Abstract
Alzheimer’s disease cannot be cured as of yet. Our current understanding on the causes of Alzheimer’s disease is limited. To develop treatments, experimental models that represent a particular cellular phase of the disease and more rigorous scrutiny of the cellular pathological mechanisms are crucial. In recent years, Alzheimer’s disease research underwent a paradigm shift. According to this tendency, Alzheimer’s disease is increasingly being conceived of a disease where not only neurons but also multiple cell types synchronously partake to manifest the pathology. Knowledge on every cell type adds an alternative approach and hope for the efforts towards the treatment. Neural stem cells and their neurogenic ability are making an appearance as a new aspect of the disease manifestation based on the recent findings that neurogenesis reduces dramatically in Alzheimer’s disease patients compared to healthy individuals. Therefore, understanding how neural stem cells can form new neurons in Alzheimer’s disease brains holds an immense potential for clinics. However, this provocative idea requires further evidence and tools for investigation. Recently, single cell sequencing appeared as a revolutionary tool to understand cellular programs in unprecedented resolution and it will undoubtedly facilitate comprehensive investigation of different cell types in Alzheimer’s disease. In this mini-review, we will touch upon recent studies that use single cell sequencing for investigating cellular response in Alzheimer’s disease and some consideration pertaining to the utilization of neural regeneration for Alzheimer’s disease research.
Keywords: Alzheimer's disease, mouse, neural regeneration, neural stem cell, neurogenesis, neuron, single cell sequencing, zebrafish
The Dilemma of Alzheimer’s Disease
Alzheimer disease (AD) is a progressive neurodegeneration that affects millions of people worldwide, yet has no effective treatment. Two major hallmarks of AD are amyloid plaques and neurofibrillary tangles formed by amyloid-beta42 (Aβ42) and microtubule-associated protein Tau, respectively (Lewis et al., 2000; Carter and Lippa, 2001; Hardy and Selkoe, 2002; Iqbal et al., 2005; Selkoe and Hardy, 2016). Widely articulated amyloid cascade hypothesis postulated that Aβ42 is the initiator of AD pathology, while subsequent inflammation and aggregation of Tau exacerbates the progression of the disease (Selkoe and Hardy, 2016). Based on amyloid cascade hypothesis, many clinical campaigns aimed at decreasing the production of Aβ42 or increasing its clearance (Ji et al., 2001; Tanzi et al., 2004; Schneider et al., 2014; Wisniewski and Goni, 2015). Several of the drugs or immunization protocols against amyloid were indeed able to clear Aβ in human brains but did not lead to cognitive improvement (Cummings et al., 2016; Mehta et al., 2017; Nicoll et al., 2019). A heated debate over the validity of amyloid toxicity hypothesis is prevailing. For us, it is a plausible hypothesis that Aβ42 might constitute an early pathogenic mechanism that initiates the disease at an age when we still cannot diagnose the pathology (De Strooper and Karran, 2016; Selkoe, 2019). More proximal mechanisms such as tau pathology, chronic inflammation and involvement of other cell types (e.g., neural stem cells (NSCs), oligodendrocytes and vascular components) might kick in later, potentiate the cellular phase of the disease, and cause the actual onset-related symptoms such as synaptic degeneration (Heneka et al., 2015; De Strooper and Karran, 2016; Dzamba et al., 2016; Tincer et al., 2016). Bibliography was searched in public database PubMed (www.pubmed.gov). The search was performed between May 2019 and August 2019.
Is Reduced Neurogenesis a Cause for Alzheimer’s Disease?
Regardless of how the disease starts and progresses, concrete cellular pathologies are widely observed in AD patients, such as the synaptic degeneration, chronic inflammation, vascular changes, reduced NSC plasticity and neurogenesis (Scheltens et al., 2016). Here, we would like to emphasize the neurogenesis aspect, which is still quite controversial as to whether it could be a potential intervention step towards the treatment of AD (Kizil and Bhattarai, 2018; Choi and Tanzi, 2019). It is a simple hypothesis that if neurons die in AD, and if we bring these cells back, we may restore the cognitive capacity (Ziabreva et al., 2006; Rodriguez and Verkhratsky, 2011). However, the reality is admittedly more complicated. Does neurogenesis take place in adult human brains and whether it is related to cognitive ability? Even if there is neurogenesis, can human brains form the neuronal types that are affected by AD, as the hippocampal neurogenesis can generate only a certain subset of neurons? Can human NSCs be made proliferative without causing tumors and can proliferating NSCs be directed into appropriate neuronal fates? What will be the functional consequence of forming new circuitries with new neurons in human brains? These questions clearly stem from valid skepticism; yet, we cannot know without trying whether the answers will be failure or success.
