Abstract
The main pathological feature of the neurodegenerative diseases is represented by neuronal death that represents the final step of a cascade of adverse/hostile events. Early in the neurodegenerative process, glial cells (including astrocytes, microglial cells, and oligodendrocytes) activate and trigger an insidious neuroinflammatory reaction, metabolic decay, blood brain barrier dysfunction and energy impairment, boosting neuronal death. How these mechanisms might induce selective neuronal death in specific brain areas are far from being elucidated. The last two decades of neurobiological studies have provided evidence of the main role of glial cells in most of the processes of the central nervous system, from development to synaptogenesis, neuronal homeostasis and integration into, highly specific neuro-glial networks. In this mini-review, we moved from in vitro and in vivo models of neurodegeneration to analyze the putative role of glial cells in the early mechanisms of neurodegeneration. We report changes of transcriptional, genetic, morphological, and metabolic activity in astrocytes and microglial cells in specific brain areas before neuronal degeneration, providing evidence in experimental models of neurodegenerative disorders, including Parkinson’s and Alzheimer’s diseases. Understanding these mechanisms might increase the insight of these processes and pave the way for new specific glia-targeted therapeutic strategies for neurodegenerative disorders.
Keywords: astrocytes, glial cells, microglia, neurodegenerative diseases, neuroinflammation, Parkinson's disease, reactive gliosis, selective neuronal degeneration
Introduction
The complex organization of the central nervous system (CNS) is based on the heterogeneity and reciprocal interactions of its cellular (neurons, glia, pericytes) and non-cellular (extracellular matrix) elements (Silbereis et al., 2016). Decades of studies have challenged the central and exclusive role of neurons in the neural transmission and neuroplasticity (Allen and Lyons, 2018) and nowadays glial cells, comprising about 33–66% of the human brain cells (Azevedo et al., 2009; Herculano-Houzel, 2014; Jäkel and Dimou, 2017), are key components of the synaptic machinery (Dityatev and Rusakov, 2011). Microglial cells (the resident macrophages of the CNS) provide the first line of defense against external stressors (Hanisch and Kettenmann, 2007) and are involved in the pruning and maturation of synaptic networks during neurodevelopment. On the other side, astrocytes are key regulators of neuronal homeostasis and neuro-glial network function (Verkhratsky and Nedergaard, 2016): beside their active role in the neurotransmitter reuptake (Ota et al., 2013) and ions balance (Lian and Stringer, 2004), astrocytes release neurotransmitters (gliotransmission), provide efficiency of the blood-brain barrier (BBB), provide trophic and metabolic support to neurons and neuropil, actively responding to neuronal firing in a tight metabolic coupling (Cirillo et al., 2015; Ruminot et al., 2019; Zhou et al., 2019). From circulating glucose, astrocytes produce lactate and transfer it to neurons (astrocyte-neuron lactate shuttle) for mitochondrial ATP production, thus modulating neuronal excitability and plasticity (Magistretti and Allaman, 2018).
To date, higher brain functions arise from morpho-functional activity of neuro-glial networks (Araque and Navarrete, 2010; Perea et al., 2014; Liddelow and Barres, 2017) that strictly interact with BBB and extracellular matrix, constituting the penta-partite synapse (De Luca et al., 2018).
External insults to the CNS first activate microglial cells, resulting in both morphological and functional changes underlying two different phenotypes, the proinflammatory M1 and the immunosuppressive M2 phenotype (Tang and Le, 2016). The latter is involved in the release of pro-inflammatory factors which contribute to astrocytic activation (reactive astrocytosis), neuronal dysfunction, altered synaptic plasticity and ultimately cell death (Papa et al., 2014).
Reactive astrocytes show specific alterations in cell morphology and proliferation and can cause permanent tissue rearrangement (Anderson et al., 2014). Reactive astrocytes show increased expression of glial fibrillary acidic protein, reduction of glial glutamate transporters, impaired anti-oxidant defense (reduction of glutathione synthesis), thus undermining the synaptic machinery, neurotransmitter homeostasis and neuroprotective functions (Cirillo et al., 2011).
