Key Points
Question
Can brain imaging predict future psychiatric symptoms in children?
Findings
In this 4-year longitudinal cohort study, distinct patterns of resting-state functional connectivity in healthy children predicted changes in psychiatric symptoms. Weaker positive dorsolateral prefrontal connectivity with medial prefrontal cortex predicted a better developmental trajectory for attentional symptoms, whereas weaker positive dorsolateral prefrontal connectivity with subgenual anterior cingulate cortex predicted a worse trajectory for internalizing symptoms (eg, anxiety/depression).
Meaning
Brain imaging measures can contribute to early identification of children at risk for common psychiatric disorders and thus identify children in need of preventive treatments.
Abstract
Importance
Understanding the neurodevelopmental trajectory of psychiatric symptoms is important for improving early identification, intervention, and prevention of mental disorders.
Objective
To test whether the strength of the coupling of activation between specific brain regions, as measured by resting-state functional magnetic resonance imaging (fMRI), predicted individual children’s developmental trajectories in terms of attentional problems characteristic of attention-deficit/hyperactivity disorder and internalizing problems characteristics of major depressive disorder (MDD).
Design, Setting, and Participants
A community cohort of 94 children was recruited from Vanderbilt University between 2010 and 2013. They were followed up longitudinally for 4 years and the data were analyzed from 2016 to 2019. Based on preregistered hypotheses and an analytic plan, we examined whether specific brain connectivity patterns would be associated with longitudinal changes in scores on the Child Behavior Checklist (CBCL), a parental-report assessment used to screen for emotional, behavioral, and social problems and to predict psychiatric illnesses.1,2
Main Outcomes and Measures
We used the strength of resting-state fMRI connectivity at age 7 years to predict subsequent changes in CBCL measures 4 years later and investigated the mechanisms of change by associating brain connectivity changes with changes in the CBCL.
Results
We analyzed data from a longitudinal brain development study involving children assessed at age 7 years (n = 94; 41 girls [43.6%]) and 11 years (n = 54; 32 girls [59.3%]). As predicted, less positive coupling at age 7 years between the medial prefrontal cortex and dorsolateral prefrontal cortex (DLPFC) was associated with a decrease in attentional symptoms by age 11 years (t49 = 2.38; P = .01; β = 0.32). By contrast, a less positive coupling between a region implicated in mood, the subgenual anterior cingulate cortex (sgACC), and DLPFC at age 7 years was associated with an increase in internalizing (eg, anxiety/depression) behaviors by age 11 years (t49 = −2.4; P = .01; β = −0.30). Logistic regression analyses revealed that sgACC-DLPFC connectivity was a more accurate predictor than baseline CBCL measures for progression to a subclinical score on internalization (t50 = −2.61; P = .01; β = −0.29). We then replicated and extended the sgACC-DLPFC result in an independent sample of children with (n = 25) or without (n = 18) familial risk for MDD.
Conclusions and Relevance
These resting-state fMRI metrics are promising biomarkers for the early identification of children at risk of developing MDD or attention-deficit/hyperactivity disorder.
This cohort study examines the ability of resting-state networks to predict the developmental trajectory of symptoms associated with attention-deficit/hyperactivity disorder and major depressive disorder in US children.
Introduction
The regulation of cognition and emotion is thought to depend on the top-down modulation of multiple neural circuits by the prefrontal cortex and, in particular, the dorsolateral prefrontal cortex (DLPFC).3,4,5,6,7 Prefrontal-dependent cognitive control mechanisms that regulate attention and mood likely play a key role in mental health.7,8 There is ample evidence of attenuation or failure of top-down control mechanisms in adults with depression,9,10,11 anxiety,12 and attention-deficit/hyperactivity disorder (ADHD).13 Given that these prevalent mental health problems often emerge during childhood and adolescence,14,15,16 it is important to know whether dysregulated top-down control can be detected even before behavioral symptoms are evident.
The strength of coupling between regions involved in top-down control and their targets can be measured with resting-state functional magnetic resonance imaging (rs-fMRI). Brain regions that are highly temporally correlated during rest-form resting-state networks (RSNs), which are intrinsic, spontaneous, low-frequency fluctuations in the fMRI blood oxygen level–dependent signal that define specific networks of the brain in the absence of any task.17 They reveal great heterogeneity in the functional organization of the brain. In fact, they may be considered “fingerprints” of the human brain, as they can accurately identify an individual from a large group (N = 126).18 Furthermore, RSN profiles are known to be robust and reliable.18,19,20,21,22,23,24
Resting-state networks are particularly relevant for studying psychiatric and pediatric populations because they are (1) task-independent, so individual differences in task performance cannot explain differences observed in the blood oxygen level–dependent data, (2) easy and fast to acquire, which make them accessible to many people, including young children and various clinical populations, and (3) and plastic, been shown to change during typical development,25 and can be modulated by behavioral26,27 or pharmacological interventions.28,29
An RSN that is particularly relevant for mental health is the Central Executive Network (CEN), of which the DLPFC is a key node. The CEN has been associated with externally focused attention30 and goal-directed behavior.31,32,33 In neurotypical adults, the CEN is negatively correlated (ie, anticorrelated) with the default mode network (DMN), an RSN associated with internal mentation and self-referential processing, whose key nodes include the medial prefrontal cortex (MPFC).34,35,36,37,38
The decoupling of these RSNs has been found to be adaptive: stronger MPFC-DLPFC anticorrelations are associated with superior cognitive control and cognitive performance in adults, such as greater working memory capacity.39,40,41,42 In addition, there is an increase with age in the magnitude of anticorrelations between the MPFC and DLPFC in typically developing children,25 which is consistent with the findings that top-down control mechanisms improve markedly over childhood and adolescence.43 Resting-state fMRI studies have also shown an association between diminished MPFC-DLPFC anticorrelations and cognitive impairment in ADHD.44
The CEN also plays a role in regulating mood through its interactions with the subgenual anterior cingulate cortex (sgACC). The sgACC is part of the affective network, which is involved in emotion processing45,46,47,48,49,50,51,52 and has anatomical connections to the hypothalamus, amygdala, entorhinal cortex, nucleus accumbens, and other limbic structures.49 There are several lines of evidence showing that top-down modulation of the sgACC is dysregulated in adults with major depressive disorder (MDD). Neuroimaging studies have reported decreased metabolisms and decreased gray matter volumes53 and a decreased number of glia in sgACC54 in patients with MDD. Furthermore, deep brain stimulation of the sgACC results in an attenuation of hyperactivation in sgACC and increased activation in previously underactive DLPFC in adults with MDD.55 In addition, the left DLPFC region that shows maximal anticorrelation with the sgACC in rs-fMRI has been identified as an optimal target for transcranial magnetic stimulation (TMS) of MDD.56 The sgACC has also been shown to exhibit decreased connectivity with cognitive control regions in children with a history of preschool depression.57 Finally, left DLPFC and sgACC exhibit anticorrelation in children at familial risk for MDD.58
In sum, prior research on patient and familial high-risk populations reveals that atypically strong functional connectivity between DLPFC and MPFC is characteristic of ADHD, whereas atypically weak connectivity between DLPFC and the sgACC is a characteristic of MDD. Here, we build on this prior work by asking whether the strength of the connectivity between these regions can predict a progression toward attentional or mood disorders in a longitudinal study of a community pediatric sample not selected for risk of ADHD or MDD.
