Key Points
Question
Do patients who receive clinical care sooner after concussion recover faster?
Findings
In this cross-sectional study of 162 adolescent and young adult athletes with concussion, those who initiated clinical care earlier (within 7 days) recovered faster and were less likely to have prolonged (≥30-day) recovery times than those who initiated care later (at 8-20 days).
Meaning
Per this study, early initiation of care after a concussion may be warranted to expedite recovery time.
This cross-sectional study investigates the association of the time from a sports injury to the initiation of clinical care with recovery time after concussion in adolescents and young adults.
Abstract
Importance
Recovery after concussion varies, with adolescents taking longer (approximately 30 days) than adults. Many factors have been reported to influence recovery, including preinjury factors, perceptions about recovery, comorbid conditions, and sex. However, 1 factor that may play a role in recovery but has received little attention from researchers is the timeliness of clinical evaluation and care.
Objective
To investigate the association of time since injury with initiation of clinical care on recovery time following concussion.
Design, Setting, and Participants
This retrospective, cross-sectional study was conducted in a sports medicine clinic between August 2016 and March 2018. Eligible participants were aged 12 to 22 years and had a diagnosed, symptomatic concussion; patients were excluded if recovery data were incomplete. Participants were divided into 2 groups: those seen within 7 days of the injury (early) vs between 8 and 20 days of the injury (late). Data were analyzed between June 2019 and August 2019.
Exposures
Time from injury (concussion) to initiation of clinical care.
Main Outcomes and Measures
Recovery time; testing with the Post-Concussion Symptom Scale, Immediate Post-Concussion Assessment and Cognitive Testing, and Vestibular/Ocular Motor Screening instruments; demographic factors, medical history, and injury information.
Results
A total of 416 individuals were eligible, and 254 (61.1%) were excluded, leaving 162 (38.9%) in analyses. The early group (98 patients) and late group (64 patients) did not differ in age (mean [SD] age, early, 15.3 [1.6] years; late, 15.4 [1.6] years), number of female patients (early, 51 of 98 [52.0%]; late, 40 of 64 [62.5%]), or other demographic, medical history, or injury information. The groups also were similar on symptom severity, cognitive, ocular, and vestibular outcomes at the first clinic visit. Results from a logistical regression supported being in the late group (adjusted odds ratio, 5.8 [95% CI, 1.9-17.6]; P = .001) and visual motion sensitivity symptoms greater than 2 (adjusted odds ratio, 4.5 [95% CI, 1.1-18.0]; P = .04) as factors significantly associated with recovery time.
Conclusions and Relevance
Findings suggest that earlier initiation of clinical care is associated with faster recovery after concussion. Other factors may also influence recovery time. Further research is needed to determine the role of active rehabilitation and treatment strategies, as well as demographic factors, medical history, and injury characteristics on the current findings.
Introduction
Concussion is a major public health concern that affects millions of adolescent and young adult athletes each year.1 Recovery following concussion, defined as the return to normal, preinjury status and experiencing no symptoms after exertion, can be variable because adolescents take a longer mean time to recover (approximately 30 days) than adults.2 Many other factors have been reported to be associated with recovery, including preinjury factors, perceptions about recovery, comorbid conditions, and sex.3,4,5,6,7,8,9,10,11 However, 1 factor that may play a role in recovery but has received little attention from researchers is the timeliness of clinical evaluation and care.
Researchers report that 57% of athletes with a concussion do not receive care for their injury beyond an initial evaluation or diagnosis at or near the time of injury.12 This lack of secondary prevention via follow-up clinical evaluation and care may result in prolonged recovery for those who do not seek care. Provision of care in the first few days after a concussion, especially active interventions that target specific symptoms and impairment, may play a pivotal role in influencing recovery.7,13,14,15,16 However, there has been a general reticence among clinicians treating concussion to engage in earlier active intervention because of perceptions that it may result in a prolonged recovery. Recent research suggests that earlier (ie, within the first few days following injury) subsymptom threshold aerobic exercise is not only safe but can also enhance and accelerate recovery.17 This active approach to treating concussion is dependent on timely access to care to facilitate earlier and more effective interventions. In spite of these findings, researchers have yet to examine the role of time from injury to clinical care on recovery and associated clinical outcomes.