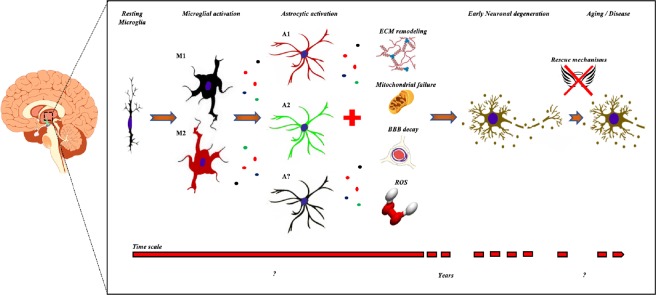
Therefore, it is conceivable that regional neuroinflammatory microglial reaction, reactive astrocytosis, and mitochondrial dysfunction might produce a persistent neuro-glial metabolic decay, causing progressive neuronal dysfunction and failure of the rescue mechanisms, leading to neuronal dysfunction and, finally, to neuronal degeneration. Microgliosis and astrocytosis, for example, usually precede of several weeks the motor symptoms onset and motor neuron degeneration in a mouse model of amyotrophic lateral sclerosis (Acevedo-Arozena et al., 2011). Therefore, it is conceivable that malfunctioning glial cells might create a maladaptive environment that negatively impact neuronal physiology, leading to neuronal death in neurodegenerative disorders but also in the physiological brain aging (Figure 1).
Figure 1.
Time-course of the mechanisms leading to selective neuronal degeneration.
Microglial and astrocytic activation occurs early in the neurodegenerative process and produces maladaptive changes of many components of the pentapartite synapse (including extracellular matrix and blood brain barrier). The release of inflammatory and toxic factors by glial cells impairs mitochondrial energy production and is associated with the release of ROS. These slowly progressive mechanisms finally initiate neuronal degeneration, however the disease onset occurs later, after failure of rescue mechanisms and progression of neuronal degeneration. BBB: Blood-brain barrier; ECM: extracellular matrix; M1, M2, A1, A2: microglial and astrocytic type 1 and type 2 phenotypes; ROS: reactive oxygen species.
Search Strategy and Selection Criteria
We have performed a PubMed literature search of experimental articles published mainly in the last 10 years (from January 2009 to July 2019) regarding the glial-induced selective neuronal vulnerability to neurodegeneration, providing data in AD, PD and other neurodegenerative disorders.
Towards a Role of Glial Phenotype in Neurodegeneration
Despite complex and unknown mechanisms, neurodegenerative disorders are characterized by degeneration of specific neuronal populations: for example, hippocampal and cortical neurons are mainly affected in Alzheimer’s disease (AD) (Minati et al., 2009), medium spiny GABA neurons in basal ganglia degenerate in Huntington’s disease (HD) (Rikani et al., 2014), cerebellar Purkinje cells in spinocerebellar ataxia type 1 (Pérez Ortiz and Orr, 2018) and dopaminergic neurons in the substantia nigra pars compacta (SNpc) in Parkinson’s disease (PD) (Ross et al., 2004) (Figure 2).
Figure 2.
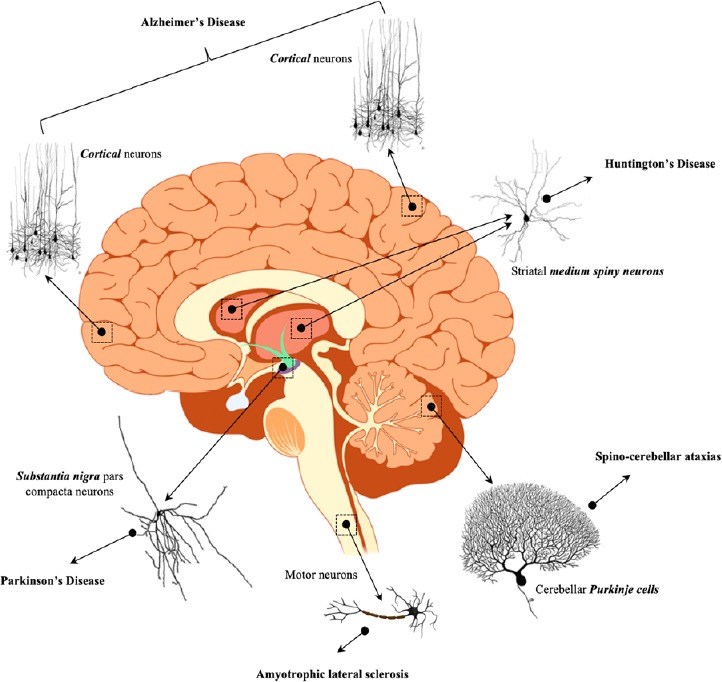
Selective neuronal degeneration and relative neurodegenerative disorders.