In humans, the adult neurogenesis has been challenged recently (Arellano et al., 2018; Paredes et al., 2018; Sorrells et al., 2018); yet, several studies proposed that human brains generate new neurons (Boldrini et al., 2018; Kempermann et al., 2018; Lima and Gomes-Leal, 2019; Moreno-Jimenez et al., 2019; Tobin et al., 2019), neurogenesis reduces dramatically in AD patients (Moreno-Jimenez et al., 2019; Tobin et al., 2019), and increased neurogenesis in animal models and humanized three-dimensional systems of AD can counteract the pathological outcomes (Bhattarai et al., 2016; Choi et al., 2016, 2018; Papadimitriou et al., 2018). These studies propose that malfunctioning neurogenesis in the brain could be one of the causes of complex neurodegenerative diseases and might lead to cognitive decline. Therefore, restoring the neurogenesis by increasing the proliferation and neurogenic outcome of NSCs would be worth to investigate as alternative therapeutic interventions.
Single-Cell Sequencing: a Game Changer
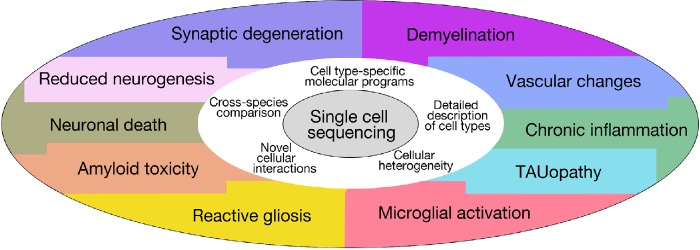
Investigation of individual cell types and their involvement in manifestation of the complex symptoms of AD is an interesting route for research, and this path is facilitated by recent developments in single cell sequencing technology that uncovers the previously unknown heterogeneity and molecular response of various cell populations during the course of AD (Figure 1). For instance, the response of the immune system to AD has been widely studied at singe cell resolution (Keren-Shaul et al., 2017; Mathys et al., 2017; Sala Frigerio et al., 2019). Keren-Shaul et al. (2017) found that the transition from homeostatic state to a new microglia state, disease-associated microglia, was achieved sequentially through Trem2-dependent and Trem2-independent pathways. In another study, the transition between microglial states during the progression of the disease was investigated using CK-p25 mouse model and transcriptional profiling (Mathys et al., 2017). During the initial stages of microglial response, the genes associated with cell proliferation are over-expressed; as microglia incorporated EdU and increased their numbers in the hippocampus. In the mid and late stage, interferon signaling is activated in the hippocampus. This finding is similar to another study (Sala Frigerio et al., 2019), where a cell type called “activated response microglia” in the hippocampus and the cortex of the mouse brains with AD was defined. This work proposed that the response in activated response microglias constitute a converging point for AD risk factors (Sala Frigerio et al., 2019).
Figure 1.
Schematic representation of the uses of single cell sequencing for Alzheimer’s disease research and its cellular pathological hallmarks.
The single-cell sequencing datasets of microglia further delineate the transition of microglial states in temporal conjunction with the progression of neurodegeneration in the animal models used. The two important conclusion from these studies are (1) the mechanisms of the activation of microglia, and stage-specific subsets of microglia during neurodegeneration are independent of the used mouse models, (2) microglial dynamics and gene expression profiles could be used to choose whether an experimental model is realistically reminiscent of human AD. A detailed comparison between microglial activation states can give further clues on the molecular mechanisms of microglial activation and its involvement in the disease progression in various models that are representing quite diverse pathological aspects of AD.
A recent study used single-nucleus RNA-Seq for the first time to comprehensively analyze gene expression in post-mortem human cells from the frontal cortex of AD and non-AD males and females (Mathys et al., 2019). This study opens a new but more comprehensive way to look AD. First of all, compared to bulk RNA-seq that use a mixture of cells, the single-nucleus RNA-Seq can detect the gene expression differences at single-cell with a higher resolution. Moreover, by comparing the AD patients to age and sex matched controls, it has been shown that disease-associated transcriptional changes at early stages of AD are cell type specific. However, the transcriptional changes at late stages of AD were common across cell types and mainly represent the global stress response.
