processed food consumption and functional gastrointestinal disorders: results from the French NutriNet-Santé cohort. Am J Gastroenterol. 2018;113(8):1217-1228. doi: 10.1038/s41395-018-0137-1 [DOI] [PubMed] [Google Scholar]
- 35.Srour B, Fezeu LK, Kesse-Guyot E, et al. . Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Santé). BMJ. 2019;365:l1451. doi: 10.1136/bmj.l1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim H, Hu EA, Rebholz CM. Ultra-processed food intake and mortality in the USA: results from the Third National Health and Nutrition Examination Survey (NHANES III, 1988-1994). Public Health Nutr. 2019;22(10):1777-1785. doi: 10.1017/S1368980018003890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gómez-Donoso C, Sánchez-Villegas A, Martínez-González MA, et al. . Ultra-processed food consumption and the incidence of depression in a Mediterranean cohort: the SUN Project. Eur J Nutr. 2019;(May). doi: 10.1007/s00394-019-01970-1 [DOI] [PubMed] [Google Scholar]
- 38.Sandoval-Insausti H, Blanco-Rojo R, Graciani A, et al. . Ultra-processed food consumption and incident frailty: a prospective cohort study of older adults [published online May 27, 2019]. J Gerontol A Biol Sci Med Sci. 2019;glz140. doi: 10.1093/gerona/glz140 [DOI] [PubMed] [Google Scholar]
- 39.Rauber F, Campagnolo PD, Hoffman DJ, Vitolo MR. Consumption of ultra-processed food products and its effects on children’s lipid profiles: a longitudinal study. Nutr Metab Cardiovasc Dis. 2015;25(1):116-122. doi: 10.1016/j.numecd.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 40.Mendonça RD, Pimenta AM, Gea A, et al. . Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) cohort study. Am J Clin Nutr. 2016;104(5):1433-1440. doi: 10.3945/ajcn.116.135004 [DOI] [PubMed] [Google Scholar]
- 41.Mendonça RD, Lopes AC, Pimenta AM, Gea A, Martinez-Gonzalez MA, Bes-Rastrollo M. Ultra-processed food consumption and the incidence of hypertension in a mediterranean cohort: the Seguimiento Universidad de Navarra Project. Am J Hypertens. 2017;30(4):358-366. doi: 10.1093/ajh/hpw137 [DOI] [PubMed] [Google Scholar]
- 42.Hercberg S, Castetbon K, Czernichow S, et al. . The Nutrinet-Santé Study: a web-based prospective study on the relationship between nutrition and health and determinants of dietary patterns and nutritional status. BMC Public Health. 2010;10:242. doi: 10.1186/1471-2458-10-242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vergnaud AC, Touvier M, Méjean C, et al. . Agreement between web-based and paper versions of a socio-demographic questionnaire in the NutriNet-Santé study. Int J Public Health. 2011;56(4):407-417. doi: 10.1007/s00038-011-0257-5 [DOI] [PubMed] [Google Scholar]
- 44.Lassale C, Péneau S, Touvier M, et al. . Validity of web-based self-reported weight and height: results of the Nutrinet-Santé study. J Med Internet Res. 2013;15(8):e152. doi: 10.2196/jmir.2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Touvier M, Méjean C, Kesse-Guyot E, et al. . Comparison between web-based and paper versions of a self-administered anthropometric questionnaire. Eur J Epidemiol. 2010;25(5):287-296. doi: 10.1007/s10654-010-9433-9 [DOI] [PubMed] [Google Scholar]
- 46.Craig CL, Marshall AL, Sjöström M, et al. . International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381-1395. doi: 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 47.Touvier M, Kesse-Guyot E, Méjean C, et al. . Comparison between an interactive web-based self-administered 24 h dietary record and an interview by a dietitian for large-scale epidemiological studies. Br J Nutr. 2011;105(7):1055-1064. doi: 10.1017/S0007114510004617 [DOI] [PubMed] [Google Scholar]
- 48.Lassale C, Castetbon K, Laporte F, et al. . Correlations between fruit, vegetables, fish, vitamins, and fatty acids estimated by web-based nonconsecutive dietary records and respective biomarkers of nutritional status. J Acad Nutr Diet. 2016;116(3):427-438.e5. doi: 10.1016/j.jand.2015.09.017 [DOI] [PubMed] [Google Scholar]
