This cross-sectional study uses Minimum Data Set assessments to investigate the hospital transfer rates among nursing home residents in the United States with advanced illness between 2011 and 2017 after the introduction of national initiatives to reduce hospitalizations.
Key Points
Question
How did the hospital transfer rates change between 2011 and 2017 among nursing home residents diagnosed with advanced illness and limited life expectancy before and after the introduction of national initiatives to reduce hospitalizations?
Findings
In this US nationwide cross-sectional study of 6 cohorts of nursing home residents with advanced illness, such as dementia, congestive heart failure, and chronic obstructive pulmonary disease, although hospital transfers for all causes and for potentially avoidable conditions were common, such transfers were found to have declined considerably from 2011 to 2017, and concurrent hospice use was low across all cohorts.
Meaning
The findings of this study suggest that hospital transfer rates among nursing home residents with advanced illness declined from 2011 to 2017 and that opportunities remain to reduce unnecessary hospital transfers among residents with advanced illness.
Abstract
Importance
Hospital transfers among nursing home residents in the United States who have been diagnosed with advanced illnesses and have limited life expectancy are often burdensome, costly, and of little clinical benefit. National initiatives, introduced since 2012, have focused on reducing such hospitalizations, but little is known about the consequences of these initiatives in this population.
Objective
To investigate the change in hospital transfer rates among nursing home residents with advanced illnesses, such as dementia, congestive heart failure (CHF), and chronic obstructive pulmonary disease (COPD), from 2011 to 2017—before and after the introduction of national initiatives to reduce hospitalizations.
Design, Setting, and Participants
In this cross-sectional study, nationwide Minimum Data Set (MDS) assessments from January 1, 2011, to December 31, 2016 (with the follow-up for transfer rates until December 31, 2017), were used to identify annual inception cohorts of long-stay (>100 days) nursing home residents who had recently progressed to the advanced stages of dementia, CHF, or COPD. The data were analyzed from October 24, 2018, to October 3, 2019.
Main Outcomes and Measures
The number of hospital transfers (hospitalizations, observation stays, and emergency department visits) per person-year alive was calculated from the MDS assessment from the date when residents first met the criteria for advanced illness up to 12 months afterward using Medicare claims from 2011 to 2017. Transfer rates for all causes, potentially avoidable conditions (sepsis, pneumonia, dehydration, urinary tract infections, CHF, and COPD), and serious bone fractures (pelvis, hip, wrist, ankle, and long bones of arms or legs) were investigated. Hospice enrollment and mortality were also ascertained.
Results
The proportions of residents in the 2011 and 2016 cohorts who underwent any hospital transfer were 56.1% and 45.4% of those with advanced dementia, 77.6% and 69.5% of those with CHF, and 76.2% and 67.2% of those with COPD. The mean (SD) number of transfers per person-year alive for potentially avoidable conditions was higher in the 2011 cohort vs 2016 cohort: advanced dementia, 2.4 (14.0) vs 1.6 (11.2) (adjusted risk ratio [aRR], 0.73; 95% CI, 0.65-0.81); CHF, 8.5 (32.0) vs 6.7 (26.8) (aRR, 0.72; 95% CI, 0.65-0.81); and COPD, 7.8 (30.9) vs 5.5 (24.8) (aRR, 0.64; 95% CI, 0.57-0.72). Transfers for bone fractures remained unchanged, and mortality did not increase. Hospice enrollment was low across all illness groups and years (range, 23%-30%).
Conclusions and Relevance
The findings of this study suggest that concurrent with new initiatives aimed at reducing hospitalizations, hospital transfers declined between 2011 and 2017 among nursing home residents with advanced illnesses without increased mortality rates. Opportunities remain to further reduce unnecessary hospital transfers in this population and improve goal-directed care for those residents who opt to forgo hospitalization.
Introduction
A central objective of the Patient Protection and Affordable Care Act (ACA) of 2010 was to transform health care by ensuring that patients receive high-value, effective care.1 Toward this objective, there has been particular focus on reducing potentially avoidable hospital transfers from the long-term care setting. The opportunity and need to reduce avoidable hospitalizations are greatest among people with advanced chronic illnesses2 for whom the burdens of hospitalization often outweigh the benefits.3,4,5 Little is currently known about how hospital transfer rates have changed among this population since broad initiatives were introduced.
Approximately 50% of nursing home residents experience 1 or more hospitalization in their last year of life.6 At least half of these hospitalizations are estimated to be potentially avoidable because the acute condition could be managed effectively in the nursing home or the hospital-level care is not aligned with patient preferences.7,8,9,10,11 Decisions regarding hospital transfers should be guided by the primary goal of care (eg, prolongation of life vs comfort), which is comfort for most nursing home residents with advanced illness.12,13,14,15 With rare exceptions (eg, serious bone fractures), hospitalization seldom promotes the goal of comfort.
Since 2012, several new approaches have emerged from the ACA to try to reduce hospital transfers of nursing home residents. Care models that enhance the nursing home’s on-site capability to manage specific targeted conditions (sepsis, pneumonia, urinary tract infections, dehydration, congestive heart failure [CHF], and chronic obstructive pulmonary disease [COPD]) have shown promise through the quality initiative program of the Centers for Medicare & Medicaid Services (CMS) to reduce avoidable hospitalizations.9,10 Evaluation of the initiative’s first phase in 7 states demonstrated estimated net reductions in 2015 of 2.2% to 9.3% in the probability of an all-cause hospitalization and 1.4% to 7.2% in the probability of a potentially avoidable hospitalization for participating residents compared with comparison groups.1 Alternative payment models, such as accountable care organizations and bundled payments, also create financial incentives for nursing homes to reduce hospital transfers.16 The Hospital Readmissions Reduction Program (HRRP) implemented financial penalties for hospitals with excess readmissions for target conditions including pneumonia, CHF, and COPD.17,18 These concurrent initiatives raised national awareness and forged collaborations around transitional care to avoid unnecessary hospitalizations.19 The consequences of such initiatives on hospital transfer rates, specifically among residents with advanced illnesses, have not been reported, to our knowledge.
The present study used Minimum Data Set (MDS) assessments linked to Medicare data to examine the national trends of hospital transfer rates from 2011 to 2017 among long-stay, fee-for-service nursing home residents with advanced dementia, CHF, or COPD. We specifically examined transfers for conditions that were deemed to be potentially avoidable and were the target of recent policy initiatives. For comparison, we also examined hospital transfer rates for serious bone fractures, which we hypothesized would be less affected by such initiatives. Finally, we assessed mortality and hospice enrollment rates.
Methods
Advanced Illness Cohorts
In this cross-sectional study, the MDS version 3.0 assessments20 from federally licensed nursing homes in the United States were used from January 1, 2011, to December 31, 2016, to construct annual inception cohorts of long-stay residents (ie, >100 days) aged 65 years or older who were diagnosed with advanced dementia, CHF, or COPD. The MDS version 3.0 is a standardized, comprehensive assessment conducted on all nursing home residents at admission and at routine intervals (eg, quarterly) and was implemented in 2011.20 Key differences between MDS 3.0 and MDS 2.0 precluded us from using MDS before 2011.21 To construct these cohorts, we identified residents from the first MDS assessment at which they met the full criteria for advanced dementia, CHF, or COPD (hereafter, baseline assessment) in each calendar year. Residents may have had the illness, such as CHF, on a prior MDS assessment but were not included in the cohort until they first met the criteria for advanced disease, as described below. Residents had to be continuously enrolled in the Medicare Parts A and B fee-for-service program for up to 12 months after their baseline assessment and could belong to more than 1 advanced illness group. Residents enrolled in hospice at the baseline assessment were excluded. The institutional review board at Brown University approved this study under expedited review and waived informed consent under 45 CFR 46.116.
We defined advanced dementia, CHF, and COPD using criteria adapted from previous studies5,22,23 using MDS to approximate hospice eligibility criteria, thus implying limited life expectancy. Advanced dementia criteria included the following: diagnosis of Alzheimer disease or other dementia, advanced cognitive impairment as defined by a score of 3 (moderate impairment) or 4 (severe impairment) on the Cognitive Function Scale,24 and extensive or total assistance needed for eating and transferring. Advanced CHF was defined as follows: CHF diagnosis, shortness of breath while sitting or supine, and extensive or total assistance needed for transferring. Advanced COPD was defined as follows: diagnosis of COPD (including emphysema and asthma), shortness of breath while sitting or supine, and extensive or total assistance needed for transferring.
