This case series reports the clinical and symptomatic response following oral sirolimus and erlotinib treatment in 4 children with Olmsted syndrome.
Key Points
Question
Does inhibiting the epidermal growth factor receptor and mammalian target of rapamycin signaling pathways have therapeutic utility in Olmsted syndrome?
Findings
In this case series of 4 children with Olmsted syndrome, skin biopsies performed in 2 patients revealed increased activation of mammalian target of rapamycin signaling and epidermal growth factor receptor signaling. After treatment with oral sirolimus and/or erlotinib, all 4 patients showed substantial clinical and symptomatic improvement.
Meaning
Findings from this study suggest that oral sirolimus and erlotinib may represent a novel, promising, and well-tolerated therapy for Olmsted syndrome.
Abstract
Importance
Olmsted syndrome is a rare and disabling genodermatosis for which no successful treatment is currently available.
Objective
To evaluate the clinical response to the mammalian target of rapamycin (mTOR) inhibitor sirolimus and/or the epidermal growth factor receptor (EGFR) inhibitor erlotinib among patients with Olmsted syndrome.
Design, Setting, and Participants
This case series focused on 4 children with treatment-refractory Olmsted syndrome. These children received treatments (initiated in 2017 and 2018) at the outpatient dermatology clinic at the Children’s Hospital of Wisconsin in Milwaukee, Wisconsin; Children’s National Hospital in Washington, DC; and Hospital Infantil Pequeno Príncipe, Curitiba in Paraná, Brazil.
Exposures
Immunohistochemical analyses for mTOR and EGFR activation were performed on skin biopsy specimens from 2 patients. Oral sirolimus was administered to these 2 patients at a dosage of 0.8 mg/m2 twice daily, titrated to a goal trough whole-blood concentration of 10 to 15 ng/mL. Erlotinib was administered to all 4 patients at a dosage of 2 mg/kg/d.
Main Outcomes and Measures
Clinical responses were assessed with visual analog scales for pruritus and pain and/or the Children’s Dermatology Life Quality Index. Adverse effects were monitored throughout treatment.
Results
Four patients (mean [SD] age, 7 [6] years; 2 boys and 2 girls) were analyzed. Lesional skin immunostaining showed increased phosphorylated ribosomal protein S6 (RPS6) and phosphorylated EGFR staining in the epidermis, indicating enhanced mTOR and EGFR signaling activation. Patients 1 and 2 were initially treated with sirolimus, displaying substantial clinical improvement in erythema and periorificial hyperkeratosis afterward. When switched to erlotinib, these patients showed substantial palmoplantar keratoderma (PPK) improvement. Patients 3 and 4 were treated with erlotinib only and later showed rapid and near complete resolution of PPK and substantial improvement in Children’s Dermatology Life Quality Index scores. All 4 patients had sustained improvements in pruritus and pain. No severe adverse effects were reported.
Conclusions and Relevance
This study’s findings suggest that the EGFR-mTOR cascade may play a substantial role in the pathophysiological process of Olmsted syndrome and may serve as a major therapeutic target. Oral sirolimus and erlotinib may be a promising, life-altering treatment for pediatric patients with Olmsted syndrome.
Introduction
Olmsted syndrome is a rare disabling genodermatosis characterized by severe palmoplantar keratoderma (PPK) and periorificial hyperkeratotic plaques with no satisfactory treatment.1,2 Pruritus and pain play major roles in morbidity, with PPK potentially restricting ambulation. Mutations in transient receptor potential vanilloid-3 gene (TRPV3; OMIM is 607066) are the most common causes of Olmsted syndrome.1 In keratinocytes, the TRPV3 channel forms a signaling complex with epidermal growth factor receptor (EGFR).3 Amplification of EGFR leads to the activation of many downstream kinases, including mammalian target of rapamycin (mTOR).4
In this case series of 4 patients, we investigated mTOR and EGFR pathway activation in skin with Olmsted syndrome. The goal was to ascertain whether the oral mTOR inhibitor sirolimus and the EGFR inhibitor erlotinib hydrochloride could be therapeutic options for Olmsted syndrome.
