Key Points
Question
Is age associated with survival in the US Centers for Medicare & Medicaid Services (CMS) beneficiary population with myelodysplastic syndrome (MDS) who undergo allogeneic hematopoietic stem cell transplantation (HCT)?
Findings
In this prospective observational study that included 1280 patients with MDS undergoing HCT, age alone was not associated with survival.
Meaning
Based on these findings, we would recommend coverage of HCT for MDS by the CMS.
This nonrandomized clinical trial examines the association of age with survival among Medicare beneficiaries with myelodysplastic syndrome who underwent allogeneic hematopoietic stem cell transplantation.
Abstract
Importance
In 2010, the US Centers for Medicare & Medicaid Services (CMS) indicated that data regarding efficacy of allogeneic hematopoietic stem cell transplantation (HCT) in the CMS beneficiary population with myelodysplastic syndrome (MDS) were currently insufficient, but that coverage would be provided for patients enrolled in a clinical study that met its criteria for Coverage with Evidence Development (CED).
Objective
The Center for International Bone Marrow Transplant Research (CIBMTR) submitted a study concept comparing the outcomes of patients aged 55 to 64 years vs aged 65 years or older who met those criteria, effectively providing coverage by CMS for HCT for MDS.
Design, Setting, and Participants
Data on patients aged 65 years or older were prospectively collected and their outcomes compared with patients aged 55 to 64 years. Patients were enrolled in the study from December 15, 2010, to May 14, 2014. The results reported herein were analyzed as of September 4, 2017, with a median follow-up of 47 months. The study was conducted by the CIBMTR. It comprises a voluntary working group of more than 420 centers worldwide that contribute detailed data on allogeneic and autologous HCT and cellular therapies.
Interventions
Patients with MDS received HCT according to institutional guidelines and preferences.
Main Outcomes and Measures
The primary outcome was overall survival (OS); secondary outcomes included nonrelapse mortality (NRM), relapse-free survival, and acute and chronic graft vs host disease.
Results
During the study period, 688 patients aged 65 years or older underwent HCT for MDS and were compared with 592 patients aged 55 to 64 years. Other than age, there were no differences in patient and disease characteristics between the groups. On univariate analysis, the 3-year NRM rate was 28% vs 25% for the 65 years or older group vs those aged 55 to 64 years, respectively. The 3-year OS was 37% vs 42% for the 65 years or older group vs the 55 to 64 years age group, respectively. On multivariable analysis after adjusting for excess risk of mortality in the older group, age group had no significant association with OS (HR, 1.09; 95% CI, 0.94-1.27; P = .23) or NRM (HR, 1.19; 95% CI, 0.93-1.52; P = .16).
Conclusions and Relevance
Older patients with MDS undergoing HCT have similar OS compared with younger patients. Based on current data, we would recommend coverage of HCT for MDS by the CMS.
Trial Registration
ClinicalTrials.gov identifier: NCT01166009
Introduction
Myelodysplastic syndromes (MDS) are a group of clonal hematological disorders characterized by progressive cytopenias and leukemic transformation. More than 15 000 patients are diagnosed with MDS annually in the United States, and 80% of those patients are older than 65 years. The median age at diagnosis is 70 years in western countries and incidence increases with age. The incidence rate is 0.22/100 000 in those younger than 49 years, 4.8/100 000 between the ages of 50 and 70 years, and 22.8/100 000 in those older than 70 years.1
The only available therapy with the potential to cure MDS is allogeneic hematopoietic stem cell transplantation (HCT). It is the treatment of choice for younger patients with high-risk MDS. Prior to the introduction of reduced intensity conditioning (RIC) regimens, regimen-related morbidity and mortality limited the utility of HCT in older patients. Although RIC regimens allow HCT to be offered more safely to older patients, HCT is underused in the older population. This was evident in a study2 by the Center for International Bone Marrow Transplant Research (CIBMTR), where only 10% of patients with acute myelogenous leukemia (AML) or MDS who underwent HCT were older than 65 years. In that study, age had no significant impact on outcome in multivariable analysis in a cohort of patients between 40 and 70 years. The 100-day mortality rate was about 20% and the 2-year probability of survival was approximately 40%.
