Abstract
Alzheimer’s disease is one of the most frequent neurodegenerative diseases. This pathology is characterized by protein aggregates, mainly constituted by amyloid peptide and tau, leading to neuronal death and cognitive impairments. Drugs currently proposed to treat this pathology do not prevent neurodegenerative processes and are mainly symptomatic therapies. However, stilbenes presenting multiple pharmacological effects could be good potential therapeutic candidates. The aim of this review is to gather the more significant papers among the broad literature on this topic, concerning the beneficial effects of stilbenes (resveratrol derivatives) in animal models of Alzheimer’s disease. Indeed, numerous studies focus on cellular models, but an in vivo approach remains of primary importance since in animals (mice or rats, generally), bioavailability and metabolism are taken into account, which is not the case in in vitro studies. Furthermore, examination of memory ability is feasible in animal models, which strengthens the relevance of a compound with a view to future therapy in humans. This paper is addressed to any researcher who needs to study untested natural stilbenes or who wants to experiment the most effective natural stilbenes in largest animals or in humans. This review shows that resveratrol, the reference polyphenol, is largely studied and seems to have interesting properties on amyloid plaques, and cognitive impairment. However, some resveratrol derivatives such as gnetin C, trans-piceid, or astringin have never been tested on animals. Furthermore, pterostilbene is of particular interest, by its improvement of cognitive disorders and its neuroprotective role. It could be relevant to evaluate this molecule in clinical trials.
Keywords: Alzheimer's disease, amyloid, animal models, cognitive impairment, inflammation, natural stilbenes, neuroprotection, resveratrol, tau
Introduction
General presentation of natural stilbenes
In the last three decades, the interest in molecules of polyphenolic structure has increased markedly. Natural phenolic compounds are plant secondary metabolites, with two or more phenolic rings. In order to protect themselves, plants produce these phytochemicals in response to exogenous stimuli such as excessive heat or ultraviolet exposures, insect attacks, and infections caused by microorganisms (bacteria or fungus) (Quideau et al., 2011). More than 8000 different phenolic compounds have been identified in the vegetal world. Natural polyphenols are particularly concentrated in fruits, vegetables, in beverages such as chocolate, tea, red wine, or in olive oil (Bravo, 1998).
Due to their antioxidant properties (Fauconneau et al., 1997), they have received an increasing attention in the prevention of various pathologies associated with oxidative stress, such as cancer (Rodriguez-Garcia et al., 2019), cardiovascular diseases, aging (Silva et al., 2019) or in others pathologies such as autoimmune diseases (Khan et al., 2019), infectious diseases (Li et al., 2019) but also in neurodegenerative pathologies (Freyssin et al., 2018). Preventive effects of polyphenols are mainly due to their antioxidant activity, by scavenging free radicals, but recent lines of evidences suggest that, moreover, they can directly target have multiple signalling cascades involved in development of numerous pathologies (Sirerol et al., 2016).
Stilbenes constitutes an important group of non-flavonoid phytochemicals characterized by a 1,2-diphenylethylenenucleus (Riviere et al., 2012). Stilbenes are low molecular weight phenolics induced (phytoalexins) by biotic and abiotic stresses and act like antifungal compounds, enabling the plant to overcome pathogen attack (Bavaresco and Fregoni, 2001). There are more than 400 natural stilbenes (Shen et al., 2009), but they are observed only in a small and heterogeneous group of plants, including Vitis vinifera L., since stilbene synthase, the key enzyme involved in stilbene biosynthesis, is not ubiquitously expressed (Riviere et al., 2012).
Natural stilbenes are composed of resveratrol derivatives (Figure 1) and have been identified as trans-resveratrol (trans-3,4′,5-trihydroxistilbene), trans- and cis-piceid (trans- and cis-resveratrol 3-O-β-D-glucopyranoside), ε-viniferin (trans-resveratrol dimer), pterostilbene (trans-3,5-dimethoxy-4′-hydroxy-stilbene), piceatannol (trans-3,3′,4,5′-tetrahydroxy-stilbene) or astringinin, and pallidol (trans-resveratrol dimer) (Bavaresco et al., 2009).
Figure 1.
