Key Points
Question
For patients recently hospitalized for acute decompensated heart failure (ADHF), what is the effect of administering sacubitril/valsartan?
Findings
In this secondary analysis of PIONEER-HF, patients with ADHF were treated with either in-hospital initiation of sacubitril/valsartan (titrated to target dose, 97 mg of sacubitril with 103 mg of valsartan twice daily) continued for 12 weeks or in-hospital enalapril (titrated to target dose, 10 mg twice daily) continued for 8 weeks followed by sacubitril/valsartan for 4 weeks. Switching patients’ treatment from enalapril to sacubitril/valsartan at 8 weeks led to a further 37% reduction in N-terminal pro–B-type natriuretic peptide levels.
Meaning
Sacubitril/valsartan is associated with further decreases in N-terminal pro–B-type natriuretic peptide levels compared with enalapril in patients recently treated for ADHF.
Abstract
Importance
In PIONEER-HF, among stabilized patients with acute decompensated heart failure (ADHF), the in-hospital initiation of sacubitril/valsartan was well tolerated and led to improved outcomes compared with enalapril. However, there are limited data comparing the strategies of in-hospital vs postdischarge initiation of sacubitril/valsartan.
Objective
To describe changes in N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels in patients recently hospitalized for ADHF and switching from taking enalapril to taking sacubitril/valsartan after discharge and compare clinical outcomes for patients randomized to receive in-hospital initiation of sacubitril/valsartan vs in-hospital initiation of enalapril who later switched to taking sacubitril/valsartan during an open-label extension phase.
Interventions
Sacubitril/valsartan titrated to 97/103 mg twice daily.
Design, Setting, and Participants
The PIONEER-HF trial was a multicenter, randomized, double-blind, active-controlled trial conducted at 129 US sites between May 2016 and May 2018 that compared the in-hospital initiation of sacubitril/valsartan vs enalapril (titrated to target dose, 10 mg twice daily) for 8 weeks among patients admitted for ADHF with reduced ejection fraction and hemodynamic stability. All patients were to continue in a 4-week, open-label study of sacubitril/valsartan; of 881 patients enrolled in PIONEER-HF, 832 (94%) continued in the open-label study.
Main Outcomes and Measures
Changes in NT-proBNP levels from week 8 to 12 as well as the exploratory composite of heart failure rehospitalization or cardiovascular death from randomization through week 12.
Results
Of 881 participants, 226 (27.7%) were women, 487 (58.5%) were white, 297 (35.7%) were black, 15 (1.8%) were Asian, and 73 (8.8%) were of Hispanic ethnicity; the mean (SD) age was 61 (14) years. For patients who continued to take sacubitril/valsartan, NT-proBNP levels declined −17.2% (95% CI, −3.2 to −29.1) from week 8 to 12. The NT-proBNP levels declined to a greater extent for those switching from taking enalapril to sacubitril/valsartan after the week 8 visit (−37.4%; 95% CI, −28.1 to −45.6; P < .001; comparing changes in 2 groups). Over the entire 12 weeks of follow-up, patients that began taking sacubitril/valsartan in the hospital had a lower hazard for the composite outcome compared with patients that initiated enalapril in the hospital and then had a delayed initiation of sacubitril/valsartan 8 weeks later (hazard ratio, 0.69; 95% CI 0.49-0.97).
Conclusions and Relevance
Switching patients’ treatment from enalapril to sacubitril/valsartan at 8 weeks after randomization led to a further 37% reduction in NT-proBNP levels in patients with heart failure with reduced ejection fraction and a recent hospitalization for ADHF.
Trial Registration
ClinicalTrials.gov identifier: NCT02554890.
This secondary analysis of the PIONEER-HF trial explores the association between patients with chronic heart failure switching from taking enalapril to taking sacubitril/valsartan with N-terminal pro–B-type natriuretic peptide levels.