There are multiple reasons that older patients do not undergo transplantation.3,4 They may have comorbidities that compromise their ability to tolerate HCT. Some oncologists are reluctant to refer older patients for HCT, even if there are no clinical contraindications, because of perceived worse outcomes. Some transplant centers have arbitrary upper age limits for HCT candidates. Third-party payers do not cover HCT for MDS in older patients until there is transformation to acute leukemia. Finally, given the known morbidity and mortality associated with MDS, many older patients are apprehensive to undergo HCT. Previously, coverage of HCT for MDS by the US Centers for Medicare & Medicaid Services (CMS) depended on local coverage determinations.5 The CMS is the primary health insurer for most US adults aged 65 years or older. In 2010, CMS made a National Coverage Determination (NCD) about MDS, indicating that efficacy data in a CMS beneficiary population were incomplete but providing coverage for patients enrolled in a clinical study appropriately designed to generate data necessary to make a future determination about efficacy and effectiveness. This decision acknowledged that “the available evidence suggests that allogeneic HCT for MDS is reasonable and necessary under §1862(a) (1) (E) of the Social Security Act through Coverage with Evidence Development (CED).”6
The CIBMTR submitted an observational study concept comparing the outcomes of patients aged 55 to 64 years vs 65 years and older, leveraging its network and the requirements of the CW Bill Young Cell Transplantation Program to collect all allogeneic HCT performed in the United States. The study was approved providing coverage for HCT through CMS’s CED mechanism. Herein we report the outcomes of 1280 patients with MDS treated on this protocol.
Methods
Data Source
The CIBMTR is a research collaboration between the National Marrow Donor Program/Be The Match and the Medical College of Wisconsin. It comprises a voluntary working group of more than 420 centers worldwide that contribute detailed data on allogeneic and autologous HCT and cellular therapies. Participating centers are required to report all transplants consecutively; compliance is monitored by research staff, and patients are followed longitudinally. Computerized checks for discrepancies, physicians’ review of submitted data, and on-site audits of participating centers ensure data quality. Studies conducted by the CIBMTR are performed in compliance with all applicable US federal regulations pertaining to the protection of human research participants. The research protocol applicable to patients described in this study has had continuous institutional review board oversight from the Center for International Blood and Marrow Transplant Research, and is listed on ClinicalTrials.gov. The trial protocol is available in Supplement 1. All participants signed informed consent for research. Protected Health Information used in the performance of such research is collected and maintained in CIBMTR’s capacity as a Public Health Authority under the HIPAA Privacy Rule. This study followed the Transparent Reporting of Evaluations With Nonrandomized Designs (TREND) reporting guidelines.
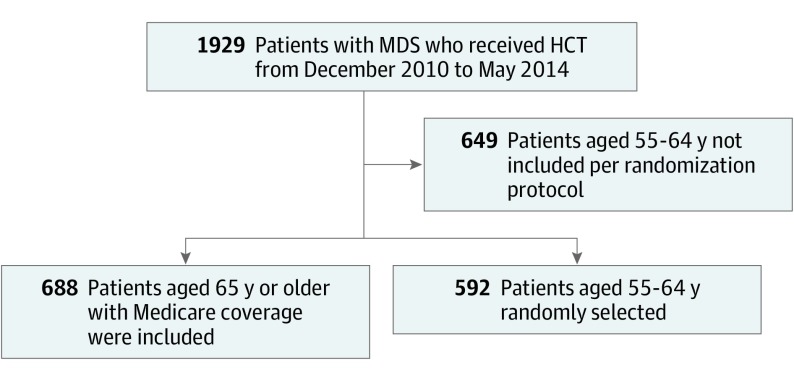
Written informed consent was obtained by the centers to collect and report data to the CIBMTR and for patients to participate in the CMS CED observational study. After signing informed consent, data for patients with Medicare coverage were prospectively collected using the standard CIBMTR forms. Race was reported as designated by the transplant center. Race was defined by either the investigator or participant depending on each institution’s standard operating procedures. Patient characteristics and outcomes were compared to a randomly selected group of patients aged 55 to 64 years with MDS undergoing allogeneic HCT and reported to CIBMTR. All patients aged 65 years or older or younger than 65 years with Medicare who gave consent for research were included in the study. Patients aged 55 to 64 years were selected based on a randomization algorithm where 50% of all patients with MDS undergoing allogeneic HCT in the United States and reported to CIBMTR were selected (Figure 1).
Figure 1. Study Flowchart.