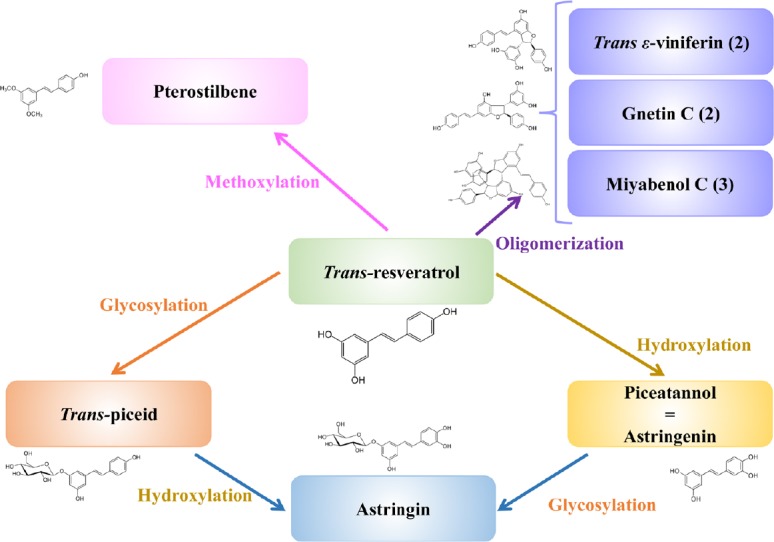
Natural stilbenes trans-resveratrol derivatives.
Alzheimer’s disease
Alzheimer’s disease (AD) is one of the most common neurodegenerative diseases. Around the world, it is estimated that there is one new case of dementia every 3 seconds (Patterson, 2018). Fifty million people worldwide were living with dementia in 2018 and this number is increasing rapidly in countries where people live longer. Indeed, this number could more than triple to 152 million in 2050 (Patterson, 2018). In AD, cerebral extracellular senile plaques and intraneuronal neurofibrillary tangles are two of the major histopathological lesions leading to the progression of the pathogenesis in this disease. Senile plaques are constituted by deposition of aggregated β amyloid (Aβ) peptides (Greenwald and Riek, 2010), mostly generated by amyloidogenic metabolism of amyloid precursor protein (APP) by the sequential activity of β- and γ secretases, β-sheet structure of Aβ leading to its aggregation. Rare familial AD are caused by a mutation in one of at least three genes, which code for presenilin 1 (PS1) and 2, two co-factors of γ secretases and for APP. Neurofibrillary tangles are composed by accumulation of hyperphosphorylated tau protein (Mietelska-Porowska et al., 2014). Moreover, these both hallmark proteins seem to present interactions and synergic effects in AD (Ittner and Gotz, 2011).
Resveratrol, one of the most studied and best known stilbene, has been associated with a wide range of pharmacological properties and is claimed to have numerous health functional properties (Thomasset et al., 2007; Szkudelska and Szkudelski, 2010, 2015; Petrovski et al., 2011), including in neuronal degenerative pathologies such as AD (Farooqui and Farooqui, 2009; Tellone et al., 2015).
This review focuses on trans-resveratrol and resveratrol derivatives, and their potential role in prevention and/or therapy specifically on one particularly worrying neurodegenerative disorder, AD, in animal models of this disease (Figure 2 and Table 1). These animal models are mainly either mice or rats but they are multiple. Some studies use transgenic mice expressing APP and/or PS1 with familial AD mutations. Other use mice, in which some symptoms of AD were induced by intracerebroventricular injection of Aβ or by bilateral injection of lipopolysaccharide (LPS) into the hippocampus or by intraperitoneal injection of LPS. Mention may also be made of models of sporadic AD, which are accelerated aging mice. Studies which used rats treat them by an injection of Aβ in their lateral ventricle, or by ovariectomy combined to treatment with D-galactose.
Figure 2.
