Key Points
Question
Do anti–programmed cell death 1 and anti–programmed cell death ligand 1 deliver different clinical outcomes?
Findings
In this systematic review and meta-analysis of 19 randomized clinical trials involving 11 379 patients, anti–programmed cell death 1 appears to exhibit significantly greater overall survival compared with anti–programmed cell death ligand 1 with a comparable safety profile in patients with solid tumors.
Meaning
Anti–programmed cell death 1 appears to exhibit favorable survival outcomes and a comparable safety profile with anti–programmed cell death ligand 1 in cancer therapy, which may provide valuable insight for future treatment strategy.
Abstract
Importance
Immune checkpoint inhibitors of programmed cell death 1 (PD-1) and its ligand (PD-L1) have led to a paradigm shift in cancer treatment. Understanding the clinical efficacy and safety profile of these drugs is necessary for treatment strategy in clinical practice.
Objective
To assess the differences between anti–PD-1 and anti–PD-L1 regarding efficacy and safety shown in randomized clinical trials across various tumor types.
Data Sources
Systematic searches of PubMed, Cochrane CENTRAL, and Embase were conducted from January 1, 2000, to March 1, 2019. In addition, abstracts and presentations from all major conference proceedings were reviewed.
Study Selection
All randomized clinical trials that compared anti–PD-1 and anti–PD-L1 with standard treatment in patients with cancer were selected as candidates. Retrospective studies, single-arm phase 1/2 studies, and trials comparing anti–PD-1 and anti–PD-L1 with other immunotherapies were excluded. Studies of anti–PD-1 and anti–PD-L1 therapy were screened and paired by the matching of clinical characteristics as mirror groups.
Data Extraction and Synthesis
Three investigators independently extracted data from each study following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guideline. Trial names, first author, year of publication, study design, National Clinical Trial identifier number, blinding status, study phase, pathologic characteristics, number of patients, patients’ age and sex distribution, Eastern Cooperative Oncology Group Performance Status, lines of treatment, study drugs, biomarker status, follow-up time, incidence of adverse events, and hazard ratios (HRs) with 95% CIs for overall survival and progression-free survival were extracted. A random-effects model was applied for data analysis.
Main Outcomes and Measures
Differences in OS between anti–PD-1 and anti–PD-L1 across different cancer types were assessed. An effect size was derived from each mirror group and then pooled across all groups using a random-effects model.
Results
Nineteen randomized clinical trials involving 11 379 patients were included in the meta-analysis. Overall, anti–PD-1 exhibited superior overall survival (HR, 0.75; 95% CI, 0.65-0.86; P < .001) and progression-free survival (HR, 0.73; 95% CI, 0.56-0.96; P = .02) compared with anti–PD-L1. No significant difference was observed in their safety profiles. Sensitivity analysis presented consistency in the overall estimates across these analyses. Consistent results were observed through frequentist and bayesian approaches with the same studies.
Conclusions and Relevance
Comprehensive analysis suggests that anti–PD-1 exhibited favorable survival outcomes and a safety profile comparable to that of anti–PD-L1, which may provide a useful guide for clinicians.
This systematic review and meta-analysis of randomized clinical trials examines use of programmed cell death 1 (PD-1) vs programmed cell death–ligand 1 (PD-L1) treatment in patients with cancer.
Introduction
Immunotherapy is one of the most important breakthroughs in cancer treatment, especially immune checkpoint inhibitors targeting programmed cell death 1 (PD-1) and PD ligand 1 (PD-L1), which significantly prolonged overall survival (OS) and possessed superior safety profile in patients with cancer compared with standard therapies across a wide range of tumor types.1,2 With the increasing studies in immunotherapy, differences between the clinical performance of anti–PD-1 and anti–PD-L1 started to be reported. For example, recent findings from the KEYNOTE-426 study demonstrated significant OS improvement with the combination of anti–PD-1 (pembrolizumab) plus axitinib vs sunitinib in previously untreated patients with advanced renal cell carcinoma (RCC)3; however, anti–PD-L1 (avelumab) plus axitinib failed to demonstrate OS superiority over sunitinib in the same settings.4 In addition, anti–PD-1 (pembrolizumab) plus carboplatin and nab-paclitaxel has been approved by the US Food and Drug Administration for first-line treatment of metastatic, squamous non–small cell lung cancer (NSCLC) based on the positive results from KEYNOTE-407,5 while anti–PD-L1 (atezolizumab) plus carboplatin and nab-paclitaxel failed to show an OS benefit compared with chemotherapy in squamous NSCLC in IMpower131.6,7 Such disparities have attracted widespread attention by clinicians, and there is a need to better understand the similarities and differences between anti–PD-1 and anti–PD-L1 for the ultimate benefit of patients with cancer.
With the lack of head-to-head comparisons available, some systematic reviews and meta-analyses have been conducted regarding the clinical performance of different immune checkpoint inhibitors through indirect comparisons.8,9,10,11,12,13,14 However, whether anti–PD-1 and anti–PD-L1 deliver different clinical outcomes remained controversial. One meta-analysis from the American Society of Clinical Oncology 2018 annual meeting suggested no significant differences regarding the efficacy and safety of anti–PD-1 vs anti–PD-L1 across different tumor types.15 Similar results were reported in another study focusing on the second-line monotherapy with nivolumab, pembrolizumab, or atezolizumab in NSCLC.11 However, other studies published in the same period suggested that anti–PD-1 exhibited superior efficacy compared with anti–PD-L1 either as monotherapy in patients with metastatic and previously treated NSCLC13 or in combination with chemotherapy as the first-line treatment of advanced squamous NSCLC.14
One main reason for the discrepancies from previous studies may be the insufficient comparability of the included studies and the lack of appropriate approach for indirect comparisons. As known, the validity of adjusted indirect comparisons depends on the internal validity and similarity of the trials involved.16 Considering the variations and inconsistencies regarding study designs and patient characteristics across different trials, a risk of bias will be introduced if comparisons of anti–PD-1 vs anti–PD-L1 were conducted between the pooled results from all related studies on each side, leaving the important issue of systematic bias or confounding unaddressed.