HCT Indicates hematopoietic stem cell transplantation; MDS, myelodysplastic syndrome. From December 2010 to May 2014, 1929 patients with MDS aged 55 years or older received allogeneic HCT. Of those, 688 patients with Medicare were included. Patients aged 65 years or older with Medicare coverage could only get allogeneic HCT under this protocol, so all patients with Medicare were included in this analysis and no patients were excluded. The characteristics and outcomes of patients aged 65 years or older were compared with a randomly selected group of patients aged 55 to 64 years with MDS undergoing allogeneic HCT and reported to the Center for International Bone Marrow Transplant Research (CIBMTR). Patients aged 55 to 64 years were selected based on a randomization algorithm where 50% of all patients with MDS undergoing allogeneic HCT in the United States and reported to CIBMTR were included.
Study End Points and Definitions
The primary outcome of the study was overall survival (OS). Survival was measured from time of transplant to death from any cause. Survivors were censored at last contact. Nonrelapse mortality (NRM) was defined as death from any cause before 28 days or death thereafter from any cause without evidence of disease progression or relapse. Relapse was defined as relapse from pre- or post-HCT complete remission (CR), progression to AML, second transplant for relapsed disease, death from relapse, and stable/persistent disease longer than 6 months from HCT. Relapse-free survival (RFS) was defined as time from allogeneic HCT (allo-HCT) to relapse.
Acute and chronic graft vs host disease (GVHD) were defined based on standardized criteria.7,8 Regimen intensity was based on previously published consensus criteria.9
Statistical Design
This study had 2 phases. The first phase was a safety phase with a 100-day mortality end point. The first phase had a target accrual of 240 patients aged 65 years or older for the 100-day mortality primary end point. Sample sizes were based on an inferiority test of the hypothesis that the 100-day mortality rate in the aged 65 years or older cohort was higher than 20%, which is the approximate 100-day mortality rate in a 55- to 64-year-old cohort. The study was designed to have approximately 80% power to detect a 6.5% or greater increase in 100-day mortality rates in the aged 65 years or older cohort. There was no difference in the 100-day mortality for patients aged 65 years or older compared with those aged 55 to 64 years when assessed in the first cohort of 240 patients.10
After demonstrating that the 100-day mortality for the aged 65 years or older group was similar to the aged 55 to 64 years group, accrual to the second phase of the study continued. The aim of the second phase was to determine the prognostic value of additional patient and disease factors on outcomes of HCT in patients aged 65 years and older. Based on the distribution of age (65-69 years vs 70 years or older), performance score, HCT Comorbidity Index (HCT-CI),11 revised international prognostic scoring system (R-IPSS),12 and disease status in the first 180 patients, approximately 700 patients aged 65 years and older were required to complete an analysis of prognostic factors.
Probabilities of acute and chronic GVHD, NRM, and relapse were calculated by using the cumulative incidence estimator.13 Relapse or progression of the primary disease was treated as a competing risk for NRM and vice versa, and death was a competing event for GVHD. Overall survival and RFS were estimated using the Kaplan-Meier method. Log transformation was used to generate 95% CIs. Proportional hazards models14 were used to estimate the hazard ratio (HR) for each outcome associated with the aged 65 years or older cohort compared with the aged 55 to 64 years group. The proportional hazards assumption was assessed for all variables using graphical methods or time-dependent covariates. The age group variable was forced into all models, and stepwise model building was used to identify additional covariates for inclusion in the regression model. Aside from age group, the following factors were considered for adjustment: sex, Karnofsky performance status at HCT, race/ethnicity, HCT-CI score at HCT, IPSS-R at Dx, disease status prior to preparative regimen, secondary MDS, time from Dx to Tx, prior transplant, blasts in BM prior to preparative regimen, therapy given between diagnosis and preparative regimen, graft type, donor type/HLA matching, unrelated donor age, donor-recipient sex match, donor-recipient CMV match, conditioning regimen intensity, GVHD prophylaxis, and use of ATG/Campath. Interactions between the main effect and other variables were assessed, but none were found significant. Subsets of the aged 65 years or older population (70 years or older vs aged 65-69 years) were also compared in the multivariable models but were not significantly different for any outcomes. Adjusted OS and DFS were estimated using a stratified Cox model, adjusting for the significant variables in the final multivariable models.15
We also assessed the contribution of age older than 65 years to mortality after transplant while accounting for the increased mortality risk in the general population for older individuals. To do this, we fit a Cox proportional hazards model for the excess hazard for death, defined as the difference between the observed hazard of the cohort and the hazard of the general population accounting for age and sex. Population mortality was obtained from the US life tables. We adjusted for the same risk factors as in the overall mortality and nonrelapse model, and focused on the effect of age older than 65 years in this model, summarized as an HR for excess mortality risk. The model was estimated using the Esteve method, implemented in the function rsadd in the package relsurv in R statistical software (R Foundation).16 All P values are 2-sided. Analyses were conducted using SAS statistical software (version 9.4; SAS Institute, Inc).
