Key Points
Question
What is the incidence of unilateral vocal fold immobility after prolonged (>12 hours) endotracheal intubation, and what clinical factors are associated with this condition?
Findings
In this single-center prospective cohort study, 7 of 100 patients intubated for more than 12 hours in a medical intensive care unit had unilateral vocal fold immobility at extubation. Hypotension requiring vasopressors, peripheral vascular disease, and coronary artery disease were associated with unilateral vocal fold immobility.
Meaning
Unilateral vocal fold immobility after prolonged intubation is more common than previously observed and is associated with vascular risk factors; these results suggest an ischemic mechanism for neural injury in unilateral vocal fold immobility after prolonged endotracheal intubation.
Abstract
Importance
Endotracheal intubation and mechanical ventilation are life-saving treatments for acute respiratory failure but are complicated by significant rates of dyspnea and dysphonia after extubation. Unilateral vocal fold immobility (UVFI) after extubation can alter respiration and phonation, but its incidence, risk factors, and pathophysiology remain unclear.
Objectives
To determine the incidence of UVFI after prolonged (>12 hours) mechanical ventilation in a medical intensive care unit and investigate associated clinical risk factors for UVFI after prolonged mechanical ventilation.
Design, Setting, and Participants
This subgroup analysis of a prospective cohort study was conducted in a single-center medical intensive care unit from August 17, 2017, through May 31, 2018, among 100 consecutive adult patients who were intubated for more than 12 hours. Patients were identified within 36 hours of extubation and recruited for study enrollment. Those with an established tracheostomy prior to mechanical ventilation, known laryngeal or tracheal pathologic characteristics, or a history of head and neck radiotherapy were excluded.
Exposure
Invasive mechanical ventilation via an endotracheal tube.
Main Outcomes and Measures
The incidence of UVFI as determined by flexible nasolaryngoscopy.
Results
One hundred patients (62 men [62%]; median age, 58.5 years [range, 19.0-87.0 years]) underwent endoscopic evaluation after extubation. Seven patients had UVFI, of which 6 cases (86%) were left sided. Patients with hypotension while intubated (odds ratio [OR], 10.8; 95% CI, 1.6 to ∞), patients requiring vasopressors while intubated (OR, 16.7; 95% CI, 2.4 to ∞), and patients with a preadmission diagnosis of peripheral vascular disease (OR, 6.2; 95% CI, 1.2-31.9) or coronary artery disease (OR, 5.1; 95% CI, 1.0-25.5) were more likely to develop UVFI.
Conclusions and Relevance
Unilateral vocal fold immobility occurred in 7 of 100 patients in the medical intensive care unit who were intubated for more than 12 hours. Unilateral vocal fold immobility was associated with inpatient hypotension and preadmission vascular disease, suggesting that ischemia of the recurrent laryngeal nerve may play a role in disease pathogenesis.
This subgroup analysis of a cohort study examines the incidence of and risk factors for unilateral vocal fold immobility after more than 12 hours of mechanical ventilation in a medical intensive care unit.
Introduction
In the United States, approximately 24 000 people, or 40% of all critically ill patients, receive endotracheal intubation and invasive mechanical ventilation in an intensive care unit (ICU) every day.1,2 Although endotracheal intubation is essential for survival during critical illness, it directly affects laryngeal structure and function. Outcomes after endotracheal intubation can range from mild edema to mucosal ulceration and fibrotic contracture.3,4,5 Unilateral vocal fold immobility (UVFI) is a known complication of endotracheal intubation, with a reported incidence of less than 0.2% for elective surgical cases.6,7,8 However, its incidence after prolonged intubation and predisposing risk factors are poorly understood.9,10
Glottic incompetence from vocal fold immobility is associated with impaired phonation, reduced pulmonary clearance, and increased risk for aspiration pneumonia.11,12 The precise mechanisms of UVFI after endotracheal intubation are unknown but may result from direct compression of the recurrent laryngeal nerve (RLN) by the endotracheal tube (ETT) cuff, dislocation or subluxation of the cricoarytenoid joint, or neurapraxia secondary to stretching during neck hyperextension.6,8,11,12 To our knowledge, few prospective studies have been performed to define the incidence of or identify risk factors for UVFI after prolonged intubation13,14,15,16,17; the available literature is limited largely to retrospective reviews of patients intubated for a short duration during elective surgical procedures.6,7,8,9,10,12,18,19 In this post hoc analysis of a single-institution prospective cohort study investigating the association of prolonged endotracheal intubation with laryngeal outcomes, we sought to define the incidence of and clinical risk factors associated with UVFI.
Methods
Study Design
The study of UVFI and potentially associated clinical factors was performed as a subgroup analysis within a prospective cohort study of acute laryngeal injury in the medical ICU. The methods and results from the primary study are described elsewhere.20 This prospective cohort study was performed in accordance with the Declaration of Helsinki21 and Good Clinical Practice and was approved by the Vanderbilt University Medical Center Institutional Review Board. Consecutive adult (≥18 years) patients intubated for more than 12 hours in the medical ICU from August 17, 2017, through May 31, 2018, at a single center were screened for enrollment. Patients with an established tracheostomy prior to mechanical ventilation, known laryngeal or tracheal pathologic characteristics, or a history of head and neck radiotherapy were excluded. Within 36 hours of extubation, patients were recruited and provided written informed consent prior to enrollment.
Laryngeal Endoscopy
Enrolled patients underwent endoscopic examination of their larynx with a flexible rhinolaryngoscope (Karl Storz SE & Co). Video and photographic evaluation was collected using an iPhone 5 (Apple Inc) with a Karl Storz Smart Scope smartphone adapter. The results of each examination were uploaded securely via Box Capture (Box Inc) for subsequent independent blinded review by 2 of us (J.R.S. and K.S.K.).
