Key Points
Question
Does early postdonation renal function offer insights into subsequent end-stage renal disease risk in living kidney donors?
Findings
In this registry-based cohort study of 71 468 living kidney donors, a 10-unit difference in early postdonation estimated glomerular filtration rate was significantly associated with a 28% higher risk of subsequent end-stage renal disease.
Meanings
Based on this analysis, donors should have their estimated glomerular filtration rate measured at 6 months postdonation, and donors with low renal function may benefit from increased surveillance or early intervention to reduce end-stage renal disease risk.
This secondary analysis of a cohort study aims to determine the association between renal function in the first 6 months postdonation and subsequent risk of end-stage renal disease in kidney donors.
Abstract
Importance
Living kidney donation is associated with increased long-term risk of end-stage renal disease (ESRD). An early postdonation marker of ESRD risk could improve postdonation risk assessment and counseling for kidney donors and allow early intervention for donors at increased risk.
Objective
To determine the association between renal function in the first 6 months postdonation and subsequent risk of ESRD in kidney donors.
Design, Setting, and Participants
This secondary analysis of a prospective national cohort uses a population-based registry of all living kidney donors in the United States between October 26, 1999, and January 1, 2018, with follow-up through December 31, 2018. All kidney donors who had donated in the date range and had serum creatinine measured at 6 months (±3 months) postdonation were included.
Exposures
Renal function as measured by estimated glomerular filtration rate 6 months after donation (eGFR6).
Main Outcomes and Measures
End-stage renal disease, ascertained via linkage to Centers for Medicare & Medicaid Services data.
Results
A total of 71 468 living kidney donors were included (of 109 065 total donors over this period). Their median (interquartile range) eGFR6 was 63 (54-74) mL/min/1.73 m2. Cumulative incidence of ESRD at 15 years postdonation ranged from 11.7 donors per 10 000 donors with eGFR6 values greater than 70 mL/min/1.73 m2 to 33.1 donors per 10 000 donors with eGFR6 values of 50 mL/min/1.73 m2 or less. Adjusting for age, race, sex, body mass index, and biological relationship, every 10 mL/min/1.73 m2 reduction in eGFR6 was associated with a 28% increased risk of ESRD (adjusted hazard ratio, 1.28 [95% CI, 1.06-1.54]; P = .009). The association between predonation eGFR and ESRD was not significant and was fully mediated by eGFR6 (adjusted hazard ratio, 1.00 [95% CI, 0.86-1.17]; P = .97). The postdonation eGFR value was a better marker of ESRD than eGFR decline after donation or the ratio of eGFR6 to predonation eGFR, as determined by the Akaike information criterion (in which a lower value indicates a better model fit; eGFR6, 1495.61; predonation eGFR − eGFR6, 1503.58; eGFR6 / predonation eGFR, 1502.30).
Conclusions and Relevance
In this study, there was an independent association of eGFR6 with subsequent ESRD risk in living kidney donors, even after adjusting for predonation characteristics. The findings support measurement of early postdonation serum creatinine monitoring in living kidney donors, and the use of these data to help identify donors who might need more careful surveillance and early intervention.
Introduction
Every year, more than 5000 healthy individuals donate a kidney in the United States.1 Several studies have demonstrated elevated risk of end-stage renal disease (ESRD) among living kidney donors compared with healthy nondonors.2,3 Although predonation risk factors for ESRD have been identified in donor populations, including older age (among nonblack donors), black race, male sex, a first-degree biological relationship to the recipient, obesity, and systolic blood pressure,4,5,6 ESRD generally takes years to develop in living kidney donors, and an early postdonation marker of ESRD risk could improve postdonation risk assessment and counseling for them.
Following an initial drop in renal function at the time of donation,7,8 renal function stabilizes within months of nephrectomy and thereafter increases over time for most living kidney donors.9,10,11 However, donors with similar baseline characteristics may not tolerate the insult of nephrectomy equally well. A study of patients who experienced acute kidney injury reported that progression to stage 4 chronic kidney disease (CKD) was more common among patients with lower postrecovery eGFR values,12 suggesting that incomplete recovery of function after acute kidney injury is an early marker of long-term risk. However, it is unknown whether the same holds true for living kidney donors, who do not experience the same pathobiology as acute kidney injury but do lose half of their nephron mass in one operation.