Introduction
Sacubitril/valsartan is an angiotensin receptor neprilysin-inhibitor recommended for use in patients with chronic heart failure with reduced ejection fraction (HFrEF).1 The PIONEER-HF trial expanded the evidence regarding the use of sacubitril/valsartan by studying patients with HFrEF who were hospitalized with acute decompensated HF (ADHF) and had achieved hemodynamic stability.2,3 Patients randomized to receive an in-hospital initiation of sacubitril/valsartan had a greater reduction in N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels and improved clinical outcomes compared with enalapril (eTable in the Supplement).3,4
In this secondary analysis, we report the results of the PIONEER-HF open-label extension study. After 8 weeks of follow-up, all patients who remained in the trial were to continue a 4-week open-label extension study and take sacubitril/valsartan (titrated to target dose, 97 mg of sacubitril with 103 mg of valsartan twice daily). This study was primarily conducted to evaluate the change in NT-proBNP levels in patients switching from taking enalapril (titrated to target dose, 10 mg twice daily) to sacubitril/valsartan. We also evaluated pooled outcomes to compare the totality of clinical events that occurred over the entire 12 weeks of follow-up in the 2 treatment arms.
Methods
Study Patients
The design and primary results of PIONEER-HF have been previously described.2,3 The key eligibility criteria included the following: a primary diagnosis of ADHF, left ventricular ejection fraction of 40% or less, NT-proBNP levels of 1600 pg/mL or greater, and hemodynamic stability, including a systolic blood pressure of 100 mm Hg for at least 6 hours before randomization. Patients enrolled in PIONEER-HF signed written informed consent before the collection of study data and institutional review board approval was obtained from all of the study institutional review boards that approved the study protocol. The PIONEER-HF study was sponsored by the Novartis Pharmaceuticals Corporation (East Hanover, New Jersey). An academic leadership committee designed the protocol and oversaw the implementation of the protocol in conjunction with the trial sponsor.
Study Procedures
Patients enrolled in PIONEER-HF were randomized to receive sacubitril/valsartan or enalapril. The first dose was administered in the hospital and then titrated at study visits. Because recruitment of participants from racial/ethnic minorities was a study goal, race/ethnicity was ascertained at the time of screening by investigators based on predefined categories. After the week 8 visit, all patients underwent a 36-hour washout and then began a 4-week open-label extension study with sacubitril/valsartan. Sites were recommended to administer sacubitril/valsartan at the week 8 dosing level and up titrate to 97/103 mg twice daily. Patients then had study visits at 10 and 12 weeks after randomization and were contacted for a safety evaluation via telephone call 30 days later.
NT-proBNP Measurements and Clinical Outcomes
Biomarker samples were frozen at local sites and shipped to a central laboratory (Clinical Reference Laboratory; Lenexa, Kansas). Plasma NT-proBNP was measured using a sandwich immunoassay (proBNP II; Roche Diagnostics). Clinical events, including rehospitalizations and mortality, were reported by sites; we later undertook a post hoc adjudication of clinical events.4
Statistical Analysis
Baseline characteristics were described by treatment arm. We tested for differences using χ2 or Fisher exact tests for categorical variables and 2-sample t tests or Wilcoxon rank-sum tests for continuous variables. We then described and compared the associated changes in NT-proBNP levels from week 8 through the end of the study by randomized treatment arm. The incidences of safety events were calculated along with relative risks (RRs) and associated 95% CIs. Cumulative clinical events over the entire study period were described and compared according to the Kaplan-Meier methods and a log-rank test. We fit a Cox proportional hazards regression model and calculated the hazard ratio (HR) and corresponding 95% CIs.
Data were managed by the United BioSource Corporation (Blue Bell, Pennsylvania), and the Duke Clinical Research Institute (Durham, North Carolina) was the data analytic center for this analysis. All analyses were performed using SAS software (version 9.4; SAS Institute). P values were 2-sided and values of less than .05 were considered significant.
Results
Of 881 patients enrolled in PIONEER-HF, 832 (94%) participated in the open-label extension study (eFigure 1 in the Supplement). Baseline characteristics are shown in Table 1. The mean (SD) age of participants was 61 years (14), 226 (27%) were women, 297 (36%) were black, and 293 (35%) had no prior diagnosis of HF. When patients began the open-label extension study, the median NT-proBNP was 1218 pg/mL (interquartile range, 522-3125) in patients continuing to take sacubitril/valsartan and 1630 pg/mL (interquartile range, 866-3423) in patients switching from taking enalapril to sacubitril/valsartan (P = .01).