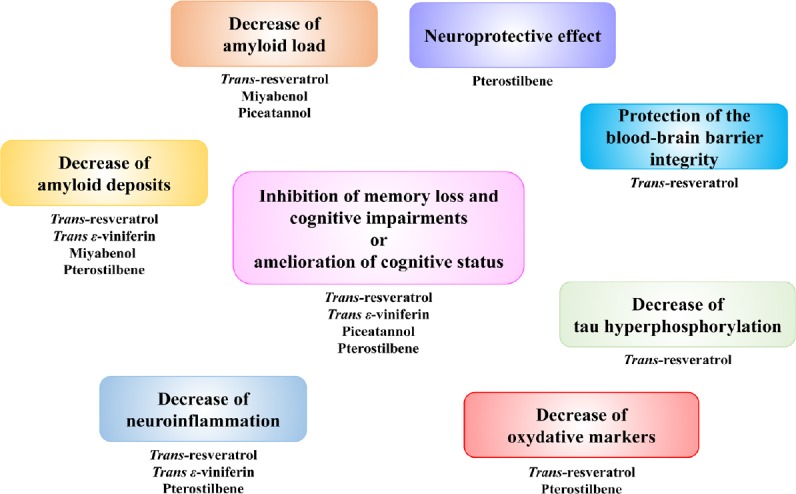
Beneficial effects of natural stilbenes.
Table 1.
Natural stilbenes effects in AD: in vivo studies cited in the paper
| Natural stilbenes | Research models | Treatments and doses Effects | References |
|---|---|---|---|
| Trans-resveratrol | Tg199589 mice: transgenic animals expressing APP695 with two familial AD mutations | Orally supplementation with trans-resveratrol at 300 mg/kg from 45 to 90 days. | Karuppagounder et al., 2009 |
| Decrease of plaque deposits, in particular in medial cortex, striatum and hypothalamus. | |||
| 15 week-old male APP/PS1 transgenic mice (B6C3-Tg(APPswe, PSEN1dE9) | Administration of diet supplemented with 0.35% trans-resveratrol during 15 weeks. | Vingtdeux et al., 2010; Capiralla et al., 2012 | |
| Lower amyloid deposition and microglial activation associated with cortical amyloid plaque formation. | |||
| SAMP8 mice (model of sporadic and age-related AD) | Administration of a supplemented with trans-resveratrol (1 g/kg), between 2 months of age and 9 months of age. | Porquet et al., 2013 | |
| Increase of life, activation of AMPK pathways and pro-survival routes (SIRT1). | |||
| Reduction of cognitive impairment. | |||
| Neuroprotective role by decreasing the amyloid burden and reducing tau hyperphosphorylation. | |||
| APP/PS1 mice | Dietary trans-resveratrol treatment. Absence of decrease plaque burden in these mice. Increase of GSK3-β phosphorylation, protein levels of transthyretin and drebrin. |
Varamini et al., 2014 | |
| Adult Sprague-Dawley rats, which are treated by an injection of Aβ25-35 in their lateral ventricle | Combination of the Aβ25–35 treatment with trans-resveratrol. Significant improvement in spatial memory. Reduction in the cellular levels of iNOS and lipid peroxidation and increase in the production of HO-1. |
Huang et al., 2011 | |
| Rat model of AD, established by ovariectomy combined injection of D-galactose (100 mg/kg) | Heart perfusion in vivo with trans-resveratrol at 20, 40 or 80 mg/kg. Decrease in the expression of GFAP at 40 and 80 mg/kg more important with the larger dose of resveratrol. |
Cheng et al., 2015 | |
| Decrease of the TNF-α levels for the three concentrations. | |||
| Rat model of AD, established by ovariectomy combined chronic treatment with D-galactose (one intraperitoneal injection per day of d-gal 100 mg/kg for 12 weeks) | Daily intragastric doses of 20, 40 and 80 mg/kg trans-resveratrol. Protection against spatial memory impairment, by decreasing oxidative stress. |
Zhao et al., 2012 | |
| Rat model of AD, established by ovariectomy combined chronic treatment with D-galactose | Chronic administration of trans-resveratrol at 20, 40 and 80 mg/kg. Decrease of the insoluble Ab42 level in hippocampus by decreasing the expression of NF-κB. Protection of the BBB integrity, by increasing the expression of Claudin-5 and decreasing RAGE and MMP-9 expressions. |
Zhao et al., 2015 | |
| Clinical study: mild to moderate AD patients | Treatment by trans-resveratrol (initially 500 mg once daily with dose escalation ending with 1000 mg twice daily) during 52 weeks. | Turner et al., 2015; Moussa et al., 2017 | |
| Passage of the BBB by resveratrol and its metabolites to exert their effects. | |||
| Safety and good tolerance of resveratrol. | |||
| Decrease of CSF Aβ42 and Aβ40 levels decline but increase of brain volume by resveratrol treatment | |||
| Modulation of neuro-inflammation and decrease of cognitive decline. | |||