The purpose of the current study was to investigate the association of time since injury to initiation of clinical care with recovery time among adolescent and young adult athletes after concussion. We compared groups of athletes who were seen within the first 7 days postinjury (the early group) with a group seen within 8 to 20 days postinjury (the late group). We expected that athletes who received care earlier would demonstrate a more rapid recovery than those who received care later. Groups were also compared for differences on demographic factors (eg, age, sex), medical history (eg, concussion, migraine, psychiatric history, or attention-deficit/hyperactivity disorder or learning disorder), injury information (eg, loss of consciousness, posttraumatic amnesia, or disorientation or confusion), and initial clinical outcomes (ie, symptoms in cognitive, ocular, and vestibular domains) at the first clinic visit. Finally, we wanted to examine the role of time to the first clinic visit relative to other factors (ie, demographic factors; medical history and injury information; cognitive, ocular, and vestibular symptoms) in prognosticating recovery time.
Methods
Design and Participants
This study involved a cross-sectional review of clinical and demographic data from ongoing research at a sport medicine concussion clinic, gathered between August 2016 and March 2018. Eligible participants included patients between the ages of 12 and 22 years with a diagnosed, symptomatic sport-associated concussion, per current consensus guidelines.18 Exclusion criteria included moderate to severe traumatic brain injury, neurological disorder, or preexisting vestibular disorder (eg, Ménière disease, vertigo).
Measures
Demographic, Medical History, and Injury Information
Participants provided demographic data including age, sex, and sports played. Medical history data included the number of previously diagnosed concussions and a history of migraine and psychiatric disorders. Injury information included the date of injury and initial signs and symptoms. All data were self-reported during a standardized clinical interview.
Concussion Symptoms
The Post-Concussion Symptom Scale (PCSS) was used to measure concussion symptom severity.19 The PCSS is a computerized self-reported symptom inventory that includes 22 items on physical, cognitive, affective, and sleep-associated concussion symptoms. Participants rate each symptom item on a 7-point Likert scale, from 0 (none) to 6 (severe). The PCSS yields a total symptom severity score across all items, ranging from 0 to 132 points. The PCSS take about 2 to 3 minutes to complete.
Neurocognitive Performance
The Immediate Post-Concussion Assessment and Cognitive Testing (IMPACT) computerized test battery was used to measure neurocognitive performance.20 The IMPACT test includes demographic information, the 22-item PCSS, and 6 cognitive subtest modules. The 6 modules include 4 composite scores for verbal memory (yielding a percentage), visual memory (yielding a percentage), visual motor processing speed (yielding a number), and reaction time (in seconds), with higher scores equaling better performance on all composites except reaction time. IMPACT takes approximately 20 to 30 minutes to complete.
Vestibular and Ocular Function
The Vestibular/Ocular Motor Screening tool was used to measure vestibular and ocular symptoms and impairment.21 This tool consists of items on smooth pursuits, horizontal and vertical saccades, the near point of convergence, horizontal and vertical vestibular ocular reflexes, and visual motion sensitivity. Prior to administration, participants are asked to rate baseline level of symptoms in 4 domains (headache, dizziness, nausea, and fogginess) on a 0 to 10 scale, with 10 representing the highest symptom level. Following each item, participants are asked to provide a subjective rating of 0 to 10 in each of the 4 symptom domains to determine the degree of symptom provocation. The near point of convergence was assessed via both symptom report and the mean near point of convergence across 3 trials. The Vestibular/Ocular Motor Screening takes about 5 to 10 minutes to administer.