Table 1. Patient Characteristics.
| Characteristics Measured at Study Enrollment | No. (%) | P Value | ||
|---|---|---|---|---|
| Overall (N = 832) | In-Hospital Followed by S/V | |||
| S/V (n = 417) | Enalapril (n = 415) | |||
| Age, mean (SD), y | 61 (14) | 61 (14) | 62 (14) | .23 |
| Women | 226 (27.2) | 104 (24.9) | 122 (29.4) | .15 |
| Race | ||||
| White | 487 (58.5) | 247 (59.2) | 240 (57.8) | .82 |
| Black | 297 (35.7) | 149 (35.7) | 148 (35.7) | |
| Asian | 15 (1.8) | 7 (1.7) | 8 (1.9) | |
| Other | 33 (4.0) | 14 (3.4) | 19 (4.6) | |
| Hispanic ethnicity | 73 (8.8) | 33 (7.9) | 40 (9.7) | .38 |
| Medical history | ||||
| Prior HF | 539 (64.8) | 281 (67.4) | 258 (62.2) | .19 |
| Atrial fibrillation | 298 (35.8) | 139 (33.3) | 159 (38.3) | .13 |
| Coronary disease | 227 (27.3) | 111 (26.6) | 116 (28.0) | .67 |
| Hypertension | 709 (85.2) | 364 (87.3) | 345 (83.1) | .09 |
| Diabetes | 157 (18.9) | 75 (18.0) | 82 (19.8) | .51 |
| CKD | 232 (27.9) | 120 (28.8) | 112 (27.0) | .57 |
| LVEF %, mean (SD) | 24 (8.0) | 24 (8.0) | 24 (7.9) | .93 |
| Vital signs, mean (SD) | ||||
| BMI | 32.1 (8.9) | 32.2 (9.0) | 32.1 (8.8) | .95 |
| BP, mm Hg | ||||
| Systolic | 122 (15.5) | 122 (14.9) | 122 (16.2) | .81 |
| Diastolic | 75 (12.7) | 76 (12.4) | 75 (13.0) | .37 |
| Laboratory data, median (IQR) | ||||
| NT-proBNP, pg/mL | 2685 (1479-5102) | 2883 (1602-5395) | 2515 (1351-4898) | .04 |
| BUN, mg/dL | 21 (17.0-28.0) | 22 (17.0-28.0) | 21 (16.0-28.0) | .96 |
| Serum Cr, mg/dL | 1.3 (1.1-1.5) | 1.3 (1.1-1.5) | 1.3 (1.1-1.5) | .84 |
| eGFR, mL/min 1.73 m2 | 59 (47.7-71.7) | 59 (48.0-71.8) | 59 (47.5-71.5) | .60 |
| Characteristics measured at week 8 before open-label extension, median (IQR) | ||||
| BP, mm Hg | ||||
| Systolic | 118 (105-131) | 118 (104-129) | 120 (108-133) | .04 |
| Diastolic | 70 (62-81) | 71 (62-82) | 70 (64-80) | .86 |
| NT-proBNP, pg/mL | 1526 (655-3334) | 1218 (522-3125) | 1630 (866-3423) | .005 |
| BUN, mg/dL | 19 (14.0-26.0) | 19 (14.0-26.0) | 20 (15.0-25.0) | .53 |
| Serum Cr, mg/dL | 1.2 (1.0-1.5) | 1.2 (1.0-1.5) | 1.2 (1.0-1.5) | .67 |
| eGFR, mL/min 1.73 m2 | 61 (47.2-76.1) | 63 (47.2-78.1) | 61 (47.1-74.4) | .21 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; BUN, blood urea nitrogen; CKD, chronic kidney disease; Cr, creatinine; eGFR, estimated glomerular filtration rate; HF, heart failure; LVEF, left ventricular ejection fraction; NT-proBNP, amino-terminal pro–B-type natriuretic peptide; S/V, sacubitril/valsartan.