Early postdonation identification of donors at increased risk of ESRD could improve surveillance and provide opportunities for intervention to reduce ESRD risk. Consensus recommendations for postdonation care for living kidney donors include annual evaluations of postdonation eGFR.13 Since 2013, the Organ Procurement and Transplantation Network (OPTN) has required transplant programs to measure serum creatinine levels at 6 months, 1 year, and 2 years postdonation.14 However, this follow-up incurs substantial burden for transplant programs; barriers include donors not wanting to return for follow-up, out-of-date contact information, and lack of reimbursement to donors or transplant programs for the costs of follow-up.15 Consequently, many programs have struggled to meet required targets for follow-up rates.16 Since the clinical utility of eGFR measured in the first 2 years postdonation is unknown and thought by many to be premature and of modest value for anticipating long-term outcomes, the mandate for early donor follow-up is controversial in the transplant community.17 To better understand the association between early postdonation renal function and subsequent ESRD risk in living kidney donors, we conducted a cohort study using national registry data on all living kidney donors in the United States.
Methods
Data Source
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere.18 The Health Resources and Services Administration (HRSA) of the US Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
This study was a secondary analysis of a deidentified dataset and was classified as exempt and not human subjects research by the institutional review board of Johns Hopkins University. As a result, informed consent procedures were not required.
Study Population
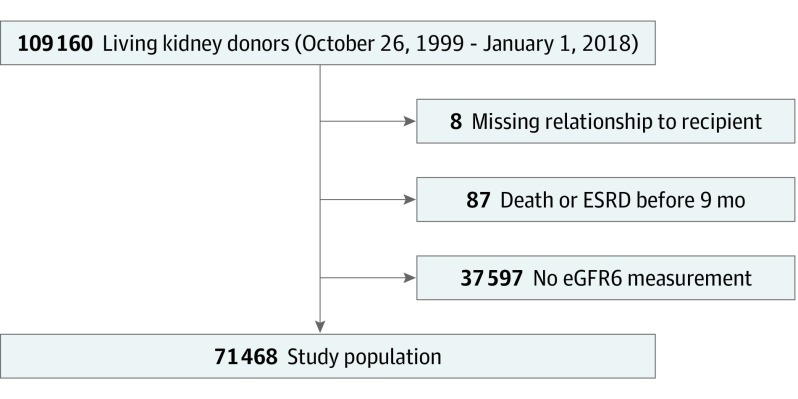
The study population consisted of living kidney donors donating between October 26, 1999, and January 1, 2018, whose familial relationship to the recipient was reported to the OPTN, who were alive and free of ESRD at 9 months postdonation, and who had at least 1 serum creatinine value reported to the OPTN at 6 months postdonation (±3 months, to allow a range of reporting from the transplant program) (Figure 1). To compare characteristics of individuals who experienced ESRD with those who did not, we used rank sum tests for continuous variables and χ2 tests for categorical variables.
Figure 1. Flow Diagram.
Flow diagram illustrating study population and exclusion criteria. eGFR6 indicates the estimated glomerular filtration rate at 6 months postdonation; ESRD, end-stage renal disease.
Exposure and Outcome Ascertainment
Measurements of eGFR predonation and at 6 months postdonation were calculated using the Chronic Kidney Disease–Epidemiology Collaboration (CKD-EPI) equation.19 Donor race/ethnicity was classified as black or African American vs all other reported races/ethnicities based on transplant-program reporting to the OPTN. Donors were followed up from the date of donation to death, ESRD diagnosis, or the end of study (December 31, 2018). Death was ascertained through standard OPTN follow-up and linkage to the Social Security Death Master File, as previously reported.20 Incident ESRD was ascertained from the US Centers for Medicare & Medicaid Services form 2728, as previously reported.3,4
eGFR and ESRD
We modeled the association between eGFR at 6 months postdonation (eGFR6) and ESRD using Cox regression. Martingale residual plots were used to assess the functional form of the eGFR. To assess whether eGFR6 was a proxy for predonation eGFR, we constructed an additional model with predonation eGFR instead of eGFR6 as an exposure, as well as a model including both predonation and postdonation eGFR values. The Sobel test was used to test whether eGFR6 mediated the association between predonation eGFR and ESRD. Potential confounders were chosen based on predonation covariates previously shown to be associated with ESRD risk in living kidney donors in the United States.4 All models were adjusted for predonation characteristics: age at donation, donor sex, donor race, age/race interaction, donor body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), and the presence of a first-degree biological relationship of the donor to the kidney recipient. Multiple imputation with chained equations was used21 to impute missing BMI values (in 4.9% of all records) and predonation eGFR values (in 1.2% of all records).