Covariates
Age, sex, nonwhite race, dual-eligible status indicating beneficiaries qualifying for both Medicare and Medicaid, MDS 3.0 mortality risk score (MRS3),25 and cohort year were ascertained from the baseline MDS assessment. The MRS3 (0-39) is a validated risk score based on demographic, clinical, and functional characteristics, with higher values indicating greater 30- and 60-day mortality risk. An MRS3 score of 8 or higher is associated with a 5.4 times increased odds of 30-day mortality compared with scores lower than 8.25
Outcomes
Residents were followed for up to 12 months from their baseline MDS assessment using Medicare claims from January 1, 2011, to December 31, 2017, to ascertain the occurrence of hospital transfers for all causes, potentially avoidable conditions, and serious bone fractures. Thus, person-time and outcomes spanned 2 calendar years (eg, for the 2016 cohort, outcomes were assessed in both 2016 and 2017). Hospital transfers included acute hospitalizations, observation stays, and emergency department (ED) visits. Hospitalizations were identified from the Medicare Provider Analysis and Review file.26 Observation stays were defined as any outpatient facility claim for observation services using Healthcare Common Procedure Codes G0378-G0379,27 Current Procedural Terminology codes 99217-99220,28 or hospital outpatient revenue center code 0762. Emergency department visits were ascertained using outpatient facility claims with revenue center codes 0450-0459 or 0981, Healthcare Common Procedure Codes G0380-G0385, or carrier claims for ED services using Current Procedural Terminology codes 99281-99285. Observation stays that became admissions were considered to be hospitalizations. Emergency department visits that became hospitalizations or observation stays were classified accordingly.
Potentially avoidable transfers were identified using ambulatory care–sensitive conditions,7,8,9,10,11 including sepsis, pneumonia, dehydration, urinary tract infections, heart failure, and COPD (including emphysema and asthma) (eTable 1 in the Supplement). These conditions account for 78% of the potentially avoidable hospitalizations among nursing home residents.9 Serious bone fractures were examined as counterfactual conditions that usually result in hospital transfers regardless of existing policies and included fractures of the pelvis, hip, long bones of arms (humerus, ulna, and radius) and legs (femur, tibia, and fibula), wrists, and ankles (eTable 1 in the Supplement). We classified hospital transfers for these conditions using the claim’s principal diagnosis. Mortality and hospice enrollment within 12 months after the baseline assessment were identified from the Medicare Master Beneficiary Summary File29 and the 2011 to 2017 hospice claims, respectively.
Statistical Analysis
The data were analyzed from October 24, 2018, to October 3, 2019. Descriptive analyses were performed for resident characteristics and outcomes using means with SDs for continuous variables and proportions for categorical variables. Descriptive results were generated for each advanced illness group stratified by cohort year. Hospital transfers were further described by type (hospitalizations, observation stays, and ED visits) and by condition (all causes, potentially avoidable, and serious bone fractures). The proportion of potentially avoidable transfers attributable to specific diagnoses (eg, sepsis, pneumonia) were also calculated and presented graphically. Outliers defined as residents with all-cause hospital transfer rates exceeding 365 transfers per person-year alive across 12 months were removed (<1% of residents across all cohorts combined). Hospital transfer outcomes across 12 months were measured as the proportion of residents who experienced at least 1 transfer and the number of transfers per person-year alive. Mortality and hospice enrollment outcomes across 12 months were measured as the proportion of residents who experienced the event and the time to event. For all models, the main independent variable was the cohort year, with 2011 as the referent category. All models were adjusted for age, sex, nonwhite race, and MRS3 score. All models were fitted using generalized estimating equations to account for clustering within nursing homes and included an offset for log-transformed person-time.
Binary outcomes (any hospital transfers, hospice enrollment, and mortality) were analyzed using log-linked binomial models to estimate relative risk with cohort year. Zero-inflated Poisson regression models were used to analyze outcomes measured as number of transfers per person-year alive to allow for overdispersion owing to the high proportion of residents without a hospital transfer.30 Adjusted risk ratios (aRRs) and 95% CIs were generated for these analyses. Finally, Cox proportional hazards regression models were used to analyze time-to-event outcomes (hospice enrollment, mortality) across 12 months of follow-up. In the hospice model, death without hospice was considered to be a competing risk. Adjusted hazard ratios and 95% CIs were estimated from these analyses. Analyses were performed using SAS, version 9.4 (SAS Institute Inc).
Results
Table 1 gives the characteristics of the nursing home residents by cohort year and advanced illness group. The 2011 cohort sizes were smaller for all illness groups compared with those of other years because residents had to be in the nursing home for at least 100 days and MDS data before 2011 were not used. Nonetheless, the overall distributions of age and sex were similar across cohort years within illness groups. Residents with advanced CHF (10.1-10.9) and COPD (9.0-9.6) generally had higher MRS3 scores (higher mortality risk) compared with residents with advanced dementia (7.5-8.4). All cohorts were predominately female, white, and dually eligible for Medicare and Medicaid.
Table 1. Characteristics of Nursing Home Residents With Advanced Illness by Cohort Yeara.
| Characteristic | Cohort Yeara | |||||
|---|---|---|---|---|---|---|
| 2011b | 2012 | 2013 | 2014 | 2015 | 2016 | |
| Advanced Dementia | ||||||
| Residents, No. | 18 178b | 44 385 | 50 725 | 53 078 | 53 418 | 52 221 |
| Follow-up, mean (SD), d | 232.5 (146.0) | 238.2 (143.6) | 242.8 (143.0) | 241.8 (142.4) | 243.6 (142.7) | 238.7 (143.5) |
| Age, mean (SD), y | 85.0 (7.6) | 85.4 (7.6) | 85.6 (7.8) | 85.8 (7.8) | 85.9 (7.9) | 85.8 (8.0) |
| Female, No. (%) | 12 400 (68.2) | 30 760 (69.3) | 35 630 (70.2) | 37 303 (70.3) | 37 906 (71.0) | 36 572 (70.0) |
| Nonwhite race, No. (%) | 3196 (17.6) | 7312 (16.5) | 8140 (16.0) | 8508 (16.0) | 8467 (15.9) | 8464 (16.2) |
| Dual eligible, No. (%)c | 12 654 (69.6) | 32 238 (72.6) | 37 522 (74.0) | 39 462 (74.3) | 39 558 (74.1) | 39 280 (75.2) |
| MRS3, mean (SD)d | 8.4 (3.6) | 8.1 (3.6) | 7.9 (3.5) | 7.7 (3.4) | 7.6 (3.4) | 7.5 (3.3) |
| Advanced Congestive Heart Failure | ||||||
| Residents, No. | 8866b | 16 667 | 17 782 | 17 392 | 17 562 | 18 931 |
| Follow-up, mean (SD), d alive | 207.8 (149.3) | 217.8 (148.1) | 228.5 (146.7) | 226.1 (146.1) | 223.9 (148.0) | 218.9 (149.5) |
| Age, mean (SD), y | 84.2 (8.1) | 84.5 (8.2) | 84.5 (8.3) | 84.5 (8.5) | 84.5 (8.6) | 84.3 (8.6) |
| Female, No. (%) | 5994 (67.6) | 11 484 (68.9) | 12 247 (68.9) | 11 999 (69.0) | 12 183 (69.4) | 12 865 (68.0) |
| Nonwhite race, No. (%) | 1417 (16.0) | 2429 (14.6) | 2544 (14.3) | 2454 (14.1) | 2338 (13.3) | 2812 (14.9) |
| Dual eligible, No. (%)c | 6447 (73.1) | 12 665 (76.0) | 13 437 (75.6) | 13 216 (76.0) | 13 097 (74.6) | 14 224 (75.1) |
| MRS3, mean (SD)d | 10.7 (3.3) | 10.9 (3.1) | 10.8 (3.0) | 10.5 (3.0) | 10.7 (3.0) | 10.1 (2.9) |
| Advanced Chronic Obstructive Pulmonary Disease | ||||||
| Residents, No. | 8467b | 16 597 | 17 806 | 17 166 | 17 409 | 19 154 |
| Follow-up, mean (SD), d alive | 220.5 (148.3) | 233.8 (144.8) | 243.7 (143.3) | 241.9 (142.7) | 242.1 (143.9) | 239.5 (144.8) |
| Age, mean (SD), y | 82.8 (8.2) | 83.1 (8.2) | 83.1 (8.4) | 83.2 (8.6) | 83.1 (8.5) | 83.1 (8.6) |
| Female, No. (%) | 5584 (64.6) | 10 818 (65.2) | 11 788 (66.2) | 11 303 (65.8) | 11 573 (66.5) | 12 668 (66.1) |
| Nonwhite race, No. (%) | 1386 (16.0) | 2422 (14.6) | 2523 (14.2) | 2441 (14.2) | 2310 (13.3) | 2775 (14.5) |
| Dual eligible, No. (%)c | 6566 (75.9) | 12 933 (77.9) | 14 019 (78.7) | 13 499 (78.6) | 13 414 (77.1) | 15 121 (78.9) |
| MRS3, mean (SD)d | 9.6 (3.3) | 9.6 (3.2) | 9.6 (3.0) | 9.4 (3.0) | 9.3 (2.9) | 9.0 (3.0) |
Abbreviations: MDS, Minimum Data Set; MRS3, MDS 3.0 mortality risk score.