Study Population Data
Baseline characteristics, including age, sex, race/ethnicity, and body mass index (calculated as weight in kilograms divided by height in meters squared) were recorded for each case. Data on race/ethnicity were gathered from the options defined within the electronic medical record for the purposes of characterizing our study population. Weighted comorbidities as a measure of general health were assessed with the Charlson Comorbidity Index22 and the Modified Frailty Index Score.23 The details of intubation, including ETT size, number of attempts, Cormack-Lehane grade of view, Mallampati score, intubation history, and types of specialists performing the intubation were abstracted from the electronic medical record.24,25 Data on hypotension, vasopressor therapy, acute kidney injury, delirium, pneumonia, acute respiratory distress syndrome, systemic corticosteroid use, and nasogastric or orogastric tube use during intubation were recorded. Delirium and hypotension were defined by standardized criteria.26,27 Hypotension was defined as a mean arterial pressure of less than 60 mm Hg in at least 2 consecutive invasive or noninvasive measurements that were preceded by at least 2 measurements that were more than 60 mm Hg.27 Vasopressor therapy was defined as the requirement of vasopressors as hemodynamic support for at least 8 hours.
Outcomes
The presence of UVFI was determined by direct visualization of the larynx through endoscopic evaluation within 36 hours of extubation. Unilateral vocal fold immobility was distinguished from glottic mucosal ulceration or glottic granulation tissue. The findings of mucosal ulceration and granulation tissue are reported separately.20 Vocal fold hypomobility was not included as UVFI. A video of each case was recorded and independently reviewed by 2 blinded investigators (J.R.S. and K.S.K.). If there was disagreement, a third reviewer (A.G.) determined the final classification. Patients with UVFI who had postextubation computed tomography scans of the neck or chest were assessed for evidence of cricoarytenoid joint dislocation using previously validated radiographic criteria.28 Overall survival was assessed 15 weeks after extubation during a follow-up telephone call.
Statistical Analysis
Patient characteristics were summarized using descriptive statistics. Frequencies were used for categorical variables, and continuous variables were summarized by the sample median and range. Univariate logistic regression was used to calculate an odds ratio (OR) with 95% CI for each patient characteristic or exposure and to evaluate which were associated with the development of UVFI. Statistical analyses were conducted using Stata, version 15.1 (StataCorp LLC).
Results
Cohort Characteristics
During the study period, 833 patients received mechanical ventilatin in the medical ICU. Of these patients, 166 were palliatively extubated, 76 were tracheostomy dependent, and 42 died of cardiopulmonary arrest prior to extubation. A total of 487 patients (59%) survived to extubation, 65 of whom were intubated for less than 12 hours. Therefore, 422 patients were screened for inclusion. Of these patients, 100 (24%) consented to be in the study and underwent nasolaryngoscopy within 36 hours after extubation (eFigure in the Supplement).
For these 100 patients, the median age was 58.5 years (range, 19.0-87.0 years). Most patients were male (62 [62%]), white (81 [81%]), and obese (median body mass index, 30.2 [range, 14.4-77.0]) (eTable 1 in the Supplement). The most common comorbid conditions were hypertension requiring medical therapy (50 [50%]), type 1 and 2 diabetes (36 [36%]), chronic pulmonary disease (35 [35%]), and congestive heart failure (28 [28%]) (eTable 2 in the Supplement). Most patients were self-reported to be functionally independent (69 [69%]) prior to ICU admission. The median Charlson Comorbidity Index was 5 (range, 0-13), and the median Modified Frailty Index Score was 0.18 (range, 0-0.91) (eTable 2 in the Supplement). Additional details of comorbidities, admission laboratory test values, mechanical ventilation, and active conditions can be found in eTable 1 and eTable 2 in the Supplement. The median length of intubation was 3 days (range, 1-18 days). The characteristics of the patients and the durations of intubation in our study closely mirror those of published medical ICU populations.29,30
Incidence of UVFI
Seven patients in the cohort had UVFI at the time of endoscopic evaluation. Of these, 6 cases of UVFI (86%) were left sided and 1 was right sided (14%). No cases of bilateral vocal fold immobility were observed. Only 1 patient with UVFI (14%) had other findings of acute laryngeal injury, specifically, posterior glottic mucosal injury found on examination. There was complete agreement between the 2 blinded reviewers in the diagnosis of UVFI (κ = 1.0).
Factors Associated With the Development of UVFI
There was no association between UVFI and patient age, sex, race/ethnicity, height, weight, or body mass index (Table 1). There were also no differences in select admission laboratory test values between patients with and patients without UVFI. The presence of hypotension during intubation (OR, 10.8; 95% CI, 1.6 to ∞) and the use of vasopressors during intubation (OR, 16.7; 95% CI, 2.4 to ∞) were associated with the development of UVFI. Hypotension occurred in all 7 patients with UVFI and in 43 of 93 patients (46%) without UVFI. All 7 patients with UVFI required vasopressors while intubated, compared with 33 patients (35%) without UVFI. There were no associations between any other characteristics of the patients’ hospital course, including delirium, pneumonia, and acute respiratory stress syndrome, and the development of UVFI. The administration of systemic corticosteroids during intubation was not associated with the development of UVFI because rates of corticosteroid use were similar between patients with and patients without UVFI (4 [57%] vs 28 [30%]). Similarly, procedural factors (duration of intubation, ETT size, Mallampati score, laryngeal exposure, number of intubation attempts, and type of specialist performing intubation) were not associated with UVFI (Table 2). Patients who required reintubation at least once during their stay in the medical ICU were more likely to develop UVFI (OR, 34.4; 95% CI, 5.0-235.1). No patient in either group was documented as having an ETT cuff pressure greater than 30 mm Hg, likely as a result of an institutional protocol mandating cuff pressures less than 30 mm Hg.
