Key Points
Question
In patients with advanced heart failure, do outcomes with left ventricular assist device implantation differ by the initial intended goal of therapy as a bridge to transplant or destination therapy?
Findings
In this randomized clinical trial, the composite end point of survival free of disabling stroke or reoperation to remove or replace a malfunctioning device at 2 years was significantly better with the magnetically levitated centrifugal-flow HeartMate 3 than the mechanical-bearing axial-flow HeartMate II, irrespective of preimplant therapeutic intent. Event-free survival was not different between patients in the bridge to transplant or destination therapy groups treated with the HeartMate 3 pump.
Meaning
Per this randomized clinical trial, use of categorizations based on current or future transplant eligibility should be abandoned in favor of a single treatment indication for use of left ventricular assist devices.
This prespecified secondary analysis of the MOMENTUM 3 randomized clinical trial aims to determine whether clinical outcomes of patients with 2 different left ventricular assist devices differed based on preoperative categories of bridge to transplant/bridge to candidacy vs destination therapy.
Abstract
Importance
Left ventricular assist devices (LVADs) are well established in the treatment of advanced heart failure, but it is unclear whether outcomes are different based on the intended goal of therapy in patients who are eligible vs ineligible for heart transplant.
Objective
To determine whether clinical outcomes in the Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy With HeartMate 3 (MOMENTUM 3) trial differed by preoperative categories of bridge to transplant (BTT) or bridge to transplant candidacy (BTC) vs destination therapy (DT).
Design, Setting, and Participants
This study was a prespecified secondary analysis of the MOMENTUM 3 trial, a multicenter randomized clinical trial comparing the magnetically levitated centrifugal-flow HeartMate 3 (HM3) LVAD to the axial-flow HeartMate II (HMII) pump. It was conducted in 69 centers with expertise in managing patients with advanced heart failure in the United States. Patients with advanced heart failure were randomized to an LVAD, irrespective of the intended goal of therapy (BTT/BTC or DT).
Main Outcomes and Measures
The primary end point was survival free of disabling stroke or reoperation to remove or replace a malfunctioning device at 2 years. Secondary end points included adverse events, functional status, and quality of life.
Results
Of the 1020 patients with implants (515 with HM3 devices [50.5%] and 505 with HMII devices [49.5%]), 396 (38.8%) were in the BTT/BTC group (mean [SD] age, 55 [12] years; 310 men [78.3%]) and 624 (61.2%) in the DT group (mean [SD] age, 63 [12] years; 513 men [82.2%]). Of the patients initially deemed as transplant ineligible, 84 of 624 patients (13.5%) underwent heart transplant within 2 years of LVAD implant. In the primary end point analysis, HM3 use was superior to HMII use in patients in the BTT/BTC group (76.8% vs 67.3% for survival free of disabling stroke and reoperation; hazard ratio, 0.62 [95% CI, 0.40-0.94]; log-rank P = .02) and patients in the DT group (73.2% vs 58.7%; hazard ratio, 0.61 [95% CI, 0.46-0.81]; log-rank P < .001). For patients in both BTT/BTC and DT groups, there were not significantly different reductions in rates of pump thrombosis, stroke, and gastrointestinal bleeding with HM3 use relative to HMII use. Improvements in quality of life and functional capacity for either pump were not significantly different regardless of preimplant strategy.
Conclusions and Relevance
In this trial, the superior treatment effect of HM3 over HMII was similar for patients in the BTT/BTC or DT groups. It is possible that use of arbitrary categorizations based on current or future transplant eligibility should be clinically abandoned in favor of a single preimplant strategy: to extend the survival and improve the quality of life of patients with medically refractory heart failure.