In this study, we aimed to assess the differences between anti–PD-1 and anti–PD-L1 in a systematic review and meta-analysis through adjusted indirect comparisons based on a well-designed mirror principle to minimize the potential bias. In brief, studies of anti–PD-1 and anti–PD-L1 were screened and paired with mirrored trial characteristics, including tumor types, treatment lines, intervention regimens, control groups, and biomarker status, into individual mirror groups for further comparisons.
Methods
Search Strategy and Selection Criteria
We searched PubMed, Cochrane CENTRAL, and Embase from January 1, 2000, to March 1, 2019, for randomized clinical trials of immune checkpoint inhibitors (anti–PD-1, anti–PD-L1) that compared anti–PD-1 and anti–PD-L1 with standard treatment in solid tumors. We also reviewed abstracts and presentations from all major conference proceedings, including the American Society of Clinical Oncology and the European Society for Medical Oncology, until March 1, 2019. Key words for the literature search included randomized, PD-1, PD-L1, nivolumab, pembrolizumab, atezolizumab, durvalumab, cemiplimab-rwlc, avelumab, and programmed death receptor 1 (eTable 1 in the Supplement).
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline and the PRISMA extension statement. A prospective protocol was created in advance and uploaded to the PROSPERO online platform.
All randomized clinical trials that had compared the efficacy of anti–PD-1 or anti–PD-L1 as monotherapy or in combination with standard treatment in patients with solid tumors were selected. We excluded retrospective studies, single-arm phase 1 or 2 clinical studies, and randomized trials that compared anti–PD-1 and anti–PD-L1 treatment with other immunotherapies. When duplicate publications for the same study were identified, we included only the most recent and complete reports or the ones supporting the approval by the US Food and Drug Administration.
Eligible studies with either anti–PD-1 or anti–PD-L1 were selected and paired based on comparable characteristics and used as one mirror group. More specifically, the mirrored studies referred to the paired trials with anti–PD-1 and anti–PD-L1 with accurate matching of clinical designs and patient characteristics, including pathologic types, treatment lines, intervention types (immune checkpoint inhibitor monotherapy or combination therapy), design of control groups (standard therapies), and biomarker status (PD-L1 expression level) (Figure 1). Only successful paired clinical studies were included for further analysis. Owing to the lack of standard therapy for third-line or later treatment in gastric or gastroesophageal junction cancer (GC), the ATTRACTION-217 with anti–PD-1 vs placebo and JAVELIN Gastric 30018 trials with anti–PD-L1 vs physician's choice of chemotherapy or best supportive care were also eligible for this study. Two of us (L.C. and X.Z.) independently searched and reviewed the results to determine whether the trials met the inclusion criteria.
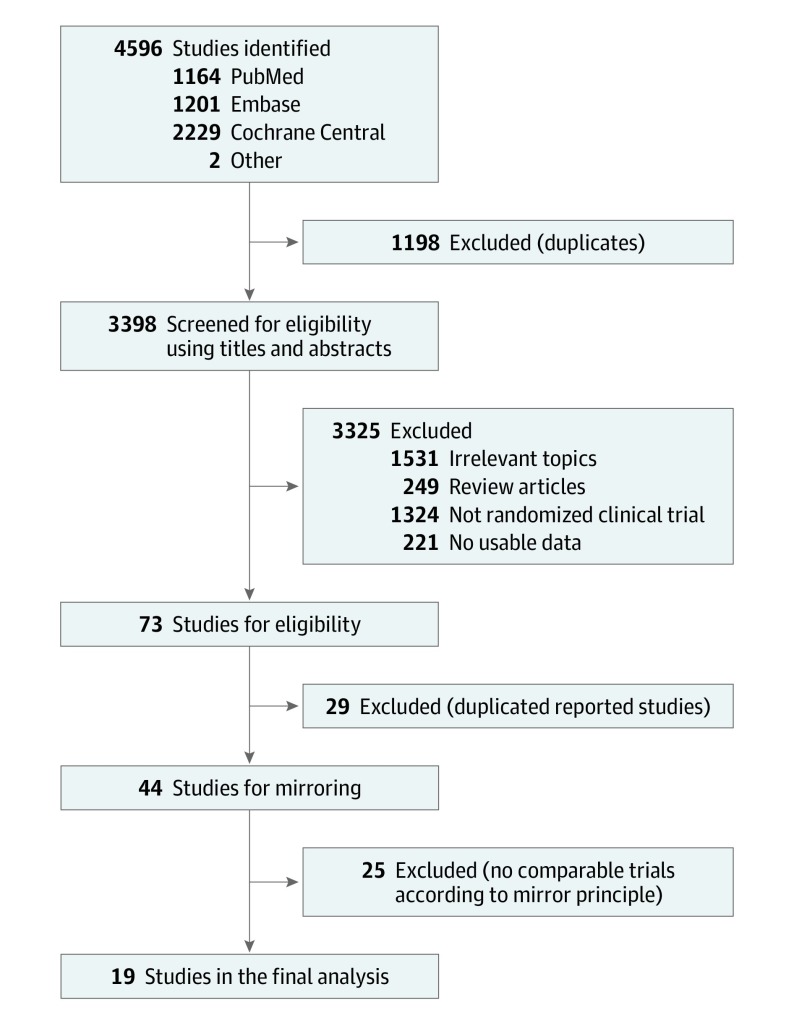
Figure 1. Study Selection .
Studies selected based on the mirror principle.
The primary outcome was the difference in efficacy between anti–PD-1 and anti–PD-L1, measured in terms of the OS difference. The secondary outcome was the differences in progression-free survival (PFS) and adverse events (AEs). For each study, 3 of us (J.D., L.C., and Z.W.) independently extracted data from the studies. The study name, first author, year of publication, study design, National Clinical Trials identification number, blinding status, study phase, pathologic characteristics, number of patients, patients’ age and sex distribution, Eastern Cooperative Oncology Group performance status, lines of treatment, study drugs, follow-up time, biomarker status, incidence of AEs, and hazard ratios (HRs) with 95% CIs for OS and PFS were extracted.
The methodologic quality for each study was evaluated using the tool recommended by the Cochrane Collaboration handbook19 based on the original study or its update and the supplementary materials. The adequacy of the following aspects was assessed: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Each aspect was evaluated, with an assessment index associated with the risk of bias classified as low, high, or unknown. All disagreements in study selection, data extraction, and quality assessment were resolved by discussion to achieve consensus among all investigators.