Methods
The 4 patients analyzed in this case series received treatments at the outpatient dermatology clinic at the Children’s Hospital of Wisconsin in Milwaukee, Wisconsin; at the Children’s National Hospital in Washington, DC; and at the Hospital Infantil Pequeno Príncipe, Curitiba in Paraná, Brazil. After parents provided written informed consent, lesional skin samples from 2 patients were obtained for biopsies and tested for phosphorylation of the downstream mTOR pathway target ribosomal protein S6 (RPS6), a hallmark of mTOR pathway activation, and phosphorylation of EGFR, an indicator of EGFR activation.5 Oral sirolimus and/or erlotinib were initiated after informed consent was received. This study was approved by the institutional review board of the Children’s Hospital of Wisconsin.
Clinical response and adverse events were monitored throughout the treatment periods. For patients 1 and 2, oral sirolimus was initially administered on December 12, 2017, and discontinued on October 1, 2018, while erlotinib treatment began on October 17, 2018, and continues in the present. For patients 3 and 4, erlotinib was first administered on October 19, 2018, and continues today. Response to treatment was evaluated by the size, thickness, and inflammation of lesions; pain and pruritus scores; and the Children’s Dermatology Life Quality Index scores.
Results
Four patients (mean [SD] age, 7 [6] years; 2 boys and 2 girls) were included in the study. Patients 1 and 2 initially received a dosage of oral sirolimus 0.8 mg/m2 twice daily, followed by erlotinib at a dosage of 2 mg/kg/d. Patients 3 and 4 were treated with erlotinib only.
Although the symptoms in patients 1 and 2 did not clear completely, all 4 patients showed rapid improvement within weeks after erlotinib initiation, including substantially decreased pruritus and pain as well as enhanced physical activity. All patients experienced mild transient hair loss and acneiform eruptions, which responded well to topical medications. No other adverse events were reported.
Cases 1 and 2
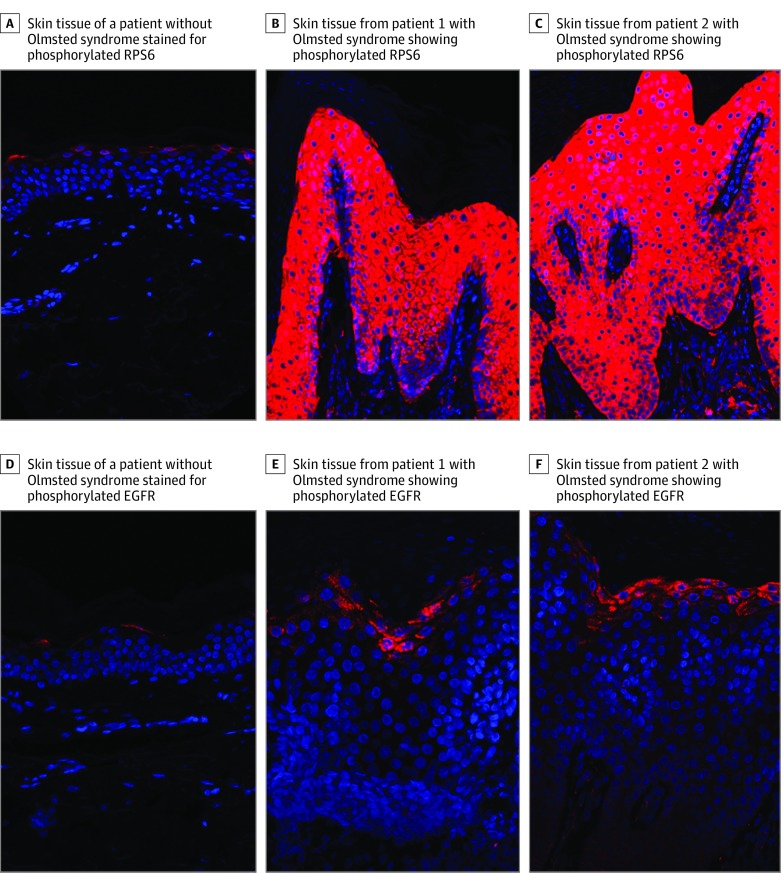
Shortly after birth, twin girls presented with hyperkeratotic plaques on the scalp that progressed to involve the periorbital and perioral skin, ears, neck, axillae, and genital area. They also developed severe erythema on the head and neck and diffuse alopecia. Genetic testing revealed the heterozygous TRPV3 variant c.1718 G>T (p.Gly573Val) in both patients. The twins were initially treated with topical corticosteroids and tazarotene cream, but disease progression continued. At 6 months of age, they developed painful PPK and began to receive oral acitretin, which was discontinued after 6 months owing to lack of improvement. Skin biopsy specimens were then obtained for immunostaining using DAPI (4′,6-diamidino-2-phenylindole) nuclear counterstain. Compared with control skin, lesional skin from the twins showed markedly increased RPS6 phosphorylation and a less pronounced but still substantial increase in EGFR phosphorylation, indicating abnormal enhancement of the mTOR pathway and EGFR activation (Figure 1).