Recovery Time and Status
Clearance for a full return to play was defined per current consensus guidelines as being asymptomatic (or back to preinjury levels of symptoms) and back to preinjury levels of cognitive, ocular, and vestibular performance at rest and experiencing no increase in symptoms after exertion.18 Recovery time (in days) was calculated by subtracting the date of injury from the date of clearance for a full return to play. Recovery time was then divided into the recovery group categories 30 days or less or more than 30 days.
Procedures
The research was approved by the University of Pittsburgh institutional review board for human subjects research. Participants were informed about the risks and benefits of the study and completed informed written consent (adults) and informed parental consent with child assent (adolescents) prior to participating in the study. Participants were enrolled at their first clinic visit after a sports-associated concussion. At this visit, participants completed all assessments in the following order: (1) demographic factors and injury information, (2) PCSS, (3) IMPACT, and (4) Vestibular/Ocular Motor Screening. Assessments were administered by trained clinicians as part of a comprehensive clinical examination and interview. Participants were followed up at regular clinical intervals (approximately 5-7 days apart) to assess recovery status as a part of ongoing research.
Data Analysis
Descriptive statistics were used to describe the demographic factors, medical history, and injury information for the overall sample. Groups were compared on all descriptive and clinical outcome data using independent-sample t tests, with Bonferroni corrections for multiple comparisons for continuous variables or χ2 analyses for categorical variables. A logistic regression was used to examine the association of demographic, medical history, injury information, clinical outcomes, and time to the first clinic visit variables to recovery time (≤30 or >30 days). We used SPSS version 24 (IBM) with a P value less than .05 for all analyses. Data were analyzed between June 2019 and August 2019.
Results
Descriptive Data
A total of 416 participants met inclusion criteria. Of the 416 eligible participants, 254 (61.1%) were excluded because of incomplete recovery data, leaving 162 of 416 (38.9%) in the analyses (mean [SD] age, 15.3 [1.6] years; 91 of 162 participants [56.2%] were female). A total of 98 of 162 participants (60.5%) were seen in the clinic within 7 days (the early group), whereas 64 (39.5%) were seen after 8 to 20 days (the late group). Among participants included in the analyses, 46 concussions occurred during recreational sports (eg, playground activities, skateboarding, cycling) (early group, n = 21; late group, n = 25), while 116 occurred during organized sports (early group, n = 77; late group, n = 39). Sports played during injury included football (early group, n = 15; late group, n = 9), soccer (early group, n = 17; late: n = 9), hockey (early group, n = 18; late group, n = 6), basketball (early group, n = 6; late group, n = 3), cheerleading (early group, n = 3; late group, n = 4), and volleyball (early group, n = 5; late group, n = 2), among others (ie, baseball/softball, wrestling, field hockey, lacrosse, dance; n < 5 for each).
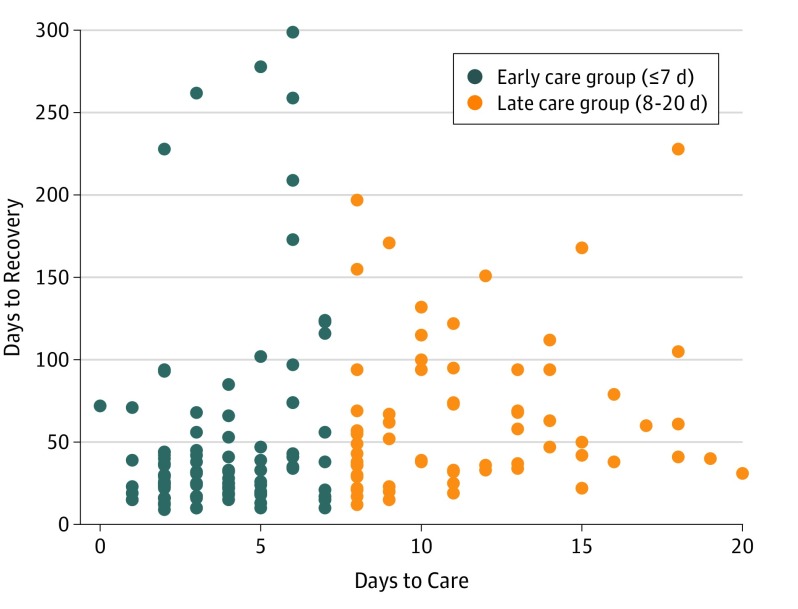
Recovery time for the sample ranged from 9 to 299 days, with a mean (SD) of 57.0 (55.9) days. A total of 63 of 162 participants (38.9%) recovered within 30 days, whereas 99 participants (61.1%) took more than 30 days to recover. A scatterplot of recovery time by days to the first clinical visit is provided in the Figure. A summary of all descriptive data for the overall sample is provided in Table 1. The groups did not differ on any demographic, medical history, or injury information variables. In addition, the groups were not different in the time from the first clinic visit to recovery.