Cohort year refers to the calendar year of the inception cohort constructed for each advanced illness using MDS 3.0 assessments from 2011 to 2016.
Cohort sizes from 2011 were smaller compared with those of other years because residents had to be in the nursing home for at least 100 days and MDS data before 2011 were not used.
Beneficiaries who qualify for both Medicare and Medicaid benefits.
MDS 3.0 mortality risk score, range 0-39; higher scores indicate higher risk of mortality.
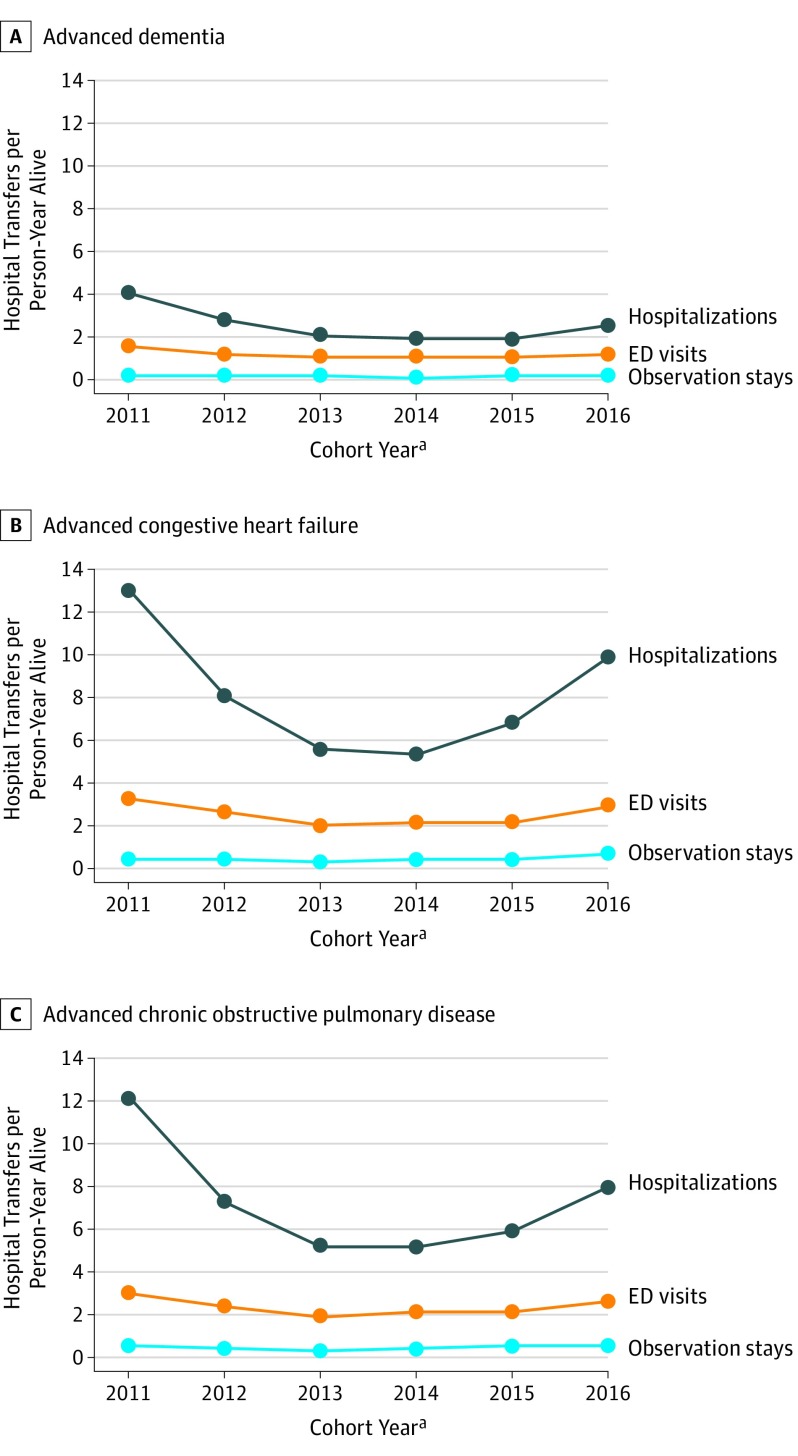
Table 2 provides hospital transfer outcomes. All-cause hospital transfers were common in all illness groups across all years and were consistently lower for residents with advanced dementia compared with other groups. The most pronounced reduction in transfers occurred from the 2011 to 2012 cohort and then from the 2012 to 2013 cohort. Data showed no further reduction after 2013 and higher rates in 2016 than in 2015, particularly in the CHF and COPD cohorts. The adjusted proportions of residents who had at least 1 hospital transfer for any cause in the 2011 and 2016 cohorts were 56.1% and 45.4% for those with advanced dementia, 77.6% and 69.5% for those with CHF, and 76.2% and 67.2% for those with COPD. The mean (SD) number of hospital transfers per person-year alive for any cause was higher in the 2011 vs 2016 cohorts for nursing home residents with advanced dementia, 5.8 (22.7) vs 3.9 (17.9); CHF, 16.8 (43.9) vs 13.5 (38.9); and COPD, 15.6 (42.4) vs 11.2 (35.8). For each illness, the decrease in transfer rates between the 2011 and 2016 cohorts remained significant (advanced dementia [aRR, 0.72; 95% CI, 0.67-0.78]; CHF [aRR, 0.74; 95% CI, 0.68-0.80]; and COPD [aRR, 0.66; 95% CI, 0.61-0.72]) after adjustment for baseline characteristics. As demonstrated in Figure 1, reductions in hospital transfer rates over time were almost entirely attributable to declines in acute hospitalizations in each illness group.