Neuronal degeneration, however, is only the final step of a cascade of complex events, including also neuroinflammation, mitochondrial dysfunction, oxidative stress and excitotoxicity (Yang et al., 2009). The regional selective vulnerability of specific brain regions in the neurodegenerative process might be correlated to the heterogeneous neuronal responses to damaging mechanisms (Wang and Michaelis, 2010) or to a different cellular and extracellular context that becomes unsupportive to neurons during the disorder development.
Astrocytic with variable phenotypes can be identified across the brain and within a brain region in normal conditions as well as in response to external injury (lipopolysaccharide, LPS – injection or middle cerebral artery occlusion) or aging (Sofroniew and Vinters, 2010; Nash et al., 2011). For example, LPS induced microglial activation and consequently reactive astrocytes, that show a detrimental phenotype (A1) for neurons, via secretion of neurotoxins, interleukin-1α, tumor necrosis factor-α, and C1q (Liddelow et al., 2017); in contrast, the ischemia-induced A2 phenotype displayed a molecular profile associated with a beneficial or protective response and promote neuronal survival (Okada et al., 2006). Activated A1 astrocytes, through the release of toxic and other (unknown) factors, induce also neuronal and oligodendrocyte degeneration in a mouse model of age-related cognitive decline (Clarke et al., 2018).
Although astrocytes from two mature brain circuits (such as striatum and hippocampus) share many similarities, they present morphological, molecular and functional differences underlying specific roles and specializations within neuronal circuits.
Accordingly, proteomic and transcriptome analysis have revealed that µ-crystallin is mainly expressed by striatal astrocytes (Khakh, 2019), while pro-brain derived neurotrophic factor and aberrant GABA represent molecular markers of active and reactive astrocytes in hippocampus, respectively (Chun et al., 2018). Striatal and hippocampal astrocytes differ in the territory size, in the number of neurons they contact and the synapses covered within their region (Chai et al., 2017).
Moreover, the astrocytic responses to the same type of injury also vary across the brain. Recently, a heterogeneous response of astrocytes from cortex, hippocampus, and striatum to a mechanical injury was reported in vitro (Cragnolini et al., 2018). Using a scratch injury model, striatum astrocytes proliferate and occupied the injured area faster than astrocytes from the other two brain areas. In the same study, the response to the neurotrophins (that promote injury recovery) significantly variated among astrocytes from the three different brain areas.
In conclusion, the heterogeneous expression of proteins determining astrocytic morphology and function might underlie the selective vulnerability of brain regions to specific diseases (Matias et al., 2019).
Glial-Based Vulnerability of Dopaminergic Neurons in Substantia Nigra Pars Compacta
Dysfunction and successive degeneration of the brain dopaminergic system strictly correlates with the evolution of the clinical picture of the patients affected by PD. However, not all dopaminergic neurons are equally affected in PD (Double et al., 2010). Neurons in the ventral and lateral SNpc, for example, are much more susceptible than dopaminergic neurons in the ventral tegmental area (VTA) or in the hypothalamus (Hirsch et al., 1988). Administration of the complex I inhibitors 1-methyl 4-phenyl 1, 6-tetrahydropyridine, rotenone, or 6-hydroxydopamine to experimental animals causes preferential neurodegeneration of SNpc neurons compared to VTA neurons (Dawson et al., 2002). Despite the exposure of all dopaminergic neurons to toxins, degeneration is commonly localized to a specific anatomic region, suggesting that other mechanisms need to be involved and are far from being fully elucidated.
In experimental models of PD, the balance of astrocytes and microglia within the midbrain is a key factor underlying the selective vulnerability of SNpc neurons. Evidence suggests that SNpc astrocytes show a different transcriptional activity compared to VTA astrocytes. Astrocytes in VTA, but not in SNpc, release “factors” which mediate protection of induced pluripotent stem cell and dopaminergic neurons in VTA and SNpc (Oksanen et al., 2019). Moreover, increased microglial reactivity has been observed in both human patients and animal models of PD, thus increasing the release of pro-inflammatory factors. The activation/stimulation of the midbrain SNpc microglia with the LPS produces degeneration of dopaminergic neurons (Gao et al., 2002; Batista et al., 2019), suggesting a role for microglia and the derived factors in disease progression.