Trial Registration
ClinicalTrials.gov identifier: NCT02224755
Introduction
The first left ventricular assist device (LVAD) for treatment of advanced heart failure was approved by the US Food and Drug Administration 25 years ago for patients using it as a bridge to transplant (BTT). Thereafter, evidence emerged that LVAD therapy improved survival time to heart transplant, allowed better use of donor organs, and enhanced posttransplant survival.1,2,3 Bolstered by observations of prolonged support with LVADs, the concept that such devices could be used as destination or lifetime therapy in patients ineligible for transplant was investigated in the landmark Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial.4 This trial randomized 129 patients with advanced heart failure who were ineligible for transplant to a pulsatile, first-generation LVAD (HeartMate XVE [Thoratec]) or continued medical management (mainly continuous use of intravenous inotropic therapy). This trial demonstrated improved 2-year survival with long-term use of LVADs, leading to approval for destination therapy (DT). Clinicians, regulatory agencies, and industry all subsequently began to view these devices through the prism of 2 distinct indications,5,6 and separate clinical trials within these discrete categories were conducted to obtain FDA approval for either BTT7,8 or DT indications.9,10
Widespread adoption of LVAD therapy ensued as the device technology transitioned to continuous-flow pumps, which were smaller, possessed fewer moving parts, and exhibited greater durability.11 However, concerns over the blurred distinction between DT and BTT emerged, especially in regions with fewer organ donors and prolonged wait times on the transplant list. Clinicians also encountered patients for whom decisions regarding transplant candidacy could not be ascertained until after a device was implanted, thus creating the gray zone of the bridge to transplant candidacy (BTC) category.12 As these discrete categories of therapeutic intent were questioned, the need was established for a pivotal trial with a single set of inclusive patient selection criteria, irrespective of the initial goal of therapy.13
The Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy With HeartMate 3 (MOMENTUM 3) randomized clinical trial was launched with the goal of enrolling patients irrespective of their intended goal of therapy. This large clinical trial compared a fully magnetically levitated centrifugal-flow pump, the HeartMate 3 (HM3 [Abbott Laboratories]), against an established axial-flow pump, the HeartMate II (HMII [Abbott Laboratories]). The trial’s innovative study design evaluated outcomes in both the short term (6 months) and the long term (24 months).14 The primary analysis15 of the full 1028 patient cohort demonstrated superiority of the HM3 device compared with the HMII device on survival free of a disabling stroke or reoperation to remove or replace a malfunctioning pump. The HM3 arm also demonstrated a reduction in hemocompatibility-associated adverse events, including pump thrombosis, stroke of any type or severity, and nonsurgical bleeding.15
The MOMENTUM 3 study protocol prespecified an analysis to evaluate whether the overall trial outcomes were differentially influenced by the patient subgroups of BTT, BTC, or DT. In this analysis, we sought to assess the effect of therapeutic intent on the primary and secondary end points and their dynamic reclassification over the trial duration.
Methods
Trial Design
The MOMENTUM 3 trial design, sample-size calculation, and protocol have been previously described in detail.14,15,16 Patients with advanced heart failure who remained symptomatic despite medical therapy were randomized 1:1 to either the HM3 or HMII device at 69 participating centers within the United States. The protocol was approved by the institutional review board at each participating center. Written informed consent was obtained from all patients or their authorized representatives. Abbott Laboratories, the trial sponsor, selected the sites, provided the devices, and performed the data analysis.
Trial Population
The MOMENTUM 3 trial enrolled patients with advanced-stage heart failure who were deemed to be candidates for LVAD therapy, irrespective of whether the intended goal of support was BTT/BTC or DT. Based on transplant eligibility at time of enrollment, each patient was assigned a preimplant strategy by the investigator: BTT, BTC (with a high, moderate, or low probability of transplant), or DT. In this prespecified subgroup analysis, patients eligible for transplant or with the possibility of eventual transplant eligibility were combined into 1 group (BTT/BTC) and compared with those deemed ineligible for transplantation (DT) at enrollment. Only patients who underwent implant with their assigned device were included in this subgroup analysis (the per-protocol population).
Left Ventricular Assist Systems
Descriptions and images of the fully magnetically levitated centrifugal-flow HM3 pump and the mechanical-bearing axial-flow HMII pump have been previously published.16 The recommended antithrombotic treatment in both treatment groups includes aspirin at a dosage of 81 to 325 mg daily and warfarin to a target range for an international normalized ratio of 2.0 to 3.0.
Primary and Secondary End Points
The primary end point was survival free of disabling stroke (defined by a modified Rankin score more than 3, on a scale in which scores range from 0 to 6, with higher scores indicating more severe disability, including death) or reoperation to remove or replace a malfunctioning device at 2 years. Secondary end points included actuarial survival, adverse events, all-cause rehospitalizations, functional status, and quality of life. In this prespecified analysis, the primary and secondary end points were evaluated to assess treatment safety and efficacy within the BTT/BTC and DT cohorts.
Two main categories of adverse events were analyzed: hemocompatibility-associated events (bleeding, stroke, and pump thrombosis) and nonhemocompatibility-associated events (infection, arrhythmias, and right heart failure), using prespecified definitions.16 An independent clinical events committee blinded to treatment assignment adjudicated the adverse events. Functional status was assessed by New York Heart Association classification and a 6-minute walk test. Quality of life was determined with the European Quality of Life–5 dimensions–5 level visual analog scale and the Kansas City Cardiomyopathy Questionnaire. Assessments were performed at enrollment and throughout the 24 months postimplant.
Statistical Analysis
Continuous variables are depicted as means (SDs) or medians (ranges), and categorical variables are shown in counts and percentages. Comparisons of continuous and categorical data between groups were performed using unpaired t tests and Fisher exact tests, respectively. The Kaplan-Meier method and log-rank test were used for time-to-event analysis. Competing risk analysis was performed using the Fine-Gray model to calculate the hazard of death while accounting for the competing outcome of heart transplant.