Specifically, we tested whether DLPFC-MPFC and DLPFC-sgACC connectivity at age 7 years predict scores at age 11 years on a questionnaire used to screen children for behavioral problems, the Child Behavior Checklist (CBCL). The goals of this research were 2-fold: first, to better understand how changes in brain connectivity over childhood are associated with cognitive and affective development, and second, to evaluate the predictive validity of DLPFC-MPFC and DLPFC-sgACC connectivity for future mental health problems in children who have not been identified previously as being at elevated risk for developing a psychiatric disorder.
Numerous studies have demonstrated high reliability between the CBCL scales and actual psychiatric diagnosis.59,60 For example, CBCL attention problem scores are used for the screening and prediction of ADHD.61,62 A subthreshold elevation on the anxiety/depression subscale of the CBCL in preadolescence predicts future development of MDD.63 However, in conjunction with behavioral measures, neuroimaging measures may identify children at the greatest risk for developing psychiatric disorders with greater confidence and at an earlier age.
Therefore, in this study, we investigated whether rs-fMRI data could predict future CBCL scores in a community sample of 54 children. Specifically, we tested whether the individual differences in MPFC-DLPFC connectivity at age 7 years predict subsequent changes in attention 4 years later, as measured by the CBCL attentional problems measure at age 11 years. Additionally, we performed an exploratory analysis to investigate whether individual differences in sgACC-DLPFC connectivity at age 7 years predict subsequent changes in anxiety/depression 4 years later, as measured by the CBCL “internalization” and anxiety/depressed subscale at age 11 years. We preregistered our hypotheses through the Open Science Framework (OSF; https://osf.io/6cgbs/). Because of space limitations, we report only major results here; the Supplement reports additional findings, as well as a null result based on the preregistered hypotheses.
Methods
Participants
Ninety-four participants were included who were enrolled in a developmental longitudinal study, “Predicting Late-Emerging Reading Disability” (Vanderbilt University; principal investigator, L.C.). In this sample, 77 children (82%) met behavioral criteria for typical development; 17 children (18%) were identified as being at risk for a late-emerging reading disability. Time 1 (or baseline) data were collected from participants at age 7 years (n = 94; 41 girls [43.6]) and subsequently at 1-year intervals for 4 years. Data at time 4 were available for 54 of the original participants (57.4%) (see eMethods in the Supplement for exclusion criteria). The CBCL subscale scores at baseline did not differ significantly between those who did and did not complete the study (attentional problems, t91 = 1.0; P = .33; internalization, t91 = 0.51; P = .61; anxiety/depression, t91 = 0.41; P = .68; Table). The study was approved by the institutional review board at Vanderbilt University and written informed consent was received from all participants.
Table. Child Behavior Checklist Measures for Time 1 at Age 7 Years and Time 4 at Age 11 Yearsa.
| Time | Attention | Internalization | Anxiety/Depression | Withdrawn | Somatic |
|---|---|---|---|---|---|
| Time 1 | |||||
| Mean (SD) | 56.29 (8.13) | 160.02 (13.02) | 53.27 (5.3) | 53.59 (5.48) | 53.37 (5.48) |
| Subclinical (>60), No. (%) | 24 (25) | 10 (11) | 13 (14) | 14 (15) | 9 (10) |
| Time 4 | |||||
| Mean (SD) | 54 (7.46) | 160.13 (14.02) | 53.11 (5.54) | 53.15 (6.32) | 53.87 (4.86) |
| Subclinical (>60), No. (%) | 10 (20) | 6 (11) | 9 (17) | 8 (15) | 9 (17) |
| P (time 1/time 4) | 0.09 | 0.9 | 0.87 | 0.88 | 0.89 |
| Mean Change | −1.4 | 1.27 | 0.5 | −0.17 | 1.17 |
| Range Change | [−16 to 12] = 28 | [−41 to 32] = 73 | [−17 to 12] = 29 | [−12 to 15] = 27 | [−17 to 12] = 29 |
Higher scores indicate worse problems. A Child Behavior Checklist score of 60 to 70 (>1 SD, <2 SD) is generally considered to represent a medium level of symptoms (subclinical or subthreshold).