Figure. Scatterplot of Number of Days to Recovery and Number of Days Until the First Clinical Visit.
Table 1. Comparison of Demographic, Medical History, and Injury Information Data Between Groups With Early and Late First Clinic Visitsa.
| Characteristic | Patients, No./Total No. (%) | ||
|---|---|---|---|
| Early Group | Late Group | Total | |
| Age, mean (SD), y | 15.3 (1.6) | 15.4 (1.6) | 15.3 (1.6) |
| Female | 51/98 (52.6) | 40/64 (62.5) | 91/162 (56.2) |
| Concussion history | 41/98 (41.8) | 20/64 (31.3) | 61/162 (37.7) |
| Migraine history | 32/98 (32.7) | 25/64 (39.1) | 57/162 (35.2) |
| Psychiatric history | 13/98 (13.3) | 6/64 (9.4) | 19/162 (11.7) |
| Attention-deficit/hyperactivity disorder or learning disorder | 7/97 (7.2) | 2/64 (3.1) | 7/161 (4.3) |
| Loss of consciousness | 9/98 (9.2) | 8/64 (12.5) | 17/162 (10.5) |
| Posttraumatic amnesia | 13/87 (14.9) | 13/63 (20.6) | 26/150 (17.3) |
| Confusion or disorientation | 20/87 (23.0) | 10/64 (15.6) | 30/151 (19.9) |
| First clinic visit to recovery, mean (SD), d | 47.4 (60.1) | 54.5 (46.7) | 51.0 (53.4) |
The early group attended a first clinic visit within 7 days (n = 98); the late group, between 8 and 20 days (n = 64).
Comparison of Initial Clinical Outcomes
A comparison of clinical outcome data between early and late clinic visit groups and for the overall sample is provided in Table 2. The groups did not differ on symptom severity, cognitive, ocular, and vestibular clinical outcomes. However, the late group had an adjusted odds ratio of 4.7 (95% CI, 2.2-9.9; P < .001) for a recovery of longer than 30 days than the early group (Table 3).
Table 2. Comparison of Clinical Outcomes Between Groups With Early and Late First Clinic Visitsa.
| Test | Early Group | Late Group | Total |
|---|---|---|---|
| Total symptom severity, mean (SD) | 32.0 (23.1) | 28.0 (18.5) | 30.4 (21.4) |
| Verbal memory, mean (SD), % | 77.9 (14.2) | 78.0 (15.6) | 77.9 (14.8) |
| Visual memory, mean (SD), % | 66.9 (13.7) | 66.8 (15.7) | 66.9 (14.5) |
| Visual motor processing speed, mean (SD), No. | 32.5 (7.3) | 33.2 (8.2) | 32.7 (7.6) |
| Reaction time, mean (SD), s | 0.70 (0.15) | 0.73 (0.21) | 0.71 (0.18) |
| Patients with scores of ≥2, No./total No. (%)b | |||
| Smooth pursuits | 65/83 (78.3) | 39/56 (69.6) | 104/139 (74.8) |
| Horizontal saccades | 66/83 (79.5) | 40/56 (71.4) | 106/139 (77.9) |
| Vertical saccades | 66/83 (79.5) | 41/56 (73.2) | 107/139 (77.0) |
| Convergence | 67/83 (80.7) | 41/56 (73.2) | 108/139 (77.8) |
| Horizontal vestibular ocular reflex | 67/82 (81.7) | 43/56 (76.8) | 110/138 (79.7) |
| Vertical vestibular ocular reflex | 64/80 (80.0) | 42/56 (75.0) | 106/136 (77.9) |
| Visual motion sensitivity | 67/80 (83.8) | 43/56 (76.8) | 114/136 (83.8) |
| Near point of convergence distance >5 cm, No./total No. (%) | 28/90 (31.1) | 17/57 (29.8) | 48/147 (32.7) |
| Recovery, mean (SD), d | 51.1 (60.5) | 66.0 (47.0) | 57.0 (55.9) |
The early group attended a first clinic visit within 7 days (n = 98); the late group, between 8 and 20 days (n = 64).