Results
Patients
From December 2010 to May 2014, 688 patients aged 65 years or older were enrolled in the study and their outcomes were compared with 592 randomly selected patients aged 55 to 64 years treated during the same time period (eFigure 1 in Supplement 2). There was no difference in the outcome of the randomly selected sample of patients included in this study compared with the rest of the patients aged 55 to 64 years treated during the same time period. With a data cutoff date of September 4, 2017, the median follow-up was 47 months (range, 13-73 months) for the 1280 patients. In the aged 65 years or older group, 76%, 22%, and 2% were aged 65 to 69 years, 70 to 74 years, and older than 74 years old, respectively. Otherwise, patient and disease characteristics were similar in both groups. Patient characteristics in this cohort were similar to prior studies reported by the CIBMTR.17,18 Patient, disease, and treatment characteristics are detailed in Table 1 (eTable 1 in Supplement 2).
Table 1. Patient-, Disease-, and Transplant-Related Characteristics.
| Variable | No. (%) | |
|---|---|---|
| Age ≥65, y | Age 55-64, y | |
| Patients, No. | 688 | 592 |
| Age, median (range), y | 68 (65-79) | 61 (55-64) |
| Female | 212 (31) | 221 (37) |
| Karnofsky score ≥80 prior to preparative regimen | 618 (90) | 526 (89) |
| Recipient race: white, non-Hispanic | 624 (91) | 536 (91) |
| HCT-CI | ||
| 0 | 156 (23) | 119 (20) |
| 1-2 | 187 (27) | 159 (27) |
| 3 | 127 (18) | 129 (22) |
| ≥4 | 218 (32) | 185 (31) |
| Disease status prior to preparative regimen | ||
| Never treated | 45 (7) | 55 (9) |
| Complete remission | 68 (10) | 73 (12) |
| Hematologic improvement | 120 (17) | 90 (15) |
| No response/stable disease | 372 (54) | 308 (52) |
| Progression to HI/relapse from CR | 51 (7) | 37 (6) |
| Not assessed/missing | 32 (5) | 29 (5) |
| Therapy related MDS | 160 (23) | 157 (27) |
| R-IPSS at diagnosis | ||
| Very low | 48 (7) | 30 (5) |
| Low | 115 (17) | 89 (15) |
| Intermediate | 161 (23) | 144 (24) |
| High | 101 (15) | 101 (17) |
| Very high | 75 (11) | 84 (14) |
| Missing | 188 (27) | 144 (24) |
| Blasts in BM prior to preparative regimen, % | ||
| <5 | 443 (64) | 406 (69) |
| 5-10 | 134 (19) | 93 (16) |
| 11-20 | 75 (11) | 45 (8) |
| Missing | 36 (5) | 48 (8) |
| Preparative regimen intensity | ||
| Myeloablative | 197 (29) | 288 (49) |
| Reduced intensity conditioning | 491 (71) | 304 (51) |
Abbreviations: BM, bone marrow; CR, complete remission; HCT-CI, Hematopoietic Cell Transplantation-Comorbidity Index; HI, hematologic improvement; MDS, myelodysplastic syndrome; R-IPSS, Revised International Prognostic Scoring System.
Overall Survival
On univariate analysis, there was no statistically significant difference in the probability of OS at 100 days, 1 day, and 3 years. There was a trend for better 3-year adjusted probability of OS for patients aged 55 to 64 years (42%) vs those aged 65 years or older (37%); however, it did not reach statistical significance (P = .06) (eTable 2 in Supplement 2) (Figure 2).
Figure 2. Overall Survival by Age.
Multivariable analysis for OS identified high/very high R-IPSS, blasts in bone marrow (bBM) greater than 11% prior to HCT, non–age-adjusted HCT-CI of 4 or greater, and GVHD prophylaxis with calcineurin inhibitor plus methotrexate were independently associated with inferior outcome (Table 2). Age group 65 years or older vs those aged 55 to 64 years had no statistically significant association with (hazard ratio [HR], 1.09; 95% CI, 0.94-1.27; P = .23) or without (HR, 1.13; 95% CI, 0.98-1.3; P = .08) adjustment for excess population-based risk of mortality in the older group (Table 2).