| Trans ε-viniferin | Memory loss induced by intracerebroventricular injection with Ab25–35 in mice | Chronic treatment for 7 days with methanol extract (containing notably trans ε-viniferin) at the concentrations of 50 and 100 mg/kg per os. Inhibition of memory loss. |
Jeong et al., 2010 |
| Transgenic APPswePS1dE9 mice | Weekly intraperitoneal injection of trans ε-viniferin at the dose of 10 mg/kg or its vehicle from 3 to 6 months of age. | Caillaud et al., 2019 | |
| Decrease of amyloid deposits and inflammation in the brain of mice. | |||
| Gnetin C Miyabenol C |
Absence of published in vivo studies 12-month-old transgenic APP/PS1 mice |
Intracerebroventricular injection into the lateral ventricle for 3 days at the dose of 0.6 μg/g. | Hu et al., 2015 |
| Reduction of both sAPPβ and soluble Aβ42 and Aβ40 levels in the cortex and hippocampus. | |||
|
Trans-piceid Piceatannol = Astringenin |
Absence of published in vivo studies AD induced in adult male Swiss albino mice by unique intraperitoneal injection of LPS at the dose of 0.8 mg/kg |
Daily intraperitoneal injection of piceatannol at 2.5 mg/kg for 6 days. Amelioration of cognitive status and decrease of cerebral Aβ42 concentration. |
Hassaan et al., 2014 |
| Astringin | Absence of published studies | ||
| Pterostilbene | SAMP8 mice (model of sporadic and age-related AD) | Diet-achievable supplementation of resveratrol or pterostilbene during 2 months | Chang et al., 2012 |
| Improvement by pterostilbene of cognitive status in these mice and decreasing of cellular stress, inflammation and AD markers. | |||
| Learning and memory impairment and changes of microglia and neurons induced in male C57BL/6 mice by bilaterally intrahippocampal injection of LPS | Daily oral administration of pterostilbene at 20 or 40 mg/kg from 7 days before intrahippocampal administration of LPS. Decrease of cognitive disorders. |
Hou et al., 2014 | |
| Anti-inflammatory and neuroprotective role. |
AD: Alzheimer’s disease; Aβ: amyloid-β; AMPK: AMP-activated protein kinase; APP: amyloid precursor protein; BBB: blood-brain barrier; CSF: cerebrospinal fluid; GFAP: glial fibrillary acidic protein; GSK3: glycogen synthase kinase-3; HO-1: heme oxygenase-1; iNOS: induible nitric oxide synthase; LPS: lipopolysaccharide; MMP-9: matrix metalloproteinase 9; NF-κB: nuclear factor κB; RAGE: receptor for advanced glycation end products; sAPPβ: soluble β-fragment of amyloid precusor protein.
Search Strategy and Selection Criteria
Database: PubMed. Date: 1980 – August 2019. Eligibility criteria: reviews, in vivo studies, studies conducted on humans and animals and published in English. Keywords/keyterms: Stilbenes, Alzheimer’disease, animal models, in vivo, Trans-resveratrol, Trans ε-viniferin, Gnetin C, Miyabenol C, Trans-piceid, Piceatannol, Astringenin, Astringin, Pterostilbene.
Beneficial Effects of Natural Stilbenes in Alzheimer’s Disease
Trans-resveratrol
Most of studies concerning beneficial in vivo roles of stilbenes for AD concern trans-resveratrol, the reference polyphenol, largely quoted in the literature. The neuroprotective effects of this stilbene are mainly due to its capacity to 1) activate the signaling pathways implicated in cellular survival mediated by AMP-activated protein kinase (AMPK), phosphoinositide 3-kinase and Akt, 2) promote synaptic plasticity by extracellular signal-regulated kinase (ERK) 1/2, 3) inhibit pathways involved in apoptosis by decreasing caspase 3 and 12, Bax and cytochrome c expressions, 4) reduce amyloidogenesis and 5) enhance the clearance Aβ. Moreover, reseveratrol has 6) antioxidant and 7) anti- inflammatory actions (Cicero et al., 2019).