Table 1. Clinical Characteristics of Patients With vs Without UVFIa.
| Characteristic | UVFI (n = 7) | No UVFI (n = 93) | OR (95% CI) |
|---|---|---|---|
| Demographics | |||
| Age, median (range), y | 62.0 (39.0-77.0) | 58.0 (19.0-87.0) | 1.0 (0.9-1.1) |
| Male sex | 4 (57) | 58 (62) | 0.8 (0.2-3.8) |
| White race | 5 (71) | 76 (82) | 0.9 (0.1-7.9) |
| BMI, median (range) | 34.7 (22.1-60.3) | 29.6 (14.4-77.0) | 1.0 (0.9-1.1) |
| Comorbidities | |||
| Myocardial infarction | 2 (29) | 16 (17) | 1.9 (0.3-10.8) |
| Percutaneous coronary intervention, stent, or angina | 3 (43) | 12 (13) | 5.1 (1.0-25.5) |
| Chronic pulmonary disease | 3 (43) | 32 (34) | 1.4 (0.3-6.8) |
| Congestive heart failure | 3 (43) | 25 (27) | 2.0 (0.4-9.8) |
| Hypertension requiring medical therapy | 3 (43) | 47 (51) | 0.7 (0.2-3.5) |
| Peripheral vascular disease | 3 (43) | 10 (11) | 6.2 (1.2-31.9) |
| Transient ischemic attack or cerebrovascular accident | 1 (14) | 7 (8) | 2.0 (0.2-19.5) |
| Diabetes | 2 (29) | 34 (37) | 0.7 (0.1-3.8) |
| Charlson Comorbidity Index, median (range) | 6 (3-8) | 5 (0-13) | 1.0 (0.8-1.3) |
| Modified Frailty Index Score, median (range) | 0.18 (0-0.64) | 0.18 (0-0.91) | 5.5 (0.2-161.0) |
| Active problems during intubation | |||
| Hypotension | 7 (100) | 43 (46) | 11 (1.6-∞) |
| Vasopressor requirement | 7 (100) | 33 (35) | 17 (2.4-∞) |
| Acute kidney injury | 5 (71) | 44 (47) | 2.8 (0.5-15) |
| Systemic corticosteroids | 4 (57) | 28 (30) | 3.1 (0.6-15) |
| Delirium | 5 (71) | 63 (68) | 1.2 (0.2-6.5) |
| Pneumonia | 2 (29) | 35 (38) | 0.7 (0.1-3.6) |
| Acute respiratory distress syndrome | 1 (14) | 10 (11) | 1.4 (0.2-12.7) |
| Nasogastric or orogastric tube | 4 (57) | 58 (62) | 0.8 (0.2-3.8) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); OR, odds ratio; UVFI, unilateral vocal fold immobility.
Data are presented as number (percentage) of patients unless otherwise indicated.
Table 2. Intubation Characteristics of Patients With vs Without UVFIa.
| Characteristic | UVFI (n = 7) | No UVFI (n = 93) | OR (95% CI) |
|---|---|---|---|
| Duration of intubation, median (range), d | 5 (2-9) | 3 (1-18) | 1.1 (0.9-1.3) |
| Endotracheal tube size, mm | |||
| 7.0 | 1 (14) | 14 (15) | 0.9 (0.1-8.0) |
| 7.5 | 3 (43) | 37 (40) | |
| 8.0 | 3 (43) | 42 (45) | |
| Mallampati score | |||
| 1 | 2 (29) | 26 (28) | 0.8 (0.3-2.1) |
| 2 | 2 (29) | 31 (33) | |
| 3 | 2 (29) | 29 (31) | |
| 4 | 0 | 6 (6) | |
| Laryngeal exposure | |||
| 1 | 4 (57) | 54 (58) | 1.4 (0.3-6.4) |
| 2 | 2 (29) | 10 (11) | |
| 3 | 0 | 3 (3) | |
| Intubation attempts, No. | |||
| 1 | 2 (29) | 58 (62) | 3.4 (0.3-41) |
| 2 | 1 (14) | 12 (13) | |
| Intubating provider | |||
| Emergency department | 1 (14) | 39 (42) | 1 [Reference] |
| Anesthesia | 3 (43) | 23 (25) | 5.1 (0.5-51.8) |
| Critical care | 1 (14) | 13 (14) | 3.0 (0.2-51.4) |
| Emergency medical services | 1 (14) | 9 (10) | 4.3 (0.2-76.0) |
| Outside hospital and other | 1 (14) | 9 (10) | 4.3 (0.2-76.0) |
| At least 1 reintubation | 4 (57) | 5 (5) | 34.4 (5.0-235.1) |
Abbreviations: OR, odds ratio; UVFI, unilateral vocal fold immobility.
Data are presented as number (percentage) of patients unless otherwise indicated.
In terms of overall comorbidity status, there were no differences between the patients with and the patients without UVFI (Table 1). The median Charlson Comorbidity Index was 6 (range, 3-8) for patients with UVFI and 5 (range, 0-13) for patients without UVFI. The median Modified Frailty Index Score was 0.18 (range, 0-0.64) for patients with UVFI and 0.18 (range, 0-0.91) for patients without UVFI. Preadmission peripheral vascular disease (OR, 6.2; 95% CI, 1.2-31.9) and coronary artery disease (OR, 5.1; 95% CI, 1.0-25.5) were associated with the development of UVFI. Peripheral vascular disease was present in 3 patients (43%) with UVFI and 10 patients (11%) without UVFI, and coronary artery disease was present in 3 patients (43%) with UVFI and 12 patients (13%) without UVFI. No other comorbidities or differences in functional status were associated with UVFI.