- 49.Lassale C, Castetbon K, Laporte F, et al. . Validation of a Web-based, self-administered, non-consecutive-day dietary record tool against urinary biomarkers. Br J Nutr. 2015;113(6):953-962. doi: 10.1017/S0007114515000057 [DOI] [PubMed] [Google Scholar]
- 50.Julia C, Martinez L, Allès B, et al. . Contribution of ultra-processed foods in the diet of adults from the French NutriNet-Santé study. Public Health Nutr. 2018;21(1):27-37. doi: 10.1017/S1368980017001367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monteiro CA, Cannon G, Moubarac JC, Levy RB, Louzada MLC, Jaime PC. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018;21(1):5-17. doi: 10.1017/S1368980017000234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moubarac JC, Parra DC, Cannon G, Monteiro CA. Food classification systems based on food processing: significance and implications for policies and actions: a systematic literature review and assessment. Curr Obes Rep. 2014;3(2):256-272. doi: 10.1007/s13679-014-0092-0 [DOI] [PubMed] [Google Scholar]
- 53.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219-242. doi: 10.1177/0962280206074463 [DOI] [PubMed] [Google Scholar]
- 54.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 55.Julia C, Méjean C, Touvier M, et al. . Validation of the FSA nutrient profiling system dietary index in French adults-findings from SUVIMAX study. Eur J Nutr. 2016;55(5):1901-1910. doi: 10.1007/s00394-015-1006-y [DOI] [PubMed] [Google Scholar]
- 56.Julia C, Etilé F, Hercberg S. Front-of-pack Nutri-Score labelling in France: an evidence-based policy. Lancet Public Health. 2018;3(4):e164. doi: 10.1016/S2468-2667(18)30009-4 [DOI] [PubMed] [Google Scholar]
- 57.Deschasaux M, Huybrechts I, Murphy N, et al. . Nutritional quality of food as represented by the FSAm-NPS nutrient profiling system underlying the Nutri-Score label and cancer risk in Europe: results from the EPIC prospective cohort study. PLoS Med. 2018;15(9):e1002651. doi: 10.1371/journal.pmed.1002651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons; New York, New York. 2004. [Google Scholar]
- 59.Rubin DB. Inference and missing data. Biometrika. 1976;63(3):581-592. doi: 10.1093/biomet/63.3.581 [DOI] [Google Scholar]
- 60.PROC MIANALYZE The MIANALYZE Procedure: SAS/STAT(R) 9.2 User’s Guide, Second Edition. https://support.sas.com/documentation/cdl/en/statug/63033/HTML/default/viewer.htm#mianalyze_toc.htm. Accessed December 19, 2018.
- 61.Fardet A. Minimally processed foods are more satiating and less hyperglycemic than ultra-processed foods: a preliminary study with 98 ready-to-eat foods. Food Funct. 2016;7(5):2338-2346. doi: 10.1039/C6FO00107F [DOI] [PubMed] [Google Scholar]
- 62.Allison DB. Liquid calories, energy compensation and weight: what we know and what we still need to learn. Br J Nutr. 2014;111(3):384-386. doi: 10.1017/S0007114513003309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hall KD, Ayuketah A, Brychta R, et al. . Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30(1):67-77.e3. doi: 10.1016/j.cmet.2019.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhattacharyya S, Feferman L, Tobacman JK. Carrageenan inhibits insulin signaling through GRB10-mediated decrease in Tyr(P)-IRS1 and through inflammation-inducediIncrease in Ser(P)307-IRS1. J Biol Chem. 2015;290(17):10764-10774. doi: 10.1074/jbc.M114.630053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gourd E. Ultra-processed foods might increase cancer risk. Lancet Oncol. 2018;19(4):e186. doi: 10.1016/S1470-2045(18)30184-0 [DOI] [PubMed] [Google Scholar]
- 66.European CHemical Agency (ECHA) Member State Committee support document for identification of 4,4’-isopropylidenediphenol (bisphenol a) as a substance of very high concern because of its toxic for reproduction (Article 57 c) properties. Adopted on 2 December 2016. https://echa.europa.eu/documents/10162/b10d6a00-8e47-9b14-4f61-c779a8dc8450. Accessed February 18, 2019.