Trans-resveratrol (trans-3,4′,5-trihydroxystilbene) is a natural polyphenol, firstly insolated in 1940 and found in abundance in red wine. It is largely studied for its beneficial effects on the health, not only in AD but also in many other pathologies such as diabetes, obesity, and cancer. Only significant papers concerning in vivo effects of this stilbene for AD will be taken into account in this review.
Many studies showed that dietary supplementation of different AD model reduced some markers of this disease but results differ according to the studies.
One study evaluated effects of this supplementation on Tg199589 mice, transgenic animals expressing APP 695 with two familial AD mutations. These AD mice were orally supplemented with trans-resveratrol at 300 mg/kg from 45 to 90 days. After this treatment, neither trans-resveratrol nor its metabolites were detectable in brain. However, this supplementation induced decrease of plaque deposits, in particular in medial cortex, striatum and hypothalamus, without detectable activation of silent mating type information regulation 2 homolog (Sirtuin) 1, encoded by the SIRT1 gene, that deacetylates proteins that contribute to cellular regulation (Karuppagounder et al., 2009).
Orally administration of trans-resveratrol was also tested on 15 week-old male APP/PS1 transgenic mice (B6C3-Tg(APPswe, PSEN1dE9), a mouse model of cerebral amyloid deposition. After an administration of diet supplemented with 0.35% trans-resveratrol during 15 weeks, it was shown a reduction of Aβ levels and amyloid deposition in the cerebral cortex, quantified by ELISA and immunofluorescence respectively (Vingtdeux et al., 2010). Moreover, a lower microglial activation, evaluated by ionized calcium binding adaptor molecule 1 (Iba-1) labelling, associated with cortical amyloid plaque formation, was demonstrated, suggesting anti-inflammatory effect of this polyphenol (Capiralla et al., 2012).
In other study, trans-resveratrol was orally administrated in the SAMP8 mice, which are a model of accelerated aging and consequently a model of sporadic and age-related AD. For this study, these mice received a diet supplemented with trans-resveratrol (1 g/kg), between 2 months of age and 9 months of age. This long-term dietary treatment has extended the average life expectancy and maximum shelf life in SAMP8. Moreover, it activated AMPK and pro-survival pathways such as SIRT1, reduced cognitive deficiency and had a neuroprotective effect by decreasing the amyloid load and reducing tau hyperphosphorylation (Porquet et al., 2013).
The reduction of amyloid load is not found in all studies. Dietary trans-resveratrol treatment of APP/PS1 mice did not decrease plaque burden in these mice. However, it increased glycogen synthase kinase 3 beta (GSK3-β) phosphorylation on serine 9, associated with its inhibition and consequently inhibited abnormal phosphorylation of tau (Varamini et al., 2014). Moreover, it increased transthyretin level, an Aβ scavenger, and also raised drebrin, a key post-synaptic protein critical to maintaining proper synaptic function, which is decreased in AD (Varamini et al., 2014).
Effects of trans-resveratrol were also studied in rat models of AD. A first rat model of AD was established by the injection of Aβ25–35 in the lateral ventricle on adult Sprague-Dawley rats leading to a significant alteration in spatial memory and an increase of oxidative stress markers. In this model, the combination of the treatment with trans-resveratrol induced a significant improvement in spatial memory, a reduction in the cellular levels of inducible nitric oxide synthase and lipid peroxidation and an increase in the production of heme oxygenase-1, suggesting anti-oxidative role of this stilbene (Huang et al., 2011).
Another rat model of AD was established by ovariectomy combined injection of D galactose (100 mg/kg). Then, 12 weeks later, a heart perfusion in vivo with trans-resveratrol was done. This study established that treatments with 40 and 80 mg/kg of trans-resveratrol induced a decrease in the expression of glial fibrillary acidic protein, more important with the larger dose of trans-resveratrol. Moreover, treatments with 20, 40 and 80 mg/kg of trans-resveratrol decreased the levels of tumor necrosis factor-alpha (TNF-α) (Cheng et al., 2015).