Absolute Postdonation eGFR vs Change in eGFR
Postdonation eGFR may be considered as an absolute measurement or as the change in eGFR from predonation to postdonation values. To determine whether absolute postdonation eGFR value was a better marker of postdonation ESRD than change in eGFR, we compared 3 Cox models: one of absolute eGFR6; one of predonation eGFR − eGFR6 (ie, the difference between the 2 measurements); and one of eGFR6 / predonation eGFR (ie, the ratio of the 2 measurements or the percentage decrement). All models were adjusted for predonation characteristics. We compared the 3 models using the Akaike information criterion (AIC) to determine which model best fit the observed data. (A lower AIC indicates better model fit.) Since the multiple imputation with chained equations procedure does not yield an AIC, these models did not use multiple imputation. Instead, only for the purposes of comparing model fit, BMI was removed and individuals missing predonation eGFR data were excluded.
Statistical Analysis
All analyses were performed using Stata version 15.0/MP for Linux (StataCorp). All P values less than .05 were considered statistically significant. Data were analyzed from March 2017 to October 2019.
Results
Study Population
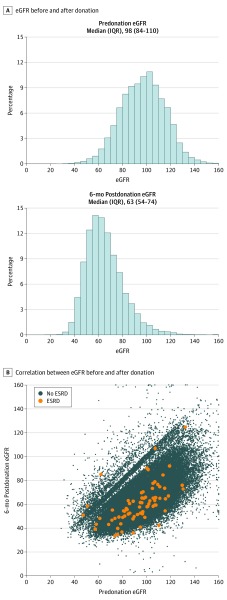
A total of 71 468 living kidney donors were included (of 109 065 total donors over this period [65.5%]). The median age of the included donors was 42 (interquartile range [IQR], 33-51) years; 44 193 (61.8%) were women. Characteristics of donors in the study population were comparable with characteristics of donors excluded because of the nonreporting of eGFR6 values (Table 1). Among the 71 468 living kidney donors, the median eGFR levels were 98 (IQR, 84-110) mL/min/1.73 m2 predonation and 63 (54-74) mL/min/1.73 m2 at 6 months postdonation (Figure 2A). The correlation (r2) between predonation eGFR and eGFR6 values was 0.63 (Figure 2B). Living kidney donors who experienced ESRD had lower eGFR6 levels (median, 58.5 [IQR, 49.7-66.0] mL/min/1.73 m2 vs 63.1 [IQR, 54.1-73.9 mL/min/1.73 m2]; P = .005) (eTable in the Supplement). Those who experienced ESRD postdonation were also more likely to be black (17 of 75 [22.7%] vs 7768 of 71 393 [10.9%]; P = .001) and first-degree relatives of their recipients (51 of 75 [68.0%] vs 33 675 of 71 373 [47.2%]; P < .001).
Table 1. Comparison of Study Participants vs Those Excluded Because of Nonreporting of Estimated Glomerular Filtration Rate (eGFR) 6 Months After Donation.
| Characteristic | Individuals, No. (%) | |
|---|---|---|
| Study Population (n = 71 468) | Excluded (n = 37 597) | |
| Age at donation, median (IQR), y | 42 (33-51) | 40 (31-48) |
| Predonation eGFR, median (IQR), mL/min/1.73 m2 | 97.6 (84.2-109.9) | 98.4 (84.7-111.0) |
| Male | 27 275 (38.2) | 15 895 (42.3) |
| Black | 7785 (10.9) | 5303 (14.1) |
| First-degree relative to recipient | 33 726 (47.2) | 20 551 (54.7) |
Abbreviation: IQR, interquartile range.
Figure 2. Distribution of Predonation and 6-Month Postdonation Estimated Glomerular Filtration Rate (eGFR) in Living Kidney Donors.