CBCL Scoring and Data Acquisition
The CBCL assesses behavioral problems and competencies of children ages 6 to 18 years based on parental reports (eMethods in the Supplement). Data were acquired at Vanderbilt University Institute of Imaging Science on a 3-T Philips Achieva magnetic resonance spectroscopy scanner with a 32-channel head coil. One 5.9-minute resting-state echoplanar imaging scan was collected with the following parameters: TR = 2200 milliseconds, TE = 30 milliseconds, 35 slices, 3-mm isotropic voxels.
rs-fMRI Analyses
The rs-fMRI data were analyzed in CONN (NeuroImaging Tools & Resources Collaboratory),64 which incorporates methods to minimize the association of head motion artifacts and allow for valid identification of correlated and anticorrelated networks22 (see eMethods in the Supplement for a complete description of image preprocessing/denoising, seed selection, bivariate correlation, and independent component analysis).
Longitudinal Analyses
Fisher-transformed r-maps from the MPFC seed were submitted to a second-level analysis of covariance regressing the changes in the CBCL measures (time 4–time 1) onto brain responses, controlling for the effect of initial severity (baseline CBCL). To create a robust prediction model that could be generalized to new cases, we performed leave-1-out cross-validation, which minimizes potential biases due to voxel selection in our predictive models (eMethods in the Supplement).
We previously found that the magnitude of MPFC-DLPFC anticorrelations grow during typical development,25 as does executive function, so we first implemented a whole-brain MPFC seed-based approach to determine whether MPFC-DLPFC correlations at time 1 were associated with future change in CBCL attentional symptoms after controlling for the baseline attentional score. Second, we implemented a more targeted approach by testing whether the baseline connectivity between the MPFC and the DLPFC mask derived from the previous study25 predicts future attentional symptoms. Finally, we ran an exploratory analysis using an independent component analysis–defined DLPFC-sgACC component to test whether the connectivity of this component predicted change of internalization symptoms and subsequently examined the internalization subscales separately: (1) anxiety/depression, (2) withdrawn behavior, and (3) somatic complaints.
Logistic Regression for CBCL Internalization (and Anxiety/Depression Subscale)
As per CBCL diagnostic category definitions, we subdivided participants into a “subclinical” category for individuals with a CBCL internalization (and anxiety/depression) score of 55 or greater and a “typical” category for those whose scores on this subscale fell below this cutoff based on the literature.63 We used a logistic regression of initial severity (baseline CBCL scores) and baseline resting-state measures combined with leave-1-out cross-validation. We did not have enough participants with subclinical scores for the CBCL attentional problems at time 4 to perform this logistic regression for that CBCL scale. Finally, we correlated the changes in connectivity with changes in CBCL measures over 4 years (from age 7 years to age 11 years).
Conceptual Replication/Clinical Extension
We tested the prediction model on an independent sample of 25 youths between ages 8 to 14 years identified as being at familial risk for MDD as well as 18 age-matched children without familial risk for MDD. We used baseline sgACC-DLPFC connectivity to predict the progression of CBCL anxiety/depression 3 years later (eTable in the Supplement).
Results
Behavioral Results and Head Motion
Between ages 7 and 11 years, 14 children (26%) had significant changes in internalizing scores (9 [17%] showing more internalizing problems at age 11 years and 5 [9%] showing fewer) and 8 children (15%) had significant changes in attentional problem scores (1 [2%] showing more attentional problems at age 11 years and 17 [3%] showing fewer). The mean (SD) number of outliers across all points was 17 of 160 (21) points. Excluding these points preserved enough data to achieve a stable estimate of RSNs65 (eMethods in the Supplement).
Neuroimaging Results
Cross-sectional analyses at time 1 (N = 94) revealed that, on average, children age 7 years did not exhibit the significant negative MPFC-DLPFC correlations that are evident in adults but rather exhibited positive MPFC-DLPFC correlations on the whole, consistent with prior findings from children ages 8 to 12-years25 (eFigure 1 in the Supplement). We had predicted in our preregistration that there would be insufficient variance in the CBCL attentional scores to establish a significant brain-CBCL association in this sample. Indeed, we did not observe any significant correlations between the MPFC-DLPFC connectivity and CBCL attentional scores at a height threshold of P < .001 (t92 > 3.40) uncorrected (or even at a liberal threshold of P < . 01 uncorrected).