Therefore, these extrinsic mechanisms could explain the protective nature of astrocytes and the deleterious functions of microglia in the disease pathogenesis, and represent putative targets for future therapeutics in PD.
Neurodegeneration as a Consequence of Glial Mitochondrial Function Decay
Early changes leading to neuronal degeneration include neuroinflammation, reactive micro and macro-gliosis, BBB decay, metabolic stress, production of ROS and mitochondrial energy impairment (Papa et al., 2014; Reeve et al., 2018). Stimulation of Na+/K+ exchanger isoform 1 in astrocytes caused ionic dysregulation under ischemic condition, while the selective knockout of this astrocytic exchanger isoform reduced astrogliosis, BBB damage, infarction and improve neurological function after ischemic stroke (Begum et al., 2018). Expression of the Na+/K+ exchanger, therefore, could be involved in astrocytic activation and metabolic acidosis after ischemic stroke.
Morphological (Papa et al., 2014) as functional changes (Kaminsky et al., 2016) of glial cells results in pathological neuro-glial interactions that, in turn, generate hostile environment that first induces neuronal dysfunction and later neurodegeneration.
Regional specific perturbations in astrocyte mitochondrial functions (Voloboueva et al., 2007) might adversely impact their neuroprotective properties and the response to neuronal activity. Morpho-functional heterogeneity of astrocytes in different brain regions might play a key role in the astrocytic responses to an external injury (Cragnolini et al., 2018). Recently, in a rat model of HD, mitochondrial failure by subchronic treatment with 3-nitropropionic acid (a suicide inhibitor of the mitochondrial complex II succinate dehydrogenase) first induced a loco-regional glial reaction and then a selective neuronal degeneration in basal ganglia of treated animals (Cirillo et al., 2019). Moreover, striatal vulnerability correlated with astrocytic metabolic reprogramming of mitochondrial functions. A reduction of striatal glucose levels leads astrocytic mitochondria to switch from ordinary glucose metabolism to fatty acid oxidation, thus increasing oxygen-induced neuronal degeneration (Polyzos et al., 2019).
Selective vulnerability of basal ganglia is also reported in humans after metabolic, toxic or ischemic insults to basal ganglia, resulting in bilateral striatal necrosis (Wang and Cheng, 2003). Recently, we have reported the case of a patient with an acquired bilateral striatal necrosis after metabolic acidosis, hypothesizing that neuronal degeneration might be the consequence of the selective vulnerability of striatal BBB and decay of mitochondrial energy production (Cirillo et al., 2019).
These results prompted us to suppose a strict morpho-metabolic-energetic coupling of reactive astrocytes: external injuries induce selective mitochondrial decay in specific astrocytes of a susceptible brain region, leading to reactive gliosis and progressive neurodegeneration.
Transcriptional and Genetic Profiles of Astrocytic Populations Involved in Neurodegenerative Mechanisms
The development of new genetic tools have allowed the transcriptome analysis of reactive astrocytes from multiple brain regions (Zamanian et al., 2012) and the differentiation of the two main astrocytic phenotypes (A1 and A2).
The neurotoxic type A1 phenotype is triggered by microglia (Liddelow et al., 2017; Yun et al., 2018) and can be detected in many neurodegenerative conditions including PD (Yun et al., 2018), AD, amyotrophic lateral sclerosis (Li et al., 2019) and after traumatic brain injury (Clark et al., 2019).
A1 astrocytes in PD experimental models have been also induced by pre-formed fibrils of α-synuclein (α-syn PFF) and can be blocked by administration of NLY01, a glucagon-like peptide-1 receptor (GLP1R) agonist. Glp1r mRNA levels show a brain-region heterogeneity, being predominantly expressed in non-cortical areas associated to the progression/ neurodegeneration in PD. On the other hand, GLP1R is mainly expressed in microglia and astrocytes after α-syn PFF administration, suggesting that astrocytes mediate α-syn PFF toxicity and NLY01 might exert its glial-mediated activity in non-cortical brain regions.