ESRD indicates end-stage renal disease; IQR, interquartile range.
eGFR and ESRD
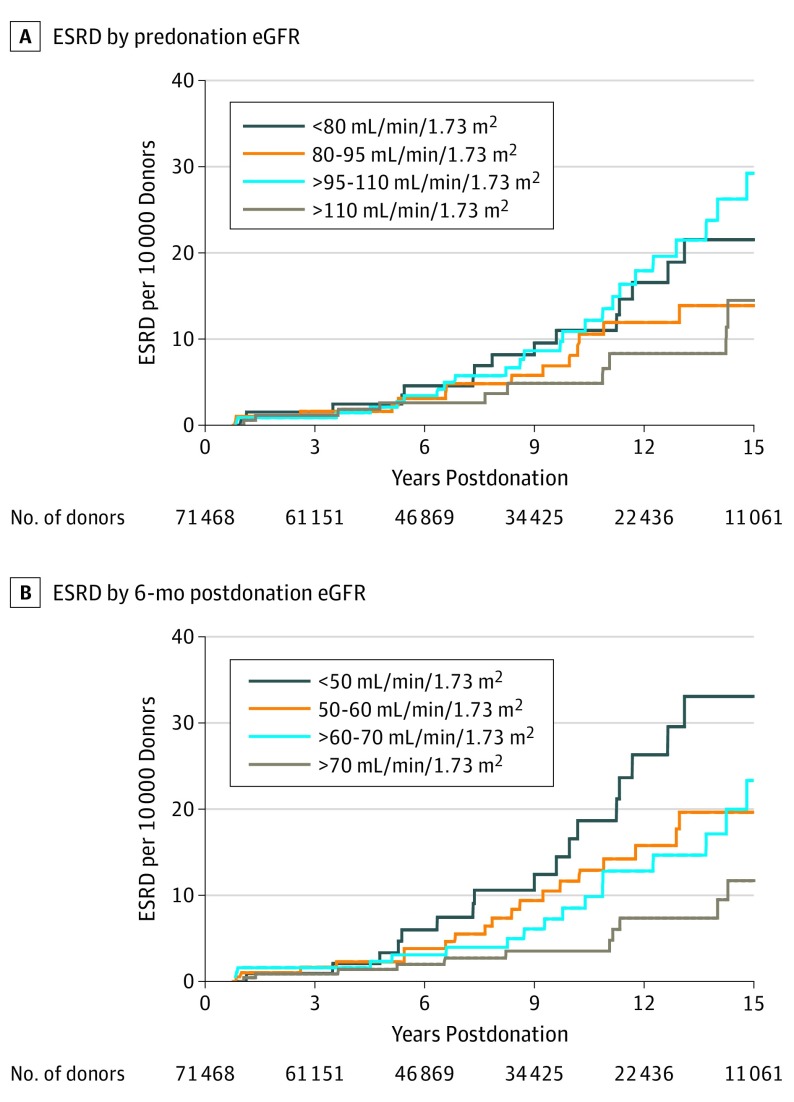
Predonation eGFR was not associated with postdonation ESRD in either an unadjusted analysis (Figure 3A) or an adjusted model (adjusted hazard ratio [aHR] associated with 10-mL/min/1.73 m2 lower eGFR level, 1.10 [95% CI, 0.96-1.26]; P = .18). However, postdonation eGFR was associated with ESRD: stratified by eGFR6, the 15-year cumulative incidence of ESRD ranged from 11.7 donors per 10 000 donors with eGFR6 values greater than 70 mL/min/1.73 m2 to 33.1 donors per 10 000 donors with eGFR6 values of 50 mL/min/1.73 m2 or less (P = .049) (Figure 3B), and in an adjusted model, a 10-mL/min/1.73 m2–lower eGFR6 was significantly associated with a 28% increased risk of ESRD (aHR, 1.28 [95% CI, 1.06-1.54]; P = .009). Postdonation eGFR values also strongly dominated predonation eGFR values in prognosticative value: in an adjusted model containing both predonation eGFR and eGFR6 values, the hazard ratio for the predonation eGFR value was very close to null (aHR, 1.00 [95% CI, 0.86-1.17]; P = .97), while the association between the eGFR6 value and ESRD remained significant (aHR, 1.28 [95% CI, 1.04-1.57]; P = .02) (Table 2). The Sobel test of mediation was statistically significant (t = −0.0109 [SE, 0.0048]; P = .02).
Figure 3. Cumulative Incidence of End-Stage Renal Disease (ESRD) in Living Kidney Donors .
Stratified by predonation estimated glomerular filtration rate (eGFR) (A) and postdonation eGFR (B).
Table 2. Association Between Estimated Glomerular Filtration Rate (eGFR) Predonation and at 6 Months Postdonation, and End-Stage Renal Disease Riska.
| eGFR Measurement | Hazard Ratio (95% CI) | ||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| Predonation | 1.10 (0.96-1.26) | NA | 1.00 (0.86-1.17) |
| 6 mo Postdonation | NA | 1.28 (1.06-1.54) | 1.28 (1.04-1.57) |
Abbreviation: NA, not applicable.