Neural Regeneration: Can It Counteract Alzheimer’s Disease?
The studies from mouse and humans convincingly demonstrated that understanding the contribution of every subtype or physiological state of cells to the onset and progression of AD will help elucidate particular cellular contributions to the complex makeup of the disease. We believe that all possible aspect of AD must be considered and investigated on the road to therapies because various cell types, when malfunctioning, contribute to the exacerbation of AD symptoms in time. Neural stem cells and their neurogenic response is no exception to this statement (Gage and Temple, 2013; Kizil and Bhattarai, 2018; Tincer et al., 2016). However, we see an inherent problem in pursuing the ways to “regenerate” a neural circuit in an organism (e.g., mouse or human) where regeneration is historically defined as “poor” (Goss, 1991; Tanaka and Ferretti, 2009). Maybe understanding how nature endowed some animals to efficiently regenerate neurons through neural stem cell plasticity could help us to decipher candidate mechanisms by which we may tweak mammalian brains to boost regenerative outcome. Definitely, this provocative hypothesis requires experimental models that would (1) mimic some particular pathological symptoms of AD, (2) react to those pathologies with increased neural stem cell plasticity and neurogenesis, and (3) provide developed molecular biology and genetic tools. We believe that the teleost zebrafish offers an excellent opportunity to tackle the NSC response to AD and can furnish us with natural candidates by which we might develop “regenerative” strategies or identify novel drug targets for humans. Recently, we generated a zebrafish model of amyloid toxicity where cell death, synaptic degeneration, inflammation and cognitive decline were observed after aggregation of human Aβ42 (Bhattarai et al., 2016). This model was also used successfully to validate the efficacy of the drugs that counteract the decline in synaptic integrity (Reinhardt et al., 2019). A neuroinflammatory crosstalk mediated by interleukin-4 enhanced the neurogenic outcome independent of aging (Bhattarai et al., 2017). This mechanism is sufficient to restore neurogenic ability of human NSCs in a three-dimensional culture of amyloid toxicity model (Papadimitriou et al., 2018). We used this zebrafish amyloid toxicity model to further analyze the NSC response at single-cell level (Cosacak et al., 2019), and observed that only particular subtypes of NSCs respond to amyloid toxicity and can form new neurons by activating certain cellular interactions that do not prevail in homeostatic conditions. This single cell sequencing approach also helped to design hypothesis that helped elucidating the role of individual cell types in various diseases such as epilepsy (Diaz Verdugo et al., 2019). These findings suggest that uncovering novel mechanisms in experimental models such as zebrafish can be used as a starting point to study the cellular phase of AD.
Stem-cell heterogeneity in mouse hippocampus (Shin et al., 2015) and sub-ventricular zone (Dulken et al., 2017; Llorens-Bobadilla et al., 2015) were studied; however, the NSC response in AD in mouse and human at single-cell resolution was not investigated in detail. As specific cells respond to amyloid toxicity in zebrafish, it would be interesting to search the “responsive” stem cells in mouse and human. A recent study also supports the importance of finding the homologous cell types in different model organisms to elucidate the universal role of a particular cell type (Hodge et al. 2019). An intriguing question is whether the “regenerative” zebrafish NSCs also exist in humans and whether the molecular programs underlying the regenerative response of zebrafish cells could coax the non-regenerative NSCs into neurogenic state. There are clearly many stages where this approach may fail, but what if it does not?
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: This work was supported by Helmholtz Association (Helmholtz Young Investigator Award), Deutsche Forschungsgemeinschaft (DFG), German Center for Neurodegenerative Diseases (DZNE), and TU Dresden (all to CK).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by Helmholtz Association (Helmholtz Young Investigator Award), Deutsche Forschungsgemeinschaft (DFG), German Center for Neurodegenerative Diseases (DZNE), and TU Dresden (all to CK).