Recently, using the Ribo-Tag technique, ribosomes were genetically tabbed and astrocyte-associated mRNA purified from different brain areas of adult and aged mouse (Boisvert et al., 2018). Astrocytes from aged brains showed a regional specific upregulation of the genes involved in eliminating synapses, highlighting the role of astrocytes in damaging synapses.
Could the gene expression profile of each brain region explain the selective vulnerability to neurodegeneration?
In recent studies, the transcriptional profiles of astrocyte populations were linked to tumors and neurodegenerative diseases (Cuevas Diaz Duran et al., 2019). Through an advanced comparative bioinformatic analysis, it has been shown that gene signatures of astrocytic subpopulations correlate with glioma subtypes. In addition, several genes linked with neurodegenerative diseases such as AD, HD or PD were enriched in subpopulations of astrocytes (Sofroniew and Vinters, 2010).
Does astrocytic dysfunction precede neurodegeneration in specific brain regions?
The progression of astrocytosis and Aβ deposition has been examined using in vivo positron emission tomography in a transgenic mouse model of AD (APPswe) (Rodriguez-Vieitez et al., 2015). In 6-month-old APPswe, authors observed intense cortical astrocytosis but not Aβ plaques deposition, suggesting that astrocytosis occurs early in AD and precedes Aβ plaque deposition. Furthermore, a pan-cortical transcriptomic network analysis provided a comprehensive assessment of the critical molecular pathways associated with AD pathology. It offered new insights into the molecular mechanisms underlying selective regional vulnerability to AD at different stages of the progression of cognitive deficits and development of the canonical neuro-pathological lesions characterizing this disorder (Wang et al., 2016). Many of the gene expression changes, including those of oligodendrocytes, occurred early in the progression of the disease, making them potential treatment targets and unlikely to be mere bystander result of degeneration.
Conclusions
More extensive studies of the time course of gliosis in neurodegenerative diseases models should be carried out to determine whether astrogliosis is a previous phenomenon triggering neurodegeneration. Understanding the responses of astrocytes in diseases represents a new challenge for the diagnostic and the development of successful strategies against specific neurological disorders by interfering with the astrocytic subpopulations.
Footnotes
Conflicts of interest: We declare no conflicts of interest.
Financial support: This work was supported by grants from Regione Campania (L.R. N.5 Bando 2003, to MP), the Italian Minister of Research and University (PRIN 2007, to MP; PRIN 2017, to GC and MP), UNIMIB (Progetto ID 2019-ATESP-0001 and Progetto ID 2018-CONV-0056, to AV).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by grants from Regione Campania (L.R. N.5 Bando 2003, to MP), the Italian Minister of Research and University (PRIN 2007, to MP; PRIN 2017, to GC and MP), UNIMIB (Progetto ID 2019-ATESP-0001 and Progetto ID 2018-CONV-0056, to AV).
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Acevedo-Arozena A, Kalmar B, Essa S, Ricketts T, Joyce P, Kent R, Rowe C, Parker A, Gray A, Hafezparast M, Thorpe JR, Greensmith L, Fisher EM. A comprehensive assessment of the SOD1G93A low-copy transgenic mouse, which models human amyotrophic lateral sclerosis. Dis Model Mech. 2011;4:686–700. doi: 10.1242/dmm.007237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen N, Lyons D. Glia as architects of central nervous system formation and function. Science. 2018;362:181–185. doi: 10.1126/science.aat0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson MA, Ao Y, Sofroniew MV. Heterogeneity of reactive astrocytes. Neurosci Lett. 2014;565:23–29. doi: 10.1016/j.neulet.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araque A, Navarrete M. Glial cells in neuronal network function. Philos Trans R Soc B Biol Sci. 2010;365:2375–2381. doi: 10.1098/rstb.2009.