Reported as hazard ratio per 10-unit lower eGFR level; all models adjusted for donor age, race, sex, body mass index, and first-degree relationship to recipient.
Absolute Postdonation eGFR Value vs Change in eGFR Value
The median change in eGFR from predonation eGFR to eGFR6 values was 33 (IQR, 24-41) mL/min/1.73 m2. The median ratio of eGFR6 to predonation eGFR was 0.65 (IQR, 0.59-0.74). Based on the model that best fit the observed data, eGFR6 was a better marker of postdonation eGFR than change from predonation eGFR to eGFR6 values, based on model fit (AIC: eGFR6, 1495.61; predonation eGFR − eGFR6, 1503.58; eGFR6 / predonation eGFR, 1502.30).
Discussion
In this national study of postdonation eGFR as an early marker of ESRD risk in 71 468 living kidney donors, we found that eGFR in the first 6 months postdonation was associated with subsequent ESRD risk (aHR per 10-mL/min/1.73 m2 lower eGFR, 1.28 [95% CI, 1.06-1.54]). This association dominated predonation eGFR, which appeared to be fully mediated by postdonation eGFR (aHR of predonation eGFR after adjusting for postdonation eGFR, 1.00 [95% CI, 0.86-1.17]; P = .97). Absolute postdonation eGFR6 value (AIC, 948.86) was a better marker of subsequent ESRD risk than the drop from predonation eGFR to postdonation eGFR.
These results allow better understanding of the association between donor eGFR and long-term ESRD risk. Our findings suggest that donors with similar predonation eGFR values may not equally tolerate the 50% nephron mass reduction resulting from donor nephrectomy. Our study expands and elaborates on previous reports of an association between predonation eGFR and subsequent ESRD risk in living kidney donors.22,23,24 In this first study (to our knowledge) to evaluate the role of postdonation eGFR in subsequent ESRD risk, we show that any association between predonation eGFR value and postdonation ESRD risk is dominated by what happens postdonation. In other words, while the predonation eGFR is of value when the postdonation eGFR is not known, its greatest value is as a surrogate of postdonation eGFR value.
Other risk factors for ESRD in donors include older age (in nonblack donors only), male sex, black race, BMI, a first-degree biological relationship to the kidney recipient, and a history of cigarette smoking.4,5,24 Older donors and black donors are also at greater risk of rehospitalization and postdonation hypertension.25,26
Prior studies suggest that after nephrectomy, an initial drop in eGFR is generally followed by an increasing eGFR in time among living donors; eGFR increases more gradually among donors with a family history of ESRD.11,27 This is in contrast with eGFR trajectories after nephrectomy associated with cancer, in which eGFR tends to rise over the first 3 to 6 months postnephrectomy and then stay constant.28 It is likely that trajectories of eGFR over time would confer additional information about ESRD risk. Nevertheless, eGFR6 provides an early and useful biomarker for postdonation risk in donors. Donors with higher baseline risk based on predonation characteristics may particularly benefit from a 6-month postdonation follow-up. Donors with lower eGFR6 values may particularly benefit from close monitoring to optimize their long-term health profile and outcomes.
Although we have demonstrated an increased risk of ESRD for donors with reduced eGFR6 values, these findings should be interpreted in the context of low overall risk of ESRD postdonation; for example, in this study, the mean unadjusted 15-year risk of ESRD ranged from 11.7 per 10 000 donors with eGFR6 levels greater than 70 min/mL/1.73 m2 to 33.1 per 10 000 donors with eGFR6 levels less than or equal to 50 min/mL/1.73 m2.
This study demonstrated a strong association between eGFR6 values and ESRD. Although the OPTN has also attempted to collect 12-month and 24-month postdonation creatinine levels since 1999 and 2009, respectively, this study was not able to estimate the added value of these measurements. Loss to follow-up at 12 months has historically been higher than at 6 months,29 and the registry does not yet contain enough 12-month or 24-month follow-up values to ascertain a separate association between 12-month or 24-month postdonation eGFR levels and ESRD. Nevertheless, it is likely that longitudinal measurement of postdonation eGFR levels will further improve risk assessment for living donors. These results strongly support the continued collection of early postdonation eGFR measurements.