- 67.Rancière F, Lyons JG, Loh VHY, et al. . Bisphenol A and the risk of cardiometabolic disorders: a systematic review with meta-analysis of the epidemiological evidence. Environ Health. 2015;14:46. doi: 10.1186/s12940-015-0036-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gayrard V, Lacroix MZ, Grandin FC, et al. . Oral systemic bioavailability of bisphenol A and bisphenol S in pigs. Environ Health Perspect. 2019;127(7):77005. doi: 10.1289/EHP4599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buckley JP, Kim H, Wong E, Rebholz CM. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013-2014. Environ Int. 2019;131:105057. doi: 10.1016/j.envint.2019.105057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S. Phthalates and diet: a review of the food monitoring and epidemiology data. Environ Health. 2014;13(1):43. doi: 10.1186/1476-069X-13-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin C-Y, Lin Y-C, Kuo H-K, et al. . Association among acrylamide, blood insulin, and insulin resistance in adults. Diabetes Care. 2009;32(12):2206-2211. doi: 10.2337/dc09-0309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feroe AG, Attanasio R, Scinicariello F. Acrolein metabolites, diabetes and insulin resistance. Environ Res. 2016;148:1-6. doi: 10.1016/j.envres.2016.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stallings-Smith S, Mease A, Johnson TM, Arikawa AY. Exploring the association between polycyclic aromatic hydrocarbons and diabetes among adults in the United States. Environ Res. 2018;166:588-594. doi: 10.1016/j.envres.2018.06.041 [DOI] [PubMed] [Google Scholar]
- 74.Fromberg A, Mariotti MS, Pedreschi F, Fagt S, Granby K. Furan and alkylated furans in heat processed food, including home cooked products. Czech J Food Sci. 2014;32(5):443-448. doi: 10.17221/341/2013-CJFS [DOI] [Google Scholar]
- 75.EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen HK, Alexander J, et al. . Risks for public health related to the presence of furan and methylfurans in food. EFSA J. 2017;15(10). doi: 10.2903/j.efsa.2017.5005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kiage JN, Merrill PD, Judd SE, et al. . Intake of trans fat and incidence of stroke in the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort. Am J Clin Nutr. 2014;99(5):1071-1076. doi: 10.3945/ajcn.113.075713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Willett WC, Mozaffarian D. Trans fats in cardiac and diabetes risk: an overview. Curr Cardiovasc Risk Rep. 2007;1(1):16-23. doi: 10.1007/s12170-007-0004-x [DOI] [Google Scholar]
- 78.Fagot-Campagna A, Fosse S, Roudier C, Romon I Prévalence et Incidence Du Diabète, et Mortalité Liée Au Diabète En France.; 2010. https://invs.santepubliquefrance.fr/Publications-et-outils/Rapports-et-syntheses/Maladies-chroniques-et-traumatismes/2010/Prevalence-et-incidence-du-diabete-et-mortalite-liee-au-diabete-en-France. Accessed May 23, 2019.
- 79.Song M, Giovannucci E. Substitution analysis in nutritional epidemiology: proceed with caution. Eur J Epidemiol. 2018;33(2):137-140. doi: 10.1007/s10654-018-0371-2 [DOI] [PubMed] [Google Scholar]
- 80.Andreeva VA, Salanave B, Castetbon K, et al. . Comparison of the sociodemographic characteristics of the large NutriNet-Santé e-cohort with French Census data: the issue of volunteer bias revisited. J Epidemiol Community Health. 2015;69(9):893-898. doi: 10.1136/jech-2014-205263 [DOI] [PubMed] [Google Scholar]
- 81.Andreeva VA, Deschamps V, Salanave B, et al. . Comparison of dietary intakes between a large online cohort study (Etude NutriNet-Santé) and a nationally representative cross-sectional study (Etude Nationale Nutrition Santé) in France: addressing the issue of generalizability in E-epidemiology. Am J Epidemiol. 2016;184(9):660-669. doi: 10.1093/aje/kww016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haut Conseil de La Santé Publique Avis Relatif à La Révision Des Repères Alimentaires Pour Les Adultes Du Futur Programme National Nutrition Santé 2017-2021.; 2017. https://www.hcsp.fr/Explore.cgi/Telecharger?NomFichier=hcspa20170216_reperesalimentairesactua2017.pdf. Accessed January 10, 2019.
- 83.Ministry of Health of Brazil Dietary Guidelines for the Brazilian Population.; 2014. http://189.28.128.100/dab/docs/portaldab/publicacoes/guia_alimentar_populacao_ingles.pdf. Accessed May 23, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eFigure 1. Distribution of the main exposure (proportion of ultra-processed food in the diet) in the study sample (N=104,707), NutriNet-Santé, France
eFigure 2. Cox model proportional risk assumption testing (Schoenfeld residuals)
eFigure 3. Spline plot for the linearity assumption of the association between the proportion of ultra-processed food in the diet and the risk of Type-2 Diabetes using Restricted cubic spline (RCS) SAS Macro® developed by Desquilbet and Mariotti
eResults.
eReferences.