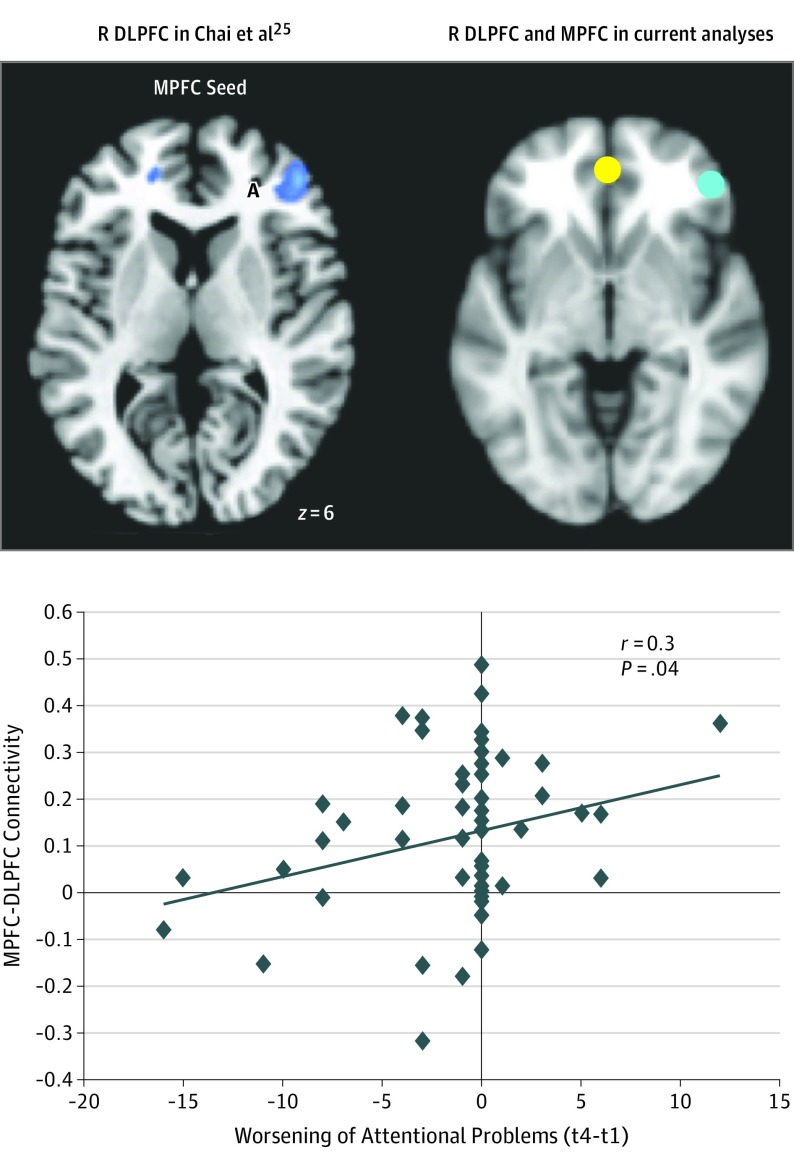
Although there was minimal average change in CBCL scores, there was considerable interparticipant variability in the change of CBCL scores across 4 years. Here, we used baseline neuroimaging data at age 7 years to predict CBCL change over 4 years. Less positive MPFC-DLFPC correlations at time 1 were associated with improvement of attentional problems 4 years later (t49 = 2.38, P = .01, controlling for medication; t49 = 1.02, P = .03, controlling for those children who received a diagnosis of ADHD; t50 = 2.36, P = .01 without controlling for participants with ADHD participants; reported P values are 1-sided because of our a priori and preregistered hypotheses) (eFigure 2 in the Supplement). Because we implemented this analysis using leave-1-out cross-validation, this is a prediction as opposed to a simple correlation, a distinction that is frequently lost in the neuroimaging literature.66 Furthermore, we found that less positive MPFC-DLPFC (a priori mask25) correlations at time 1 were associated with an improvement of attentional problems 4 years later (r = 0.3; P = .04; Figure 125).
Figure 1. Longitudinal Prediction of Change in Attentional Problems From Ages 7 to 11 Years.
Baseline medial prefrontal cortex and dorsolateral prefrontal cortex (MPFC-DLPFC) (a priori mask) anticorrelations were associated with changes in attentional problems 4 years later. Negative change scores indicate improvement and positive change scores indicate decline over 4 years. The peak MPFC coordinates are −1, 47, −4 and the peak coordinates for the DLPFC mask are 46, 46, 6. R indicates right; time 1, t 1; time 4, t 4.
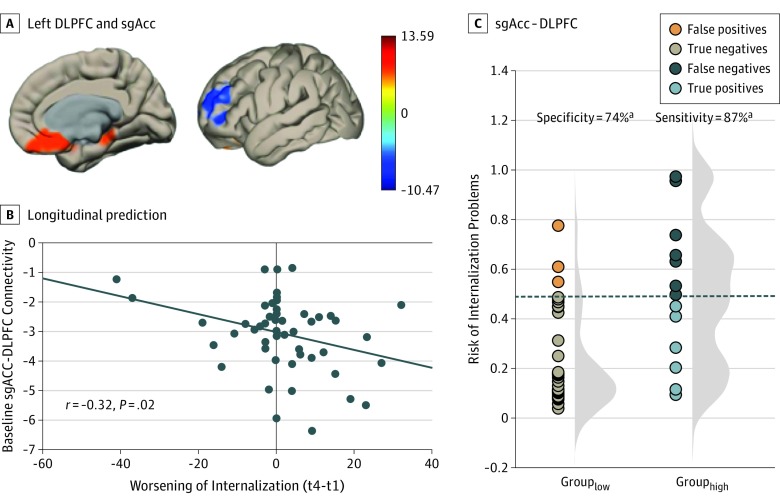
Weaker left DLPFC-sgACC connectivity at baseline predicted a greater worsening of internalization CBCL scores by time 4 (t49 = −2.4; P = .01; controlling for medication; Figure 2) and (t49 = −2.15; P = .02, controlling for ADHD) and (t50 = −2.61; P = .01, not controlling for ADHD or medication). Specifically, weaker left DLPFC-sgACC connectivity (or stronger anticorrelations) predicted greater worsening on the internalization subscales of anxiety/depression (t49 = −2.64; P = .005, controlling for medication) and withdrawn (t49 = −2.38; P = .01, controlling for medication). By contrast, left DLPFC-sgACC connectivity was not associated with changes in somatic complaints (t49 = −0.88; P = .19, controlling for medication). Based on our previous work,58 we had hypothesized that the sgACC-DMN connectivity would predict a worsening of internalization in our preregistration; however, this analysis did not reach current statistical threshold standards (eMethods in the Supplement).
Figure 2. Longitudinal Prediction of Change in Internalization Problems From Ages 7 to 11 Years.
A, Left dorsolateral prefrontal cortex (DLPFC) and subgenual anterior cingulate cortex (sgACC) predicted change in internalization (and anxiety/depression and withdrawn subscales) such that a greater anticorrelation at time 1 (age 7 years) predicted a worsening of internalization 4 years later (age 11 years). B, Scatterplot of longitudinal prediction. Negative change scores indicate improvement and positive change scores indicate decline over 4 years. C, Logistic regression using sgACC-DLPFC to predict internalization problems at time 4 (≥55), controlling for internalization scores at time 1 (t 1). The histograms represent the distribution of the risk of internalization problems at time 4 (t 4) (as predicted by sgACC-DLPFC connectivity at time 1) displayed separately for those participants with low (left) vs high (right) internalization problem scores at time 4.