Statistical Analysis
Hazard ratio was used as the effect size for OS or PFS, and risk ratio (RR) was used as the effect size of AEs. Hazard ratios or RRs were pooled using the inverse variance method.19,20 Effects from the interventions with 2 different doses in KEYNOTE-010 were combined with Review Manager, version 5.3 (RevMan; Cochrane Collaboration) according to the Cochrane Collaboration handbook recommendation to form a single effect.19 Frequentist and bayesian approaches are well-known and commonly used in indirect comparisons. A frequentist P value is an expectation of a long-run frequency, whereas a bayesian posterior is an expression of a degree of belief. Previous methodologic studies have demonstrated that results derived from these 2 approaches usually agree with each other and rarely differ in the direction or treatment rankings.19,21 In our study, to avoid the potential discrepancies from the statistical models, we applied both frequentist and bayesian approaches in the indirect comparisons.
As the main results, indirect comparison of immune checkpoint inhibitors was carried out with a frequentist approach using the R package netmeta, version 1.0-1 (R Foundation for Statistical Computing), for each mirror group based on the generic inverse variance method using a random-effects model.22,23 When multiple studies were present for one intervention within the group, effect sizes of these studies were first combined through the same approach. The effect sizes derived from each group were then pooled across different mirror groups using Review Manager with a random-effects model.24 During this step, a fixed-effects model was applied as a sensitivity test. Statistical heterogeneity was evaluated using the Q test and inconsistency index (I2). To assess the stability of results, preplanned subgroup analyses, by tumor types or intervention types, and sensitivity analyses, by exclusion of each type of tumor, were performed for OS and PFS outcomes. All reported P values are 2-sided, with findings at P < .05 considered significant. Both types of effect sizes are reported with 95% CIs.
In addition, the consistency of OS and PFS outcomes was assessed with a bayesian framework approach with the same trials screened using the mirror principle. Hazard ratios and 95% credible intervals (CrIs) were computed with a hierarchical model by Markov chain Monte Carlo (MCMC) methods with JAGS software, version 4.3.0, and R, version 3.5.3 package gemtc, version 0.8-2, for each mirror group.21,25,26 The effect sizes were then pooled through the same approach. The deviance information criterion was used to choose the effects model, and the model with the lowest deviance information criterion was considered to provide the best data fit.21,27
The Begg and Egger tests were used to assess publication bias.28,29 A P value <.10 indicates significant asymmetry and publication bias. A meta-regression analysis was applied following the instruction in the Cochrane Collaboration handbook (Stata, version 15; StataCorp) to examine the heterogeneity between studies and the influence of potential confounders on effect sizes.
Results
Systematic Review and Characteristics
A total of 4596 publications were retrieved through the initial literature search, and 3398 studies remained after duplications were excluded. With title and abstract review, 3325 publications were excluded because the topics were irrelevant, the articles were reviews, the studies were nonrandomized controlled trials, or no usable data were reported. Seventy-three potentially relevant articles were identified for detailed review. After a full-text review, 29 duplicate studies were removed, and 25 of 44 studies were excluded owing to a lack of comparability based on the mirror principle. Following this process, 19 randomized clinical trials3,4,5,7,17,18,30,31,32,33,34,35,36,37,38,39,40,41,42 involving 11 379 patients were identified as eligible to be included in the meta-analysis (Figure 1). These studies were divided into 7 mirror groups with matched tumor types, treatment lines, biomarker status, and intervention types for adjusted indirect comparison. Illustration of the mirror principle is shown in Figure 2.
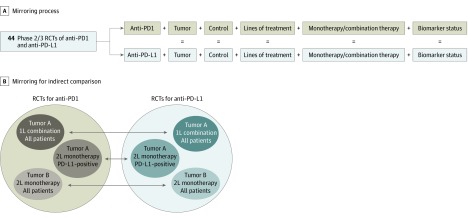
Figure 2. Illustration of Selection Based on the Mirror Principle.
Illustrations of study mirroring (A) and mirroring for indirect comparison (B). 1L indicates first-line treatment; 2L, second-line treatment; PD-1, programmed cell death 1; PD-L1, PD-ligand 1; and RCTs, randomized clinical trials.
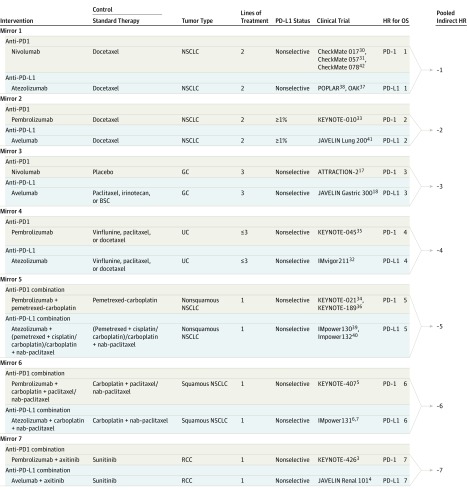
The selected studies covered 10 trials with anti–PD-1 (including 3 with nivolumab and 3 with pembrolizumab in monotherapy settings, and 4 with pembrolizumab in combination with standard therapy) and 9 trials with anti–PD-L1 (including 2 with avelumab and 3 with atezolizumab in monotherapy settings, and 1 with avelumab and 3 with atezolizumab in combination with standard therapy) compared with control groups receiving standard therapies. Thirteen trials were done in patients with NSCLC, 2 trials in patients with GC, 2 trials in patients with urothelial cancer (UC), and 2 trials in patients with RCC (Figure 3; eTable 2 in the Supplement). All included trials were well-designed with well-defined main outcomes. Data from 3 trials (IMpower130,39 IMpower131,6,7 and IMpower13240) were retrieved from conference presentations. The assessment of risk of bias of each included study is provided in eTable 3 in the Supplement. The Begg test and Egger test were carried out for the evaluation of publication bias,28,29 and P values of .58 and .48 were obtained, respectively, indicating that no bias exists for the selected studies (eFigure 1 in the Supplement).
Figure 3. Trial Characteristics and Mirror Design.
BSC indicates best supportive care; GC, gastric or gastroesophageal junction cancer; HR, hazard ratio; NSCLC, non–small cell lung cancer; PD-1, programmed cell death 1; PD-L1, PD-ligand 1; RCC, renal cell carcinoma; and UC, urothelial carcinoma.