Figure 1. Mammalian Target of Rapamycin (mTOR) and Epidermal Growth Factor Receptor (EGFR) Pathway Activation in Patients With Olmsted Syndrome .
Compared with tissue from the skin of a control patient without Olmsted syndrome (A), tissue from patient 1 (B) and patient 2 (C) that underwent immunostaining showed substantially stronger level of phosphorylated ribosomal protein S6 (RPS6; Ser240/244), a hallmark of mTOR signaling pathway activation, expanded to all layers of the epidermis. Compared with control tissue (D), tissue from patient 1 (E) and patient 2 (F) that underwent immunostaining showed a substantially stronger level of phosphorylated EGFR (Tyr1173), a hallmark of EGFR pathway activation, slightly expanded in the epidermis. DAPI (4′,6-diamidino-2-phenylindole) nuclear counterstain is shown in blue.
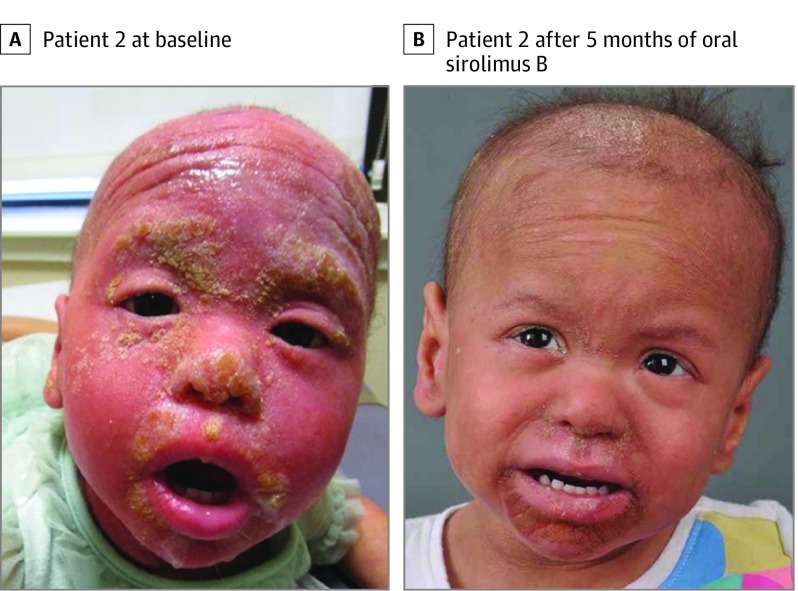
The twins began receiving treatment with oral sirolimus at a dosage of 0.8 mg/m2 twice daily, titrated to a trough concentration of 10 to 15 ng/mL.6 They improved rapidly and, 5 months later, showed resolution of their erythema and substantial reduction of their periorificial hyperkeratotic plaques (Figure 2; eFigure 1 in the Supplement). Parent-reported pruritus and pain scores, assessed with a visual analog scale, decreased from 10 to 2 for pruritus and 10 to 5 for pain in patient 1, and from 10 to 2 for pruritus and 9 to 5 for pain in patient 2. (Pain and pruritus scores for patients 1 and 2 were reported by their parents because of the patients’ young age.) No adverse effects were reported. However, the twins’ PPK continued to progress while taking sirolimus. After 9 months, sirolimus was discontinued and erlotinib 2 mg/kg/d was administered, and rapid improvement of the PPK followed. After 6 months, pruritus and pain scores decreased to 0 and 2, respectively, for both girls, and patient 2 was able to start walking 2 weeks after the initial administration of erlotinib.