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Arellano JI, Harding B, Thomas JL. Adult human hippocampus: no new neurons in sight. Cereb Cortex. 2018;28:2479–2481. doi: 10.1093/cercor/bhy106. [DOI] [PubMed] [Google Scholar]
- 2.Bhattarai P, Thomas AK, Cosacak MI, Papadimitriou C, Mashkaryan V, Froc C, Reinhardt S, Kurth T, Dahl A, Zhang Y, Kizil C. IL4/STAT6 signaling activates neural stem cell proliferation and neurogenesis upon Amyloid-β42 aggregation in adult zebrafish brain. Cell Rep. 2016;17:941–948. doi: 10.1016/j.celrep.2016.09.075. [DOI] [PubMed] [Google Scholar]
- 3.Bhattarai P, Thomas AK, Zhang Y, Kizil C. The effects of aging on Amyloid-β42-induced neurodegeneration and regeneration in adult zebrafish brain. Neurogenesis (Austin) 2017;4:e1322666. doi: 10.1080/23262133.2017.1322666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, Rosoklija GB, Stankov A, Arango V, Dwork AJ, Hen R, Mann JJ. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018;22:589–599.e585. doi: 10.1016/j.stem.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter J, Lippa CF. Beta-amyloid, neuronal death and Alzheimer’s disease. Curr Mol Med. 2001;1:733–737. doi: 10.2174/1566524013363177. [DOI] [PubMed] [Google Scholar]
- 6.Choi SH, Bylykbashi E, Chatila ZK, Lee SW, Pulli B, Clemenson GD, Kim E, Rompala A, Oram MK, Asselin C, et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science. 2018 doi: 10.1126/science.aan8821. doi:10.1126/science. aan8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi SH, Kim YH, Quinti L, Tanzi RE, Kim DY. 3D culture models of Alzheimer’s disease: a road map to a “cure-in-a-dish”. Mol Neurodegener. 2016;11:75. doi: 10.1186/s13024-016-0139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi SH, Tanzi RE. Is Alzheimer’s disease a neurogenesis disorder? Cell Stem Cell. 2019;25:7–8. doi: 10.1016/j.stem.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Cosacak MI, Bhattarai P, Reinhardt S, Petzold A, Dahl A, Zhang Y, Kizil C. Single-cell transcriptomics analyses of neural stem cell heterogeneity and contextual plasticity in a zebrafish brain model of amyloid toxicity. Cell Rep. 2019;27:1307–1318.e1303. doi: 10.1016/j.celrep.2019.03.090. [DOI] [PubMed] [Google Scholar]
- 10.Cummings J, Aisen PS, DuBois B, Frölich L, Jack CR, Jr, Jones RW, Morris JC, Raskin J, Dowsett SA, Scheltens P. Drug development in Alzheimer’s disease: the path to 2025. Alzheimers Res Ther. 2016;8:39. doi: 10.1186/s13195-016-0207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Strooper B, Karran E. The cellular phase of Alzheimer’s disease. Cell. 2016;164:603–615. doi: 10.1016/j.cell.2015.12.056. [DOI] [PubMed] [Google Scholar]
- 12.Diaz Verdugo C, Myren-Svelstad S, Aydin E, Van Hoeymissen E, Deneubourg C, Vanderhaeghe S, Vancraeynest J, Pelgrims R, Cosacak MI, Muto A, Kizil C, Kawakami K, Jurisch-Yaksi N, Yaksi E. Glia-neuron interactions underlie state transitions to generalized seizures. Nat Commun. 2019;10:3830. doi: 10.1038/s41467-019-11739-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dulken BW, Leeman DS, Boutet SC, Hebestreit K, Brunet A. Single-cell transcriptomic analysis defines heterogeneity and transcriptional dynamics in the adult neural stem cell lineage. Cell Rep. 2017;18:777–790. doi: 10.1016/j.celrep.2016.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dzamba D, Harantova L, Butenko O, Anderova M. Glial cells - the key elements of Alzheimer´s disease. Curr Alzheimer Res. 2016;13:894–911. doi: 10.2174/1567205013666160129095924. [DOI] [PubMed] [Google Scholar]
- 15.Gage FH, Temple S. Neural stem cells: generating and regenerating the brain. Neuron. 2013;80:588–601. doi: 10.1016/j.neuron.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 16.Goss RJ. Cambridge, UK: Cambridge University Press; 1991. The natural history (and mystery) of regeneration. [Google Scholar]