Table 2. Hospital Transfer Outcomes Across 12 Months Among Nursing Home Residents With Advanced Illness by Cohort Yeara.
| Outcome | Cohort Yeara | |||||
|---|---|---|---|---|---|---|
| 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | |
| Advanced Dementia | ||||||
| Any transfer, adjusted % (95% CI)b | ||||||
| All causes | 56.1 (55.4-56.9) | 51.1 (50.5-51.4) | 45.9 (45.6-46.4) | 45.1 (44.7-45.5) | 44.5 (44.1-44.9) | 45.4 (44.9-45.8) |
| Potentially avoidablec | 26.5 (25.9-27.2) | 23.2 (22.8-23.6) | 20.0 (19.6-20.3) | 19.7 (19.4-20.1) | 19.5 (19.2-19.8) | 20.4 (20.1-20.7) |
| Fracturesd | 2.4 (2.2-2.6) | 2.3 (2.2-2.5) | 2.3 (2.1-2.4) | 2.3 (2.2-2.5) | 2.4 (2.2-2.5) | 2.4 (2.3-2.6) |
| Hospital transfers per person-year alive | ||||||
| All causes, mean (SD) | 5.8 (22.7) | 4.1 (16.7) | 3.3 (14.4) | 3.1 (13.6) | 3.2 (14.2) | 3.9 (17.9) |
| Adjusted RRb | 1 [Reference] | 0.71 (0.66-0.77) | 0.58 (0.54-0.62) | 0.56 (0.52-0.60) | 0.57 (0.53-0.62) | 0.72 (0.67-0.78) |
| Potentially avoidable, mean (SD)c | 2.4 (14.0) | 1.6 (10.2) | 1.2 (7.8) | 1.2 (7.6) | 1.2 (8.0) | 1.6 (11.2) |
| Adjusted RRb | 1 [Reference] | 0.67 (0.60-0.75) | 0.52 (0.47-0.58) | 0.51 (0.46-0.57) | 0.53 (0.48-0.59) | 0.73 (0.65-0.81) |
| Fractures, mean (SD)d | 0.06 (0.78) | 0.06 (0.87) | 0.05 (0.82) | 0.06 (0.72) | 0.07 (1.08) | 0.08 (1.24) |
| Adjusted RR | 1 [Reference] | 0.98 (0.77-1.24) | 0.85 (0.67-1.07) | 0.93 (0.75-1.16) | 1.03 (0.81-1.30) | 1.28 (1.00-1.61) |
| Advanced Congestive Heart Failure | ||||||
| Any transfer, adjusted % (95% CI)b | ||||||
| All causes | 77.6 (76.7-78.5) | 69.3 (68.6-70.0) | 63.6 (62.9-64.3) | 63.2 (62.5-63.9) | 64.7 (63.9-65.4) | 69.5 (68.9-70.2) |
| Potentially avoidablec | 50.0 (49.9-51.1) | 41.5 (40.7-42.2) | 36.4 (35.7-37.1) | 36.6 (35.8-37.3) | 38.1 (37.3-38.8) | 42.9 (42.2-43.6) |
| Fracturesd | 2.0 (1.8-2.3) | 1.9 (1.8-2.2) | 2.0 (1.8-2.2) | 2.1 (1.9-2.3) | 2.0 (1.8-2.2) | 2.0 (1.8-2.2) |
| Hospital transfers per person-year alive | ||||||
| All causes, mean (SD) | 16.8 (43.9) | 11.1 (33.6) | 8.0 (24.1) | 8.0 (24.3) | 9.6 (28.3) | 13.5 (38.9) |
| Adjusted RRb | 1 [Reference] | 0.69 (0.64-0.75) | 0.49 (0.45-0.53) | 0.49 (0.45-0.53) | 0.57 (0.53-0.62) | 0.74 (0.68-0.80) |
| Potentially avoidable, mean (SD)c | 8.5 (32.0) | 5.2 (23.5) | 3.5 (15.8) | 3.4 (15.1) | 4.5 (19.6) | 6.7 (26.8) |
| Adjusted RRb | 1 [Reference] | 0.64 (0.58-0.72) | 0.42 (0.38-0.47) | 0.42 (0.38-0.47) | 0.53 (0.48-0.59) | 0.72 (0.65-0.81) |
| Fractures, mean (SD)d | 0.08 (1.20) | 0.08 (1.59) | 0.08 (1.01) | 0.07 (1.01) | 0.07 (1.21) | 0.90 (2.37) |
| Adjusted RR | 1 [Reference] | 0.99 (0.65-1.51) | 0.86 (0.60-1.24) | 0.84 (0.58-1.21) | 0.82 (0.55-1.21) | 1.02 (0.63-1.63) |
| Advanced Chronic Obstructive Pulmonary Disease | ||||||
| Any transfer, adjusted % (95% CI)b | ||||||
| All causes | 76.2 (75.3-77.1) | 69.1 (68.4-69.9) | 63.3 (62.6-64.0) | 63.1 (62.3-63.8) | 63.9 (63.2-64.7) | 67.2 (66.6-67.9) |
| Potentially avoidablec | 49.2 (48.2-50.2) | 41.2 (40.4-41.9) | 35.9 (35.2-36.6) | 36.0 (35.3-36.7) | 37.2 (36.4-37.9) | 40.7 (40.0-41.4) |
| Fracturesd | 2.3 (2.0-2.6) | 2.2 (2.0-2.5) | 2.3 (2.1-2.6) | 2.4 (2.2-2.6) | 2.2 (2.0-2.5) | 2.2 (2.0-2.5) |
| Hospital transfers per person-year alive | ||||||
| All causes, mean (SD) | 15.6 (42.4) | 9.9 (30.2) | 7.3 (22.1) | 7.7 (24.8) | 8.6 (27.4) | 11.2 (35.8) |
| Adjusted RRb | 1 [Reference] | 0.60 (0.55-0.65) | 0.43 (0.39-0.46) | 0.46 (0.41-0.49) | 0.50 (0.46-0.55) | 0.66 (0.61-0.72) |
| Potentially avoidable, mean (SD)c | 7.8 (30.9) | 4.6 (21.1) | 3.1 (14.3) | 3.2 (15.5) | 3.8 (17.5) | 5.5 (24.8) |
| Adjusted RRb | 1 [Reference] | 0.55 (0.49-0.62) | 0.36 (0.32-0.41) | 0.37 (0.33-0.42) | 0.44 (0.39-0.49) | 0.64 (0.57-0.72) |
| Fractures, mean (SD)d | 0.09 (1.19) | 0.07 (0.98) | 0.08 (0.98) | 0.09 (1.08) | 0.07 (0.74) | 0.95 (2.16) |
| Adjusted RR | 1 [Reference] | 0.79 (0.55-1.11) | 0.82 (0.58-1.14) | 0.88 (0.63-1.23) | 0.69 (0.50-0.96) | .095 (0.63-1.46) |
Abbreviations: COPD, chronic obstructive pulmonary disease; MDS, Minimum Data Set; MRS3, MDS 3.0 mortality risk score; RR, relative risk.
Cohort year refers to the calendar year of the inception cohort constructed for each advanced illness using MDS assessments from 2011 to 2016. Medicare data from 2011 to 2017 were used to identify transfers occurring within 12 months of meeting the criteria for advanced illness for each cohort. Thus, person-time and transfers were assessed for up to 12 months and spanned 2 calendar years (eg, for the 2016 cohort, transfers were assessed in both 2016 and 2017).
All models were adjusted for age, sex, nonwhite race, and MRS3 and included an offset for log(person-time).
Transfers for primary diagnosis of sepsis, pneumonia, dehydration, urinary tract infections, heart failure, and COPD or asthma.
Fractures of pelvis, hip, wrist, ankle, and long bone fractures of arms (humerus, ulna, and radius) and legs (femur, tibia, and fibula).
Figure 1. Transfer Rates for Hospitalizations, Emergency Department (ED) Visits, and Observation Stays for Illness Groups by Cohort Year.
aCohort year refers to the calendar year of the inception cohort constructed for each advanced illness using Minimum Data Set assessments from 2011 to 2016. Medicare data from 2011 to 2017 were used to identify transfers occurring within 12 months of meeting the criteria for advanced illness for each cohort. Thus, person-time and transfers were assessed for up to 12 months and spanned 2 calendar years (eg, for the 2016 cohort, transfers were assessed in both 2016 and 2017).