A similarity of clinical characteristics was observed between the anti–PD-1 and anti–PD-L1 trials within each mirror group (eTable 2 in the Supplement), which supported the possible comparability of the trials involved with a minimal risk of bias and apparent transitivity of effect size across different groups, further supporting the validity of adjusted indirect comparisons.16,43
OS Comparison: Frequentist Approach
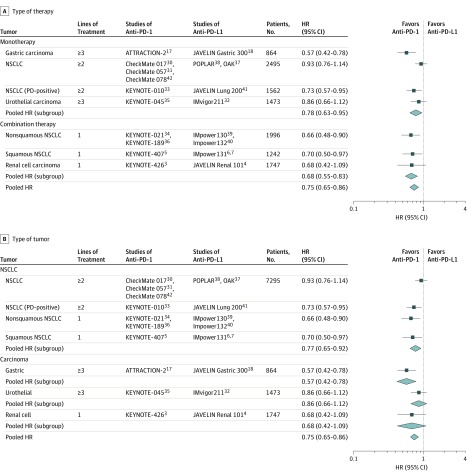
The primary outcome of the analysis was the difference in OS between studies with anti–PD-1 and anti–PD-L1. The pooled results across all mirror groups suggested that, overall, patients obtained greater OS benefit from treatments containing anti–PD-1 compared with anti–PD-L1 with either a random-effects model (HR, 0.75; 95% CI, 0.65-0.86; P < .001) (Figure 4A) for heterogeneity (I2 = 37%; P = .15) or a fixed-effects model (HR, 0.77; 95% CI, 0.69-0.85; P < .001) (eFigure 2 in the Supplement).
Figure 4. Overall Survival Outcomes in Patients Who Received Therapies Based on Anti–Programmed Cell Death 1 (PD-1) vs Anti–PD-Ligand 1 (PD-L1).
A, Survival outcomes by type of therapy. Squares represent adjusted indirect effect size (hazard ratio [HR]). Horizontal lines indicate 95% CIs. Diamonds indicate the meta-analytic pooled HRs, calculated separately by monotherapy and combination therapy subgroups, and the overall pooled HRs (95% CIs) in patients with cancer. B, Survival outcomes by tumor type. Squares represent subgroup-specific pooled HRs. Horizontal lines indicate 95% CIs. Diamonds indicate the meta-analytic pooled HRs, calculated separately by tumor types, and the overall pooled HRs (95% CIs) in patients with cancer. NSCLC indicates non-small cell lung cancer.
Preplanned subgroup analysis was performed to examine the potential source of heterogeneities. When stratified by intervention types, anti–PD-1 appeared to show better OS than anti–PD-L1 as monotherapy (HR, 0.78; 95% CI, 0.63-0.95; P = .01) and combination therapy (HR, 0.68; 95% CI, 0.55-0.83; P < .001) (Figure 4A). Interstudy heterogeneity was observed among studies with immune checkpoint inhibitors as monotherapy (I2 = 61%; P = .05), but not as combination therapy (I2 = 0%; P = .97). For tumor types, the OS values were significantly prolonged for patients treated with anti–PD-1 compared with anti–PD-L1 with a random-effects model in NSCLC (HR, 0.77; 95% CI, 0.65-0.92; P < .001; for heterogeneity, I2 = 38%; P = .18) and GC (HR, 0.57; 95% CI, 0.42-0.78; P < .001), but not in UC (HR, 0.86; 95% CI, 0.66-1.12; P = .26) or RCC (HR, 0.68; 95% CI, 0.42-1.09; P = .11) (Figure 4B). Sensitivity analysis with a fixed-effects model in NSCLC noted consistently favorable outcome in OS for anti–PD-1 vs anti–PD-L1 (HR, 0.79; 95% CI, 0.69-0.90; P < .001) (eFigure 3 in the Supplement). Data were available from only 2 studies for comparison of GC, UC, or RCC, providing insufficient power to draw reliable conclusions within these tumor types.
To assess the stability of our results, another sensitivity analysis was conducted by repeating the analyses and omitting 1 tumor type each time. The overall estimates remained consistent across these analyses (eTable 4 in the Supplement). In addition, meta-regression analysis revealed no significant effect of PS or age on OS effect sizes (eTable 5 in the Supplement).
PFS Comparison: Frequentist Approach
Analyses of PFS between anti–PD-1 and anti–PD-L1 were conducted using the frequentist approach. Six groups of indirect comparison covering 17 studies with available PFS data were included in the analysis. Consistent with the results for OS, patients receiving anti–PD-1 appeared to exhibit better PFS than those receiving anti–PD-L1 (HR, 0.73; 95% CI, 0.56-0.96; P = .02) (eFigure 4 in the Supplement). For subgroup analysis, anti–PD-1 seemed to lead to borderline significantly superior PFS than anti–PD-L1 as monotherapy (HR, 0.62; 95% CI, 0.37-1.05; P = .08) and significant superior PFS as combination therapy (HR 0.86; 95% CI, 0.74-0.99; P = .04) (eFigure 4 in the Supplement). Substantial heterogeneity was observed for overall PFS (I2 = 83%; P < .001) and in studies with immune checkpoint inhibitors as monotherapy (I2 = 91%; P < .001), but not in studies with immune checkpoint inhibitors as combination therapy (I2 = 0%; P = .37). Results of sensitivity analysis repeated with 1 tumor type omitted at each evaluation are shown in eTable 6 in the Supplement.
Indirect Comparisons of OS and PFS With the Bayesian Approach
Consistent results were observed when the bayesian framework was used with the model fit assessed with deviance information criterion scores (eTable 7 and eTable 8 in the Supplement). The data suggested that anti–PD-1 exhibited significant or borderline significant OS and PFS superiority compared with anti–PD-L1 across different tumor types in either overall population (OS: HR, 0.79; 95% CrI, 0.71-0.88; PFS: HR, 0.80; 95% CrI, 0.69-0.93), as monotherapy (OS: HR, 0.85; 95% CrI, 0.74-0.97; PFS: HR, 0.77; 95% CrI, 0.58-1.02), or combined with standard treatment (OS: HR, 0.67; 95% CrI, 0.55-0.82; PFS: HR, 0.82; 95% CrI, 0.69-0.97) (eFigure 5 and eFigure 6 in the Supplement). Sensitivity analyses by omitting 1 tumor type each time are reported in eTable 9 and eTable 10 in the Supplement.