- 17.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 18.Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodge RD, Bakken TE, Miller JA, Smith KA, Barkan ER, Graybuck LT, Close JL, Long B, Johansen N, Penn O, Yao Z, Eggermont J, Höllt T, Levi BP, Shehata SI, Aevermann B, Beller A, Bertagnolli D, Brouner K, Casper T, et al. Conserved cell types with divergent features in human versus mouse cortex. Nature. 2019 doi: 10.1038/s41586-019-1506-7. doi: 10.1038/s41586-019-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iqbal K, Alonso Adel C, Chen S, Chohan MO, El-Akkad E, Gong CX, Khatoon S, Li B, Liu F, Rahman A, Tanimukai H, Grundke-Iqbal I. Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta. 2005;1739:198–210. doi: 10.1016/j.bbadis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Ji Y, Permanne B, Sigurdsson EM, Holtzman DM, Wisniewski T. Amyloid beta40/42 clearance across the blood-brain barrier following intra-ventricular injections in wild-type, apoE knock-out and human apoE3 or E4 expressing transgenic mice. J Alzheimers Dis. 2001;3:23–30. doi: 10.3233/jad-2001-3105. [DOI] [PubMed] [Google Scholar]
- 22.Kempermann G, Gage FH, Aigner L, Song H, Curtis MA, Thuret S, Kuhn HG, Jessberger S, Frankland PW, Cameron HA, Gould E, Hen R, Abrous DN, Toni N, Schinder AF, Zhao X, Lucassen PJ, Frisén J. Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell. 2018;23:25–30. doi: 10.1016/j.stem.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M, Amit I. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell. 2017;169:1276–1290.e1217. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 24.Kizil C, Bhattarai P. Is Alzheimer’s also a stem cell disease? –The zebrafish perspective. Front Cell Dev Biol. 2018;6:159. doi: 10.3389/fcell.2018.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Paul Murphy M, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- 26.Lima SMA, Gomes-Leal W. Neurogenesis in the hippocampus of adult humans: controversy “fixed” at last. Neural Regen Res. 2019;14:1917–1918. doi: 10.4103/1673-5374.259616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Llorens-Bobadilla E, Zhao S, Baser A, Saiz-Castro G, Zwadlo K, Martin-Villalba A. Single-cell transcriptomics reveals a population of dormant neural stem cells that become activated upon brain injury. Cell Stem Cell. 2015;17:329–340. doi: 10.1016/j.stem.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Mathys H, Adaikkan C, Gao F, Young JZ, Manet E, Hemberg M, De Jager PL, Ransohoff RM, Regev A, Tsai LH. Temporal tracking of microglia activation in neurodegeneration at single-cell resolution. Cell Rep. 2017;21:366–380. doi: 10.1016/j.celrep.2017.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathys H, Davila-Velderrain J, Peng Z, Gao F, Mohammadi S, Young JZ, Menon M, He L, Abdurrob F, Jiang X, Martorell AJ, Ransohoff RM, Hafler BP, Bennett DA, Kellis M, Tsai LH. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature. 2019;570:332–337. doi: 10.1038/s41586-019-1195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta D, Jackson R, Paul G, Shi J, Sabbagh M. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010-2015. Expert Opin Investig Drugs. 2017;26:735–739. doi: 10.1080/13543784.2017.1323868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moreno-Jiménez EP, Flor-García M, Terreros-Roncal J, Rábano A, Cafini F, Pallas-Bazarra N, Ávila J, Llorens-Martín M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med. 2019;25:554–560. doi: 10.1038/s41591-019-0375-9. [DOI] [PubMed] [Google Scholar]
- 32.Nicoll JAR, Buckland GR, Harrison CH, Page A, Harris S, Love S, Neal JW, Holmes C, Boche D. Persistent neuropathological effects 14 years following amyloid-beta immunization in Alzheimer’s disease. Brain. 2019;142:2113–2126. doi: 10.1093/brain/awz142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papadimitriou C, Celikkaya H, Cosacak MI, Mashkaryan V, Bray L, Bhattarai P, Brandt K, Hollak H, Chen X, He S, Antos CL, Lin W, Thomas AK, Dahl A, Kurth T, Friedrichs J, Zhang Y, Freudenberg U, Werner C, Kizil C. 3D culture method for Alzheimer’s disease modeling reveals interleukin-4 rescues Abeta42-induced loss of human neural stem cell plasticity. Dev Cell. 2018;46:85–101,e108. doi: 10.1016/j.devcel.