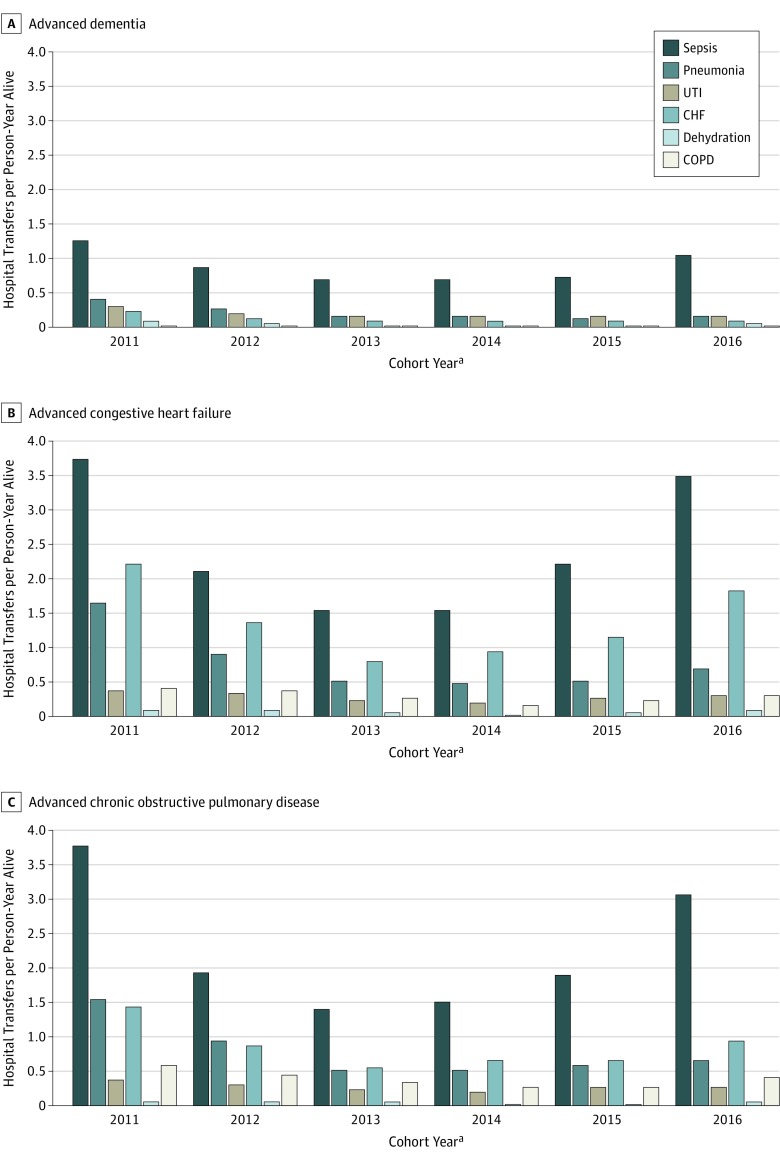
Transfers for potentially avoidable conditions were common and decreased over time in each illness group in patterns similar to those of transfers for all causes (Table 2). The adjusted proportions of residents having at least 1 hospital transfer for a potentially avoidable condition in the 2011 and 2016 cohorts, respectively, were 26.5% and 20.4% for those with advanced dementia; 50.0% and 42.9% for those with CHF; and 49.2% and 40.7% for those with COPD. The mean (SD) number of hospital transfers per person-year alive for potentially avoidable conditions for the 2011 vs 2016 cohorts were 2.4 (14.0) vs 1.6 (11.2) (aRR, 0.73; 95% CI, 0.65-0.81) for those with advanced dementia; 8.5 (32.0) vs 6.7 (26.8) (aRR, 0.72; 95% CI, 0.65-0.81) for those with CHF; and 7.8 (30.9) vs 5.5 (24.8) (aRR, 0.64; 95% CI, 0.57-0.72) for those with COPD. Sepsis was the most frequent condition attributed to potentially avoidable transfers in all cohort years for all groups (Figure 2). The next most frequent conditions documented for potentially avoidable transfers were pneumonia and urinary tract infections for advanced dementia and CHF and pneumonia for both advanced CHF and COPD.
Figure 2. Hospital Transfer Rates for Potentially Avoidable Conditions by Cohort Year .
CHF indicates congestive heart failure; COPD, chronic obstructive pulmonary disease; and UTI, urinary tract infection.
aCohort year refers to the calendar year of the inception cohort constructed for each advanced illness using Minimum Data Set assessments from 2011 to 2016. Medicare data from 2011 to 2017 were used to identify transfers occurring within 12 months of meeting the criteria for advanced illness for each cohort. Thus, person-time and transfers were assessed for up to 12 months and spanned 2 calendar years (eg, for the 2016 cohort, transfers were assessed in both 2016 and 2017).
Hospital transfers for a primary diagnosis of serious bone fractures were relatively uncommon and, as hypothesized, remained unchanged for all advanced illness groups across all years (Table 2). Table 3 gives the mortality and hospice enrollment outcomes. Hospice enrollment was low across all illness groups and years (range, 23%-30%). Mortality within 1 year exceeded 50% for all advanced illness groups across all years but did not increase over time in any illness group. Hospice use was low for all illnesses across all years but increased slightly for the 2016 cohort of residents with advanced dementia.
Table 3. Mortality and Hospice Enrollment Across 12 Months Among Nursing Home Residents With Advanced Illness by Cohort Yeara.
| Outcome | Cohort Yeara | |||||
|---|---|---|---|---|---|---|
| 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | |
| Advanced Dementia | ||||||
| 12-mo Mortality adjusted | ||||||
| % (95% CI)b | 52.8 (51.6-53.1) | 51.8 (51.3-52.3) | 50.5 (50.1-51.0) | 52.0 (51.6-52.5) | 51.0 (50.5-51.4) | 53.3 (52.8-53.7) |
| HR (95% CI)c | 1 [Reference] | 0.97 (0.95-1.00) | 0.94 (0.92-0.96) | 0.98 (0.95-1.00) | 0.95 (0.93-0.98) | 1.02 (0.99-1.04) |
| Hospice in 12 mo adjusted | ||||||
| % (95% CI)b | 27.2 (26.5-27.8) | 26.9 (26.5-27.4) | 27.1 (26.7-27.4) | 27.2 (26.9-27.6) | 27.9 (27.6-28.3) | 30.0 (29.6-30.4) |
| HR (95% CI)d | 1 [Reference] | 0.97 (0.94-1.01) | 0.97 (0.94-1.00) | 0.98 (0.95-1.02) | 1.01 (0.98-1.04) | 1.11 (1.08-1.15) |
| Advanced CHF | ||||||
| 12-mo Mortality adjusted | ||||||
| % (95% CI)b | 61.6 (60.6-62.6) | 58.9 (58.1-59.6) | 55.4 (54.7-56.2) | 56.8 (56.0-57.5) | 56.3 (55.5-57.0) | 57.3 (56.6-58.0) |
| HR (95% CI)c | 1 [Reference] | 0.94 (0.90-0.99) | 0.87 (0.83-0.91) | 0.87 (0.84-0.90) | 0.87 (0.84-0.90) | 0.89 (0.86-0.92) |
| Hospice in 12 mo adjusted | ||||||
| % (95% CI)b | 27.9 (27.0-28.9) | 27.1 (26.4-27.8) | 25.8 (25.2-26.5) | 26.9 (26.2-27.5) | 27.4 (26.7-28.0) | 29.9 (29.2-30.5) |
| HR (95% CI)d | 1 [Reference] | 0.94 (0.89-0.99) | 0.87 (0.83-0.91) | 0.91 (0.87-0.95) | 0.93 (0.89-0.98) | 1.03 (0.98-1.08) |
| Advanced COPD | ||||||
| 12-mo Mortality adjusted | ||||||
| % (95% CI)b | 57.8 (56.7-58.9) | 53.6 (52.8-54.3) | 49.9 (49.0-50.5) | 51.3 (50.6-52.1) | 50.7 (50.0-51.5) | 52.2 (51.4-52.9) |
| HR (95% CI)c | 1 [Reference] | 0.88 (0.85-0.91) | 0.80 (0.77-0.82) | 0.82 (0.79-0.85) | 0.82 (0.79-0.85) | 0.85 (0.82-0.88) |
| Hospice in 12 mo adjusted | ||||||
| % (95% CI)b | 24.7 (23.8-25.6) | 23.9 (23.2-24.6) | 23.1 (22.5-24.0) | 23.3 (22.7-24.0) | 24.1 (23.4-24.7) | 26.2 (25.6-26.9) |
| HR (95% CI)d | 1 [Reference] | 0.92 (0.87-0.97) | 0.87 (0.82-0.91) | 0.87 (0.83-0.92) | 0.91 (0.86-0.95) | 1.00 (0.95-1.05) |
Abbreviations: CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; MDS, Minimum Data Set; MRS3, MDS 3.0 mortality risk score.