On a 0 to 10 scale via the Vestibular/Ocular Motor Screening tool.
Table 3. Comparison of Recovery Between Groups With Early vs Late First Clinic Visitsa.
| Recovery Time | Patients, No. (%) | ||
|---|---|---|---|
| Early Group | Late Group | Total | |
| ≤30 d | 51 (52.0) | 12 (19.0) | 63 (38.9) |
| >30 d | 47 (48.0) | 52 (81.3) | 99 (61.1) |
| Total | 98 (60.5) | 64 (39.5) | 162 (100.0) |
The early group attended a first clinic visit within 7 days (n = 98); the late group, between 8 and 20 days (n = 64).
Logistic Regression for Recovery Time
The logistic regression for recovery time was significant (Nagelkerke R2,0.29; P < .001). Table 4 provides a summary of all significant factors in the logistic regression model. As is evident from Table 4, the late group for the first clinical visit was associated with a 5.8-times increased likelihood of a recovery longer than 30 days (adjusted odds ratio, 5.8 [95% CI, 1.9-17.6]; P = .002). Additionally, a visual motion sensitivity score of more than 2 points was associated with a 4.5-times increased likelihood of a recovery longer than 30 days (adjusted odds ratio, 4.5 [95% CI, 1.1-18.0]; P = .04). All other demographic, medical history, injury information, and clinical outcome variables were not significantly associated with the logistic regression model for recovery time groups.
Table 4. Factors Significantly Associated With Recovery Time Groups, per Logistic Regressiona.
| Constant | Adjusted Odds Ratio | P Value |
|---|---|---|
| Late initiation of clinical care | 5.8 (1.9-17.6) | .002 |
| Visual motion sensitivity symptoms over clinical cutoff | 4.5 (1.1-18.0) | .04 |
The recovery time groups were those with 30 days or less or more than 30 days to recovery; the total group was 122 individuals.
Discussion
This study sought to investigate the association of time since injury to initiation of a first clinic visit with recovery time among adolescent and young adult athletes after concussion. Our hypothesis that earlier clinical care would be associated with a shorter recovery time was supported. Specifically, athletes who presented for evaluation within the first week of injury recovered faster than athletes who initially presented 2 to 3 weeks postinjury. When collectively examining demographic, medical history, injury information and clinical outcomes as factors associated with protracted recovery (>30 days), only time to clinical care and history of migraine were factors significantly associated with the outcome.
Our results support the notion that early clinical care is associated with faster recovery, similar to findings from other more severe forms of brain injury (eg, stroke).22 In this study, athletes who were evaluated within a week of injury recovered in a mean of 20 days faster than those athletes seen 2 to 3 weeks postinjury. One reasonable explanation for this difference is the earlier initiation of active rehabilitation strategies, including exertion progression17 and opportunity to start structured physical therapies (eg, on vestibular, vision, and cervical systems).23 Further, without clinical guidance and behavioral management recommendations postinjury, athletes may have been engaging in counterproductive recovery strategies, such as strict rest24 or excessive physical activity. This explanation is supported by the fact that athletes recovered in a similar amount of time after that first evaluation; as such, the days before initial evaluation were primarily accounting for the longer recovery duration, rather than time in which patients were under clinical care with a treatment plan. There is no reason to believe that group differences in clinical presentation (eg, a group with more severe injury or a greater likelihood of vestibular dysfunction) accounted for discrepancy in recovery time, because the groups did not differ on any clinical outcomes, including symptom severity, neurocognitive test performance, and the presence of vestibular or oculomotor dysfunction at the first visit. In short, it appears that all athletes had similar impairments and recovery time after they had received initial clinical care, highlighting the importance of clinical care as soon as possible.