Patient Follow-up and Outcomes
A total of 4 patients (57%) with UVFI died before the 15-week follow-up, compared with 9 patients (10%) with normal glottic mobility. The causes of death for 3 of the patients with UVFI were vancomycin-resistant enterococcal sepsis in the setting of chronic immunosuppression, sepsis secondary to bacterial peritonitis, and hypoxic respiratory failure secondary to aspiration. The cause of death for the fourth patient is unknown.
Discussion
Among patients mechanically ventilated for more than 12 hours, 7% were found to have UVFI within 36 hours of extubation. Of these patients, 6 (86%) had left-sided UVFI. Development of UVFI was associated with multiple vascular factors, including hypotension while intubated, vasopressor requirement while intubated, peripheral vascular disease, and coronary artery disease. No cases of UVFI occurred in the absence of hypotension and vasopressor use during intubation.
Reported incidences of UVFI after intubation have ranged from 0.03% to 0.23% for patients undergoing short elective surgical procedures.6,7,8 However, studies of critically ill patients receiving longer durations of intubation have reported incidences of UVFI as high as 41%.9,10 These studies may overestimate the prevalence of UVFI in the ICU population by failing to evaluate the degree of mucosal injury in the posterior glottis at extubation (likely including a subset of patients with glottic immobility secondary to fibrotic contracture in the group of patients with UVFI)10 and including patients with even minor vocal fold motion abnormalities.9 In our prospective cohort, we identified UVFI in 7% of patients. Although some prior literature suggests that longer intubation times and larger ETTs may be associated with higher rates of UVFI,7,10 we found that duration of intubation and ETT size were not associated with the development of UVFI.
Left-sided UVFI was more common than right-sided UVFI in our cohort. Although the reasons for this observation remain unclear, previous studies by Kikura et al7 and Colton House et al9 reported similar lateral asymmetry. Unilateral vocal fold immobility after intubation has frequently been considered a compressive neuropraxic injury, which is the mildest form of nerve injury caused by focal segmental demyelination without axonal or connective tissue damage.31,32 Working to explore factors that explain the vulnerability of the RLN to compressive injury after endotracheal intubation, Cavo33 performed a series of cadaveric dissections to identify the portion of the RLN most vulnerable to compression. He noted that, within the larynx, the RLN was most vulnerable at the junction of the vocal process of the arytenoid cartilage and the membranous true vocal cord, approximately 6 to 10 mm below the level of the vocal cord. At this point, the RLN could be compressed by the ETT or inflated ETT cuff medially and the thyroid lamina laterally.
Working under this “compressive” model, several factors have been hypothesized to account for the higher proportion of affected left RLNs: (1) clinicians preferentially securing the ETT tube to the right side of the patient’s mouth (causing increased pressure on the left side of the larynx and trachea by the ETT)7; (2) clinicians predominantly using their right hand to intubate, which may predispose the left side of the airway to more initial trauma from contact with the ETT; and (3) the longer course of the left RLN (originating at the aortic arch, passing inferior to the ligamentum arteriosum, and ascending superiorly toward the larynx in the tracheoesophageal groove [Figure 1]),34 rendering it more vulnerable to compression within the tracheoesophageal groove.
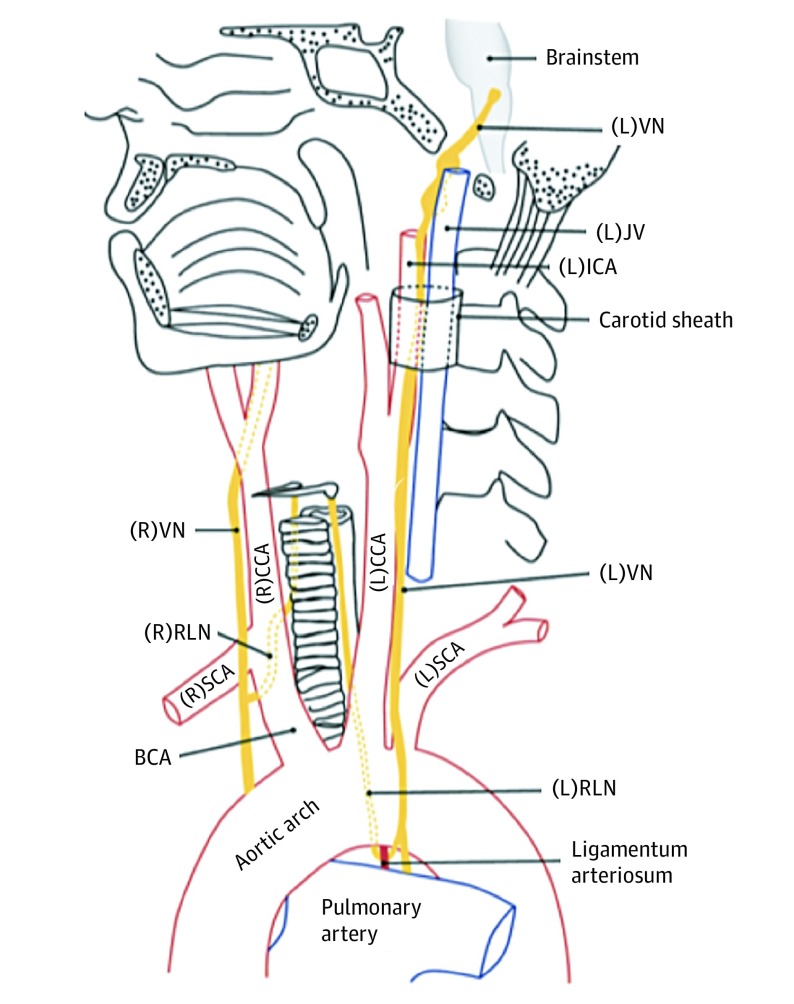
Figure 1. Normal Anatomy of the Vagus Nerves (VNs) and Recurrent Laryngeal Nerves (RLNs).