SI conversion factors: To convert Cr to μmol/L, multiply by 76.25; and urea nitrogen to mmol/L, multiply by 0.357.
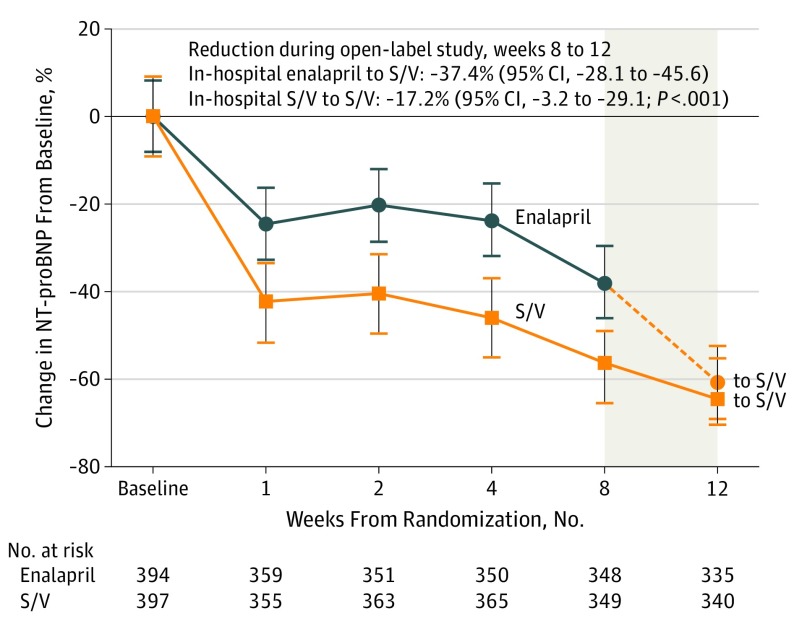
During the open-label extension study, the rates of safety events were similar in patients switching from taking enalapril to sacubitril/valsartan compared with patients continuing to take sacubitril/valsartan (Table 2). For patients continuing to take sacubitril/valsartan, the NT-proBNP level declined to 1009 pg/mL (interquartile range, 368-3077), a change of −17.2% (95% CI, −3.2 to −29.1). For patients switching from taking enalapril to open-label sacubitril/valsartan, the NT-proBNP level declined to 981 pg/mL (interquartile range, 443-2185), a change of −37.4% (95% CI, −28.1 to −45.6; P < .001; comparing changes in 2 groups) (Figure).
Table 2. Safety Events During the Open-label Extension Study.
| Event | In-Hospital Followed by S/V | Relative Risk (95% CI) | |
|---|---|---|---|
| S/V | Enalapril | ||
| Worsening renal function | 8.6 | 9.6 | 0.89 (0.58-1.37) |
| Hyperkalemia | 2.4 | 4.1 | 0.59 (0.27-1.26) |
| Symptomatic hypotension | 3.4 | 4.6 | 0.73 (0.37-1.44) |
| Angioedema | 0 | 0 | 0 |
Abbreviation: S/V, sacubitril/valsartan.
Figure. Changes in N-terminal Pro–b-Type (NT-proBNP) From Baseline Over 12 Weeks.
This figure displays changes in NT-proBNP by randomized treatment arm during the double-blind period (weeks 0-8) and the open-label extension study (weeks 8-12; gray box). The vertical lines indicate 95% CIs and the dotted line represents patients switching from taking sacubitril/valsartan (S/V) after the week 8 visit.
Over the entire 12 weeks of follow-up, patients that had received sacubitril/valsartan initially in the hospital had a lower incidence of HF rehospitalization or cardiovascular death than those that started to take enalapril in the hospital and then had a delayed initiation of sacubitril/valsartan 8 weeks later (13.0% vs 18.1%; HR 0.69; 95% CI, 0.49-0.97; P = .03) (eFigure 2 in the Supplement).