Moreover, long-term trans-resveratrol consumption protected ovariectomized rats chronically treated with D-galactose against spatial memory impairment, by decreasing oxidative stress. For this study, intragastric doses of 20, 40 or 80 mg/kg trans-resveratrol were administred daily (Zhao et al., 2012).
Another study of the same authors has evaluated effect of trans-resveratrol on the integrity of blood brain-barrier (BBB). They showed that trans-resveratrol reduced the insoluble Aβ42 level in hippocampus, by decreasing the expression of nuclear factor-kappa B. It also protected the integrity of BBB in these rats, by 1) increasing the expression of claudin-5, a protein implicated in tight junctions, 2) decreasing receptor for advanced glycation end products (RAGE), a protein involved in amyloid influx, and 3) reducing matrix metallopeptidase (MMP)-9, a member of extracellular matrix enzymes which degrade junction proteins and modify the permeability of the BBB (Zhao et al., 2015).
Trans ε-viniferin
Trans ε-viniferin is a trans-resveratrol dimer, notably found in Vitis vinifera grapevines and in wines. Only two in vivo studies concerning its effects on AD are described in the literature. The first evaluated its beneficial effects on memory loss, by using a methanol extract from the leaf and stem of Vitis amurensis, which notably contained trans ε-viniferin. Memory loss induced by intracerebroventicular injection with Aβ25–35 in mice was inhibited by chronic treatment for 7 days with this extract at the concentrations of 50 and 100 mg/kg per os (Jeong et al., 2010).
More recently, purified trans ε-viniferin was tested in our lab, on a mouse transgenic model of AD. APPswePS1dE9 mice were treated by weekly intraperitoneal injection of this stilbene at the dose of 10 mg/kg or its vehicle from 3 to 6 months of age. This treatment decreased amyloid deposits, astrogliosis and microglial activation, evaluated by immunofluorescence using W0-2, glial fibrillary acidic protein and Iba-1 respectively, in the brain of mice, reflecting a preventive role for this polyphenol (Caillaud et al., 2019).
Gnetin C
To our knowledge, no in vivo study was described in the literature.
Miyabenol C
Miyabenol C is a trans-resveratrol trimer which can be isolated from the stem and leaf extracts of the small-leaf grape Vitisthunbergii var. taiwaniana. Its beneficial effects on 12-month-old transgenic APP/PS1 mice by intracerebroventricular injection at the dose of 0.6 μg/g into the lateral ventricle for three days (Hu et al., 2015). This treatment with miyabenol C treatment induced reduction of soluble β-fragment of amyloid precusor protein and a reduction of both soluble toxic Aβ42 and Aβ40 levels, in cortex and hippocampus without modification of insoluble Aβ42 nor Aβ40 levels (Hu et al., 2015).
Trans-piceid
To our knowledge, no in vivo study was described in the literature.
Piceatannol = Astringenin
Piceatannol, also named astringenin, is a metabolite of trans-resveratrol, especially found in red wine, grapes, or white tea. in vivo effects of this hydroxide of trans-resveratrol for AD have been described in only one study (Hassaan et al., 2014), in which AD was induced in adult male Swiss albino mice by unique intraperitoneal injection of LPS at the dose of 0.8 mg/kg. Authors showed that treatment of these mice by daily intraperitoneal injection of piceatannol at 2.5 mg/kg for 6 days ameliorated cognitive status, evaluated by Y maze and object recognition. Moreover, Aβ42 concentration was significantly reduced in the brain of animals that were treated by this stilbene (Hassaan et al., 2014).
Astringin
No study describing effects of this stilbene, neither in vitro nor in vivo, was published to our knowledge.
Pterostilbene
Pterostilbene is a naturally-derived stilbenoid structurally related to resveratrol. It was initially isolated from sandalwood, but is also found in fruits, such as grapes and blueberries.