Cox proportional hazards modeling was used to identify factors independently associated with primary end point success. First, a series of univariable analyses on patients implanted with either device was performed to screen for potential clinically relevant factors. Baseline variables found to be potentially significant (P < .10) were included in a multivariable Cox proportional hazards model. Highly correlated variables, such as multiple parameters describing renal function and hemodynamic filling pressures, were excluded to avoid multicollinearity. The variables included in the model were pump type, age, intended use, race, ischemic cause of heart failure, estimated glomerular filtration rate, pulmonary capillary wedge pressure, and prior cardiac surgery. Hazard ratios (HRs) are presented with the corresponding 95% CIs.
Adverse event rates are presented as a percentage of patients and as events per patient-year (EPPY). Major adverse event rates were compared as EPPY between the 2 treatment groups using Poisson regression. The rate differences are described as relative risks (RRs) with 95% CIs. Rehospitalizations attributable to any cause were analyzed using the Andersen-Gill model and the Wilcoxon rank sum test. Longitudinal changes in functional status and quality of life were analyzed by means of linear mixed-effects modeling.
The net clinical benefit of reduction in hemocompatibility-associated adverse events with the HM3 device was derived from the EPPY for pump thrombosis, stroke, and bleeding. The total number of events averted for every 100 patients implanted with the HM3 device compared with the HMII device over a 2-year period was calculated for the BTT/BTC and DT groups.
All reported P values for the primary end point are 2-tailed, and P values less than .05 are considered significant. Statistical analyses were performed with SAS software version 9.4 (SAS Institute). Additional details are in the protocol and statistical plan in Supplement 2.
Results
Patient Characteristics
The MOMENTUM 3 study cohort included 1028 patients randomized 1:1 to receive either the HM3 or HMII devices (eFigure 1 in Supplement 1). Eight patients withdrew from the study before implant, resulting in 1020 patients in the per-protocol population, of whom 515 (50.5%) received the HM3 device and 505 (49.5%) received the HMII device (Table 1). Of these patients, 396 (38.8%) were designated by the investigator as BTT or BTC (transplant eligible or potentially eligible, respectively; 310 men [78.3%]) and 624 (61.1%) as DT (transplant ineligible; 513 men [82.2%]) (eFigure 1 in Supplement 1; Table 1). Among the BTT/BTC group, the same number of patients (198) were implanted with each device, while in the DT cohort, 317 patients (50.8%) received the HM3 pump and 307 (49.2%) were implanted with the HMII device (eFigure 1 in Supplement 1).
Table 1. Investigator Designation of Intended Goal of Therapy PreImplant.
| Intended Goal of Therapy | Patients, No. (%) | ||
|---|---|---|---|
| HeartMate 3 (n = 515) | HeartMate II (n = 505) | Total (N = 1020)a | |
| Bridge to transplant | 112 (21.7) | 120 (23.8) | 232 (22.7) |
| Bridge to candidacy | 86 (16.7) | 78 (15.4) | 164 (16.1) |
| Likely to become eligible for transplant | 45 (8.7) | 43 (8.5) | 88 (8.6) |
| Moderately likely to become transplant eligible | 32 (6.2) | 33 (6.5) | 65 (6.4) |
| Unlikely to become transplant eligible | 9 (1.7) | 2 (0.4) | 11 (1.1) |
| Bridge to transplant and bridge to candidacy subtotal | 198 (38.4) | 198 (39.2) | 396 (38.8) |
| Destination therapy | 317 (61.6) | 307 (60.8) | 624 (61.2) |
A total of 1028 patients were in the intent-to-treat population. One randomized to the HeartMate 3 and 7 randomized to HeartMate II did not receive a left ventricular assist device and were not included in the per-protocol population (n = 1020).
Baseline characteristics for the BTT/BTC and DT cohorts are depicted in Table 2. Compared with the BTT/BTC group, patients in the DT group were older (mean [SD] age: BTT/BTC group, 55 [12] years; DT group, 63 [12] years; P < .001) and more likely to have an ischemic cause of heart failure (BTT/BTC group, 155 of 396 [39.1%]; DT group, 297 of 624 [47.6%]; P = .01), prior coronary artery bypass grafting (BTT/BTC group, 48 of 396 [12.1%]; DT group, 165 of 624 [26.4%]; P < .001), a history of atrial fibrillation (BTT/BTC group, 158 of 396 [39.9%]; DT group, 292 of 624 [46.8%]; P = .03), and worse renal function (mean [SD] estimated glomerular filtration rate: BTT/BTC group, 64.9 [24.6] mL/min/1.73 m2; DT group, 57.7 [21.2] mL/min/1.73 m2; P < .001).