Figure 2. Response to the Mammalian Target of Rapamycin Pathway Inhibitor Sirolimus.
Compared with baseline (A), patient 2 showed resolution of erythema, regrowth of hair, and substantial reduction of periorificial hyperkeratotic plaques after 5 months of oral sirolimus treatment (B).
Case 3
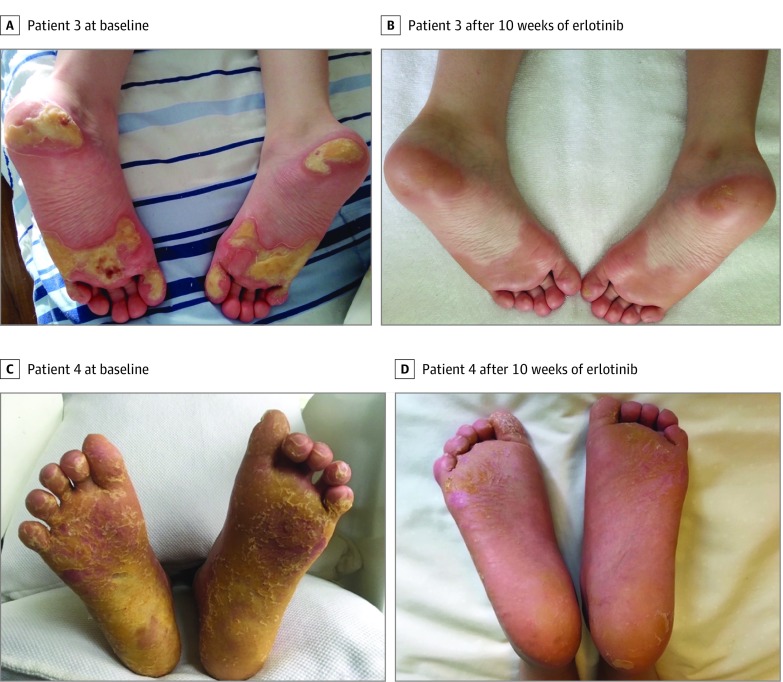
A boy about 10 years of age presented with painful PPK primarily involving pressure areas of the feet from 2 years of age. He had hyperkeratosis of the external auditory canals but no other features of Olmsted syndrome. The patient and his affected mother were both heterozygous for the c.1717G>T (p.Gly573Cys) TRPV3 variant. He had been treated with acitretin 10 to 15 mg daily since age 5 years with limited efficacy. The patient began treatment with erlotinib 2 mg/kg/d. Rapid and sustained clinical improvement occurred within the first week, including decreased pain, pruritus, and Children’s Dermatology Life Quality Index scores (Figure 3A and 3B; eFigure 2A in the Supplement).
Figure 3. Response to the Epidermal Growth Factor Receptor Pathway Inhibitor Erlotinib.
Compared with patient 3 (A) and patient 4 (C) at baseline, patient 3 (B) and patient 4 (D) showed near complete resolution of hyperkeratotic plantar plaques after 10 weeks of erlotinib therapy.