The left VN exits the skull base through the jugular foramen and descends through the neck posterolateral to the internal carotid arteries (ICAs) and common carotid arteries (CCAs). As the left VN passes anterolateral to the aortic arch, the left RLN branches off and passes below the arch posterior to the ligamentum arteriosum. It then ascends within the left tracheoesophageal groove to enter the larynx posteriorly at the level of the cricoarytenoid joint. The right VN descends posterolateral to the right ICA and CCA from the right jugular foramen, giving rise to the right RLN as it passes anterior to the right subclavian artery (SCA). The right RLN then passes posterior to the right brachiocephalic artery (BCA) before ascending to the larynx within the right tracheoesophageal groove. Adapted with permission from the Radiological Society of North America. JV indicates jugular vein; L, left; and R, right.
The specifics of intubation practices in our cohort offer evidence refuting several proposed mechanisms for left-sided injury bias to the RLN after prolonged intubation. In our medical ICU, ETT tubes are secured in the midline using an AnchorFast oral endotracheal tube fastener (Hollister Inc). Despite this practice (which should prevent excessive pressure on the left endolarynx or trachea), an increased incidence of left-sided UVFI was still observed. In addition, even though all clinicians who intubated patients who developed UVFI were right handed (theoretically predisposing the left side of the airway to more initial trauma from contact with the ETT), the intubations were not traumatic (only 1 of 7 patients with UVFI had findings of acute laryngeal mucosal injury), and the number of attempts required for successful intubation was not associated with UVFI. Finally, while the anatomical course of the left RLN certainly could render it more susceptible to compression in the tracheoesophageal groove, the presence of a nasogastric feeding tube should theoretically increase the risk of a compressive neural injury in this location; however, this association was not observed in our study.
In a separate study investigating acute laryngeal mucosal injury (defined as glottic mucosal ulceration or granulation tissue) in this same cohort of patients, the development of acute laryngeal mucosal injury was associated with the use of larger ETTs (8.0 vs 7.5 or 7.0 mm), type 2 diabetes, and high body mass index.20 A history of vascular disease and the use of vasopressors were not found to increase the risk of developing acute laryngeal mucosal injury. Thus, the risk factors for acute laryngeal mucosal injury do not overlap with the factors associated with UVFI, which suggests that they are distinct entities with different causes and patient populations.
Although laryngeal electromyography was not performed on the patients in our cohort with UVFI, 5 of these patients underwent a postextubation computed tomography scan of the neck, and none had radiographic evidence of cricoarytenoid joint dislocation.28 Therefore, we propose an alternate mechanism underlying UVFI: RLN injury secondary to axonal ischemia. Although the precise mechanism of RLN injury in our cohort remains speculative, the strong association with vascular comorbidities (preadmission vascular disease and coronary artery disease, as well as inpatient hypotension requiring vasopressors) suggests an underlying microvascular pathologic characteristic of neural injury.
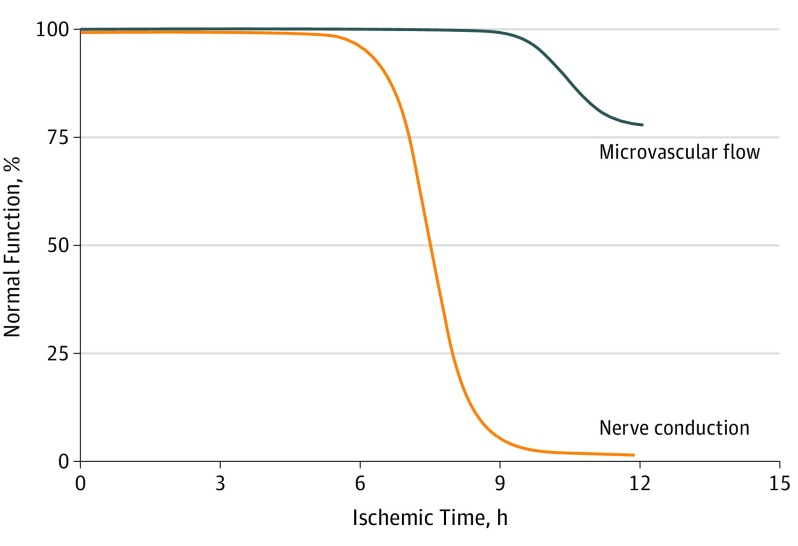
Animal models exploring the cellular mechanism of peripheral nerve injury suggest that ischemia is a critical inciting event. Few signs of tissue injury (vasa nervorum granulocytosis, microthrombi, or microemboli) are observed after 4 hours of neuronal ischemia. However, when ischemia extends beyond 6 hours, Wallerian degeneration and severe axonal injury occur, resulting in the loss of nerve conduction (Figure 2).35,36,37 This ischemic model may account for the higher incidence of UVFI observed in patients with prolonged intubation compared with short-duration intubation during elective surgical procedures. It also may account for the absence of association between duration of intubation and UVFI risk in our cohort because, by definition, all patients in our cohort were intubated for longer than the threshold generally required for loss of nerve conduction. The explanation for the association between reintubation and UVFI is unclear, but it could be associated with repeated exposure to neural hypoperfusion during the intubation procedure. Given the lack of laryngeal mucosal injury observed after an endoscopic examination, mucosal trauma is thought to be an unlikely mechanism.
Figure 2. Intraneural Microvascular Flow and Nerve Conduction During Ischemia.