Table 2. Baseline Characteristics.
| Characteristic | Patients, No. (%) | P Valuea | |
|---|---|---|---|
| Bridge to Transplant/Bridge to Candidacy (n = 396) | Destination Therapy (n = 624) | ||
| Age | |||
| Mean (SD), y | 55 (12) | 63 (12) | <.001 |
| Median (range), y | 58 (18-73) | 65 (22-84) | |
| Male | 310 (78.3) | 513 (82.2) | .12 |
| Race | |||
| White | 266 (67.2) | 437 (70.0) | .37 |
| Black or African American | 113 (28.5) | 151 (24.2) | |
| Asian | 2 (0.5) | 9 (1.4) | |
| Native Hawaiian or Pacific Islander | 1 (0.3) | 3 (0.5) | |
| Other | 14 (3.5) | 24 (3.8) | |
| Ischemic cause of heart failure | 155 (39.1) | 297 (47.6) | .01 |
| History of atrial fibrillation | 158 (39.9) | 292 (46.8) | .03 |
| Intravenous inotropic agents | 327(82.6) | 534 (85.6) | .22 |
| Coronary bypass surgery | 48 (12.1) | 165 (26.4) | <.001 |
| Intra-aortic balloon pump | 63 (15.9) | 76 (12.2) | .09 |
| Serum creatinine, mean (SD), mg/dL | 1.3 (0.4) | 1.4 (0.4) | <.001 |
| Estimated glomerular filtration rate, mean (SD), mL/min/1.73 m2 | 64.9 (24.6) | 57.7 (21.2) | <.001 |
| Albumin, mean (SD), g/dL | 3.7 (0.5) | 3.6 (0.5) | <.001 |
| Blood urea nitrogen, mean (SD), mg/dL | 25.9 (12.5) | 29.2 (13.6) | <.001 |
| INTERMACS profile | |||
| 1 | 15 (3.8) | 12 (1.9) | .15 |
| 2 | 112 (28.3) | 187 (30.0) | |
| 3 | 198 (50.0) | 325 (52.1) | |
| 4 | 59 (14.9) | 89 (14.3) | |
| 5-7 | 11 (2.8) | 7 (1.1) | |
| Not provided | 1 (0.3) | 4 (0.6) | |
Abbreviation: INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support.
SI conversion factors: To convert albumin to g/L, multiply by 10; blood urea nitrogen to mmol/L, multiply by 0.357; serum creatinine to μmol/L, multiply by 88.4.
Data were compared between groups using unpaired t tests or Fisher exact tests and χ2 tests, as appropriate.
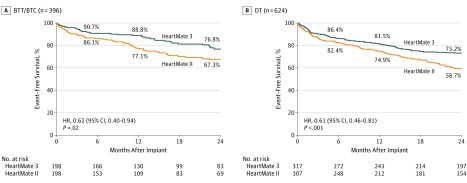
Primary End Point
The primary end point of the trial for HM3 compared with HMII devices is shown in Figure 1, stratified by the intended goal of therapy. The individual components leading to failure of the composite end point are shown in eTable 1 in Supplement 1. Primary end point success was significantly greater with HM3 compared with HMII devices, irrespective of a BTT/BTC or DT preimplant designation (HRs: 0.62 [95% CI, 0.40-0.94] and 0.61 [95% CI, 0.46-0.81], respectively), indicating a comparable treatment effect. The interaction P value between treatment arm and therapeutic intent was not significant. Event-free survival for patients with HM3 devices (76.8% for the BTT/BTC group and 73.2% for the DT group by Kaplan-Meier analysis; log-rank P = .19) was not significantly different between groups.
Figure 1. Primary End Point Analysis.
Survival at 2 years free of disabling stroke (defined as a modified Rankin score greater than 3) or reoperation to replace or remove a malfunctioning device in the bridge to transplant (BTT)/bridge to transplant candidacy (BTC) and destination therapy (DT) cohorts. HR indicates hazard ratio.
A multivariable Cox proportional hazards model found that HM3 pump type (HR, 0.64 [95% CI, 0.51-0.82]; P < .001), better baseline renal function (HR per 1-mL/min/1.73 m2 increase, 0.99 [95% CI, 0.99-1.00]; P = .01), and nonwhite race (HR, 0.73 [95% CI, 0.55-0.97]; P = .03) were factors independently associated with primary end point success. Preimplant designation of BTT/BTC vs DT was not significantly associated with this outcome (HR, 0.83 [95% CI, 0.64-1.09]; P = .18) (eTable 2 in Supplement 1).
Survival and Competing Outcomes
Survival was not different between pumps for patients in the BTT/BTC group (HR, 0.93 [95% CI, 0.55-1.59]) or patients in the DT group (HR, 0.87 [95% CI, 0.63-1.20]) (eFigure 2 in Supplement 1). Within the HMII arm, survival was higher in patients in the BTT/BTC group compared with patients in the DT group (HR, 0.61 [95% CI, 0.39-0.95]; log-rank P = .03). Similar trends for survival were noted between groups that received HM3 devices, but statistical significance was not achieved (HR, 0.66 [95% CI, 0.43-1.03]; log-rank P = .07).