Cohort year refers to the calendar year of the inception cohort constructed for each advanced illness using MDS assessments from 2011 to 2016. Medicare data from 2011 to 2017 were used to identify outcomes occurring within 12 months of meeting the criteria for advanced illness for each cohort. Thus, person-time and outcomes were assessed for up to 12 months and spanned 2 calendar years (eg, for the 2016 cohort, outcomes were assessed in both 2016 and 2017).
Adjusted for age, sex, nonwhite race, and MRS3 and offset for person-time.
Adjusted for age, sex, nonwhite race, and MRS3. Mortality: HR <1.00 indicates lower rate of death relative to rate in 2011.
Adjusted for age, sex, nonwhite race, and MRS3. Death was considered a competing risk. Hospice: HR <1.00 indicates lower rate of hospice enrollment relative to rate in 2011.
Discussion
We examined changes in national hospital transfer rates among long-stay nursing home residents with advanced dementia, CHF, and COPD from 2011 to 2017, a period during which national initiatives were introduced to reduce hospital transfers across care settings, including the long-term care setting. Transfers for all causes and potentially avoidable conditions declined for each advanced illness group during these years without adversely affecting mortality. This decline was almost entirely attributable to a reduction in hospitalizations rather than in observation stays or ED visits. In contrast, transfer rates for serious bone fractures, which are less likely to be associated with policy changes, remained stable. Sepsis and pneumonia were common reasons for potentially avoidable hospital transfers across all groups. Although the hospital transfer rates for all causes and potentially avoidable conditions declined, they remained common. In the 2016 cohorts, 45% of the residents with advanced dementia and roughly two-thirds of those with advanced CHF and COPD experienced at least 1 transfer despite the greater than 50% mortality rate across all groups. These observations, coupled with low hospice enrollment throughout all years, suggest that opportunities remain to further reduce hospital transfers and improve end-of-life care for nursing home residents with advanced illnesses.
Although several studies describe changes in hospitalization rates in the United States in 2014 and 2015,17,18,31,32,33 the present study focused on long-term care residents with advanced illness. The issue is particularly pertinent for this population because hospital transfers can be especially burdensome, of little clinical benefit, and distressing for family members.3,4,5,34 Treatment decisions should be guided by patient preferences. Thus, if the decline in transfer rates observed in this study reflect, in part, decisions to avoid hospitalizations in favor of comfort-focused care, then such care should be available in the nursing home. High-quality palliative care is currently lacking in many US nursing homes.35,36,37 Palliative care and hospice improve the quality of end-of-life care among nursing home residents, including reducing hospitalization rates.32,36,37,38,39 Although we could not ascertain whether residents received palliative care outside the Medicare Hospice Benefit, we observed low rates of hospice enrollment and no concurrent uptake of hospice as hospital transfer rates declined. Given the increased scrutiny and federal audits of nursing homes with long hospice stays, the present study suggests that most residents with advanced dementia, CHF, and COPD are not receiving hospice care near the end of life.
Potentially avoidable hospital transfers among dual-eligible beneficiaries are key quality measures in long-term care and cost Medicare an estimated $2.6 billion annually.9,40 Infections accounted for most potentially avoidable hospital transfers across advanced illness groups. Among residents with advanced CHF and COPD, exacerbations of these underlying conditions were also common reasons for transfers. Consequently, advance care planning among providers, residents, and family members presents a critical opportunity for informed decision-making about wishes for future hospitalizations in anticipation of expected clinical complications, and online resources are available to help guide these discussions.41 Proxies of residents with advanced dementia should understand that recurrent infections are a hallmark of the end stage of the disease. Among residents with advanced illness for whom curative care remains aligned with their preferences, infections and cardiopulmonary decompensations can often be managed in the nursing home.3,42,43,44,45 Of note, mortality did not increase across the cohort years in any group despite the decline in hospital transfers.
Programmatic initiatives, such as Interventions to Reduce Acute Care Transfers (INTERACT),46 have been adopted widely to avoid unnecessary hospitalizations by improving the nursing home’s capacity to provide on-site evaluation and management of acute changes through early recognition, monitoring, and staff training. Although a recent cluster randomized clinical trial of INTERACT demonstrated no effect on hospitalizations or ED visits, the trial highlighted the complexities of implementing and sustaining such interventions in nursing homes.16
This study’s findings are consistent with those of studies documenting prominent declines in hospitalization and readmission rates between 2010 and 2012 after the enactment of the ACA and announcement of the HRRP, a Medicare value-based purchasing program that financially penalizes hospitals with excess readmissions for specific conditions.17,18,47,48,49 Health care systems may have made systemic preparations in anticipation of the HRRP penalty phase in October 2012. Our findings also support the possible spillover effect of HRRP onto nonspecific conditions.17,18,47,48,50 Consistent with other studies, we observed a leveling off of hospital transfer rates during 2014 to 2016.17,18,47,48 Chhabra and colleagues48 suggest that this pattern may reflect a floor effect—a point at which achieving additional reductions in rates becomes more difficult. Regression to the mean may also partly explain the marked decline observed in transfer rates compared with those in 2011.33
Several factors acting in parallel may contribute to the observed declines in potentially avoidable transfers. Increased attention and quality and performance improvement initiatives have targeted unnecessary hospital transfers. Recent studies demonstrate reductions in hospital readmissions for potentially avoidable conditions after implementation of the HRRP.17,18 Although nursing homes were not subject to financial penalties under HRRP, many nursing homes across the United States adopted quality improvement programs, such as INTERACT, in anticipation of CMS quality assurance and performance improvement regulations.46 In addition, nursing homes included in accountable care organizations or other preferred referral networks were further incentivized to reduce hospitalizations to remain in network.16 The CMS is conducting demonstration projects to examine payment models that include value-based purchasing incentives as part of the initiative to reduce avoidable hospitalizations among nursing home residents.10 The Skilled Nursing Facility (SNF) value-based purchasing program took effect in October 2018 to extend penalties for excess readmissions to SNFs; preliminary data suggest that 73% of SNFs face financial penalties after failing to achieve benchmarks.51
Previous research in long-term care settings has focused almost exclusively on potentially avoidable hospitalizations. The present study extends prior work to include all potentially avoidable hospital transfers, including ED visits and observation stays. We found that declines in all-cause hospital transfer rates were almost exclusively associated with reductions in acute hospitalizations, and the rates of observation stays remained low between 2011 and 2017. Thus, concerns that hospitals may shift admissions to observation stay status to avoid HRRP penalties18 appear to be unfounded in this population. In addition, rates of decline were similar for all-cause and potentially avoidable transfers, suggesting that broader efforts are being made to reduce hospital transfers or that there is a spillover effect from the focus on potentially avoidable conditions. Although we observed reductions in potentially avoidable hospital transfers, several thousand nursing home residents with advanced illness continue to be transferred to hospitals near the end of life. We estimated 708 096 transfers for all-cause transfers across the 3 advanced illness cohorts from 2011 to 2017, of which 259 339 (36.8%) were potentially avoidable. Moreover, despite the initial reduction in potentially avoidable transfers from 39.1% in 2011 to 35.1% in 2013, the rate appears to have rebounded to 37.7% by 2016 (eTable 2 and eFigure in the Supplement).