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- 6.Batista CRA, Gomes GF, Candelario-Jalil E, Fiebich BL, de Oliveira ACP. Lipopolysaccharide-induced neuroinflammation as a bridge to understand neurodegeneration. Int J Mol Sci. 2019;20:2293. doi: 10.3390/ijms20092293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Begum G, Song S, Wang S, Zhao H, Bhuiyan MIH, Li E, Nepomuceno R, Ye Q, Sun M, Calderon MJ, Stolz DB, St Croix C, Watkins SC, Chen Y, He P, Shull GE, Sun D. Selective knockout of astrocytic Na+/H+ exchanger isoform 1 reduces astrogliosis, BBB damage, infarction, and improves neurological function after ischemic stroke. Glia. 2018;66:126–144. doi: 10.1002/glia.23232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boisvert MM, Erikson GA, Shokhirev MN, Allen NJ. The aging astrocyte transcriptome from multiple regions of the mouse brain. Cell Rep. 2018;22:269–285. doi: 10.1016/j.celrep.2017.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chai H, Diaz-Castro B, Shigetomi E, Monte E, Octeau JC, Yu X, Cohn W, Rajendran PS, Vondriska TM, Whitelegge JP, Coppola G, Khakh BS. Neural circuit-specialized astrocytes: transcriptomic, proteomic, morphological and functional evidence. Neuron. 2017;95:531–549.e9. doi: 10.1016/j.neuron.2017.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chun H, An H, Lim J, Woo J, Lee J, Ryu H, Lee CJ. Astrocytic proBDNF and tonic GABA distinguish active versus reactive astrocytes in hippocampus. Exp Neurobiol. 2018;27:155–170. doi: 10.5607/en.2018.27.3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cirillo G, Bianco MR, Colangelo AM, Cavaliere C, Daniele DL, Zaccaro L, Alberghina L, Papa M. Reactive astrocytosis-induced perturbation of synaptic homeostasis is restored by nerve growth factor. Neurobiol Dis. 2011;41:630–639. doi: 10.1016/j.nbd.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Cirillo G, Cirillo M, Panetsos F, Virtuoso A, Papa M. Selective vulnerability of basal ganglia: Insights into the mechanisms of bilateral striatal necrosis. J Neuropathol Exp Neurol. 2019;78:123–129. doi: 10.1093/jnen/nly123. [DOI] [PubMed] [Google Scholar]
- 13.Cirillo G, Colangelo AM, Berbenni M, Ippolito VM, De Luca C, Verdesca F, Savarese L, Alberghina L, Maggio N, Papa M. Purinergic modulation of spinal neuroglial maladaptive plasticity following peripheral nerve injury. Mol Neurobiol. 2015;52:1440–1457. doi: 10.1007/s12035-014-8943-y. [DOI] [PubMed] [Google Scholar]
- 14.Clark DPQ, Perreau VM, Shultz SR, Brady RD, Lei E, Dixit S, Taylor JM, Beart PM, Boon WC. Inflammation in traumatic brain injury: roles for toxic A1 astrocytes and microglial–astrocytic crosstalk. Neurochem Res. 2019;44:1410–1424. doi: 10.1007/s11064-019-02721-8. [DOI] [PubMed] [Google Scholar]
- 15.Clarke LE, Liddelow SA, Chakraborty C, Münch AE, Heiman M, Barres BA. Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci U S A. 2018;115:E1896–1905. doi: 10.1073/pnas.1800165115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cragnolini AB, Montenegro G, Friedman WJ, Mascó DH. Brain-region specific responses of astrocytes to an in vitro injury and neurotrophins. Mol Cell Neurosci. 2018;88:240–248. doi: 10.1016/j.mcn.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Cuevas-Diaz Duran R, Wang CY, Zheng H, Deneen B, Wu JQ. Brain region-specific gene signatures revealed by distinct astrocyte subpopulations unveil links to glioma and neurodegenerative diseases. eNeuro. 2019 doi: 10.1523/ENEURO.0288-18.2019. doi: 10.1523/ENEURO.0288-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawson TM, Mandir AS, Lee MK. Animal models of PD: Pieces of the same puzzle? Neuron. 2002;35:219–222. doi: 10.1016/s0896-6273(02)00780-8. [DOI] [PubMed] [Google Scholar]
- 19.De Luca C, Colangelo AM, Alberghina L, Papa M. Neuro-immune hemostasis: homeostasis and diseases in the central nervous system. Front Cell Neurosci. 2018;12:459. doi: 10.3389/fncel.2018.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dityatev A, Rusakov DA. Molecular signals of plasticity at the tetrapartite synapse. Curr Opin Neurobiol. 2011;21:353–359. doi: 10.1016/j.conb.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Double DL, Reyes R, Werry WL, Halliday HM. Selective cell death in neurodegeneration: Why are some neurons spared in vulnerable regions? Prog Neurobiol. 2010;92:316–329. doi: 10.1016/j.pneurobio.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: Relevance to Parkinson’s disease. J Neurochem. 2002;81:1285–1297. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- 23.Hanisch UK, Kettenmann H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 24.Herculano-Houzel S. The glia/neuron ratio: How it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia. 2014;62:1377–1391. doi: 10.1002/glia.22683. [DOI] [PubMed] [Google Scholar]