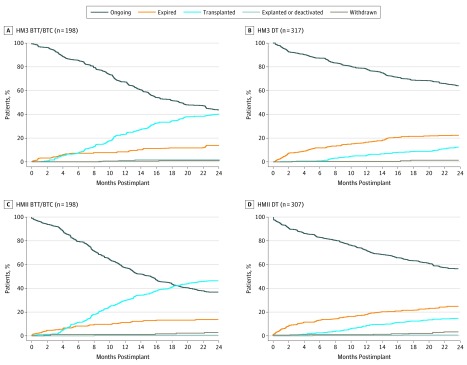
Overall, 256 patients (25.1%) in the per-protocol population underwent transplant over the 2-year follow-up period, with a slightly greater proportion of recipients of HMII devices (137 of 505 [27.1%]) transplanted compared with patients with HM3 devices (119 of 515 [23.1%]). Competing outcome plots for recipients of HM3 and HMII devices are shown in Figure 2 for the BTT/BTC and DT groups. Of the patients who received HM3 devices, 86 of 198 patients (43.4%) deemed BTT/BTC prior to implant continued to remain on LVAD support at 2 years; the rate for those who received HMII devices was 36.9% (73 of 198 patients). Meanwhile, 39 of 317 patients (12.3%) with HM3 devices and 45 of 307 patients (14.7%) with HMII devices who were deemed DT underwent heart transplant within 2 years, suggesting a dynamic change in the intended goal during LVAD support. In patients with BTC designations only, 36 of 88 patients (40.9%) of those deemed likely to be eligible, 21 of 65 patients (32.3%) with moderate likelihood, and 1 of 11 (9.1%) deemed unlikely to be eligible received transplants by 2 years. When accounting for the competing outcome of transplant with Fine-Gray modeling, the risk of death remained similar for patients receiving HM3 vs HMII devices in the BTT/BTC group (HR, 0.98 [95% CI, 0.58-1.68]) and the DT group (HR, 0.88 [95% CI, 0.64-1.22]).
Figure 2. Competing Outcomes in the Bridge to Transplant (BTT)/Bridge to Transplant Candidacy (BTC) and Destination Therapy (DT) Cohorts for Patients Implanted With HeartMate 3 (HM3) or HeartMate II (HMII).
Adverse Events
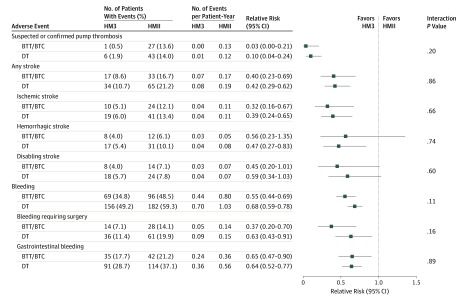
Comparisons of hemocompatibility-associated adverse event rates for HM3 vs HMII devices are depicted in Figure 3 for patients in the BTT/BTC and DT groups. Patients implanted with the HM3 device compared with those with the HMII device had lower rates of pump thrombosis (RRs: BTT/BTC group, 0.03 [95% CI, 0.00-0.21]; DT group, 0.10 [95% CI, 0.04-0.24]), stroke (RRs: BTT/BTC group, 0.40 [95% CI, 0.23-0.69]; DT group, 0.42 [95% CI, 0.29-0.62]), and bleeding (RRs: BTT/BTC group, 0.55 [95% CI, 0.44-0.69]; DT group, 0.68 [95% CI, 0.59-0.78]), irrespective of preimplant designation of intended use. Moreover, there was no difference in the treatment effect size for any of the individual hemocompatibility-associated adverse events across the different intended-use categories.
Figure 3. Comparison of Hemocompatibility-Associated Adverse Events With HeartMate 3 vs HeartMate II Devices in the Bridge to Transplant (BTT)/Bridge to Transplant Candidacy (BTC) and Destination Therapy (DT) Cohorts.
Poisson regression was used to calculate the P value and relative risk (comparing HeartMate 3 [HM3] and HeartMate II [HMII] devices).
Pump thrombosis was virtually eliminated with the HM3 device in both the BTT/BTC group (0.00 EPPY) and the DT group (0.01 EPPY) (Figure 3) and significantly reduced from the HMII device (0.13 EPPY and 0.12 EPPY, respectively). This benefit with the HM3 device was seen irrespective of preimplant strategy, with a 99.4% freedom from event among recipients designated BTT/BTC and 97.5% among recipients designated DT (per Kaplan-Meier analysis), both significantly outperforming their respective HMII cohorts (BTT/BTC: HR, 0.03 [95% CI, 0.01-0.25]; P < .001; DT: HR, 0.12 [95% CI, 0.05-0.29]; P < .001; eFigure 3 in Supplement 1).