In human peripheral nerve (tibial nerve), 4 hours of ischemia is followed by complete recovery of microvascular flow (blue) and nerve conduction (orange). After 6 hours of ischemia, there is a complete recovery of microvascular flow and almost complete recovery of nerve conduction. However, after more than 6 hours of ischemia, nerve conduction is almost completely lost. Reprinted with permission from JAMA Surgery.35
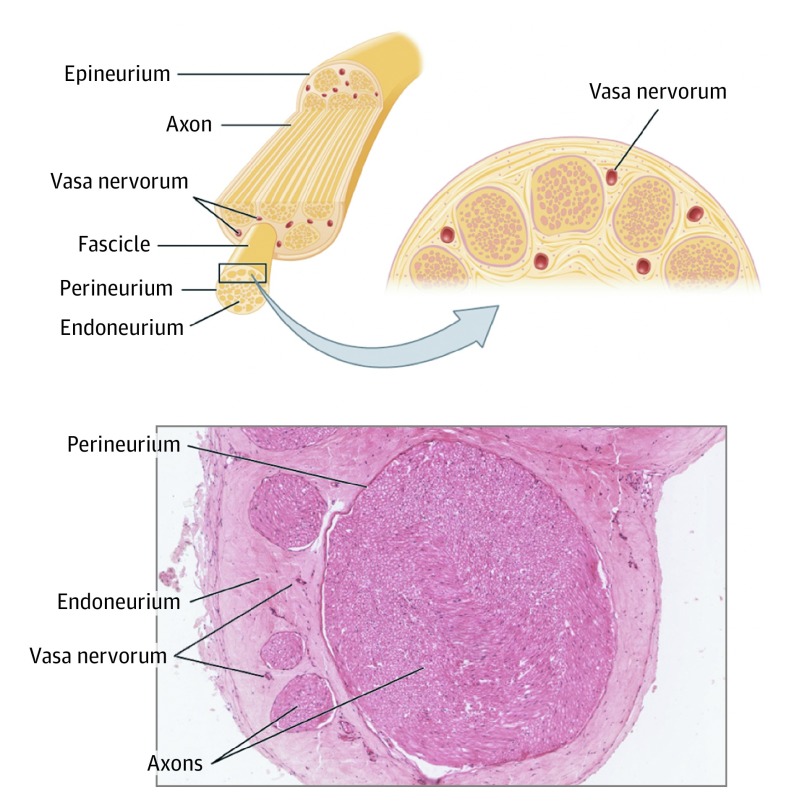
Susceptibility to axonal ischemia is associated with nerve length, and cadaveric anatomical studies demonstrate that the left RLN is nearly 50% longer than the right RLN, which may explain the higher incidence of left-sided UVFI.38,39 Although the RLN vascular supply can be variable, the constant arterial sources for the left RLN include the anterior bronchoesophageal and inferior thyroid arteries.40 The subclavian artery, first thoracic intercostal artery, and posterior aspect of the aortic arch can also provide blood supply to the left RLN.40 Perfusion pressure could be compromised by vascular disease or systemic hypotension because these arteries ascend in the neck with preferential left-sided UVFI. This compromise of perfusion pressure could be compounded by direct compression of the vasa nervorum or venous outflow of the RLN by the ETT cuff, even if the cuff is inflated to proper pressures (Figure 3). This proposed mechanism may help explain why prophylactic or early treatment with calcium channel blockers may enhance recovery in vocal fold paralysis via increased vascular flow and reduced axonal ischemia.41,42
Figure 3. Peripheral Nerve Anatomy.
The vasa nervorum is derived from adjacent blood vessels. After entering a nerve, the vessels form anastomoses at intervals throughout the course of the nerve, reinforcing the blood supply within the epineurium. Where a considerable interval between the supplying nutrient vessels occurs, there will be a natural “watershed” region susceptible to ischemia. Used under a Creative Commons Attribution 4.0 International License from Anatomy and Physiology, OpenStax at Rice University (https://openstax.org/books/anatomy-and-physiology/pages/13-4-the-peripheral-nervous-system).
Limitations
Our study has several limitations. Despite our cohort size of 100 patients, the low number of detected outcomes (7 patients with UVFI) precluded multivariate analysis. The low number of UVFI cases also negatively affects the precision of our univariate analyses, and for this reason, we think that the results of our study should be used to generate hypotheses for future work rather than be viewed as providing definitive risk factors for the development of UVFI.
In our study, long-term follow-up data were not available owing to the high mortality of patients with UVFI. Prior studies suggest that an acutely compressed RLN can recover function in weeks to months.31 Because the fiberoptic scope examination was performed within 36 hours after extubation without subsequent follow-up examinations, it is possible that some of the UVFI noted was transient. The prevalence of permanent UVFI after prolonged intubation remains unresolved. In addition, by design, our study evaluated only patients who received mechanical ventilation for at least 12 hours. It is unclear whether the results would be the same for patients intubated for less than 12 hours. Our study defined requirement of vasopressors as the receipt of vasoactive medications for at least 8 hours, and it is unclear whether the results of our study would have been different had a different definition been used. Laryngeal electromyography was not performed during the study, so our proposal of axonal ischemia as the mechanism of injury in patients with UVFI remains speculative. Although ETT cuff pressures in our study were normal, we did not routinely evaluate the position of the ETT relative to the glottis using imaging. Therefore, we were unable to evaluate the association of ETT position with the development of UVFI. We are also unable to comment on whether ETT cuff pressures lower than 30 mm Hg are protective against UVFI.
Conclusions
Although intubation-associated UVFI is rare in patients undergoing elective surgery, it occurred in 7% of patients receiving mechanical ventilation for more than 12 hours in the medical ICU. Peripheral vascular disease, coronary artery disease, and hypotension requiring vasopressors were associated with the development of UVFI. These results suggest that neural ischemia may be a mechanism of injury in UVFI.