Time to clinical care was a more robust factor associated with protracted recovery than many other constitutional risk factors and injury characteristics previously established as moderating factors of recovery.25 The early and late groups did not differ on any demographic factors, clinical characteristics, or the presence of preinjury risk factors. However, in addition to time to clinical care, a visual motion sensitivity symptom score greater than 2 points was associated with a protracted recovery time. Because there was no difference between groups in clinical outcomes, this result suggests that vestibular dysfunction may be associated with a protracted recovery regardless of the time to the first clinical visit, likely because of a referral for vestibular therapy.
Clinical Implications
There are several positive clinical implications of early clinical care reducing recovery duration. Among athletes playing at a competitive level, earlier care may lead to fewer games or competitions missed. Similarly, it is reasonable to speculate that student athletes may benefit from fewer missed days of school and may not fall behind in academic achievement if seen earlier. Beyond this, earlier care may mitigate iatrogenic outcomes of negative short-term management strategies, such as anxiety associated with excessive rest. Earlier clinical care after concussion may also have implications for the health care industry and insurance providers; it is unclear how earlier clinical care is associated with overall health care utilization throughout recovery, warranting further research.
Strengths
This novel study was, to our knowledge, the first to examine differences in recovery time and clinical outcomes among athletes who receive early and late initial clinical care. The study included a large sample with comprehensive clinical data available.
Limitations
There are notable limitations. This study was a retrospective, cross-sectional design, as such we only included those who had complete data in these analyses. Selection bias may be present because of the large number of patients excluded for incomplete recovery data. This study was not exhaustive in the examination of factors that may influence concussion recovery. Specifically, we did not report or track adherence to various rehabilitation strategies (ie, early exertion, vestibular therapy) and other treatment factors (eg, school attendance) that likely affect recovery outcomes. Another reason athletes do not receive early care postinjury is associated with the tendency to not report and remain in play through injury, which is a factor associated with protracted recovery26,27 that was not considered in this study. There are limitations to the generalizability of these findings to other populations, including military service members. Future research should evaluate if there are differences in the outcome reported in this study between younger patients (eg, children and adolescents) and older patients (eg, college-aged youths or older individuals) with concussion. In short, future research should focus on further understanding the specific mechanisms by which earlier health care postconcussion promotes faster recovery and determine if these findings apply to other subpopulations with concussion.
Conclusions
Athletes with concussions who presented for clinical care within the first week of injury recovered faster than athletes who did not receive care until 2 to 3 weeks postinjury. Visual motion sensitivity symptoms over clinical cutoff was the only significant clinical outcome with protracted recovery after considering time from injury, suggesting the time to first evaluation may be a factor robustly associated with recovery relative to other injury factors and constitutional risk factors in previously established literature. This study also demonstrated that once care was established, time to recovery did not differ for athletes evaluated within the first week of injury compared with those evaluated 2 to 3 weeks postinjury, indicating the days before initial evaluation were primarily accounting for longer recovery duration rather than time in which patients were under clinical care with a treatment plan. Education on injury and behavioral recommendations to optimize concussion recovery and earlier initiation of active rehabilitation strategies, including exertion and vestibular therapy, are plausible explanations for the association of a shorter recovery time with earlier care. However, future research should focus on the specific mechanisms by which earlier health care postconcussion promotes faster recovery and determine if these findings apply to other subpopulations, including military personnel.