Logistic Regression
Logistic regression analyses revealed that sgACC-DLPFC connectivity was a more accurate predictor than baseline CBCL measures for progression to a subclinical score on internalization (t50 = −2.61; P = .01; Figure 2). This analysis yielded 77% accuracy, 87% sensitivity, and 74% specificity.
Association of Brain Connectivity Changes With Changes in the CBCL and Conceptual Replication/Clinical Extension
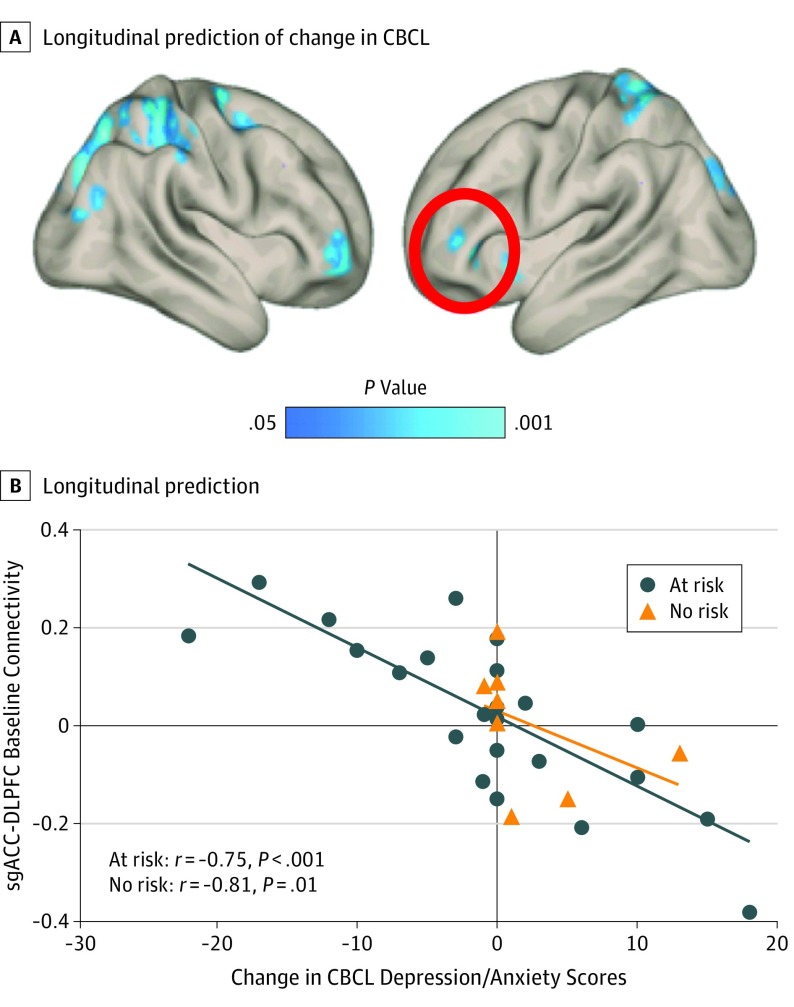
An increase in MPFC-DLPFC anticorrelations correlated with an improvement of CBCL attentional scores. and an increase in sgACC-DLPFC anticorrelations correlated with a worsening of CBCL anxiety/depression scores over 4 years (eFigures 3 and 4 in the Supplement). Weaker DLPFC-sgACC connectivity (or stronger anticorrelations) at baseline predicted worsening on the internalization subscale of anxiety/depression 3 years later for children at familial risk for MDD as well as a new sample of typically developing children (at-risk: r = −0.75; P < .001; controls: r = −0.81; P = .01; Figure 3 and eFigure 3 in the Supplement).
Figure 3. Conceptual Replication/Clinical Extension.
A, Longitudinal prediction of change in Child Behavior Checklist (CBCL) anxiety/depression symptoms over 3 years in children with (blue) or without (red) familial risk for depression. Baseline resting-state subgenual anterior cingulate cortex–dorsolateral prefrontal cortex (sgACC-DLPFC) connectivity predicted a change of anxiety/depression, such that less positive correlations at time 1 predicted a worsening of anxiety/depression scores 3 years later. B, Scatterplot of longitudinal prediction. Negative change scores indicate improvement, and positive change scores indicate decline over 3 years.
Discussion
The RSNs at age 7 years in a community sample of children predicted the developmental trajectory of symptoms associated with ADHD and MDD at age 11 years. The variations in functional connectivity occurred in neural systems that are known to be salient for attention or mood. Weaker positive MPFC-DLPFC correlations at age 7 years predicted improved attention scores at age 11 years, whereas weaker positive sgACC-left DLPFC correlations at age 7 years predicted a worsening of MDD symptoms (internalization) at age 11 years. It is noteworthy that most children with attentional problems at age 7 years exhibited reduced symptoms at age 11 years, whereas most children with internalizing symptoms at age 7 years exhibited more symptoms at age 11 years. Thus, our functional connectivity measures appear to be sensitive to resilience and vulnerability.
The associations between specific neural networks and specific longitudinal declines are consistent with prior findings. That a stronger positive MPFC-DLPFC coupling was associated with a worse developmental trajectory for attention is consistent with the hypothesis that anticorrelated MPFC-DLPFC activation is associated with the ability to selectively focus attention on internal thoughts vs external stimuli.67 Weaker anticorrelations between MPFC and DLPFC, which are core nodes of DMN and CEN, respectively, may reflect an attenuation of top-down control mechanisms and an inability to allocate resources away from internal thoughts and feelings and toward external stimuli to adaptively perform difficult tasks.67,68,69 Thus, children age 7 years who exhibit MPFC-DLPFC anticorrelations may have the capacity to toggle between internal and external foci of attention more readily than those who do not. The failure to decouple these networks may be an early indicator of attentional problems or may preclude the development of age-appropriate attentional skills.