Case 4
A teenage boy presented with painful diffuse PPK starting at 2 years of age. He did not have periorificial hyperkeratosis. The patient was heterozygous for the c.2017C>T (p.Leu673Phe) variant in TRPV3. The patient had been treated with acitretin 10 mg daily from 4 years of age but showed limited improvement. After starting erlotinib 2mg/kg/d, the patient experienced almost complete resolution of his PPK with decreased pain, pruritus, and Children’s Dermatology Life Quality Index scores (Figure 3C and 3D; eFigure 2B in the Supplement). Acitretin was discontinued after 7 weeks. His weight also improved, moving him from the first to the tenth weight percentile.
Discussion
Olmsted syndrome is an extremely disabling genodermatosis with no current effective treatment. The syndrome is primarily defined by PPK and periorificial hyperkeratotic plaques, but considerable clinical heterogeneity exists. Most cases of Olmsted syndrome, including for all 4 patients in this study, result from pathogenic mutations in TRPV3. Three of the patients carried mutations in the mutational hotspot Gly573, which is the most frequent TRPV3 mutation identified to date in Olmsted syndrome. The familial variant identified in patient 3 and the de novo variants identified in patients 1, 2, and 4 have all been previously described.1,2,7,8 Each of these substitutions changes a highly conserved amino acid residue across species and is predicted to be damaging by Polyphen-2, SIFT, and MutationTaster software. Moreover, functional studies have shown that Gly573Cys is a gain-of-function mutation that increases ion-channel activity in vitro and that Gly573Ser is associated with pruritus and/or dermatitis in transgenic mice in vivo.8,9
The TRPV3 channel interacts with and regulates EGFR signaling. This interaction is essential for the proliferation and terminal differentiation of keratinocytes, hair morphogenesis, and skin barrier maintenance.3 In murine skin, increased EGFR signaling promotes skin cancer and hairless phenotypes.10,11 The EGFR is a potent activator of many downstream kinases, including the serine/threonine kinase mTOR, a major regulator of cell growth and proliferation. Activation of the mTOR pathway has been observed in various inflammatory and neoplastic skin diseases, including pachyonychia congenita.12,13 Similar to Olmsted syndrome, pachyonychia congenita is characterized by painful PPK and has been reported to respond to mTOR inhibition.14
In the present study, oral sirolimus was used to treat Olmsted syndrome in patients 1 and 2, which was supported on immunohistochemistry by enhanced mTOR signaling activation. Although the patients experienced substantial reduction of their periorificial plaques, their PPK did not improve. This response could be associated with their massive degree of PPK and/or sirolimus having a greater effect on the inflammatory component of the hyperkeratotic plaques, which was more prominent on the head than on the hands and feet.
For the PPK aspect of Olmsted syndrome, all 4 patients were treated with the EGFR inhibitor erlotinib. Erlotinib has been reported to treat PPK in an adult patient with Olmsted syndrome, but it had not been used in pediatric patients.15 Increased EGFR phosphorylation in the skin of patients 1 and 2 appears to support the theory that TRPV3 mutations activate EGFR signaling and that EGFR could serve as the missing link between TRPV3 mutations and mTOR pathway activation.
Limitations
This study has some limitations, including the absence of a control group and the small number of cases, with only 2 of the 4 patients receiving sirolimus. Although the preliminary observations were promising, larger studies with longer follow-up periods can further assess clinical advantages, long-term safety, clinical course after drug discontinuation, and responses in patients with Olmsted syndrome with different mutations. In addition, no standardized rating scales for Olmsted syndrome were available.
Conclusions
This case series demonstrates that EGFR appears to play a critical role in the pathogenesis of Olmsted syndrome through its interaction with the TRPV3 channel, which is associated with increased mTOR activation and downstream outcomes, including skin inflammation and hyperproliferation. We identified the EGFR-mTOR cascade as a therapeutic target for Olmsted syndrome. Administration of the oral mTOR inhibitor sirolimus to pediatric patients with Olmsted syndrome was followed by substantial improvement of skin inflammation and periorificial hyperkeratotic plaques, whereas the dramatic reduction of PPK and its associated functional limitations was the clinical response to the use of EGFR inhibitor erlotinib. These findings suggest that these medications represent promising, life-altering treatments for this disabling disease.