References
- 1.Bryan MA, Rowhani-Rahbar A, Comstock RD, Rivara F; Seattle Sports Concussion Research Collaborative . Sports-and recreation-related concussions in US youth. Pediatrics. 2016;138(1):e20154635. doi: 10.1542/peds.2015-4635 [DOI] [PubMed] [Google Scholar]
- 2.Davis GA, Anderson V, Babl FE, et al. . What is the difference in concussion management in children as compared with adults? a systematic review. Br J Sports Med. 2017;51(12):949-957. doi: 10.1136/bjsports-2016-097415 [DOI] [PubMed] [Google Scholar]
- 3.Bock S, Grim R, Barron TF, et al. . Factors associated with delayed recovery in athletes with concussion treated at a pediatric neurology concussion clinic. Childs Nerv Syst. 2015;31(11):2111-2116. doi: 10.1007/s00381-015-2846-8 [DOI] [PubMed] [Google Scholar]
- 4.Tanveer S, Zecavati N, Delasobera EB, Oyegbile TO. Gender differences in concussion and postinjury cognitive findings in an older and younger pediatric population. Pediatr Neurol. 2017;70:44-49. doi: 10.1016/j.pediatrneurol.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 5.McLean SA, Kirsch NL, Tan-Schriner CU, et al. . Health status, not head injury, predicts concussion symptoms after minor injury. Am J Emerg Med. 2009;27(2):182-190. doi: 10.1016/j.ajem.2008.01.054 [DOI] [PubMed] [Google Scholar]
- 6.Novak Z, Aglipay M, Barrowman N, et al. ; Pediatric Emergency Research Canada Predicting Persistent Postconcussive Problems in Pediatrics (PERC 5P) Concussion Team . Association of persistent postconcussion symptoms with pediatric quality of life. JAMA Pediatr. 2016;170(12):e162900. doi: 10.1001/jamapediatrics.2016.2900 [DOI] [PubMed] [Google Scholar]
- 7.Zemek RL, Farion KJ, Sampson M, McGahern C. Prognosticators of persistent symptoms following pediatric concussion: a systematic review. JAMA Pediatr. 2013;167(3):259-265. doi: 10.1001/2013.jamapediatrics.216 [DOI] [PubMed] [Google Scholar]
- 8.Sandel NK, Lovell MR, Kegel NE, Collins MW, Kontos AP. The relationship of symptoms and neurocognitive performance to perceived recovery from sports-related concussion among adolescent athletes. Appl Neuropsychol Child. 2013;2(1):64-69. doi: 10.1080/21622965.2012.670580 [DOI] [PubMed] [Google Scholar]
- 9.Kostyun RO, Hafeez I. Protracted recovery from a concussion: a focus on gender and treatment interventions in an adolescent population. Sports Health. 2015;7(1):52-57. doi: 10.1177/1941738114555075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuen KM, Tsai YH, Lin WC, Yang CC, Huang SJ. Retrospectively evaluated preinjury personality traits influence postconcussion symptoms. Appl Neuropsychol Adult. 2016;23(5):322-332. doi: 10.1080/23279095.2015.1057638 [DOI] [PubMed] [Google Scholar]
- 11.Field M, Collins MW, Lovell MR, Maroon J. Does age play a role in recovery from sports-related concussion? a comparison of high school and collegiate athletes. J Pediatr. 2003;142(5):546-553. doi: 10.1067/mpd.2003.190 [DOI] [PubMed] [Google Scholar]
- 12.O’Kane JW, Spieker A, Levy MR, Neradilek M, Polissar NL, Schiff MA. Concussion among female middle-school soccer players. JAMA Pediatr. 2014;168(3):258-264. doi: 10.1001/jamapediatrics.2013.4518 [DOI] [PubMed] [Google Scholar]
- 13.Zuckerbraun NS, Atabaki S, Collins MW, Thomas D, Gioia GA. Use of modified acute concussion evaluation tools in the emergency department. Pediatrics. 2014;133(4):635-642. doi: 10.1542/peds.2013-2600 [DOI] [PubMed] [Google Scholar]