Safety Analysis
The overall safety profiles of anti–PD-1 and anti–PD-L1 were comparable for both any AE (grades 3-5: RR, 1.04; 95% CI, 0.78-1.39; P = .78) and immune-related AEs (grades 3-5: RR, 0.88; 95% CI, 0.46-1.68; P = .69) (Table). The risk of AEs leading to death or discontinuation was also comparable between anti–PD-1 and anti–PD-L1 (any AE leading to death: RR, 1.01; 95% CI, 0.53-1.93; P = .98; any AE leading to discontinuation: RR, 1.20; 95% CI, 0.95-1.52; P = .13; immune-related AEs leading to death: RR, 1.38; 95% CI, 0.11-16.89; P = .80) (Table). Significant heterogeneity across the studies was observed in any AE of grades 3 to 5 (I2 = 90%; P < .001), but not in any AE leading to discontinuation (I2 = 0%; P = .81) or death (I2 = 0%; P = .68), or in any immune-related AE of grades 3 to 5 (I2 = 0%; P = .80) or leading to death (I2 = 0%, P = .59).
Table. Adjusted Risk Ratios Comparing Treatment-Related AEs in Patients Who Received Anti–PD– vs Anti–PD-L1–Based Therapies.
| Tumor | Lines of Treatment, No. | Studies | Any AEs | Immune-Related AEs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| With Anti–PD-1 | With Anti–PD-L1 | Grade | Outcome | Grade | Outcome | |||||
| Any | 3-5 | Death | Discontinuation | Any | 3-5 | Death | ||||
| Monotherapy | ||||||||||
| Gastric carcinoma | ≥3 | ATTRACTION-217 | JAVELIN Gastric 30018 | 2.42 (1.74-3.38) | 7.28 (3.09-17.11) | 3.81 (0.09-155.32) | 1.47 (0.32-6.71) | NA | NA | NA |
| NSCLC | ≥2 | CheckMate 017,30 CheckMate 057,31 CheckMate 07842 | POPLAR,38 OAK37 | 1.00 (0.91-1.09) | 0.56 (0.42-0.74) | 0.88 (0.06-12.62) | 1.03 (0.62-1.71) | NA | NA | NA |
| NSCLC (PD-L1–positive) | ≥2 | KEYNOTE-01033 | JAVELIN Lung 20041 | 1.08 (0.96-1.21) | 2.05 (1.38-3.04) | 2.00 (0.40-9.92) | 0.92 (0.48-1.76) | NA | NA | NA |
| Urothelial carcinoma | ≤3 | KEYNOTE-04535 | IMvigor21132 | 0.87 (0.77-0.98) | 0.65 (0.45-0.96) | 2.23 (0.36-13.73) | 2.04 (0.90-4.62) | 1.66 (0.95-2.88) | 1.13 (0.32-4.02) | 9.00 (0.09-906.39) |
| Pooled subgroup RR | 1.15 (0.92-1.44) | 1.42 (0.59-3.40) | 1.92 (0.67-5.50) | 1.15 (0.81-1.63) | 1.66 (0.95-2.88) | 1.13 (0.32-4.02) | 9.00 (0.09-906.39) | |||
| Combination therapy | ||||||||||
| Nonsquamous NSCLC | 1 | KEYNOTE-021,34 KEYNOTE-18936,a | IMpower130,39 IMpower13240 | 0.97 (0.93-1.01) | 0.84 (0.71-0.98) | 0.73 (0.26-2.04) | 1.13 (0.69-1.86) | 0.80 (0.48-1.31) | 0.98 (0.32-2.95) | 1.16 (0.02-75.55) |
| Squamous NSCLC | 1 | KEYNOTE-4075,a | IMpower1316,7 | 0.96 (0.91-1.01) | 0.85 (0.73-0.99) | 0.97 (0.19-4.83) | 1.24 (0.67-2.28) | 1.47 (0.91-2.39) | 0.67 (0.24-1.90) | 0.34 (0.00-23.69) |
| Renal cell carcinoma | 1 | KEYNOTE-4263 | JAVELIN Renal 1014 | 1.00 (0.96-1.04) | 1.06 (0.90-1.24) | 0.19 (0.01-2.43) | 1.42 (0.79-2.54) | NA | NA | NA |
| Pooled subgroup RR | 0.98 (0.96-1.00) | 0.91 (0.79-1.05) | 0.68 (0.30-1.55) | 1.24 (0.90-1.71) | 1.09 (0.60-1.98) | 0.80 (0.38-1.71) | 0.63 (0.03-12.45) | |||
| Pooled results | 1.01 (0.95-1.08) | 1.04 (0.78-1.39) | 1.01 (0.53-1.93) | 1.20 (0.95-1.52) | 1.24 (0.79-1.93) | 0.88 (0.46-1.68) | 1.38 (0.11-16.89) | |||
Abbreviations: AEs, adverse events; NA, not available; NSCLC, non–small cell lung cancer; PD-1, programmed cell death 1; PD-L1, PD ligand 1; RR, risk ratio.
Data provided were all-cause AEs, without attribution to any treatment.
Discussion
To our knowledge, this is the first systematic review and meta-analysis that compared treatment outcomes and safety profiles between anti–PD-1 and anti–PD-L1 in patients with solid tumors. The results suggest that anti–PD-1 is associated with statistically significant improved survival outcomes and comparable AEs with anti–PD-L1.