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Paredes MF, Sorrells SF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, James D, Mayer S, Chang J, Auguste KI, Chang EF, Gutierrez Martin AJ, Kriegstein AR, Mathern GW, Oldham MC, Huang EJ, Garcia-Verdugo JM, Yang Z, Alvarez-Buylla A. Does adult neurogenesis persist in the human hippocampus? Cell Stem Cell. 2018;23:780–781. doi: 10.1016/j.stem.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reinhardt L, Kordes S, Reinhardt P, Glatza M, Baumann M, Drexler HCA, Menninger S, Zischinsky G, Eickhoff J, Fröb C, Bhattarai P, Arulmozhivarman G, Marrone L, Janosch A, Adachi K, Stehling M, Anderson EN, Abo-Rady M, Bickle M, Pandey UB. Dual inhibition of GSK3beta and CDK5 protects the cytoskeleton of neurons from neuroinflammatory-mediated degeneration in vitro and in vivo. Stem Cell Reports. 2019;12:502–517. doi: 10.1016/j.stemcr.2019.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodríguez JJ, Verkhratsky A. Neurogenesis in Alzheimer’s disease. J Anat. 2011;219:78–89. doi: 10.1111/j.1469-7580.2011.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sala Frigerio C, Wolfs L, Fattorelli N, Thrupp N, Voytyuk I, Schmidt I, Mancuso R, Chen WT, Woodbury ME, Srivastava G, Möller T, Hudry E, Das S, Saido T, Karran E, Hyman B, Perry VH, Fiers M, De Strooper B. The major risk factors for Alzheimer’s disease: age, sex, and genes modulate the microglia response to abeta plaques. Cell Rep. 2019;27:1293–1306.e1296. doi: 10.1016/j.celrep.2019.03.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM. Alzheimer’s disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 39.Schneider LS, Mangialasche F, Andreasen N, Feldman H, Giacobini E, Jones R, Mantua V, Mecocci P, Pani L, Winblad B, Kivipelto M. Clinical trials and late-stage drug development for Alzheimer’s disease: an appraisal from 1984 to 2014. J Intern Med. 2014;275:251–283. doi: 10.1111/joim.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selkoe DJ. Alzheimer disease and aducanumab: adjusting our approach. Nat Rev Neurol. 2019;15:365–366. doi: 10.1038/s41582-019-0205-1. [DOI] [PubMed] [Google Scholar]
- 41.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shin J, Berg DA, Zhu Y, Shin JY, Song J, Bonaguidi MA, Enikolopov G, Nauen DW, Christian KM, Ming GL, Song H. Single-cell RNA-Seq with waterfall reveals molecular cascades underlying adult neurogenesis. Cell Stem Cell. 2015;17:360–372. doi: 10.1016/j.stem.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, James D, Mayer S, Chang J, Auguste K, Chang EF, Gutierrez AJ, Kriegstein AR, Mathern GW, Oldham MC, Huang EJ, Garcia-Verdugo JM, Yang Z, Alvarez-Buylla A. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555:377–381. doi: 10.1038/nature25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka EM, Ferretti P. Considering the evolution of regeneration in the central nervous system. Nat Rev Neurosci. 2009;10:713–723. doi: 10.1038/nrn2707. [DOI] [PubMed] [Google Scholar]
- 45.Tanzi RE, Moir RD, Wagner SL. Clearance of Alzheimer’s Abeta peptide: the many roads to perdition. Neuron. 2004;43:605–608. doi: 10.1016/j.neuron.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 46.Tincer G, Mashkaryan V, Bhattarai P, Kizil C. Neural stem/progenitor cells in Alzheimer’s disease. Yale J Biol Med. 2016;89:23–35. [PMC free article] [PubMed] [Google Scholar]
- 47.Tobin MK, Musaraca K, Disouky A, Shetti A, Bheri A, Honer WG, Kim N, Dawe RJ, Bennett DA, Arfanakis K, Lazarov O. Human hippocampal neurogenesis persists in aged adults and Alzheimer’s disease patients. Cell Stem Cell. 2019;24:974–982.e973. doi: 10.1016/j.stem.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wisniewski T, Goni F. Immunotherapeutic approaches for Alzheimer’s disease. Neuron. 2015;85:1162–1176. doi: 10.1016/j.neuron.2014.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ziabreva I, Perry E, Perry R, Minger SL, Ekonomou A, Przyborski S, Ballard C. Altered neurogenesis in Alzheimer’s disease. J Psychosom Res. 2006;61:311–316. doi: 10.1016/j.jpsychores.2006.07.017. [DOI] [PubMed] [Google Scholar]