A first in vivo study compared diet-achievable supplementation of trans-resveratrol or pterostilbene during two months to improve functional impairments and markers of AD in the SAMP8 mice (Chang et al., 2012). Authors showed that, unlike resveratrol, pterostilbene improved cognitive status, evaluated by radial arm water maze, in these mice. Moreover, it decreased markers of 1) cellular stress, such as manganese superoxide dismutase, an endogenous antioxidant defense protein, 2) inflammation such as peroxisome proliferator-activated receptor alpha receptor and 3) AD such as phosphorylated tau. However, neither trans-resveratrol nor pterostilbene increased SIRT1 expression and activation in this model of sporadic AD (Chang et al., 2012).
Another study evaluated the effects of pterostilbene on learning and memory impairment and changes of microglia and neurons induced in male C57BL/6 mice by bilaterally intrahippocampal injection of LPS (Hou et al., 2014). Pterostilbene, orally administrated at 20 or 40 mg/kg everyday from 7 days before intrahippocampal administration of LPS decreased cognitive disorders, evaluated by Y-maze and Morris water maze. Moreover, it significantly decreased the number of microglial Iba-1 positive cells and neuronal precursor doublecortin positive cells and increased neuronal nuclear antigen-stained area of neurons the hippocampus of these mice, suggesting anti-inflammatory and neuroprotective role (Hou et al., 2014).
Discussion
As described above, most studies about beneficial effects of natural stilbenes in animal models concern trans-resveratrol (Table 1 and Figure 2).
The other natural stilbenes are much less studied. Thus, some stilbenes, such as gnetin C (Seino et al., 2018), trans-piceid (Riviere et al., 2007) or piceatannol, also named astringenin (Fu et al., 2016), are described only for their in vitro effects. For other, such as trans ε-viniferin (Riviere et al., 2007; Jeong et al., 2010; Richard et al., 2011, 2013; Pinho et al., 2013; Schuck et al., 2015; Vion et al., 2018) or pterostilbene (Hou et al., 2014; Fu et al., 2016; Li et al., 2016, 2018), most papers describe in vitro experiments and in vivo studies remain rare.
In the opposite, trans-resveratrol was largely described for its effects both in vitro and in vivo, in murine and rat models of AD. However, these encouraging results need to be confirmed in human AD. Although many clinical trials investigating the effect of trans-resveratrol on AD or other conditions associated with this pathology are listed in the NIH clinicaltrials.gov registry, to our knowledge, results of only one clinical study are described in the literature. In this one, mild to moderate AD patients received placebo or trans-resveratrol (initially 500 mg once daily with dose escalation ending with 1000 mg twice daily) during 52 weeks. Authors showed that trans-resveratrol and its metabolites were measurable in plasma and cerebrospinal fluid (CSF) and obviously penetrated the BBB to exert their effects. Moreover, trans-resveratrol was safe and well-tolerated. But results of this clinical study were ambivalent. Indeed, CSF Aβ42 and Aβ40 levels declined more in the placebo group than in the trans-resveratrol group. However, brain volume loss was increased in the trans-resveratrol treatment group (Turner et al., 2015). This same study showed that trans-resveratrol had effect on some inflammatory proteins. Indeed, it markedly reduced CSF matrix metallopeptidase MMP-9 and increased macrophage-derived chemokine, interleukin (IL)-4, and fibroblast growth factor 2. In the plasma, it increased MMP-10 and decreased IL-12P40, IL-12P70, and chemokine (C-C motif) ligand 5 (CCL5). All these results suggest that trans-resveratrol modulated neuro-inflammation, and induced adaptive immunity. Moreover, this treatment attenuated declines evaluated by mini-mental status examination scores (Moussa et al., 2017). Indeed, a significant decrease in mini-mental status examination score was observed at 52 weeks compared to baseline in the placebo group, but no significant change was detected for this test in the trans-resveratrol treatment group. Alzheimer’s Disease Assessment Scale-activities of daily living scores showed a decline at 52 weeks compared to control in both placebo and trans-resveratrol groups, but the decrease in the placebo group twice as large as that in the trans-resveratrol group at week 52. These results suggest that trans-resveratrol could slow progressive cognitive and functional decline in mild to moderate AD subjects (Moussa et al., 2017).