The HM3 event rates for any stroke were similar for the BTT/BTC group (0.07 EPPY) and the DT group (0.08 EPPY) and significantly reduced from the HMII event rates (0.17 and 0.19 EPPY, respectively) (Figure 3). Actuarial freedom from any stroke at 2 years was also significantly better for patients receiving the HM3 device vs the HMII device, irrespective of preimplant designation of intended use (eFigure 4 in Supplement 1). Moreover, freedom from any stroke with the HM3 pump was similar between the BTT/BTC and DT cohorts (89.2% and 88.1%, respectively, by Kaplan-Meier analysis) with absolute improvements compared with the HMII group of 11% in patients in the BTT/BTC group and 14% in the patients in the DT group (by Kaplan-Meier analysis). Hazard ratios were similar (BTT/BTC: HR, 0.48 [95% CI, 0.27-0.86]; P = .01; DT: HR, 0.47 [95% CI, 0.31-0.70]; P < .001), indicating a greater than 50% reduction in stroke with the HM3 device regardless of preimplant strategy.
The patients in the DT group had a higher incidence of gastrointestinal bleeding rates compared with patients in the BTT/BTC group (HMII device: DT, 0.56 EPPY; BTT/BTC, 0.36 EPPY; HM3 device: DT, 0.36 EPPY; BTT/BTC, 0.24 EPPY) (Figure 3; eFigure 5 in Supplement 1). However, the size of the HM3 device treatment effect showed similar reductions of gastrointestinal bleeding rates in the BTT/BTC and DT groups (RRs, 0.65 [95% CI, 0.47-0.90] and 0.64 [95% CI, 0.52-0.77], respectively).
The incidence of nonhemocompatibility-associated adverse events was balanced between pumps and preimplant strategy, except for any right heart failure, which was greater for HM3 in patients in the BTT/BTC group (HR, 1.58 [95% CI, 1.14-2.21]) but not the DT group (HR, 0.93 [95% CI, 0.71-1.21]; interaction P = .01). However, no differences in severe right heart failure requiring right ventricular assist device support were noted between pumps (eFigure 6 in Supplement 1). The overall incidence of major infection was not significantly different between pumps and among the 3 infection categories of sepsis, driveline infection, and non-LVAD infection.
Rehospitalizations
The days out of the hospital and duration of rehospitalization did not differ between the groups receiving HM3 vs HMII pumps, for patients with BTT/BTC designations. However, among patients designated DT, recipients of HM3 devices had an additional 24 days out of hospital over a 2-year period (median [interquartile range], 668 [438-700] days) compared with patients with HMII devices (median [interquartile range], 644 [342-693] days) and a 12% reduction in rehospitalization rates over that period (HR, 0.88 [95% CI, 0.81-0.96]; eTable 3 in Supplement 1).
Functional Capacity and Quality of Life
There were significant improvements on functional capacity as measured by New York Heart Association class (classes I or II: BTT/BTC group: baseline, 0 of 198 patients; 24 months, 70 of 84 patients; DT group: baseline, 0 of 316 patients; 24 months, 149 of 191 patients; both P < .001) and 6-minute walk distance (mean [SD] distance: BTT/BTC group: baseline, 134 [152] m; 24 months, 332 [126] m; DT group: baseline, 137 [164] m; 24 months, 320 [166] m; both P < .001), and health-related quality of life by the Kansas City Cardiomyopathy Questionnaire (mean [SD] score: BTT/BTC group: baseline, 38 [20] points; 24 months, 69 [20] points; DT group: baseline, 40 [21] points; 24 months, 69 [23] points; both P < .001), and the European Quality of Life–5 dimensions–5 level visual analog scale (mean [SD] score: BTT/BTC group: baseline, 47 [24] points; 24 months, 75 [17] points; DT group: baseline, 51 [24] points; 24 months, 77 [19] points; both P < .001) for patients in both the BTT/BTC and DT groups with the HM3 pump (eFigure 7 and 8 in Supplement 1). Improvements in the patients implanted with the HMII were highly similar.
Net Clinical Benefit
The net clinical benefit observed in the improvement of hemocompatibility-associated adverse events with the HM3 pump was similar regardless of intended use. Based on the observed adverse event rates, we calculate that for every 100 patients implanted with the HM3 device compared with the HMII device over a 2-year period, 118 events (26 incidents of pump thrombosis, 20 strokes, and 72 bleeding events) are averted in those designated BTT/BTC, while 110 events (22 incidents of pump thrombosis, 22 strokes, and 66 bleeding events) are averted for each of 100 patients in the DT intended-goal category.
Discussion
The principal findings of this prespecified analysis of the MOMENTUM 3 trial demonstrate the superiority of the HM3 pump over the HMII LVAD for survival free of a disabling stroke or reoperation to replace or remove a malfunctioning device in either patients who are transplant eligible (or candidates likely to become transplant eligible) or those deemed ineligible by the treating clinician at the time of enrollment. The absolute benefit of the HM3 pump was not altered by the intended goal of therapy.15 The primary end point was also successfully achieved at similar event rates in patients implanted with the HM3 device, irrespective of the intended goal of therapy. An equally important observation from this analysis pertains to the fact that the initial intended goal of therapy is not static, as has been observed by other researchers,8 since nearly 15% of those initially deemed transplant ineligible were eventually transplanted within 2 years of follow-up. These combined findings provide strong rationale for abandoning discrete transplant eligibility labels in clinical assessments and especially in the context of designing clinical trials for future device approvals.