- 25.Hirsch E, Graybiel AM, Agid YA. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature. 1988;334:345–348. doi: 10.1038/334345a0. [DOI] [PubMed] [Google Scholar]
- 26.Jäkel S, Dimou L. Glial cells and their function in the adult brain: a journey through the history of their ablation. Front Cell Neurosci. 2017;11:24. doi: 10.3389/fncel.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaminsky N, Bihari O, Kanner S, Barzilai A. Connecting malfunctioning glial cells and brain degenerative disorders. Genomics, Proteomics Bioinforma. 2016;14:155–165. doi: 10.1016/j.gpb.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khakh BS. Astrocyte-neuron interactions in the striatum: insights on identity, form, and function. Trends Neurosci. 2019 doi: 10.1016/j.tins.2019.06.003. doi: 10.1016/j.tins.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li K, Li J, Zheng J, Qin S. Reactive astrocytes in neurodegenerative diseases. Aging Dis. 2019;10:664. doi: 10.14336/AD.2018.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lian XY, Stringer JL. Astrocytes contribute to regulation of extracellular calcium and potassium in the rat cerebral cortex during spreading depression. Brain Res. 2004;1012:177–184. doi: 10.1016/j.brainres.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liddelow SA, Barres BA. Reactive astrocytes: production, function, and therapeutic potential. Immunity. 2017;46:957–967. doi: 10.1016/j.immuni.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Magistretti PJ, Allaman I. Lactate in the brain: From metabolic end-product to signalling molecule. Nat Rev Neurosci. 2018;19:235–249. doi: 10.1038/nrn.2018.19. [DOI] [PubMed] [Google Scholar]
- 34.Matias I, Morgado J, Gomes FCA. Astrocyte heterogeneity: impact to brain aging and disease. Front Aging Neurosci. 2019;11:59. doi: 10.3389/fnagi.2019.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minati L, Edginton T, Grazia Bruzzone M, Giaccone G. Current concepts in alzheimer’s disease: A multidisciplinary review. Am J Alzheimers Dis Other Demen. 2009;24:95–121. doi: 10.1177/1533317508328602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nash B, Ioannidou K, Barnett SC. Astrocyte phenotypes and their relationship to myelination. J Anat. 2011;219:44–52. doi: 10.1111/j.1469-7580.2010.01330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med. 2006;12:829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- 38.Oksanen M, Lehtonen S, Jaronen M, Goldsteins G, Hämäläinen RH, Koistinaho J. Astrocyte alterations in neurodegenerative pathologies and their modeling in human induced pluripotent stem cell platforms. Cell Mol Life Sci. 2019;76:2739–2760. doi: 10.1007/s00018-019-03111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ota Y, Zanetti AT, Hallock RM. The role of astrocytes in the regulation of synaptic plasticity and memory formation. Neural Plast. 2013;2013:1–11. doi: 10.1155/2013/185463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papa M, DeLuca C, Petta F, Alberghina L, Cirillo G. Astrocyte-neuron interplay in maladaptive plasticity. Neurosci Biobehav Rev. 2014;42:35–54. doi: 10.1016/j.neubiorev.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Perea G, Sur M, Araque A. Neuron-glia networks: integral gear of brain function. Front Cell Neurosci. 2014;8:378. doi: 10.3389/fncel.2014.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pérez Ortiz JM, Orr HT. Spinocerebellar ataxia type 1: Molecular mechanisms of neurodegeneration and preclinical studies. Adv Exp Med Biol. 2018;1049:135–145. doi: 10.1007/978-3-319-71779-1_6. [DOI] [PubMed] [Google Scholar]