That stronger sgACC-left DLPFC anticorrelations predicted a future worsening of internalization, characteristic of MDD, is consistent with the MDD and at-risk literature.70 One study found a reduction of left DLPFC-sgACC rs-fMRI connectivity in children at familial risk for MDD, for which the at-risk group had significant anticorrelations while the not-at-risk group had positive correlations.58 Furthermore, left DLPFC-sgACC anticorrelations have been used to identify individually specific targets for TMS in patients with MDD.56 Stronger sgACC-left DLPFC anticorrelations at this young age may already reflect an attenuation or failure of top-down control mechanisms that are evident in adult MDD. Thus, the functional connectivity of specific neural systems in middle childhood forecasts individuals’ vulnerability or resilience in cognition and emotion over the ensuing 4 years of development.
These findings extend the use of neuroimaging to identify childhood neuromarkers of risk for psychopathology from highly selected children, such as those with identified familial risk, to a sample of children more representative of the population as a whole. Although children with parents who have had depression are at an elevated risk for developing depression, most children who develop depression do not come from families with an identified history of depression.63 Further, the longitudinal nature of this study supports the validity of using RSNs to predict the worsening of psychiatric symptoms in childhood.
Although variation in RSNs forecasts the developmental trajectory of attentional and emotional symptoms, there is strong evidence that such networks are plastic, and thus may be altered by supportive interventions. Resting-state functional connectivity is thought to reflect habitual network activations that can be remodeled by various long-term71 and even brief behavioral interventions26,27,72,73 and pharmacological interventions.29
Limitations
First, although some children developed subclinical scores on CBCL measures by age 11 years, we do not know which children have subsequently converted to psychiatric diagnoses. However, elevated scores on the CBCL measures, such as internalization (including anxiety/depression), are highly predictive of near-term conversion to psychiatric diagnoses.63 Second, the current sample size was too small to make any meaningful interpretations for the subset of participants who moved between clinical categories over time. Third, our targeted, hypothesized-driven approach could be viewed by some readers as a limitation of the study.
Conclusions
These findings not only further our understanding of the neurobiological vulnerabilities that foster the deterioration of mental health, but also could inform early identification and preventative treatment. Identification of risk at the level of individual children may be strengthened by large multilevel data sets that integrate multimodal neuroimaging, genetics, and social factors with new statistical tools.74 These findings illustrate the idea that the neurobiological seeds of future psychopathology are becoming visible and measurable in children.
eMethods.
eTable. Table of cluster statistics and locations from Figure 3
eFigure 1. On average, children 7 years of age exhibit positive MPFC-DLPFC resting state connectivity
eFigure 2. Longitudinal prediction of progression of attentional problems over four years (ages 7-11)
eFigure 3. Increase in MPFC-DLPFC anticorrelations correlates with improvement of CBCL attentional scores over 4 years.
eFigure 4. Increase sgACC-DLPFC anticorrelations correlates with a worsening of CBCL anxiety/depression scores over 4 years.
References
- 1.Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- 2.Petty CR, Rosenbaum JF, Hirshfeld-Becker DR, et al. The child behavior checklist broad-band scales predict subsequent psychopathology: A 5-year follow-up. J Anxiety Disord. 2008;22(3):532-539. doi: 10.1016/j.janxdis.2007.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stuss D, Knight R. Principles of Frontal Lobe Functions. 2nd ed New York, NY: Oxford University Press; 2012. [Google Scholar]
- 4.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9(5):242-249. doi: 10.1016/j.tics.2005.03.010 [DOI] [PubMed] [Google Scholar]
- 5.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167-202. Review. doi: 10.1146/annurev.neuro.24.1.167 [DOI] [PubMed] [Google Scholar]
- 6.Uchida M, Biederman J, Gabrieli JD, et al. Emotion regulation ability varies in relation to intrinsic functional brain architecture. Soc Cogn Affect Neurosci. 2015;10(12):1738-1748. doi: 10.1093/scan/nsv059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Etkin A, Büchel C, Gross JJ. The neural bases of emotion regulation. Nat Rev Neurosci. 2015;16(11):693-700. doi: 10.1038/nrn4044 [DOI] [PubMed] [Google Scholar]
- 8.Cole MW, Repovš G, Anticevic A. The frontoparietal control system: a central role in mental health. Neuroscientist. 2014;20(6):652-664. doi: 10.1177/1073858414525995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27(33):8877-8884. doi: 10.1523/JNEUROSCI.2063-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12(8):467-477. doi: 10.1038/nrn3027 [DOI] [PubMed] [Google Scholar]
- 11.Kaiser RH, Whitfield-Gabrieli S, Dillon DG, et al. Dynamic resting-state functional connectivity in major depression. Neuropsychopharmacology. 2016;41(7):1822-1830. doi: 10.1038/npp.2015.352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mogg K, Bradley BP. Anxiety and attention to threat: cognitive mechanisms and treatment with attention bias modification. Behav Res Ther. 2016;87:76-108. doi: 10.1016/j.brat.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 13.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69(4):680-694. doi: 10.1016/j.neuron.2011.01.020 [DOI] [PubMed] [Google Scholar]
- 14.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9(12):947-957. doi: 10.1038/nrn2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keshavan MS, Giedd J, Lau JY, Lewis DA, Paus T. Changes in the adolescent brain and the pathophysiology of psychotic disorders. Lancet Psychiatry. 2014;1(7):549-558. doi: 10.1016/S2215-0366(14)00081-9 [DOI] [PubMed] [Google Scholar]