Evidence from registry analyses17,18 has suggested that patients deemed to be transplant ineligible (those in the DT group) also have worse overall survival and have an increased burden of adverse events with LVADs used prior to the introduction of the HM3 pump. It has been thought that these patients, by virtue of older age and greater comorbidity levels, are more prone to adverse outcomes. However, this trial analysis reassuringly demonstrates that by using the MOMENTUM 3 patient selection criteria, 2-year survival for patients designated DT who received the control HMII pump has continued to improve in the current era. Slaughter et al9 documented a 58% overall survival in the HMII DT trial, while Rogers and colleagues10 more recently reported a 67.6% survival with the HMII device in the The HeartWare Ventricular Assist System as Destination Therapy of Advanced Heart Failure (ENDURANCE) comparative trial (NCT01166347) in patients ineligible for transplant. This incremental improvement of 15.4% over a decade highlights the importance of better patient selection and medical management strategies and suggests that ongoing and future experience with the study HM3 device may lead to enhanced benefits as well. In this regard, we have also shown that the survival rates achieved with either the HM3 or HMII pump (which were no different) in patients categorized as DT are now approaching rates expected with transplant at the 2-year point, although the rates still do not exactly mirror one another.16
Of interest in our analysis is the demonstration of a markedly lower burden of adverse events in patients implanted with the HM3 pump, especially within the domain of hemocompatibility-associated events, such as pump thrombosis, stroke, and bleeding. The selection of the HM3 LVAD nearly abrogates the risk for pump thrombosis and achieves stroke rates (whether disabling or nondisabling) that are the lowest reported in any LVAD trial. These are uniquely similar irrespective of intended goal of therapy. While bleeding rates are also markedly improved with the HM3 device compared with the HMII device in either category, it is apparent that patients in the DT group have a higher burden of bleeding, particularly gastrointestinal bleeding, compared with patients who are transplant candidates or potential candidates at the outset. The improvements noted with the HM3 pump are also responsible for greater days spent out of the hospital in patients in the DT category. Taken in aggregate, these findings should open the door to greater confidence in consideration of LVAD therapy in patients deemed to be transplant ineligible. Our multivariable Cox model provides further support that once the choice of the pump centers on the HM3 LVAD, arbitrary designations of BTT/BTC or DT may lose relevance. The net clinical benefit observed in events averted are also similar across these separate categories.
Limitations
There are some limitations in this analysis. The trial was not blinded, which may have introduced bias. However, the outcomes are of a nature that are less prone to clinician bias, and medical management with anticoagulation and antihypertensive medications, follow-up visits, and outcome ascertainment were similar between groups. Also, the sample size of the trial was not powered directly for this prespecified analysis, and there was no adjustment made for multiplicity.
Conclusions
In summary, this prespecified analysis of the MOMENTUM 3 trial suggests that the use of DT or BTT/BTC designations based on current or uncertain future transplant eligibility is not necessary. Patients with medically refractory heart failure can be successfully treated under a single preimplant strategy with the goal of extending survival and improving quality of life.
eFigure 1. CONSORT diagram.
eFigure 2. Overall survival for patients receiving the HeartMate 3 and HeartMate II, stratified by intended use.
eFigure 3. Freedom from pump thrombosis in BTT/BTC and DT patients.
eFigure 4. Freedom from any stroke in BTT/BTC and DT patients.
eFigure 5. Freedom from gastrointestinal bleeding in BTT/BTC and DT patients.
eFigure 6. Comparison of non-hemocompatibility-associated adverse events in BTT/BTC and DT patients.
eFigure 7. Functional status and quality of life for BTT/BTC and DT patients implanted with HeartMate 3.
eFigure 8. Functional Status and quality of life for BTT/BTC and DT patients implanted with HeartMate II.
eTable 1. Components of the primary endpoint.
eTable 2. Cox proportional hazards model for the primary endpoint.
eTable 3. Days out of the hospital and rehospitalizations.
Protocol and Statistical Plan.