Discussion
Among hospitalized patients with HFrEF who were discharged and treated with enalapril for 8 weeks, the switch to taking sacubitril/valsartan led to an incremental reduction in NT-proBNP levels of 37% over the subsequent 4 weeks. This decrease was greater in patients switching from taking enalapril to sacubitril/valsartan than in patients who were discharged and continued to take sacubitril/valsartan. Over 12 weeks of follow-up, we also observed lower rates of HF rehospitalization or cardiovascular death for patients who were treated with a strategy of in-hospital sacubitril/valsartan compared with those who were treated with upfront angiotensin-converting enzyme inhibitor for 8 weeks followed by an initiation of treatment with sacubitril/valsartan. These data suggest that while a strategy of in-hospital vs delayed sacubitril/valsartan treatment initiation led to a significantly greater early reduction of NT-proBNP with in-hospital initiation of sacubitril/valsartan, NT-proBNP levels in the 2 groups were similar at 12 weeks when all patients were being treated with sacubitril/valsartan. In contrast, the benefits of in-hospital initiation of sacubitril/valsartan on cardiovascular events were observed early after discharge at 8 weeks and remained substantial at 12 weeks.
Changes in NT-proBNP levels were described in prior studies of sacubitril/valsartan but in different patient populations.5,6,7,8 The PARADIGM-HF study enrolled ambulatory patients with chronic HFrEF and patients with ADHF were excluded.9,10 Patients randomized to receive sacubitril/valsartan vs enalapril had a greater reduction in NT-proBNP levels, and 48% vs 26% of patients had a reduction of more than 30% from baseline to 1 month after randomization.5 The TRANSITION study that evaluated patients hospitalized for ADHF and receiving sacubitril/valsartan predischarge led to a 28% decrease in NT-proBNP levels by the time of discharge.6 This study is unique in that patients were randomized in the hospital to receive sacubitril/valsartan or an active comparator and we reported changes in NT-proBNP levels weeks after treatment for ADHF. The data from PARADIGM-HF and TRANSITION, along with this study, demonstrate that sacubitril/valsartan treatment is associated with a rapid decrease in NT-proBNP levels regardless of whether it is initiated in the hospital when patients are congested, prior to discharge from the hospital, soon after discharge, or as stable outpatients.
This study also reinforces the safety profile of initiating sacubitril/valsartan in various clinical settings. The eligibility criteria for PIONEER-HF were different than those for PARADIGM-HF. The patients enrolled in PIONEER-HF who were in the hospital for ADHF and the patient characteristics were notable for more black patients and no recent treatment with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, including those with a new diagnosis of HFrEF. Among this population, sacubitril-valsartan was well tolerated during in-hospital initiation and during the open-label extension study.
Clinical Implications
Reductions in NT-proBNP levels in patients with HFrEF who were treated with sacubitril/valsartan are associated with future improvements in cardiac structure and function.7 Also, a strategy of in-hospital vs delayed initiation of sacubitril/valsartan is safe, well tolerated, and led to an early improvement in postdischarge outcomes that was sustained over 12 weeks. The magnitude of benefit is comparable with the findings from PARADIGM-HF in which within 30 days of randomization patients randomized to receive sacubitril/valsartan had a lower hazard for HF hospitalization compared with enalapril (HR, 0.60; 95% CI, 0.38-0.94).10,11
Prior clinical trials and observational data suggest the hospital is a unique setting in which to initiate guideline-directed medical therapy and that in-hospital initiation is associated with short-term adherence, long-term persistence, and potentially improved postdischarge outcomes.12,13 As such, HF guidelines recommend that evidence-based medications are instituted before hospital discharge.14 The current analysis extends these recommendations by demonstrating that in-hospital initiation of sacubitril/valsartan leads to an early improvement in postdischarge outcomes that may be lost by delaying the initiation of sacubitril/valsartan in the outpatient setting.
Limitations
This study has limitations, including the fact that 49 of 881 eligible patients (6%) did not participate in the open-label extension phase. Also, PIONEER-HF was specifically designed to evaluate changes in NT-proBNP, not clinical outcomes. Finally, follow-up for the open-label extension study was only for 4 weeks and different results may have been observed with longer follow-up.