Table 2. Overall Survival Multivariable Analysis.
| Variable | Patients, No. | HR (95% CI) | P Value |
|---|---|---|---|
| Age, y | |||
| Overall | .09 | ||
| 55-64 | 592 | 1 [Reference] | |
| ≥65 | 688 | 1.13 (0.98-1.3) | .09 |
| R-IPSS | |||
| Overall | .001 | ||
| Very low | 78 | 1 [Reference] | |
| Low | 204 | 1.08 (0.77-1.53) | .65 |
| Intermediate | 305 | 1.07 (0.77-1.48) | .70 |
| High | 202 | 1.39 (0.99-1.95) | .06 |
| Very high | 159 | 1.67 (1.18-2.37) | .004 |
| Missing | 332 | 1.30 (0.94-1.79) | .12 |
| Blasts in BM prior to start of preparative regimen | |||
| Overall | .001 | ||
| <5% | 849 | 1 [Reference] | |
| 5%-10% | 227 | 1.00 (0.83-1.2) | .99 |
| ≥11% | 120 | 1.49 (1.18-1.87) | .001 |
| Missing | 84 | 1.41 (1.08-1.85) | .01 |
| GVHD prophylaxis | |||
| Overall | <.001 | ||
| Calcineurin inhibitor + MMF +/− others | 547 | 1 [Reference] | |
| Calcineurin inhibitor + methotrexate +/− others | 430 | 1.19 (1.02-1.39) | .03 |
| Calcineurin inhibitor +/− others | 179 | 0.73 (0.58-0.91) | .006 |
| Cyclophosphamide | 52 | 1.26 (0.89-1.79) | .19 |
| T-depletion | 42 | 0.90 (0.59-1.36) | .61 |
| Other | 30 | 1.72 (1.14-2.6) | .009 |
| HCT-CI | |||
| Overall | <.001 | ||
| 0 | 275 | 1 [Reference] | |
| 1-2 | 346 | 1.15 (0.94-1.42) | .18 |
| 3 | 256 | 1.23 (0.98-1.53) | .07 |
| ≥4 | 403 | 1.50 (1.23-1.83) | <.001 |
BM, bone marrow; GVHD, graft vs host disease; HCT-CI, Hematopoietic Stem Cell Transplantation Comorbidity Index; HR, hazard ratio; MMF, mycophenolate mofetil; R-IPSS, Revised International Prognostic Scoring System.
We further analyzed OS for patients aged 70 years or older vs 55 to 64 years vs 65 to 69 years. On univariate analysis, the 3-year OS was 30%, 39%, and 42% (P = .02), respectively. On multivariable analysis, there was a trend for better OS for the aged 55 to 64 years group vs 70 years or older group (HR, 1.29; 95% CI, 1.05-1.60; P = .02); however, this was not statistically significant after adjusting for multiple comparisons. This demonstrates that allo-HCT can be reasonably considered in patients aged 70 years or older; however, only 14 (2%) patients in this study were older than 75.
Relapse-Free Survival
The adjusted RFS at 3 years was 27% vs 35% for the group aged 65 years or older vs those aged 55 to 64 years (P = .005) (eTable 2 and eFigure 2 in Supplement 2). On multivariable analysis, age group 65 years or older vs those 55 to 64 years had no significant association with RFS (HR, 1.14; CI, 0.99-1.31; P = .07) (Table 3). Overall, R-IPSS high/very high, in vivo T-cell depletion, bBM greater than 11% prior to transplant, conditioning regimen (non–fludarabine/busulfan myeloablative [MA], RIC fludarabine/cyclophosphamide/total body irradiation [TBI]), not in CR before transplant, and HCT-CI of 4 or greater were associated with worse RFS on MVA (Table 3).
Table 3. Relapse-Free Survival Multivariable Analysis.