As a strength of this work, data were obtained from 19 randomized clinical trials, which were selected based on the mirror principle to ensure the comparability of the included studies and avoid the risk of bias in this meta-analysis. One previous meta-analysis suggested that the treatment efficacy was similar between anti–PD-1 and anti–PD-L1 in patients with cancer.44 However, the reliability of this study remains inconclusive owing to the lack of comparability of the included trials. For example, for UC, head and neck carcinoma, and melanoma, only trials with anti–PD-1 were included, which will lead to a great risk of bias in the comparison. The mirror principle used in our present study may provide a valuable tool for the indirect comparative analysis with a minimal risk of bias across multiple interventions.16,43
The magnitude of possible survival benefit of anti–PD-1 compared with anti–PD-L1 is clinically relevant, which may provide important clues for treatment selection for clinicians in clinical practice. Agents used in both UC and RCC showed better outcomes with anti–PD-1, even without reaching statistical significance owing to the insufficient statistical power, with only 1 mirror group available for each indication. The results were robust according to the subsequent analysis, suggesting that both frequentist and bayesian approaches supported the superior OS outcomes with anti–PD-1 compared with anti–PD-L1. Two previous, large phase 1 studies testing the Bristol-Myers Squibb PD-1 antibody and PD-L1 antibody have provided similar evidence, with a higher overall response rate for anti–PD-1 (20%-25%) than anti–PD-L1 (6%-17%) being observed in NSCLC, RCC, and melanoma.45,46
One reason for the possibly improved efficacy of anti–PD-1 compared with anti–PD-L1 is the inherent differences between anti–PD-1 and anti–PD-L1. PD-1 antibodies can bind to PD-1 and further block the binding of PD-1 to its ligands (PD-L1 and PD-L2) at the same time. However, although PD-L1 antibodies would also inhibit the binding of PD-1 to PD-L1, the interaction of PD-1 and PD-L2 remains intact, which may inhibit activation of T cells. Therefore, the tumor might escape antitumor immune response through the PD-1/PD-L2 axis when being treated with anti–PD-L1.47 The PD-L2 expression status was also identified as a significant predictor of survival benefit to immune checkpoint inhibitor treatment independent of PD-L1 expression status.48,49 It was reported that patients with NSCLC and GC demonstrated moderate to high PD-L2 expression,48 supporting our observation of the superior clinical efficacy of anti–PD-1 compared with anti–PD-L1 in NSCLC and GC. Inhibition of PD-L1 also plays an important role in blocking the interaction between PD-L1 and CD80, which is a negative regulator of T-lymphocyte activation.50 Such blockage would be achieved with an anti–PD-L1 antibody but not an anti–PD-1 antibody, which increases the complexity of their performance in cancer treatment.
The possible survival superiority seems even stronger when anti–PD-1 was used in combination with standard therapies, which lowered the risk of death by 32% compared with anti–PD-L1 plus standard therapies as evidenced in the results of NSCLC and RCC trials. Previous studies showed that chemotherapy may enhance the expression of PD-L1,51,52 leading to the synergistic effect of immune checkpoint inhibitors with chemotherapy. As a result, T-cell activation might be inhibited by PD-L1 antibody overconsumption owing to extra PD-L1 expression, which needs to be explored in future studies.
Limitations
There are several limitations of this study. First, the clinical settings were not identical among all comparison groups. For instance, the proportion of patients with EGFR mutation and ALK rearrangement was higher in CheckMate 01730 and CheckMate 05731 than that in POPLAR38 and OAK,37 and NSCLC with such mutation was reported to be less sensitive to immune checkpoint inhibitors than wild-type NSCLCs.53,54,55 Nevertheless, these patients accounted for only about 10% of the population in these studies; thus, the risk of such bias is limited. Second data from IMpower130 (mature data),39 IMpower131 (second interim analysis),6,7 and IMpower132 (interim analysis)40 were available only from conference presentations. These data may be associated with limited peer review and immature data, which may lead to potential bias. However, considering that all of these studies were randomized clinical trials with a high level of evidence, their follow-up times were comparable with those of the other completed trials (eTable 2 in the Supplement), and the methodology and outcomes were reported in detail, we expect that these data will not differ much from the final analysis. The final results of IMpower130 were published56 after the cutoff date of our data collection, and the results were identical to those retrieved from the conference presentations as used in this analysis. In addition, because all 3 of the IMpower studies were based on combination treatment with immune checkpoint inhibitors plus chemotherapy, the reliability of the results in the monotherapy subgroup will not be affected.
Conclusions
Our meta-analysis suggests that anti–PD-1 exhibited better survival outcomes than anti–PD-L1 in patients with solid tumors in either overall, monotherapy, or combination therapy settings, with comparable safety profiles. Owing to the lack of direct evidence from randomized clinical trials, adjusted indirect comparison was adopted in the present study as a surrogate. To minimize the potential risk of bias, a mirror principle was applied to ensure the internal similarity of the included studies. Future head-to-head studies are warranted for direct comparison across alternative interventions.
eTable 1. Search Strategy
eTable 2. Trial Characteristics
eTable 3. Risk of Bias for Each Included Study
eTable 4. Sensitivity Analysis of OS Results with Frequentist Approach
eTable 5. Meta-Regression Analysis
eTable 6. Sensitivity Analysis of PFS Results With Frequentist Approach
eTable 7. Model Fit Statistics With OS, Fixed- and Random-Effects Model
eTable 8. Model Fit Statistics With PFS, Fixed- and Random-Effects Model
eTable 9. Sensitivity Analysis of OS Results With Bayesian Approach
eTable 10. Sensitivity Analysis of PFS Results With Bayesian Approach
eFigure 1. Begg’s Test for Publication Bias
eFigure 2. Forest Plot of Relative Hazard Ratios From Indirect Comparison of Overall Survival Outcomes in Patients Who Received Therapies Based on Anti–PD-1 Versus Those Based on Anti–PD-L1 With Frequentist Fixed-Effects Model
eFigure 3. Forest Plot of Pooled Hazard Ratios of Overall Survival Outcomes for Anti–PD-1 Versus Those Based on Anti–PD-L1 Stratified by Tumor Types With Frequentist Fixed-Effects Model