References
- 1.Aaronson KD, Eppinger MJ, Dyke DB, Wright S, Pagani FD. Left ventricular assist device therapy improves utilization of donor hearts. J Am Coll Cardiol. 2002;39(8):1247-1254. doi: 10.1016/S0735-1097(02)01751-5 [DOI] [PubMed] [Google Scholar]
- 2.McCarthy PM, James KB, Savage RM, et al. ; Implantable LVAD Study Group . Implantable left ventricular assist device: approaching an alternative for end-stage heart failure. Circulation. 1994;90(5, pt 2):II83-II86. [PubMed] [Google Scholar]
- 3.Frazier OH, Rose EA, McCarthy P, et al. Improved mortality and rehabilitation of transplant candidates treated with a long-term implantable left ventricular assist system. Ann Surg. 1995;222(3):327-336. doi: 10.1097/00000658-199509000-00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rose EA, Gelijns AC, Moskowitz AJ, et al. ; Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group . Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345(20):1435-1443. doi: 10.1056/NEJMoa012175 [DOI] [PubMed] [Google Scholar]
- 5.Felker GM, Rogers JG. Same bridge, new destinations rethinking paradigms for mechanical cardiac support in heart failure. J Am Coll Cardiol. 2006;47(5):930-932. doi: 10.1016/j.jacc.2005.09.070 [DOI] [PubMed] [Google Scholar]
- 6.Fang JC, Stehlik J. Moving beyond “bridges”. JACC Heart Fail. 2013;1(5):379-381. doi: 10.1016/j.jchf.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 7.Miller LW, Pagani FD, Russell SD, et al. ; HeartMate II Clinical Investigators . Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357(9):885-896. doi: 10.1056/NEJMoa067758 [DOI] [PubMed] [Google Scholar]
- 8.Aaronson KD, Slaughter MS, Miller LW, et al. ; HeartWare Ventricular Assist Device (HVAD) Bridge to Transplant ADVANCE Trial Investigators . Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation. 2012;125(25):3191-3200. doi: 10.1161/CIRCULATIONAHA.111.058412 [DOI] [PubMed] [Google Scholar]
- 9.Slaughter MS, Rogers JG, Milano CA, et al. ; HeartMate II Investigators . Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241-2251. doi: 10.1056/NEJMoa0909938 [DOI] [PubMed] [Google Scholar]
- 10.Rogers JG, Pagani FD, Tatooles AJ, et al. intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med. 2017;376(5):451-460. doi: 10.1056/NEJMoa1602954 [DOI] [PubMed] [Google Scholar]
- 11.Kirklin JK, Naftel DC, Kormos RL, et al. Third INTERMACS annual report: the evolution of destination therapy in the United States. J Heart Lung Transplant. 2011;30(2):115-123. doi: 10.1016/j.healun.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 12.Teuteberg JJ, Stewart GC, Jessup M, et al. Implant strategies change over time and impact outcomes: insights from the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support). JACC Heart Fail. 2013;1(5):369-378. doi: 10.1016/j.jchf.2013.05.006 [DOI] [PubMed] [Google Scholar]
- 13.Acker MA, Pagani FD, Stough WG, et al. Statement regarding the pre and post market assessment of durable, implantable ventricular assist devices in the United States. Circ Heart Fail. 2013;6(1):e1-e11. [DOI] [PubMed] [Google Scholar]
- 14.Heatley G, Sood P, Goldstein D, et al. ; MOMENTUM 3 Investigators . Clinical trial design and rationale of the Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy With HeartMate 3 (MOMENTUM 3) investigational device exemption clinical study protocol. J Heart Lung Transplant. 2016;35(4):528-536. doi: 10.1016/j.healun.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 15.Mehra MR, Uriel N, Naka Y, et al. ; MOMENTUM 3 Investigators . A fully magnetically levitated left ventricular assist device—the final cohort. N Engl J Med. 2019;380(17):1618-1627. doi: 10.1056/NEJMoa1900486 [DOI] [PubMed] [Google Scholar]
- 16.Mehra MR, Naka Y, Uriel N, et al. ; MOMENTUM 3 Investigators . A fully magnetically levitated circulatory pump for advanced heart failure. N Engl J Med. 2017;376(5):440-450. doi: 10.1056/NEJMoa1610426 [DOI] [PubMed] [Google Scholar]
- 17.Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34(12):1495-1504. doi: 10.1016/j.healun.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 18.The International Society for Heart and Lung Transplantation . International Thoracic Organ Transplant (TTX) registry data slides. https://ishltregistries.org/registries/slides.asp. Published 2019. Accessed December 3, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. CONSORT diagram.
eFigure 2. Overall survival for patients receiving the HeartMate 3 and HeartMate II, stratified by intended use.
eFigure 3. Freedom from pump thrombosis in BTT/BTC and DT patients.
eFigure 4. Freedom from any stroke in BTT/BTC and DT patients.
eFigure 5. Freedom from gastrointestinal bleeding in BTT/BTC and DT patients.
eFigure 6. Comparison of non-hemocompatibility-associated adverse events in BTT/BTC and DT patients.
eFigure 7. Functional status and quality of life for BTT/BTC and DT patients implanted with HeartMate 3.
eFigure 8. Functional Status and quality of life for BTT/BTC and DT patients implanted with HeartMate II.
eTable 1. Components of the primary endpoint.
eTable 2. Cox proportional hazards model for the primary endpoint.
eTable 3. Days out of the hospital and rehospitalizations.
Protocol and Statistical Plan.