Conclusions
Treatment with sacubitril/valsartan is associated with a decrease in NT-proBNP levels compared with enalapril regardless of when it is initiated. The early observed improvement in postdischarge outcomes supports the in-hospital initiation of sacubitril/valsartan in stabilized patients with ADHF.
eMethods.
eTable. Clinical outcomes from baseline over 12 weeks
eFigure 1. Flow diagram of the study design
eFigure 2. Effect of sacubitril/valsartan on clinical outcomes over 12 weeks
References
- 1.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136(6):e137-e161. doi: 10.1161/CIR.0000000000000509 [DOI] [PubMed] [Google Scholar]
- 2.Velazquez EJ, Morrow DA, DeVore AD, et al. Rationale and design of the comparison of sacubitril/valsartan versus enalapril on effect on NT-pro-BNP in patients stabilized from an acute heart failure episode (PIONEER-HF) trial. Am Heart J. 2018;198:145-151. doi: 10.1016/j.ahj.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 3.Velazquez EJ, Morrow DA, DeVore AD, et al. ; PIONEER-HF Investigators . Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380(6):539-548. doi: 10.1056/NEJMoa1812851 [DOI] [PubMed] [Google Scholar]
- 4.Morrow DA, Velazquez EJ, DeVore AD, et al. Clinical outcomes in patients with acute decompensated heart failure randomly assigned to sacubitril/valsartan or enalapril in the PIONEER-HF Trial. Circulation. 2019;139(19):2285-2288. doi: 10.1161/CIRCULATIONAHA.118.039331 [DOI] [PubMed] [Google Scholar]
- 5.Zile MR, Claggett BL, Prescott MF, et al. Prognostic Implications of changes in n-terminal pro-B-type natriuretic peptide in patients with heart failure. J Am Coll Cardiol. 2016;68(22):2425-2436. doi: 10.1016/j.jacc.2016.09.931 [DOI] [PubMed] [Google Scholar]
- 6.Pascual-Figal D, Senni M, Belohlavek J, et al. Short-term effect on cardiac biomarkers of initiation of sacubitril/valsartan in hospitalized patients with heart failure and reduced ejection fraction: results of the transition study. Circulation. 2018;138(suppl 1):A15567-A15567. [Google Scholar]
- 7.Januzzi JL Jr, Prescott MF, Butler J, et al. ; PROVE-HF Investigators . Association of change in n-terminal pro-B-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction [published online September 2, 2019]. JAMA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai AS, Solomon SD, Shah AM, et al. ; EVALUATE-HF Investigators . Effect of sacubitril-valsartan vs enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: a randomized clinical trial [published online September 2, 2019]. JAMA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMurray JJ, Packer M, Desai AS, et al. ; PARADIGM-HF Committees and Investigators . Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial (PARADIGM-HF). Eur J Heart Fail. 2013;15(9):1062-1073. doi: 10.1093/eurjhf/hft052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMurray JJV, Packer M, Desai AS, et al. ; PARADIGM-HF Investigators and Committees . Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 11.Packer M, McMurray JJ, Desai AS, et al. ; PARADIGM-HF Investigators and Coordinators . Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131(1):54-61. doi: 10.1161/CIRCULATIONAHA.114.013748 [DOI] [PubMed] [Google Scholar]
- 12.Gattis WA, O’Connor CM, Gallup DS, Hasselblad V, Gheorghiade M; IMPACT-HF Investigators and Coordinators . Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT-HF) trial. J Am Coll Cardiol. 2004;43(9):1534-1541. doi: 10.1016/j.jacc.2003.12.040 [DOI] [PubMed] [Google Scholar]
- 13.Bhagat AA, Greene SJ, Vaduganathan M, Fonarow GC, Butler J. Initiation, continuation, switching, and withdrawal of heart failure medical therapies during hospitalization. JACC Heart Fail. 2019;7(1):1-12. doi: 10.1016/j.jchf.2018.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yancy CW, Jessup M, Bozkurt B, et al. ; WRITING COMMITTEE MEMBERS; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):e240-e327. doi: 10.1161/CIR.0b013e31829e8776 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable. Clinical outcomes from baseline over 12 weeks
eFigure 1. Flow diagram of the study design
eFigure 2. Effect of sacubitril/valsartan on clinical outcomes over 12 weeks