Limitations
This study must be understood in the context of its limitations. The study population consists only of donors who had serum creatinine levels reported 6 months postdonation; however, reporting of postdonation serum creatinine levels is driven largely by factors unlikely to confound analyses, including secular trends and program-level variations in practice. Donors excluded because of a lack of serum creatinine level reported at 6 months were comparable with donors included in the study with regards to age, race, sex, predonation eGFR, and biological relationship to the kidney recipient, so selection bias is unlikely. The eGFR value is estimated via the CKD-EPI creatinine equation; although the CKD-EPI equation incorporating cystatin C levels is associated with true GFR values with greater accuracy, the OPTN does not collect cystatin C data.30 Even an optimal estimated GFR is only an estimate and may not represent true GFR. In the case of donors with low predonation eGFR values, it is likely that donor suitability was determined through other means (eg, nuclear studies). In interpreting postdonation eGFR values in donors, clinicians should remember that no estimating equations currently available for estimating GFR were developed using living donor populations, and these equations may not accurately estimate true GFR in living donors without intrinsic renal disease.31 Nevertheless, even if creatinine eGFR value estimates true GFR imperfectly in donors, this study demonstrates that postdonation eGFR value in the way that we have estimated it has clinically relevant, prognostic value for living donors.
Conclusions
In conclusion, we have shown that postdonation eGFR values, calculated from a single serum creatinine measured around 6 months postdonation, is strongly associated with subsequent risk of ESRD in living kidney donors. These findings underscore the importance of early postdonation follow-up of living kidney donors, despite the logistical challenges, and support continued national collection of postdonation serum creatinine levels by the OPTN. The transplant community should continue to work to improve postdonation compliance with existing postdonation follow-up requirements and should support efforts to expand and extend the clinical follow-up of donors. Clinicians can use these findings to reassure donors with relatively high postdonation eGFR levels and identify donors with low postdonation eGFR levels for possible increased surveillance and targeted interventions to slow their progression to chronic kidney disease.
eTable. Characteristics of donors by ESRD status.
References
- 1.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2016 annual data report: kidney. Am J Transplant. 2018;18(suppl 1):-. doi: 10.1111/ajt.14557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mjoen G, Hallan S, Hartmann A, et al. Long-term risks for kidney donors. Kidney Int. 2013. [DOI] [PubMed] [Google Scholar]
- 3.Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586. doi: 10.1001/jama.2013.285141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Massie AB, Muzaale AD, Luo X, et al. Quantifying postdonation risk of ESRD in living kidney donors. J Am Soc Nephrol. 2017;28(9):2749-2755. doi: 10.1681/ASN.2016101084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Locke JE, Reed RD, Massie A, et al. Obesity increases the risk of end-stage renal disease among living kidney donors. Kidney Int. 2017;91(3):699-703. doi: 10.1016/j.kint.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibrahim HN, Foley RN, Reule SA, et al. Renal function profile in white kidney donors: the first 4 decades. J Am Soc Nephrol. 2016;27(9):2885-2893. doi: 10.1681/ASN.2015091018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Timsit MO, Nguyen KN, Rouach Y, et al. Kidney function following nephrectomy: similitude and discrepancies between kidney cancer and living donation. Urol Oncol. 2012;30(4):482-486. doi: 10.1016/j.urolonc.2010.04.005 [DOI] [PubMed] [Google Scholar]
- 8.Kasiske BL, Anderson-Haag T, Ibrahim HN, et al. A prospective controlled study of kidney donors: baseline and 6-month follow-up. Am J Kidney Dis. 2013;62(3):577-586. doi: 10.1053/j.ajkd.2013.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi KH, Yang SC, Joo DJ, et al. Clinical assessment of renal function stabilization after living donor nephrectomy. Transplant Proc. 2012;44(10):2906-2909. doi: 10.1016/j.transproceed.2012.05.086 [DOI] [PubMed] [Google Scholar]
- 10.Kasiske BL, Anderson-Haag T, Israni AK, et al. A prospective controlled study of living kidney donors: three-year follow-up. Am J Kidney Dis. 2015;66(1):114-124. doi: 10.1053/j.ajkd.2015.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matas AJ, Vock DM, Ibrahim HN. GFR ≤25 years postdonation in living kidney donors with (vs. without) a first-degree relative with ESRD. Am J Transplant. 2018;18(3):625-631. doi: 10.1111/ajt.14525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawhney S, Marks A, Fluck N, et al. Post-discharge kidney function is associated with subsequent ten-year renal progression risk among survivors of acute kidney injury. Kidney Int. 2017;92(2):440-452. doi: 10.1016/j.kint.2017.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lentine KL, Kasiske BL, Levey AS, et al. Summary of Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline on the evaluation and care of living kidney donors. Transplantation. 2017;101(8):1783-1792. doi: 10.1097/TP.0000000000001770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Organ Procurement and Transplantation Network Policy 18: living donor data submission requirements. https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf. Published 2016. Accessed December 6, 2019.