Future work involving a larger multi-institutional prospective cohort should be undertaken to confirm the observed rate of UVFI after prolonged endotracheal intubation and obtain long-term physiological and patient-reported follow-up data. Laryngeal electromyography would facilitate investigation of the pathophysiological mechanism of injury. Future collection of rates of other postextubation neurovascular injuries (ie, acute kidney injury) associated with UVFI could also support an ischemic mechanism of injury. In addition, larger prospective studies are necessary to investigate the potential of calcium channel blockers, or other therapies that increase tissue perfusion, to aid in functional recovery from UVFI.
eFigure. CONSORT Diagram
eTable 1. Cohort Characteristics
eTable 2. Cohort Health Status and Comorbid Disease
References
- 1.Angus DC, Shorr AF, White A, Dremsizov TT, Schmitz RJ, Kelley MA; Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS) . Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations. Crit Care Med. 2006;34(4):1016-1024. doi: 10.1097/01.CCM.0000206105.05626.15 [DOI] [PubMed] [Google Scholar]
- 2.Wunsch H, Wagner J, Herlim M, Chong DH, Kramer AA, Halpern SD. ICU occupancy and mechanical ventilator use in the United States. Crit Care Med. 2013;41(12):2712-2719. doi: 10.1097/CCM.0b013e318298a139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colice GL, Stukel TA, Dain B. Laryngeal complications of prolonged intubation. Chest. 1989;96(4):877-884. doi: 10.1378/chest.96.4.877 [DOI] [PubMed] [Google Scholar]
- 4.Hawkins DB. Pathogenesis of subglottic stenosis from endotracheal intubation. Ann Otol Rhinol Laryngol. 1987;96(1, pt 1):116-117. doi: 10.1177/000348948709600126 [DOI] [PubMed] [Google Scholar]
- 5.Vila J, Bosque MD, García M, Palomar M, Quesada P, Ramis B. Endoscopic evolution of laryngeal injuries caused by translaryngeal intubation. Eur Arch Otorhinolaryngol. 1997;254(suppl 1):S97-S100. doi: 10.1007/BF02439735 [DOI] [PubMed] [Google Scholar]
- 6.Hsu YT, Hao SP. Intubation-related vocal cord palsy. Otolaryngol Head Neck Surg. 2012;147(2S):192. doi: 10.1177/0194599812451426a213 [DOI] [Google Scholar]
- 7.Kikura M, Suzuki K, Itagaki T, Takada T, Sato S. Age and comorbidity as risk factors for vocal cord paralysis associated with tracheal intubation. Br J Anaesth. 2007;98(4):524-530. doi: 10.1093/bja/aem005 [DOI] [PubMed] [Google Scholar]
- 8.Sariego J. Vocal fold hypomobility secondary to elective endotracheal intubation: a general surgeon’s perspective. J Voice. 2010;24(1):110-112. doi: 10.1016/j.jvoice.2008.05.001 [DOI] [PubMed] [Google Scholar]
- 9.Colton House J, Noordzij JP, Murgia B, Langmore S. Laryngeal injury from prolonged intubation: a prospective analysis of contributing factors. Laryngoscope. 2011;121(3):596-600. doi: 10.1002/lary.21403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santos PM, Afrassiabi A, Weymuller EA Jr. Risk factors associated with prolonged intubation and laryngeal injury. Otolaryngol Head Neck Surg. 1994;111(4):453-459. doi: 10.1177/019459989411100411 [DOI] [PubMed] [Google Scholar]
- 11.Matta R, Halan B, Sandhu K. Postintubation recurrent laryngeal nerve palsy: a review. J Laryngol Voice. 2017;7(2):25. doi: 10.4103/jlv.JLV_5_16 [DOI] [Google Scholar]
- 12.Xu W, Han D, Hu R, Bai Y, Zhang L. Characteristics of vocal fold immobility following endotracheal intubation. Ann Otol Rhinol Laryngol. 2012;121(10):689-694. doi: 10.1177/000348941212101012 [DOI] [PubMed] [Google Scholar]
- 13.Goto T, Gibo K, Hagiwara Y, et al. Factors associated with first-pass success in pediatric intubation in the emergency department. West J Emerg Med. 2016;17(2):129-134. doi: 10.5811/westjem.2016.1.28685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibuki T, Ando N, Tanaka Y. Vocal cord paralysis associated with difficult gastric tube insertion. Can J Anaesth. 1994;41(5, pt 1):431-434. doi: 10.1007/BF03009868 [DOI] [PubMed] [Google Scholar]
- 15.Marie JP, Keghian J, Mendel I, Gueit I, Dehesdin D, Andrieu-Guitrancourt J. Post-intubation vocal cord paralysis: the viral hypothesis: a case report. Eur Arch Otorhinolaryngol. 2001;258(6):285-286. doi: 10.1007/s004050100357 [DOI] [PubMed] [Google Scholar]
- 16.Hurtado Nazal C, Araneda Vilches A, Vergara Marín C, García Contreras K, Napolitano Valenzuela C, Badía Ventí P. Vocal cord paralysis after endotracheal intubation: an uncommon complication of general anesthesia [in Portuguese]. Rev Bras Anestesiol. 2018;68(6):637-640. doi: 10.1016/j.bjan.2017.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taşlı H, Kara U, Gökgöz MC, Aydın Ü. Vocal cord paralysis following endotracheal intubation. Turk J Anaesthesiol Reanim. 2017;45(5):321-322. doi: 10.5152/TJAR.2017.91297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kastanos N, Estopá Miró R, Marín Perez A, Xaubet Mir A, Agustí-Vidal A. Laryngotracheal injury due to endotracheal intubation: incidence, evolution, and predisposing factors: a prospective long-term study. Crit Care Med. 1983;11(5):362-367. doi: 10.1097/00003246-198305000-00009 [DOI] [PubMed] [Google Scholar]