eFigure 4. Forest Plot of Relative Hazard Ratios From Indirect Comparison of Progression Free Survival in Patients Who Received Therapies Based on Anti–PD-1 Versus Those Based on Anti–PD-L1
eFigure 5. Forest Plot of Relative Hazard Ratios From Indirect Comparison of overall Survival Outcomes in Patients Who Received Therapies Based on Anti–PD-1 Versus Those Based on Anti–PD-L1 With Bayesian Approach
eFigure 6. Forest Plot of Relative Hazard Ratios From Indirect Comparison of progression Free Survival in Patients Who Received Therapies Based on Anti–PD-1 Versus Those Based on Anti–PD-L1 With Bayesian Approach
References
- 1.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350-1355. doi: 10.1126/science.aar4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang J, Yu JX, Hubbard-Lucey VM, Neftelinov ST, Hodge JP, Lin Y. Trial watch: the clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nat Rev Drug Discov. 2018;17(12):854-855. doi: 10.1038/nrd.2018.210 [DOI] [PubMed] [Google Scholar]
- 3.Rini BI, Plimack ER, Stus V, et al. ; KEYNOTE-426 Investigators . Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116-1127. doi: 10.1056/NEJMoa1816714 [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103-1115. doi: 10.1056/NEJMoa1816047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paz-Ares L, Luft A, Vicente D, et al. ; KEYNOTE-407 Investigators . Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N Engl J Med. 2018;379(21):2040-2051. doi: 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- 6.Socinski MA, Rittmeyer A, Shapovalov D, et al. IMpower131: Progression-free survival (PFS) and overall survival (OS) analysis of a randomised phase III study of atezolizumab 1 carboplatin 1 paclitaxel or nab-paclitaxel vs carboplatin 1 nabpaclitaxel in 1L advanced squamous NSCLC [abstract]. Ann Oncol. 2018;29(suppl 8):mdy424.077. [Google Scholar]
- 7.Jotte RM, Cappuzzo F, Vynnychenko I, et al. IMpower131: primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC [abstract]. J Clin Oncol. 2018;36(18)(suppl):LBA9000. doi: 10.1200/JCO.2018.36.18_suppl.LBA9000 [DOI] [Google Scholar]
- 8.Xu C, Chen YP, Du XJ, et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ. 2018;363:k4226. doi: 10.1136/bmj.k4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spagnuolo A, Gridelli C. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: is there a substantial difference or not? J Thorac Dis. 2018;10(suppl 33):S4065-S4068. doi: 10.21037/jtd.2018.09.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pillai RN, Behera M, Owonikoko TK, et al. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non–small cell lung cancer: a systematic analysis of the literature. Cancer. 2018;124(2):271-277. doi: 10.1002/cncr.31043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y, Lin L, Shen Y, Wu H. Comparison between PD-1/PD-L1 inhibitors (nivolumab, pembrolizumab, and atezolizumab) in pretreated NSCLC patients: evidence from a bayesian network model. Int J Cancer. 2018;143(11):3038-3040. doi: 10.1002/ijc.31733 [DOI] [PubMed] [Google Scholar]
- 12.Passiglia F, Galvano A, Rizzo S, et al. Looking for the best immune-checkpoint inhibitor in pre-treated NSCLC patients: an indirect comparison between nivolumab, pembrolizumab and atezolizumab. Int J Cancer. 2018;142(6):1277-1284. doi: 10.1002/ijc.31136 [DOI] [PubMed] [Google Scholar]
- 13.You W, Liu M, Miao JD, et al. A network meta-analysis comparing the efficacy and safety ofanti–PD-1 withanti–PD-L1 in non–small cell lung cancer. J Cancer. 2018;9(7):1200-1206. doi: 10.7150/jca.22361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Zhou H, Zhang L. Which is the optimal immunotherapy for advanced squamous non–small-cell lung cancer in combination with chemotherapy:anti–PD-1 oranti–PD-L1? J Immunother Cancer. 2018;6(1):135. doi: 10.1186/s40425-018-0427-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koneru M, Patnaik A, Liu J, Nanda S, Thomas ZM, Li J. A meta-analysis to indirectly compare the safety and efficacy of PD-1 and PD-L1 antibodies across solid tumors using a bayesian hierarchical model [abstract]. J Clin Oncol. 2018;36(15)(suppl):3065. doi: 10.1200/JCO.2018.36.15_suppl.306530188791 [DOI] [Google Scholar]
- 16.Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ. 2003;326(7387):472. doi: 10.1136/bmj.326.7387.472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461-2471. doi: 10.1016/S0140-6736(17)31827-5 [DOI] [PubMed] [Google Scholar]
- 18.Bang YJ, Ruiz EY, Van Cutsem E, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29(10):2052-2060. doi: 10.1093/annonc/mdy264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JPT, Green S; Cochrane Collaboration . Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0). The Cochrane Collaboration; 2011. [Google Scholar]
- 20.Hartung J, Knapp G, Sinha BK. Statistical Meta-analysis With Applications. Hoboken, NJ: John Wiley & Sons; 2008. doi: 10.1002/9780470386347 [DOI] [Google Scholar]
- 21.Palmerini T, Benedetto U, Bacchi-Reggiani L, et al. Mortality in patients treated with extended duration dual antiplatelet therapy after drug-eluting stent implantation: a pairwise and bayesian network meta-analysis of randomised trials. Lancet. 2015;385(9985):2371-2382. doi: 10.1016/S0140-6736(15)60263-X [DOI] [PubMed] [Google Scholar]
- 22.Torbahn G, Hofmann H, Rücker G, et al. Efficacy and safety of antibiotic therapy in early cutaneous Lyme borreliosis: a network meta-analysis. JAMA Dermatol. 2018;154(11):1292-1303. doi: 10.1001/jamadermatol.2018.3186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarzer G, Carpenter JR, Rücker G. Use R! Meta-analysis with R. Cham: Springer; 2015. doi: 10.1007/978-3-319-21416-0 [DOI] [Google Scholar]
- 24.Fisher DJ, Carpenter JR, Morris TP, Freeman SC, Tierney JF. Meta-analytical methods to identify who benefits most from treatments: daft, deluded, or deft approach? BMJ. 2017;356:j573. doi: 10.1136/bmj.j573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synth Methods. 2012;3(4):285-299. doi: 10.1002/jrsm.1054 [DOI] [PubMed] [Google Scholar]
- 26.Wang R, Dasgupta A, Ward MM. Comparative efficacy of non-steroidal anti-inflammatory drugs in ankylosing spondylitis: a bayesian network meta-analysis of clinical trials. Ann Rheum Dis. 2016;75(6):1152-1160. doi: 10.1136/annrheumdis-2015-207677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spiegelhalter DJ, Best N, Carlin BP, van der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc B. 2002;64(4):583-639. doi: 10.1111/1467-9868.00353 [DOI] [Google Scholar]
- 28.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088-1101. doi: 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 29.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373(2):123-135. doi: 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. doi: 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391(10122):748-757. doi: 10.1016/S0140-6736(17)33297-X [DOI] [PubMed] [Google Scholar]