| Variable | Patients, No. | HR (95% CI) | P Value |
|---|---|---|---|
| Age, y | |||
| 55-64 | 586 | 1 [Reference] | |
| ≥65 | 682 | 1.14 (0.99-1.31) | .07 |
| R-IPSS | |||
| Very low | 78 | 1 [Reference] | |
| Low | 204 | 1.24 (0.90-1.71) | .20 |
| Intermediate | 302 | 1.16 (0.85-1.58) | .36 |
| High | 201 | 1.60 (1.16-2.22) | .005 |
| Very high | 159 | 1.75 (1.25-2.46) | .001 |
| Missing | 324 | 1.32 (0.97-1.8) | .08 |
| In vivo T-cell depletion | |||
| No alemtuzumab/ATG | 833 | 1 [Reference] | |
| ATG | 392 | 1.22 (1.06-1.41) | .007 |
| Alemtuzumab | 43 | 1.38 (0.95-2.02) | .09 |
| Blasts in BM prior to preparative regimen | |||
| <5% | 843 | 1 [Reference] | |
| 5%-10% | 226 | 0.97 (0.81-1.16) | .74 |
| ≥11% | 119 | 1.32 (1.05-1.66) | .02 |
| Missing | 80 | 1.36 (1.03-1.79) | .03 |
| Conditioning regimen | |||
| Fludarabine + busulfan +/– others MA | 345 | 1 [Reference] | |
| Other myeloablative | 134 | 1.33 (1.04-1.69) | .02 |
| Fludarabine + busulfan RIC | 281 | 1.14 (0.94-1.37) | .18 |
| Fludarabine + melphalan RIC | 214 | 0.79 (0.64-0.99) | .04 |
| Fludarabine + TBI + Cy RIC | 85 | 1.40 (1.06-1.86) | .02 |
| Other TBI based RIC | 136 | 1.17 (0.92-1.49) | .19 |
| Other RIC | 73 | 1.42 (1.07-1.88) | .02 |
| Status prior to preparative regimen | |||
| Complete remission | 141 | 1 [Reference] | |
| Hematological improvement | 210 | 1.32 (1.01-1.72) | .04 |
| No response/stable disease | 673 | 1.52 (1.20-1.91) | <.001 |
| Progression/relapse | 87 | 1.43 (1.01-2.01) | .04 |
| No prior therapy | 100 | 1.30 (0.94-1.79) | .11 |
| Missing | 57 | 1.08 (0.73-1.6) | .68 |
| HCT-CI | |||
| 0 | 271 | 1 [Reference] | |
| 1-2 | 343 | 1.15 (0.95-1.4) | .16 |
| 3 | 254 | 1.21 (0.99-1.49) | .07 |
| ≥4 | 400 | 1.38 (1.14-1.66) | .001 |
Abbreviations: ATG, antithymocyte globulin; BM, bone marrow; MA, myeloablative; Cy, cyclophosphamide; HCT-CI, Hematopoietic Stem Cell Transplantation Comorbidity Index; RIC, reduced intensity conditioning; R-IPSS, Revised International Prognostic Scoring System; TBI, total body irradiation.
Nonrelapse Mortality
There was no statistical difference in NRM between the age groups (eTable 2 in Supplement 2). At 3 years, NRM was 28% vs 25% for the 65 years or older vs the 55 to 64 years age group. On multivariable analysis, age group 65 years or older, non–fludarabine/busulfan MA conditioning regimen, unrelated donor/cord blood, HCT-CI of 4 or greater, and disease status not in CR before transplant were independently associated with increased NRM (eTable 3 in Supplement 2).
However, after adjusting for excess risk of NRM in the older population, there was no statistically significant difference in NRM between the aged 65 years or older group and the aged 55 to 64 years group NRM (HR, 1.19; 95% CI, 0.93-1.52; P = .16).
Acute GVHD
There was no difference between groups in rates of grades II to IV acute GVHD (aGVHD) (eTable 2 in Supplement 2). At 100 days, the incidence of grades II to IV aGVHD was 38% in both groups.
By multivariable analysis, age group had no significant association with aGVHD. Findings indicate that MA regimens other than fludarabine/busulfan, unrelated donor, HCT-CI of 4 or greater, and female sex were independently associated with increased risk of aGVHD (eTable 4 in Supplement 2).
Chronic GVHD
On univariate analysis, there was no statistical difference in chronic GVHD (cGVHD) between the age groups (eTable 2 in Supplement 2). At 1 year, the incidence of cGVHD was 44% and 47% in the aged 65 years or older and the 55 to 64 years groups, respectively.
On multivariable analysis, in vivo T-cell depletion, T depletion, BM graft source, conditioning regimen (fludarabine/busulfan RIC and other RIC), and HLA-identical sibling were associated with lower risk of cGVHD (eTable 5 in Supplement 2).
In addition, on multivariable analysis, center characteristics such as volume of allogeneic transplants, total transplant volume, and years of operation had no effect on any outcome listed above.