- 19.Peppard SB, Dickens JH. Laryngeal injury following short-term intubation. Ann Otol Rhinol Laryngol. 1983;92(4, pt 1):327-330. doi: 10.1177/000348948309200402 [DOI] [PubMed] [Google Scholar]
- 20.Shinn JR, Kimura KS, Campbell BR, et al. Incidence and outcomes of acute laryngeal injury after prolonged mechanical ventilation [published online October 17, 2019]. Crit Care Med. 2019. doi: 10.1097/CCM.0000000000004015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 23.Farhat JS, Velanovich V, Falvo AJ, et al. Are the frail destined to fail? frailty index as predictor of surgical morbidity and mortality in the elderly. J Trauma Acute Care Surg. 2012;72(6):1526-1530. doi: 10.1097/TA.0b013e3182542fab [DOI] [PubMed] [Google Scholar]
- 24.Cormack RS, Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia. 1984;39(11):1105-1111. doi: 10.1111/j.1365-2044.1984.tb08932.x [DOI] [PubMed] [Google Scholar]
- 25.Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985;32(4):429-434. doi: 10.1007/BF03011357 [DOI] [PubMed] [Google Scholar]
- 26.Pandharipande PP, Girard TD, Ely EW. Long-term cognitive impairment after critical illness. N Engl J Med. 2014;370(2):185-186. [DOI] [PubMed] [Google Scholar]
- 27.Boone MD, Massa J, Mueller A, et al. The organizational structure of an intensive care unit influences treatment of hypotension among critically ill patients: a retrospective cohort study. J Crit Care. 2016;33:14-18. doi: 10.1016/j.jcrc.2016.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffman HT, Brunberg JA, Winter P, Sullivan MJ, Kileny PR. Arytenoid subluxation: diagnosis and treatment. Ann Otol Rhinol Laryngol. 1991;100(1):1-9. doi: 10.1177/000348949110000101 [DOI] [PubMed] [Google Scholar]
- 29.Hernández G, Vaquero C, González P, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA. 2016;315(13):1354-1361. doi: 10.1001/jama.2016.2711 [DOI] [PubMed] [Google Scholar]
- 30.Miltiades AN, Gershengorn HB, Hua M, Kramer AA, Li G, Wunsch H. Cumulative probability and time to reintubation in US ICUs. Crit Care Med. 2017;45(5):835-842. doi: 10.1097/CCM.0000000000002327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menorca RM, Fussell TS, Elfar JC. Nerve physiology: mechanisms of injury and recovery. Hand Clin. 2013;29(3):317-330. doi: 10.1016/j.hcl.2013.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain. 1951;74(4):491-516. doi: 10.1093/brain/74.4.491 [DOI] [PubMed] [Google Scholar]
- 33.Cavo JW., Jr True vocal cord paralysis following intubation. Laryngoscope. 1985;95(11):1352-1359. doi: 10.1288/00005537-198511000-00012 [DOI] [PubMed] [Google Scholar]
- 34.Simon MM. Recurrent laryngeal nerve in thyroid surgery. Am J Surg. 1943;60:212-220. doi: 10.1016/S0002-9610(43)90652-X [DOI] [Google Scholar]
- 35.Lundborg G. Limb ischemia and nerve injury. Arch Surg. 1972;104(5):631-632. doi: 10.1001/archsurg.1972.04180050007001 [DOI] [PubMed] [Google Scholar]
- 36.Mäkitie J, Teräväinen H. Peripheral nerve injury and recovery after temporary ischemia. Acta Neuropathol. 1977;37(1):55-63. doi: 10.1007/BF00684541 [DOI] [PubMed] [Google Scholar]
- 37.Myers RR, Yamamoto T, Yaksh TL, Powell HC. The role of focal nerve ischemia and Wallerian degeneration in peripheral nerve injury producing hyperesthesia. Anesthesiology. 1993;78(2):308-316. doi: 10.1097/00000542-199302000-00015 [DOI] [PubMed] [Google Scholar]
- 38.Prades JM, Dubois MD, Dumollard JM, et al. Morphological and functional asymmetry of the human recurrent laryngeal nerve. Surg Radiol Anat. 2012;34(10):903-908. doi: 10.1007/s00276-012-0999-7 [DOI] [PubMed] [Google Scholar]
- 39.McDermott MM, Sufit R, Nishida T, et al. Lower extremity nerve function in patients with lower extremity ischemia. Arch Intern Med. 2006;166(18):1986-1992. doi: 10.1001/archinte.166.18.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Filaire M, Garçier JM, Harouna Y, et al. Intrathoracic blood supply of the left vagus and recurrent laryngeal nerves. Surg Radiol Anat. 2001;23(4):249-252. doi: 10.1007/s00276-001-0249-x [DOI] [PubMed] [Google Scholar]
- 41.Mattsson P, Björck G, Remahl S, et al. Nimodipine and microsurgery induced recovery of the vocal cord after recurrent laryngeal nerve resection. Laryngoscope. 2005;115(10):1863-1865. doi: 10.1097/01.mlg.0000177034.51559.50 [DOI] [PubMed] [Google Scholar]
- 42.Rosen CA, Smith L, Young V, Krishna P, Muldoon MF, Munin MC. Prospective investigation of nimodipine for acute vocal fold paralysis. Muscle Nerve. 2014;50(1):114-118. doi: 10.1002/mus.24111 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. CONSORT Diagram
eTable 1. Cohort Characteristics
eTable 2. Cohort Health Status and Comorbid Disease