- 16.Visser HA, van Minnen A, van Megen H, et al. The relationship between adverse childhood experiences and symptom severity, chronicity, and comorbidity in patients with obsessive-compulsive disorder. J Clin Psychiatry. 2014;75(10):1034-1039. doi: 10.4088/JCP.13m08825 [DOI] [PubMed] [Google Scholar]
- 17.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537-541. doi: 10.1002/mrm.1910340409 [DOI] [PubMed] [Google Scholar]
- 18.Finn ES, Shen X, Scheinost D, et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci. 2015;18(11):1664-1671. doi: 10.1038/nn.4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Damoiseaux JS, Rombouts SA, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103(37):13848-13853. doi: 10.1073/pnas.0601417103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen S, Ross TJ, Zhan W, et al. Group independent component analysis reveals consistent resting-state networks across multiple sessions. Brain Res. 2008;1239:141-151. doi: 10.1016/j.brainres.2008.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shehzad Z, Kelly AMC, Reiss PT, et al. The resting brain: unconstrained yet reliable. Cereb Cortex. 2009;19(10):2209-2229. doi: 10.1093/cercor/bhn256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103(1):297-321. doi: 10.1152/jn.00783.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuo X-N, Di Martino A, Kelly C, et al. The oscillating brain: complex and reliable. Neuroimage. 2010;49(2):1432-1445. doi: 10.1016/j.neuroimage.2009.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuo X-N, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. Neuroimage. 2010;49(3):2163-2177. doi: 10.1016/j.neuroimage.2009.10.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chai J, Ofen N, Gabrieli JD, Whitfield-Gabrieli S. Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. J Cogn Neurosci. 2014;26(3):501-513. doi: 10.1162/jocn_a_00517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan M, Zhu H, Qiu C, et al. Group cognitive behavioral therapy modulates the resting-state functional connectivity of amygdala-related network in patients with generalized social anxiety disorder. BMC Psychiatry. 2016;16:198. doi: 10.1186/s12888-016-0904-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McFadden KL, Cornier M-A, Melanson EL, Bechtell JL, Tregellas JR. Effects of exercise on resting-state default mode and salience network activity in overweight/obese adults. Neuroreport. 2013;24(15):866-871. doi: 10.1097/WNR.0000000000000013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salinas FS, Szabó CÁ. Resting-state functional connectivity changes due to acute and short-term valproic acid administration in the baboon model of GGE. Neuroimage Clin. 2017;16:132-141. doi: 10.1016/j.nicl.2017.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitfield-Gabrieli S, Fischer AS, Henricks AM, et al. Understanding marijuana’s effects on functional connectivity of the default mode network in patients with schizophrenia and co-occurring cannabis use disorder: A pilot investigation. Schizophr Res. 2018;194:70-77. doi: 10.1016/j.schres.2017.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673-9678. doi: 10.1073/pnas.0504136102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimamura AP. The role of the prefrontal cortex in dynamic filtering. Psychobiology. 2000;28:207-218. doi: 10.3758/BF03331979 [DOI] [Google Scholar]
- 32.Olson EA, Luciana M. The development of prefrontal cortex functions in adolescence: theoretical models and a possible dissociation of dorsal versus ventral subregions In: Nelson CA, Luciana M, eds. Handbook of Developmental Cognitive Neuroscience. 2nd ed Cambridge, MA: MIT Press; 2008:575-590. [Google Scholar]
- 33.Best JR, Miller PH. A developmental perspective on executive function. Child Dev. 2010;81(6):1641-1660. doi: 10.1111/j.1467-8624.2010.01499.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676-682. doi: 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self: an event-related fMRI study. J Cogn Neurosci. 2002;14(5):785-794. doi: 10.1162/08989290260138672 [DOI] [PubMed] [Google Scholar]
- 36.Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440-457. doi: 10.1016/j.neuroimage.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 37.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1-38. doi: 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- 38.Whitfield-Gabrieli S, Moran JM, Nieto-Castañón A, Triantafyllou C, Saxe R, Gabrieli JDE. Associations and dissociations between default and self-reference networks in the human brain. Neuroimage. 2011;55(1):225-232, 34. doi: 10.1016/j.neuroimage.2010.11.048 [DOI] [PubMed] [Google Scholar]
- 39.Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39(1):527-537. doi: 10.1016/j.neuroimage.2007.08.008 [DOI] [PubMed] [Google Scholar]
- 40.Hampson M, Driesen N, Roth JK, Gore JC, Constable RT. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magn Reson Imaging. 2010;28(8):1051-1057. doi: 10.1016/j.mri.2010.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barber AD, Caffo BS, Pekar JJ, Mostofsky SH. Developmental changes in within- and between-network connectivity between late childhood and adulthood. Neuropsychologia. 2013;51(1):156-167. doi: 10.1016/j.neuropsychologia.2012.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keller JB, Hedden T, Thompson TW, Anteraper SA, Gabrieli J, Whitfield-Gabrieli S. Resting-state anticorrelations between medial and lateral prefrontal cortex: association with working memory, aging, and individual differences. Cortex. 2015;64:271-280. doi: 10.1016/j.cortex.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bunge SA, Toga AW. Introduction to section II: frontal lobe development In: Stuss DT, Knight RT, eds. Principles of Frontal Lobe Function. New York, NY: Oxford University Press; 2013:93-98. [Google Scholar]
- 44.Mattfeld A, Gabrieli J, Biederman J, et al. Brain differences between persistent and remitted attention deficit hyperactivity disorder. Brain. 2014;137(pt 9):2423-2428. doi: 10.1093/brain/awu137 [DOI] [PubMed] [Google Scholar]
- 45.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215-222. doi: 10.1016/S1364-6613(00)01483-2 [DOI] [PubMed] [Google Scholar]