Discussion
Although the safety and efficacy of RIC has been established for more than a decade,19,20 older patients with hematologic malignant diseases are not routinely offered allo-HCT. In a recent large intergroup trial for patients with high-risk MDS with a median age of 70 (range, 28-93) years, only 13% of patients proceeded to HCT.21 Sekeres et al,22 conducted a cross-sectional survey between June 2005 to January 2007 of 101 physicians responsible for treating 4154 patients with MDS. The median age of the patients was 71 (range, 65-80) years, 55% were men, and less than 5% of patients were evaluated for allo-HCT. Older patients may not have routine access to allo-HCT for multiple reasons previously discussed. Since approval of the CED, the number of transplants in patients with MDS older than 65 years has quadrupled from 96 in 2010 to 361 in 2014 (eFigure 2 in Supplement 2), providing access through the CED mechanism. Clearly, insurance coverage was a factor limiting access to HCT in this population.
Results of this study showed that there was no difference in OS, RFS, NRM, aGVHD, or cGVHD in patients older than 55 years. These results are similar to the retrospective study by Shaffer et al.18 In that study, the median age of patients was 56 (range, 18-77) years and 42% of patients had intermediate- or high-risk MDS. Using a maximum likelihood method, the optimal cut points for age prognostic categories were 18 to 29, 30 to 49, and older than 50 years, which indicates that patients older than 50 years were uniformly experiencing the same rate of survival, corroborating the findings in our study.
Not unexpectedly, an HCT-CI8 score of 4 or higher was independently associated with worse outcome for OS, DFS, NRM, and aGVHD. These results indicate that chronologic age alone, which is likely a surrogate for other risk factors associated with aging, may not be an appropriate selection factor for HCT. Physiologic age and functional status, as measured by the HCT-CI, are more relevant indicators of fitness for HCT. Other factors defining patients’ functional status such as geriatric assessments have been shown to be independently associated with outcomes in older patients receiving allo-HCT.23 This study and ours demonstrate that functional status is more predictive of outcome than age alone. The newly developed BMT-CTN study—the CHARM study—is meant to develop and validate a risk score for NRM for older patients receiving allo-HCT.24
On multivariable analysis, NRM was significantly higher in the aged 65 years or older group compared with patients aged 55 to 64 years. The absolute difference in NRM between the aged 55 to 64 years and the aged 65 years or older group was 2%, 1%, 1%, and 3% at 100 days, 6 months, 1 year, and 3 years, respectively. As time from HCT increased, the difference in NRM between the aged 65 years or older and the aged 55 to 64 years group increased. This finding suggests that the excess NRM seen at year 3 may be owing to a general higher risk of dying in the older population. After applying an excess risk of mortality model to account for the standardized increased death rate in the older population, there was no significant difference in NRM between the groups. This confirmed that the excess NRM is more likely owing to age-related trends in mortality than related to HCT.
Several other important practical findings were evident. There was no difference in OS or relapse risk for patients with less than 5% blasts or 5% to 10% blasts, suggesting that achieving a blast percentage less than 11% may be an appropriate goal of pre-HCT treatment. In addition, there was no difference in OS based on conditioning regimen intensity.
Limitations
There are several limitations to our study. First, because our study only included patients who were referred for, and were able to receive HCT, these results may not be generalizable to all older patients with MDS. Second, there was no systematic collection of geriatric assessments, which may help identify older patients who can tolerate HCT. Third, we did not collect information about quality of life among these recipients. Finally, molecular data were not collected, but may be helpful to further predict which patients with MDS are most likely to benefit from allo-HCT.
Conclusions
Findings of this study suggest that chronologic age alone should not be used as a determinant for transplant consideration. Availability of insurance coverage affects access to HCT. Outcomes in patients older than 65 years are only marginally different from those in younger patients. Based on current data, we would recommend coverage of HCT for MDS by CMS.
Trial Protocol.