- 33.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non–small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550. doi: 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 34.Langer CJ, Gadgeel SM, Borghaei H, et al. ; KEYNOTE-021 investigators . Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non–small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497-1508. doi: 10.1016/S1470-2045(16)30498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellmunt J, de Wit R, Vaughn DJ, et al. ; KEYNOTE-045 Investigators . Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015-1026. doi: 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. ; KEYNOTE-189 Investigators . Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092. doi: 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 37.Rittmeyer A, Barlesi F, Waterkamp D, et al. ; OAK Study Group . Atezolizumab versus docetaxel in patients with previously treated non–small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255-265. doi: 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fehrenbacher L, Spira A, Ballinger M, et al. ; POPLAR Study Group . Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837-1846. doi: 10.1016/S0140-6736(16)00587-0 [DOI] [PubMed] [Google Scholar]
- 39.Cappuzzo F, McCleod M, Hussein M, et al. IMpower130: efficacy and safety from a randomised phase iii study of carboplatin and nab-paclitaxel with or without atezolizumab in 1L advanced non-squamous NSCLC. Presented at European Society for Medical Oncology 2018 Congress; October 22, 2018; Munich, Germany. [Google Scholar]
- 40.Papadimitrakopoulou V, Cobo M, Bordoni R, et al. OA05.07 IMpower132: PFS and safety results with 1L atezolizumab + carboplatin/cisplatin + pemetrexed in stage IV non-squamous NSCLC. J Thorac Oncol. 2018;13(10):S332-S333. doi: 10.1016/j.jtho.2018.08.262 [DOI] [Google Scholar]
- 41.Barlesi F, Vansteenkiste J, Spigel D, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non–small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol. 2018;19(11):1468-1479. doi: 10.1016/S1470-2045(18)30673-9 [DOI] [PubMed] [Google Scholar]
- 42.Wu YL, Lu S, Cheng Y, et al. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced NSCLC: CheckMate 078 randomized phase III clinical trial. J Thorac Oncol. 2019;14(5):867-875. doi: 10.1016/j.jtho.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 43.Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health. 2011;14(4):417-428. doi: 10.1016/j.jval.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 44.Weng YM, Peng M, Hu MX, Yao Y, Song QB. Clinical and molecular characteristics associated with the efficacy of PD-1/PD-L1 inhibitors for solid tumors: a meta-analysis. Onco Targets Ther. 2018;11:7529-7542. doi: 10.2147/OTT.S167865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates ofanti–PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454. doi: 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455-2465. doi: 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen L, Han X. Anti–PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125(9):3384-3391. doi: 10.1172/JCI80011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yearley JH, Gibson C, Yu N, et al. PD-L2 Expression in human tumors: relevance to anti–PD-1 therapy in cancer. Clin Cancer Res. 2017;23(12):3158-3167. doi: 10.1158/1078-0432.CCR-16-1761 [DOI] [PubMed] [Google Scholar]
- 49.George S, Papanicolau-Sengos A, Lenzo FL, et al. PD-L2 amplification and durable disease stabilization in patient with urothelial carcinoma receiving pembrolizumab. Oncoimmunology. 2018;7(12):e1460298. doi: 10.1080/2162402X.2018.1460298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti–PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563-567. doi: 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lacour M, Hiltbrunner S, Lee SY, et al. Adjuvant chemotherapy increases programmed death-ligand 1 (PD-L1) expression in non–small cell lung cancer recurrence. Clin Lung Cancer. 2019;20(5):391-396. doi: 10.1016/j.cllc.2019.05.013 [DOI] [PubMed] [Google Scholar]
- 52.Fournel L, Wu Z, Stadler N, et al. Cisplatin increases PD-L1 expression and optimizes immune check-point blockade in non-small cell lung cancer. Cancer Lett. 2019;464:5-14. doi: 10.1016/j.canlet.2019.08.005 [DOI] [PubMed] [Google Scholar]
- 53.Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non–small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22(18):4585-4593. doi: 10.1158/1078-0432.CCR-15-3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res. 2017;23(15):4242-4250. doi: 10.1158/1078-0432.CCR-16-3133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee CK, Man J, Lord S, et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non–small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):210-216. doi: 10.1001/jamaoncol.2017.4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non–small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924-937. doi: 10.1016/S1470-2045(19)30167-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Search Strategy
eTable 2. Trial Characteristics
eTable 3. Risk of Bias for Each Included Study
eTable 4. Sensitivity Analysis of OS Results with Frequentist Approach
eTable 5. Meta-Regression Analysis
eTable 6. Sensitivity Analysis of PFS Results With Frequentist Approach
eTable 7. Model Fit Statistics With OS, Fixed- and Random-Effects Model
eTable 8. Model Fit Statistics With PFS, Fixed- and Random-Effects Model
eTable 9. Sensitivity Analysis of OS Results With Bayesian Approach
eTable 10. Sensitivity Analysis of PFS Results With Bayesian Approach
eFigure 1. Begg’s Test for Publication Bias
eFigure 2. Forest Plot of Relative Hazard Ratios From Indirect Comparison of Overall Survival Outcomes in Patients Who Received Therapies Based on Anti–PD-1 Versus Those Based on Anti–PD-L1 With Frequentist Fixed-Effects Model
eFigure 3. Forest Plot of Pooled Hazard Ratios of Overall Survival Outcomes for Anti–PD-1 Versus Those Based on Anti–PD-L1 Stratified by Tumor Types With Frequentist Fixed-Effects Model
eFigure 4. Forest Plot of Relative Hazard Ratios From Indirect Comparison of Progression Free Survival in Patients Who Received Therapies Based on Anti–PD-1 Versus Those Based on Anti–PD-L1
eFigure 5. Forest Plot of Relative Hazard Ratios From Indirect Comparison of overall Survival Outcomes in Patients Who Received Therapies Based on Anti–PD-1 Versus Those Based on Anti–PD-L1 With Bayesian Approach
eFigure 6. Forest Plot of Relative Hazard Ratios From Indirect Comparison of progression Free Survival in Patients Who Received Therapies Based on Anti–PD-1 Versus Those Based on Anti–PD-L1 With Bayesian Approach