eFigure 1. Patient 1 Response to the mTOR Inhibitor Sirolimus
eFigure 2. Responses in Pain, Pruritus, and Quality of Life to the EGFR Inhibitor Erlotinib
References
- 1.Duchatelet S, Hovnanian A. Olmsted syndrome: clinical, molecular and therapeutic aspects. Orphanet J Rare Dis. 2015;10:33. doi: 10.1186/s13023-015-0246-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson NJ, Cole C, Milstone LM, et al. Expanding the phenotypic spectrum of Olmsted syndrome. J Invest Dermatol. 2015;135(11):2879-2883. doi: 10.1038/jid.2015.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng X, Jin J, Hu L, et al. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell. 2010;141(2):331-343. doi: 10.1016/j.cell.2010.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wee P, Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers (Basel). 2017;9(5):E52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruf MT, Andreoli A, Itin P, Pluschke G, Schmid P. Ribosomal protein S6 is hyperactivated and differentially phosphorylated in epidermal lesions of patients with psoriasis and atopic dermatitis. Br J Dermatol. 2014;171(6):1533-1536. doi: 10.1111/bjd.13248 [DOI] [PubMed] [Google Scholar]
- 6.Adams DM, Trenor CC III, Hammill AM, et al. Efficacy and safety of sirolimus in the treatment of complicated vascular anomalies. Pediatrics. 2016;137(2):e20153257. doi: 10.1542/peds.2015-3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhi YP, Liu J, Han JW, et al. Two familial cases of Olmsted-like syndrome with a G573V mutation of the TRPV3 gene. Clin Exp Dermatol. 2016;41(5):510-513. doi: 10.1111/ced.12833 [DOI] [PubMed] [Google Scholar]
- 8.Lin Z, Chen Q, Lee M, et al. Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am J Hum Genet. 2012;90(3):558-564. doi: 10.1016/j.ajhg.2012.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshioka T, Imura K, Asakawa M, et al. Impact of the Gly573Ser substitution in TRPV3 on the development of allergic and pruritic dermatitis in mice. J Invest Dermatol. 2009;129(3):714-722. doi: 10.1038/jid.2008.245 [DOI] [PubMed] [Google Scholar]
- 10.Montell C. Preventing a perm with TRPV3. Cell. 2010;141(2):218-220. doi: 10.1016/j.cell.2010.03.043 [DOI] [PubMed] [Google Scholar]
- 11.Schneider MR, Werner S, Paus R, Wolf E. Beyond wavy hairs: the epidermal growth factor receptor and its ligands in skin biology and pathology. Am J Pathol. 2008;173(1):14-24. doi: 10.2353/ajpath.2008.070942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buerger C, Shirsath N, Lang V, et al. Inflammation dependent mTORC1 signaling interferes with the switch from keratinocyte proliferation to differentiation. PLoS One. 2017;12(7):e0180853. doi: 10.1371/journal.pone.0180853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fogel AL, Hill S, Teng JM. Advances in the therapeutic use of mammalian target of rapamycin (mTOR) inhibitors in dermatology. J Am Acad Dermatol. 2015;72(5):879-889. doi: 10.1016/j.jaad.2015.01.014 [DOI] [PubMed] [Google Scholar]
- 14.Hickerson RP, Leake D, Pho LN, Leachman SA, Kaspar RL. Rapamycin selectively inhibits expression of an inducible keratin (K6a) in human keratinocytes and improves symptoms in pachyonychia congenita patients. J Dermatol Sci. 2009;56(2):82-88. doi: 10.1016/j.jdermsci.2009.07.008 [DOI] [PubMed] [Google Scholar]
- 15.Kenner-Bell BM, Paller AS, Lacouture ME. Epidermal growth factor receptor inhibition with erlotinib for palmoplantar keratoderma. J Am Acad Dermatol. 2010;63(2):e58-e59. doi: 10.1016/j.jaad.2009.10.052 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Patient 1 Response to the mTOR Inhibitor Sirolimus
eFigure 2. Responses in Pain, Pruritus, and Quality of Life to the EGFR Inhibitor Erlotinib