eTable 1. Patient, Disease and Treatment Related Characteristics
eTable 2. Univariate analysis of outcomes
eTable 3. Non-relapse mortality multivariable analysis
eTable 4. Grade II-IV acute GVHD multivariable analysis
eTable 5. Chronic GVHD multivariable analysis
eFigure 1. Number of HCTs Performed for Patients with MDS ≥65 years old by Year
eFigure 2. Adjusted Disease Free Survival by age
References
- 1.Ma X, Does M, Raza A, Mayne ST. Myelodysplastic syndromes: incidence and survival in the United States. Cancer. 2007;109(8):1536-1542. doi: 10.1002/cncr.22570 [DOI] [PubMed] [Google Scholar]
- 2.McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28(11):1878-1887. doi: 10.1200/JCO.2009.25.4821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appelbaum FR. What is the impact of hematopoietic cell transplantation (HCT) for older adults with acute myeloid leukemia (AML)? Best Pract Res Clin Haematol. 2008;21(4):667-675. doi: 10.1016/j.beha.2008.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Estey E, de Lima M, Tibes R, et al. Prospective feasibility analysis of reduced-intensity conditioning (RIC) regimens for hematopoietic stem cell transplantation (HSCT) in elderly patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS). Blood. 2007;109(4):1395-1400. doi: 10.1182/blood-2006-05-021907 [DOI] [PubMed] [Google Scholar]
- 5.http://www.cms.gov/mcd/viewdraftdecisionmemo.asp?id=238. Accessed July 10, 2010.
- 6.Centers for Medicare & Medicaid Services. Decision Memo for Allogeneic Hematopoietic Stem Cell Transplantation (HSCT) for Myelodysplastic Syndrome. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=238. Accessed June 12, 2019.
- 7.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97(4):855-864. doi: 10.1046/j.1365-2141.1997.1112925.x [DOI] [PubMed] [Google Scholar]
- 8.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204-217. doi: 10.1016/0002-9343(80)90380-0 [DOI] [PubMed] [Google Scholar]
- 9.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atallah E, Pedersen TL, Warlick ED, et al. The outcome of hematopoietic cell transplantation (HCT) for Myelodysplastic Syndrome (MDS) in adults ≥65 years of age: first report of the coverage with evidence development (CED) in Medicare beneficiaries. ASH Abst. 2012;120(21):1983. [Google Scholar]
- 11.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912-2919. doi: 10.1182/blood-2005-05-2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454-2465. doi: 10.1182/blood-2012-03-420489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16(8):901-910. doi: [DOI] [PubMed] [Google Scholar]
- 14.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34(2):187-220. [Google Scholar]
- 15.Zhang X, Loberiza FR, Klein JP, Zhang MJ. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88(2):95-101. doi: 10.1016/j.cmpb.2007.07.010 [DOI] [PubMed] [Google Scholar]
- 16.Pohar M, Stare J. Relative survival analysis in R. Comput Methods Programs Biomed. 2006;81(3):272-278. doi: 10.1016/j.cmpb.2006.01.004 [DOI] [PubMed] [Google Scholar]
- 17.Lindsley RC, Saber W, Mar BG, et al. Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. N Engl J Med. 2017;376(6):536-547. doi: 10.1056/NEJMoa1611604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaffer BC, Ahn KW, Hu ZH, et al. Scoring system prognostic of outcome in patients undergoing allogeneic hematopoietic cell transplantation for myelodysplastic syndrome. J Clin Oncol. 2016;34(16):1864-1871. doi: 10.1200/JCO.2015.65.0515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97(11):3390-3400. doi: 10.1182/blood.V97.11.3390 [DOI] [PubMed] [Google Scholar]
- 20.Sorror ML, Sandmaier BM, Storer BE, et al. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. JAMA. 2011;306(17):1874-1883. doi: 10.1001/jama.2011.1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sekeres MA, Othus M, List AF, et al. Randomized phase II study of azacitidine alone or in combination with lenalidomide or with vorinostat in higher-risk myelodysplastic syndromes and chronic myelomonocytic leukemia: North American Intergroup Study SWOG S1117. J Clin Oncol. 2017;35(24):2745-2753. doi: 10.1200/JCO.2015.66.2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekeres MA, Schoonen WM, Kantarjian H, et al. Characteristics of US patients with myelodysplastic syndromes: results of six cross-sectional physician surveys. J Natl Cancer Inst. 2008;100(21):1542-1551. doi: 10.1093/jnci/djn349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muffly LS, Kocherginsky M, Stock W, et al. Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica. 2014;99(8):1373-1379. doi: 10.3324/haematol.2014.103655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CHARM 2019; https://web.emmes.com/study/bmt2/protocol/1704_protocol/1704_protocol.html. Accessed June 12, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eTable 1. Patient, Disease and Treatment Related Characteristics
eTable 2. Univariate analysis of outcomes
eTable 3. Non-relapse mortality multivariable analysis
eTable 4. Grade II-IV acute GVHD multivariable analysis
eTable 5. Chronic GVHD multivariable analysis
eFigure 1. Number of HCTs Performed for Patients with MDS ≥65 years old by Year
eFigure 2. Adjusted Disease Free Survival by age