- 14.Resch JE, Brown CN, Macciocchi SN, Cullum CM, Blueitt D, Ferrara MS. A preliminary formula to predict timing of symptom resolution for collegiate athletes diagnosed with sport concussion. J Athl Train. 2015;50(12):1292-1298. doi: 10.4085/1062-6050-50.12.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dematteo C, Volterman KA, Breithaupt PG, Claridge EA, Adamich J, Timmons BW. Exertion testing in youth with mild traumatic brain injury/concussion. Med Sci Sports Exerc. 2015;47(11):2283-2290. doi: 10.1249/MSS.0000000000000682 [DOI] [PubMed] [Google Scholar]
- 16.Harmon KG, Clugston JR, Dec K, et al. . American Medical Society for Sports Medicine position statement on concussion in sport. Br J Sports Med. 2019;53(4):213-225. doi: 10.1136/bjsports-2018-100338 [DOI] [PubMed] [Google Scholar]
- 17.Leddy JJ, Haider MN, Ellis MJ, et al. . Early subthreshold aerobic exercise for sport-related concussion: a randomized clinical trial. JAMA Pediatr. 2019;173(4):319-325. doi: 10.1001/jamapediatrics.2018.4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCrory P, Meeuwisse W, Dvořák J, et al. . Consensus statement on concussion in sport-the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838-847. [DOI] [PubMed] [Google Scholar]
- 19.Lovell MR, Iverson GL, Collins MW, et al. . Measurement of symptoms following sports-related concussion: reliability and normative data for the post-concussion scale. Appl Neuropsychol. 2006;13(3):166-174. doi: 10.1207/s15324826an1303_4 [DOI] [PubMed] [Google Scholar]
- 20.Iverson GL, Lovell MR, Collins MW. Interpreting change on ImPACT following sport concussion. Clin Neuropsychol. 2003;17(4):460-467. doi: 10.1076/clin.17.4.460.27934 [DOI] [PubMed] [Google Scholar]
- 21.Mucha A, Collins MW, Elbin RJ, et al. . A brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions: preliminary findings. Am J Sports Med. 2014;42(10):2479-2486. doi: 10.1177/0363546514543775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salter K, Jutai J, Hartley M, et al. . Impact of early vs delayed admission to rehabilitation on functional outcomes in persons with stroke. J Rehabil Med. 2006;38(2):113-117. doi: 10.1080/16501970500314350 [DOI] [PubMed] [Google Scholar]
- 23.Schneider KJ, Meeuwisse WH, Nettel-Aguirre A, Boyd L, Barlow KM, Emery CA. Cervico-vestibular physiotherapy in the treatment of individuals with persistent symptoms following sport related concussion: a randomised controlled trial. Br J Sports Med. 2013;47(5):304-307. doi: 10.1136/bjsports-2012-092101.5423479489 [DOI] [Google Scholar]
- 24.Thomas DG, Apps JN, Hoffmann RG, McCrea M, Hammeke T. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135(2):213-223. doi: 10.1542/peds.2014-0966 [DOI] [PubMed] [Google Scholar]
- 25.Elbin R, Covassin T, Gallion C, Kontos AP. Factors influencing risk and recovery from sport-related concussion: reviewing the evidence. SIG 2. Perspect Neurophysiol Neurogenic Speech Lang Disord. 2015;25(1):4-16. doi: 10.1044/nnsld25.1.4 [DOI] [Google Scholar]
- 26.Elbin RJ, Sufrinko A, Schatz P, et al. . Removal from play after concussion and recovery time. Pediatrics. 2016;138(3):e20160910. doi: 10.1542/peds.2016-0910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asken BM, McCrea MA, Clugston JR, Snyder AR, Houck ZM, Bauer RM. “Playing through it”: delayed reporting and removal from athletic activity after concussion predicts prolonged recovery. J Athl Train. 2016;51(4):329-335. doi: 10.4085/1062-6050-51.5.02 [DOI] [PMC free article] [PubMed] [Google Scholar]