- 46.Johansen-Berg H, Gutman DA, Behrens TE, et al. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18(6):1374-1383. doi: 10.1093/cercor/bhm167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kennedy SH, Evans KR, Krüger S, et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry. 2001;158(6):899-905. doi: 10.1176/appi.ajp.158.6.899 [DOI] [PubMed] [Google Scholar]
- 48.Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675-682. [DOI] [PubMed] [Google Scholar]
- 49.Ongür D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460(3):425-449. doi: 10.1002/cne.10609 [DOI] [PubMed] [Google Scholar]
- 50.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54(5):515-528. doi: 10.1016/S0006-3223(03)00171-9 [DOI] [PubMed] [Google Scholar]
- 51.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192-216. doi: 10.1038/npp.2009.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010;107(24):11020-11025. doi: 10.1073/pnas.1000446107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drevets WC, Price JL, Simpson JR, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824-827. doi: 10.1038/386824a0 [DOI] [PubMed] [Google Scholar]
- 54.Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95(22):13290-13295. doi: 10.1073/pnas.95.22.13290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651-660. doi: 10.1016/j.neuron.2005.02.014 [DOI] [PubMed] [Google Scholar]
- 56.Fox MD, Liu H, Pascual-Leone A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. Neuroimage. 2013;66:151-160. doi: 10.1016/j.neuroimage.2012.10.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gaffrey MS, Luby JL, Repovš G, et al. Subgenual cingulate connectivity in children with a history of preschool-depression. Neuroreport. 2010;21(18):1182-1188. doi: 10.1097/WNR.0b013e32834127eb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chai XJ, Hirshfeld-Becker D, Biederman J, et al. Altered intrinsic functional brain architecture in children at familial risk of major depression. Biol Psychiatry. 2016;80(11):849-858. doi: 10.1016/j.biopsych.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Warnick EM, Bracken MB, Kasl S. Screening efficiency of the child behavior checklist and strengths and difficulties questionnaire: a systematic review. Child Adolesc Ment Health. 2007;13:140-147. doi: 10.1111/j.1475-3588.2007.00461.x [DOI] [PubMed] [Google Scholar]
- 60.Biederman J, Faraone SV, Doyle A, et al. Convergence of the Child Behavior Checklist with structured interview-based psychiatric diagnoses of ADHD children with and without comorbidity. J Child Psychol Psychiatry. 1993;34(7):1241-1251. doi: 10.1111/j.1469-7610.1993.tb01785.x [DOI] [PubMed] [Google Scholar]
- 61.Lampert TL, Polanczyk G, Tramontina S, Mardini V, Rohde LA. Diagnostic performance of the CBCL-Attention Problem Scale as a screening measure in a sample of Brazilian children with ADHD. J Atten Disord. 2004;8(2):63-71. doi: 10.1177/108705470400800204 [DOI] [PubMed] [Google Scholar]
- 62.Hudziak JJ, Copeland W, Stanger C, Wadsworth M. Screening for DSM-IV externalizing disorders with the Child Behavior Checklist: a receiver-operating characteristic analysis. J Child Psychol Psychiatry. 2004;45(7):1299-1307. doi: 10.1111/j.1469-7610.2004.00314.x [DOI] [PubMed] [Google Scholar]
- 63.Uchida M, Fitzgerald M, Lin K, Carrellas N, Woodworth H, Biederman J. Can subsyndromal manifestations of major depression be identified in children at risk? Acta Psychiatr Scand. 2017;135(2):127-137. doi: 10.1111/acps.12660 [DOI] [PubMed] [Google Scholar]
- 64.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125-141. doi: 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- 65.Gabrieli JDE, Ghosh SS, Whitfield-Gabrieli S. Prediction as a humanitarian and pragmatic contribution from human cognitive neuroscience. Neuron. 2015;85(1):11-26. doi: 10.1016/j.neuron.2014.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49-76. doi: 10.1146/annurev-clinpsy-032511-143049 [DOI] [PubMed] [Google Scholar]
- 67.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106(4):1279-1284. doi: 10.1073/pnas.0809141106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Northoff G. Spatiotemporal psychopathology I: no rest for the brain’s resting state activity in depression? Spatiotemporal psychopathology of depressive symptoms. J Affect Disord. 2016;190:854-866. doi: 10.1016/j.jad.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 69.Connolly CG, Wu J, Ho TC, et al. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol Psychiatry. 2013;74(12):898-907. doi: 10.1016/j.biopsych.2013.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harmelech T, Preminger S, Wertman E, Malach R. The day-after effect: long term, Hebbian-like restructuring of resting-state fMRI patterns induced by a single epoch of cortical activation. J Neurosci. 2013;33(22):9488-9497. doi: 10.1523/JNEUROSCI.5911-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taren AA, Gianaros PJ, Greco CM, et al. Mindfulness meditation training and executive control network resting state functional connectivity: a randomized controlled trial. Psychosom Med. 2017;79(6):674-683. doi: 10.1097/PSY.0000000000000466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sacchet MD, Gotlib IH. Neurofeedback training for major depressive disorder: recent developments and future directions. Expert Rev Neurother. 2016;16(9):1003-1005. doi: 10.1080/14737175.2016.1199959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paulus MP, Thompson WK. The challenges and opportunities of small effects: the new normal in academic psychiatry. JAMA Psychiatry. 2019;76(4):353-354. doi: 10.1001/jamapsychiatry.2018.4540 [DOI] [PubMed] [Google Scholar]
- 74.Chai XJ, Castañón AN, Ongür D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59(2):1420-1428. doi: 10.1016/j.neuroimage.2011.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable. Table of cluster statistics and locations from Figure 3
eFigure 1. On average, children 7 years of age exhibit positive MPFC-DLPFC resting state connectivity
eFigure 2. Longitudinal prediction of progression of attentional problems over four years (ages 7-11)
eFigure 3. Increase in MPFC-DLPFC anticorrelations correlates with improvement of CBCL attentional scores over 4 years.
eFigure 4. Increase sgACC-DLPFC anticorrelations correlates with a worsening of CBCL anxiety/depression scores over 4 years.