Limitations
This study has limitations. The application of claims-based measures of potentially avoidable conditions to nursing home populations remains controversial because they do not capture the complex health status of frail, older nursing home residents with multimorbid conditions or the specific challenges of managing acute illnesses in this population and setting.7,45 Moreover, insurance claims and MDS 3.0 assessments do not fully capture the circumstances leading to decisions for hospital transfer, including advance directives. Important drivers of decisions to transfer are associated with decisional processes and organizational factors, including communication, family preferences, and nursing home resources.52,53,54,55,56 Furthermore, the principal diagnoses listed on hospital claims are assigned at discharge as the diagnosis that was chiefly responsible for the admission, whereas the diagnosis assigned to an ED visit or observation stay is based on more limited information.57 However, we focused on long-term care residents with advanced illness for whom care decisions should align with values and goals of care and for whom the harms associated with hospital transfers usually outweigh possible benefits.3,4,5
Conclusions
Hospital transfer rates for all causes and for potentially avoidable causes declined in 2017 compared with 2011 for long-stay nursing home residents with advanced dementia, CHF, or COPD, with no increase in mortality. Reductions in potentially avoidable transfers are encouraging, yet many residents still experienced multiple transfers for infections and CHF or COPD exacerbations. These findings indicate important opportunities to improve care of nursing home residents with advanced illness through improvements in advance care planning, acute care management, and the delivery of high-quality palliative care.
eTable 1. Detailed Listing of ICD-9/ICD-10-CM Diagnosis Codes for Potentially Avoidable Conditions and for Serious Bone Fractures. (Pages 2-21)
eTable 2. Total Number of Hospital Transfers Among Nursing Home Residents With Advanced Illness by Cohort Year (Page 22)
eFigure. Percent of All Transfers That Were Potentially Avoidable by Cohort Year (Page 23)
References
- 1.Ingber MJ, Feng Z, Khatutsky G, et al. Initiative to reduce avoidable hospitalizations among nursing facility residents shows promising results. Health Aff (Millwood). 2017;36(3):441-450. doi: 10.1377/hlthaff.2016.1310 [DOI] [PubMed] [Google Scholar]
- 2.Donzé J, Lipsitz S, Bates DW, Schnipper JL. Causes and patterns of readmissions in patients with common comorbidities: retrospective cohort study. BMJ. 2013;347:f7171. doi: 10.1136/bmj.f7171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ouslander JG, Lamb G, Perloe M, et al. Potentially avoidable hospitalizations of nursing home residents: frequency, causes, and costs: see editorial comments by Drs. Jean F. Wyman and William R. Hazzard, pp 760-761. J Am Geriatr Soc. 2010;58(4):627-635. doi: 10.1111/j.1532-5415.2010.02768.x [DOI] [PubMed] [Google Scholar]
- 4.Ouslander JG, Berenson RA. Reducing unnecessary hospitalizations of nursing home residents. N Engl J Med. 2011;365(13):1165-1167. doi: 10.1056/NEJMp1105449 [DOI] [PubMed] [Google Scholar]
- 5.Gozalo P, Teno JM, Mitchell SL, et al. End-of-life transitions among nursing home residents with cognitive issues. N Engl J Med. 2011;365(13):1212-1221. doi: 10.1056/NEJMsa1100347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xing J, Mukamel DB, Temkin-Greener H. Hospitalizations of nursing home residents in the last year of life: nursing home characteristics and variation in potentially avoidable hospitalizations. J Am Geriatr Soc. 2013;61(11):1900-1908. doi: 10.1111/jgs.12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polniaszek S, Walsh EG, Wiener JM United States Department of Health and Human Services; Office of the Assistant Secretary for Planning and Evaluation; Office of Disability, Aging and Long-Term Care Policy. Hospitalizations of nursing home residents: background and options. https://aspe.hhs.gov/system/files/pdf/76296/NHResHosp.pdf. Accessed October 6, 2019.
- 8.Segal M, Rollins E, Hodges K, Roozeboom M. Medicare-Medicaid eligible beneficiaries and potentially avoidable hospitalizations. Medicare Medicaid Res Rev. 2014;4(1):E1-E10. doi: 10.5600/mmrr.004.01.b01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh EG, Wiener JM, Haber S, Bragg A, Freiman M, Ouslander JG. Potentially avoidable hospitalizations of dually eligible Medicare and Medicaid beneficiaries from nursing facility and home- and community-based services waiver programs. J Am Geriatr Soc. 2012;60(5):821-829. doi: 10.1111/j.1532-5415.2012.03920.x [DOI] [PubMed] [Google Scholar]
- 10.Ingber MJ, Feng Z, Khatutsky G, et al. Evaluation initiatives to reduce avoidable hospitalizations among nursing facility residents: final report. https://downloads.cms.gov/files/cmmi/irahnfr-finalevalrpt.pdf. Published 2017. Accessed October 6, 2019. [DOI] [PubMed]
- 11.Trask PC, Teno JM, Nash J. Transitions of care and changes in distressing pain. J Pain Symptom Manage. 2006;32(2):104-109. doi: 10.1016/j.jpainsymman.2006.03.007 [DOI] [PubMed] [Google Scholar]
- 12.Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361(16):1529-1538. doi: 10.1056/NEJMoa0902234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanson LC, Zimmerman S, Song MK, et al. Effect of the goals of care intervention for advanced dementia: a randomized clinical trial. JAMA Intern Med. 2017;177(1):24-31. doi: 10.1001/jamainternmed.2016.7031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ernecoff NC, Zimmerman S, Mitchell SL, et al. Concordance between goals of care and treatment decisions for persons with dementia. J Palliat Med. 2018;21(10):1442-1447. doi: 10.1089/jpm.2018.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell SL, Palmer JA, Volandes AE, Hanson LC, Habtemariam D, Shaffer ML. Level of care preferences among nursing home residents with advanced dementia. J Pain Symptom Manage. 2017;54(3):340-345. doi: 10.1016/j.jpainsymman.2017.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kane RL, Huckfeldt P, Tappen R, et al. Effects of an intervention to reduce hospitalizations from nursing homes: a randomized implementation trial of the INTERACT program. JAMA Intern Med. 2017;177(9):1257-1264. doi: 10.1001/jamainternmed.2017.2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desai NR, Ross JS, Kwon JY, et al. Association between hospital penalty status under the Hospital Readmission Reduction Program and readmission rates for target and nontarget conditions. JAMA. 2016;316(24):2647-2656. doi: 10.1001/jama.2016.18533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the Hospital Readmissions Reduction Program. N Engl J Med. 2016;374(16):1543-1551. doi: 10.1056/NEJMsa1513024 [DOI] [PubMed] [Google Scholar]
- 19.McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):1796-1803. doi: 10.1161/CIRCULATIONAHA.114.010270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Medicare & Medicaid Services Long-term care facility resident assessment instrument 3.0 user's manual v1.16. https://downloads.cms.gov/files/1-MDS-30-RAI-Manual-v1-16-October-1-2018.pdf. Accessed October 6, 2019.
- 21.Saliba D, Jones M, Streim J, Ouslander J, Berlowitz D, Buchanan J. Overview of significant changes in the Minimum Data Set for nursing homes version 3.0. J Am Med Dir Assoc. 2012;13(7):595-601. doi: 10.1016/j.jamda.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 22.Mor V, Volandes AE, Gutman R, Gatsonis C, Mitchell SL. Pragmatic trial of video education in nursing homes: the design and rationale for a pragmatic cluster randomized trial in the nursing home setting. Clin Trials. 2017;14(2):140-151. doi: 10.1177/1740774516685298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell SL, Mor V, Gozalo PL, Servadio JL, Teno JM. Tube feeding in US nursing home residents with advanced dementia, 2000-2014. JAMA. 2016;316(7):769-770. doi: 10.1001/jama.2016.9374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas KS, Dosa D, Wysocki A, Mor V. The Minimum Data Set 3.0 Cognitive Function Scale. Med Care. 2017;55(9):e68-e72. doi: 10.1097/MLR.0000000000000334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas KS, Ogarek JA, Teno JM, Gozalo PL, Mor V. Development and validation of the nursing home Minimum Data Set 3.0 mortality risk score (MRS3). J Gerontol A Biol Sci Med Sci. 2019;74(2):219-225. doi: 10.1093/gerona/gly044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MedPAR data documentation. Research Data Assistance Center website. https://www.resdac.org/cms-data/files/medpar/data-documentation. Accessed November 19, 2019.
- 27.2019. HCPCS codes level II. HCPCS website. https://hcpcs.codes. Accessed November 19, 2019.
- 28.American Medical Association CPT Professional 2019: Current Procedural Terminology. Chicago, IL: American Medical Association; 2019. [Google Scholar]
- 29.Master Beneficiary Summary File (MBSF) base. Research Data Assistance Center website. https://www.resdac.org/cms-data/files/mbsf-base. Accessed November 19, 2019.