- 43.Polyzos AA, Lee DY, Datta R, Hauser M, Budworth H, Holt A, Mihalik S, Goldschmidt P, Frankel K, Trego K, Bennett MJ, Vockley J, Xu K, Gratton E, McMurray CT. Metabolic reprogramming in astrocytes distinguishes region-specific neuronal susceptibility in Huntington mice. Cell Metab. 2019;29:1258–1273.e11. doi: 10.1016/j.cmet.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reeve AK, Grady JP, Cosgrave EM, Bennison E, Chen C, Hepplewhite PD, Morris CM. Mitochondrial dysfunction within the synapses of substantia nigra neurons in Parkinson’s disease. NPJ Park Dis. 2018;4:9. doi: 10.1038/s41531-018-0044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rikani AA, Choudhry Z, Choudhry AM, Rizvi N, Ikram H, Mobassarah NJ, Tulli S. The mechanism of degeeeration of striatal neuronal subtypes in Huntington disease. Ann Neurosci. 2014;21:112–114. doi: 10.5214/ans.0972.7531.210308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez-Vieitez E, Ni R, Gulyás B, Tóth M, Häggkvist J, Halldin C, Voytenko L, Marutle A, Nordberg A. Astrocytosis precedes amyloid plaque deposition in Alzheimer APPswe transgenic mouse brain: a correlative positron emission tomography and in vitro imaging study. Eur J Nucl Med Mol Imaging. 2015;42:1119–1132. doi: 10.1007/s00259-015-3047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ross GW, Petrovitch H, Abbott RD, Nelson J, Markesbery W, Davis D, Hardman J, Launer L, Masaki K, Tanner CM, White LR. Parkinsonian signs and substantia nigra neuron density in decendents elders without PD. Ann Neurol. 2004;56:532–539. doi: 10.1002/ana.20226. [DOI] [PubMed] [Google Scholar]
- 48.Ruminot I, Schmälzle J, Leyton B, Barros LF, Deitmer JW. Tight coupling of astrocyte energy metabolism to synaptic activity revealed by genetically encoded FRET nanosensors in hippocampal tissue. J Cereb Blood Flow Metab. 2019;39:513–523. doi: 10.1177/0271678X17737012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silbereis JC, Pochareddy S, Zhu Y, Li M, Sestan N. The Cellular and molecular landscapes of the developing human central nervous system. Neuron. 2016;89:248. doi: 10.1016/j.neuron.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sofroniew M V, Vinters H V. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang Y, Le W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol. 2016;53:1181–1194. doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- 52.Verkhratsky A, Nedergaard M. The homeostatic astroglia emerges from evolutionary specialization of neural cells. Philos Trans R Soc B Biol Sci. 2016;371:20150428. doi: 10.1098/rstb.2015.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Voloboueva LA, Suh SW, Swanson RA, Giffard RG. Inhibition of mitochondrial function in astrocytes: Implications for neuroprotection. J Neurochem. 2007;102:1383–1394. doi: 10.1111/j.1471-4159.2007.4634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang HC, Cheng SJ. The syndrome of acute bilateral basal ganglia lesions in diabetic uremic patients. J Neurol. 2003;250:948–955. doi: 10.1007/s00415-003-1122-0. [DOI] [PubMed] [Google Scholar]
- 55.Wang M, Roussos P, McKenzie A, Zhou X, Kajiwara Y, Brennand KJ, De Luca GC, Crary JF, Casaccia P, Buxbaum JD, Ehrlich M, Gandy S, Goate A, Katsel P, Schadt E, Haroutunian V, Zhang B. Integrative network analysis of nineteen brain regions identifies molecular signatures and networks underlying selective regional vulnerability to Alzheimer’s disease. Genome Med. 2016;8:104. doi: 10.1186/s13073-016-0355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X, Michaelis E. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang YX, Wood NW, Latchman DS. Molecular basis of Parkinson’s disease. Neuroreport. 2009;20:150–156. doi: 10.1097/WNR.0b013e32831c50df. [DOI] [PubMed] [Google Scholar]
- 58.Yun SP, Kam TI, Panicker N, Kim S, Oh Y, Park JS, Kwon SH, Park YJ, Karuppagounder SS, Park H, Kim S, Oh N, Kim NA, Lee S, Brahmachari S, Mao X, Lee JH, Kumar M, An D, Kang SU, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med. 2018;24:931–938. doi: 10.1038/s41591-018-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou B, Zuo YX, Jiang RT. Astrocyte morphology: Diversity, plasticity, and role in neurological diseases. CNS Neurosci Ther. 2019;25:665–673. doi: 10.1111/cns.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]