- 15.Waterman AD, Dew MA, Davis CL, et al. Living-donor follow-up attitudes and practices in U.S. kidney and liver donor programs. Transplantation. 2013;95(6):883-888. doi: 10.1097/TP.0b013e31828279fd [DOI] [PubMed] [Google Scholar]
- 16.Henderson ML, Thomas AG, Shaffer A, et al. The national landscape of living kidney donor follow-up in the United States. Am J Transplant. 2017;17(12):3131-3140. doi: 10.1111/ajt.14356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leichtman A, Abecassis M, Barr M, et al. ; Living Kidney Donor Follow-Up Conference Writing Group . Living kidney donor follow-up: state-of-the-art and future directions, conference summary and recommendations. Am J Transplant. 2011;11(12):2561-2568. doi: 10.1111/j.1600-6143.2011.03816.x [DOI] [PubMed] [Google Scholar]
- 18.Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant. 2014;14(8):1723-1730. doi: 10.1111/ajt.12777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Segev DL, Muzaale AD, Caffo BS, et al. Perioperative mortality and long-term survival following live kidney donation. JAMA. 2010;303(10):959-966. doi: 10.1001/jama.2010.237 [DOI] [PubMed] [Google Scholar]
- 21.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30(4):377-399. doi: 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 22.Grams ME, Sang Y, Levey AS, et al. ; Chronic Kidney Disease Prognosis Consortium . Kidney-failure risk projection for the living kidney-donor candidate. N Engl J Med. 2016;374(5):411-421. doi: 10.1056/NEJMoa1510491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wainright JL, Robinson AM, Wilk AR, Klassen DK, Cherikh WS, Stewart DE. Risk of ESRD in prior living kidney donors. Am J Transplant. 2018;18(5):1129-1139. doi: 10.1111/ajt.14678 [DOI] [PubMed] [Google Scholar]
- 24.Matas AJ, Berglund DM, Vock DM, Ibrahim HN. Causes and timing of end-stage renal disease after living kidney donation. Am J Transplant. 2018;18(5):1140-1150. doi: 10.1111/ajt.14671 [DOI] [PubMed] [Google Scholar]
- 25.Schold JD, Goldfarb DA, Buccini LD, et al. Hospitalizations following living donor nephrectomy in the United States. Clin J Am Soc Nephrol. 2014;9(2):355-365. doi: 10.2215/CJN.03820413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lentine KL, Schnitzler MA, Xiao H, et al. Consistency of racial variation in medical outcomes among publicly and privately insured living kidney donors. Transplantation. 2014;97(3):316-324. doi: 10.1097/01.TP.0000436731.23554.5e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellini MI, Charalampidis S, Stratigos I, Dor FJMF, Papalois V. The effect of donors’ demographic characteristics in renal function post-living kidney donation. analysis of a UK single centre cohort. J Clin Med. 2019;8(6):E883. doi: 10.3390/jcm8060883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel HD, Pierorazio PM, Johnson MH, et al. Renal functional outcomes after surgery, ablation, and active surveillance of localized renal tumors: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2017;12(7):1057-1069. doi: 10.2215/CJN.11941116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schold JD, Buccini LD, Rodrigue JR, et al. Critical factors associated with missing follow-up data for living kidney donors in the United States. Am J Transplant. 2015;15(9):2394-2403. doi: 10.1111/ajt.13282 [DOI] [PubMed] [Google Scholar]
- 30.Inker LA, Schmid CH, Tighiouart H, et al. ; CKD-EPI Investigators . Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20-29. doi: 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Londen M, Wijninga AB, de Vries J, et al. Estimated glomerular filtration rate for longitudinal follow-up of living kidney donors. Nephrol Dial Transplant. 2018;33(6):1054-1064. doi: 10.1093/ndt/gfx370 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Characteristics of donors by ESRD status.