- 30.Weaver CG, Ravani P, Oliver MJ, Austin PC, Quinn RR. Analyzing hospitalization data: potential limitations of Poisson regression. Nephrol Dial Transplant. 2015;30(8):1244-1249. doi: 10.1093/ndt/gfv071 [DOI] [PubMed] [Google Scholar]
- 31.Gupta A, Allen LA, Bhatt DL, et al. Association of the Hospital Readmissions Reduction Program implementation with readmission and mortality outcomes in heart failure. JAMA Cardiol. 2018;3(1):44-53. doi: 10.1001/jamacardio.2017.4265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuckerman RB, Stearns SC, Sheingold SH. Hospice use, hospitalization, and Medicare spending at the end of life. J Gerontol B Psychol Sci Soc Sci. 2016;71(3):569-580. doi: 10.1093/geronb/gbv109 [DOI] [PubMed] [Google Scholar]
- 33.Joshi S, Nuckols T, Escarce J, Huckfeldt P, Popescu I, Sood N. Regression to the mean in the Medicare Hospital Readmissions Reduction Program. JAMA Intern Med. 2019;179(9):1167-1173. doi: 10.1001/jamainternmed.2019.1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Givens JL, Selby K, Goldfeld KS, Mitchell SL. Hospital transfers of nursing home residents with advanced dementia. J Am Geriatr Soc. 2012;60(5):905-909. doi: 10.1111/j.1532-5415.2012.03919.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meier DE, Back AL, Berman A, Block SD, Corrigan JM, Morrison RS. A national strategy for palliative care. Health Aff (Millwood). 2017;36(7):1265-1273. doi: 10.1377/hlthaff.2017.0164 [DOI] [PubMed] [Google Scholar]
- 36.Miller SC, Lima JC, Intrator O, Martin E, Bull J, Hanson LC. Palliative care consultations in nursing homes and reductions in acute care use and potentially burdensome end-of-life transitions. J Am Geriatr Soc. 2016;64(11):2280-2287. doi: 10.1111/jgs.14469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller SC, Dahal R, Lima JC, et al. Palliative care consultations in nursing homes and end-of-life hospitalizations. J Pain Symptom Manage. 2016;52(6):878-883. doi: 10.1016/j.jpainsymman.2016.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng NT, Mukamel DB, Friedman B, Caprio TV, Temkin-Greener H. The effect of hospice on hospitalizations of nursing home residents. J Am Med Dir Assoc. 2015;16(2):155-159. doi: 10.1016/j.jamda.2014.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feng Z, Ingber MJ, Segelman M, et al. Nursing facilities can reduce avoidable hospitalizations without increasing mortality risk for residents. Health Aff (Millwood). 2018;37(10):1640-1646. doi: 10.1377/hlthaff.2018.0379 [DOI] [PubMed] [Google Scholar]
- 40.Walsh E, Freiman M, Haber S, Bragg A, Ouslander J, Wiener J Cost drivers for dually eligible beneficiaries: potentially avoidable hospitalizations from nursing facility, skilled nursing facility, and home and community-based services waiver programs: final task 2 report. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Reports/downloads/costdriverstask2.pdf. Accessed October 6, 2019. [DOI] [PubMed]
- 41.Mitchell SL, Catic AG, Givens JL, Knopp J, Moran JA Advanced dementia: a guide for families. https://www.marcusinstituteforaging.org/sites/default/files/files/DementiaGuideForFamilies.pdf. Accessed October 6, 2019.
- 42.Fried TR, Gillick MR, Lipsitz LA. Whether to transfer? factors associated with hospitalization and outcome of elderly long-term care patients with pneumonia. J Gen Intern Med. 1995;10(5):246-250. doi: 10.1007/BF02599879 [DOI] [PubMed] [Google Scholar]
- 43.Loeb M, Carusone SC, Goeree R, et al. Effect of a clinical pathway to reduce hospitalizations in nursing home residents with pneumonia: a randomized controlled trial. JAMA. 2006;295(21):2503-2510. doi: 10.1001/jama.295.21.2503 [DOI] [PubMed] [Google Scholar]
- 44.Givens JL, Jones RN, Shaffer ML, Kiely DK, Mitchell SL. Survival and comfort after treatment of pneumonia in advanced dementia. Arch Intern Med. 2010;170(13):1102-1107. doi: 10.1001/archinternmed.2010.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McAndrew RM, Grabowski DC, Dangi A, Young GJ. Prevalence and patterns of potentially avoidable hospitalizations in the US long-term care setting. Int J Qual Health Care. 2016;28(1):104-109. doi: 10.1093/intqhc/mzv110 [DOI] [PubMed] [Google Scholar]
- 46.Ouslander JG, Bonner A, Herndon L, Shutes J. The Interventions to Reduce Acute Care Transfers (INTERACT) quality improvement program: an overview for medical directors and primary care clinicians in long term care. J Am Med Dir Assoc. 2014;15(3):162-170. doi: 10.1016/j.jamda.2013.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Figueroa JF, Burke LG, Zheng J, Orav EJ, Jha AK. Trends in hospitalization vs observation stay for ambulatory care-sensitive conditions [published online August 26, 2019]. JAMA Intern Med. doi: 10.1001/jamainternmed.2019.3177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chhabra KR, Ibrahim AM, Thumma JR, Ryan AM, Dimick JB. Impact of Medicare readmissions penalties on targeted surgical conditions. Health Aff (Millwood). 2019;38(7):1207-1215. doi: 10.1377/hlthaff.2019.00096 [DOI] [PubMed] [Google Scholar]
- 49.Brennan N, Engelhardt T Data brief: sharp reductions in avoidable hospitalizations among long-term care facility residents. Centers for Medicare & Medicaid Services blog page. https://www.cms.gov/blog/data-brief-sharp-reduction-avoidable-hospitalizations-among-long-term-care-facility-residents. Accessed October 6, 2019.
- 50.Ferro EG, Secemsky EA, Wadhera RK, et al. Patient readmission rates for all insurance types after implementation of the Hospital Readmissions Reduction Program. Health Aff (Millwood). 2019;38(4):585-593. doi: 10.1377/hlthaff.2018.05412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Data.Medicare.gov. FY 2019. Skilled Nursing Facility Value-Based Purchasing (SNF VBP) Program. Nursing Home Compare database. https://data.medicare.gov/Nursing-Home-Compare/SNF-VBP-Facility-Level-Dataset/284v-j9fz. Accessed October 6, 2019.
- 52.Trahan LM, Spiers JA, Cummings GG. Decisions to transfer nursing home residents to emergency departments: a scoping review of contributing factors and staff perspectives. J Am Med Dir Assoc. 2016;17(11):994-1005. doi: 10.1016/j.jamda.2016.05.012 [DOI] [PubMed] [Google Scholar]
- 53.Ashcraft AS, Champion JD. Nursing home resident symptomatology triggering transfer: avoiding unnecessary hospitalizations. Nurs Res Pract. 2012;2012:495103. doi: 10.1155/2012/495103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spector WD, Limcangco R, Williams C, Rhodes W, Hurd D. Potentially avoidable hospitalizations for elderly long-stay residents in nursing homes. Med Care. 2013;51(8):673-681. doi: 10.1097/MLR.0b013e3182984bff [DOI] [PubMed] [Google Scholar]
- 55.Unroe KT, Carnahan JL, Hickman SE, Sachs GA, Hass Z, Arling G. The complexity of determining whether a nursing home transfer is avoidable at time of transfer. J Am Geriatr Soc. 2018;66(5):895-901. doi: 10.1111/jgs.15286 [DOI] [PubMed] [Google Scholar]
- 56.Unroe KT, Hickman SE, Carnahan JL, Hass Z, Sachs G, Arling G. Investigating the avoidability of hospitalizations of long stay nursing home residents: opportunities for improvement. Innov Aging. 2018;2(2):igy017. doi: 10.1093/geroni/igy017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.MedPAC Hospital and SNF use by Medicare beneficiaries who reside in nursing facilities. http://www.medpac.gov/docs/default-source/reports/jun17_ch9.pdf?sfvrsn=0. Accessed November 19, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Detailed Listing of ICD-9/ICD-10-CM Diagnosis Codes for Potentially Avoidable Conditions and for Serious Bone Fractures. (Pages 2-21)
eTable 2. Total Number of Hospital Transfers Among Nursing Home Residents With Advanced Illness by Cohort Year (Page 22)
eFigure. Percent of All Transfers That Were Potentially Avoidable by Cohort Year (Page 23)