However, this molecule is rapidly metabolized, mainly in these glucuronidated and sulfated forms and excreted in the urine. Another natural stilbene, pterostilbene, seems more promising than trans-resveratrol. Indeed, methylation of the phenolic hydroxyl could limit the glucuronidation and sulfation processes of pterostilbene, because it provides less conjugating site than resveratrol, resulting in a better metabolic stability (Wang and Sang, 2018). As described above, low doses of pterostilbene, but not resveratrol, were described to be beneficial for AD (Chang et al., 2012). Thus, pterostilbene, which is more metabolically stable and has higher pharmacological activities than resveratrol, could be interesting for clinical trials.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Ubaldo Armato, University of Verona Medical School, Italy.
P-Reviewer: Armato U; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Bavaresco C, Fregoni C. Physiological role and molecular aspects of grapevine stilbenic compounds. In: Roubelakis-Angelakis KA, editor. Molecular Biology and Biotechnology of the Grapevine. The Netherlands: Kluwer Academic Publisher, Dordrecht; 2001. pp. 153–182. [Google Scholar]
- 2.Bavaresco L, Fregoni C, de Macedo Basto Gonçalves MZ, Vezzulli S. Physiology & molecular biology of grapevine stilbenes: an update. In: Roubelakis-Angelakis KA, editor. Grapevine molecular physiology & biotechnology. Springer. Dordrecht: 2009. pp. 341–364. [Google Scholar]
- 3.Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 4.Caillaud M, Guillard J, Richard D, Milin S, Chassaing D, Paccalin M, Page G, Rioux Bilan A. Trans epsilon viniferin decreases amyloid deposits and inflammation in a mouse transgenic Alzheimer model. PLoS One. 2019;14:1–12. doi: 10.1371/journal.pone.0212663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capiralla H, Vingtdeux V, Zhao H, Sankowski R, Al-Abed Y, Davies P, Marambaud P. Resveratrol mitigates lipopolysaccharide- and Abeta-mediated microglial inflammation by inhibiting the TLR4/NF-kappaB/STAT signaling cascade. J Neurochem. 2012;120:461–472. doi: 10.1111/j.1471-4159.2011.07594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang J, Rimando A, Pallas M, Camins A, Porquet D, Reeves J, Shukitt-Hale B, Smith MA, Joseph JA, Casadesus G. Low-dose pterostilbene, but not resveratrol, is a potent neuromodulator in aging and Alzheimer’s disease. Neurobiol Aging. 2012;33:2062–2071. doi: 10.1016/j.neurobiolaging.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Cheng X, Wang Q, Li N, Zhao H. Effects of resveratrol on hippocampal astrocytes and expression of TNF-alpha in Alzheimer’s disease model rate. Wei Sheng Yan Jiu. 2015;44:610–614. [PubMed] [Google Scholar]
- 8.Cicero AFG, Ruscica M, Banach M. Resveratrol and cognitive decline: a clinician perspective. Arch Med Sci. 2019;15:936–943. doi: 10.5114/aoms.2019.85463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farooqui T, Farooqui AA. Aging: an important factor for the pathogenesis of neurodegenerative diseases. Mech Ageing Dev. 2009;130:203–215. doi: 10.1016/j.mad.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Fauconneau B, Waffo-Teguo P, Huguet F, Barrier L, Decendit A, Merillon JM. Comparative study of radical scavenger and antioxidant properties of phenolic compounds from Vitis vinifera cell cultures using in vitro tests. Life Sci. 1997;61:2103–2110. doi: 10.1016/s0024-3205(97)00883-7. [DOI] [PubMed] [Google Scholar]
- 11.Freyssin A, Page G, Fauconneau B, Rioux Bilan A. Natural polyphenols effects on protein aggregates in Alzheimer’s and Parkinson’s prion-like diseases. Neural Regen Res. 2018;13:955–961. doi: 10.4103/1673-5374.233432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu Z, Yang J, Wei Y, Li J. Effects of piceatannol and pterostilbene against beta-amyloid-induced apoptosis on the PI3K/Akt/Bad signaling pathway in PC12 cells. Food Funct. 2016;7:1014–1023. doi: 10.1039/c5fo01124h. [DOI] [PubMed] [Google Scholar]
- 13.Greenwald J, Riek R. Biology of amyloid: structure, function, and regulation. Structure. 2010;18:1244–1260. doi: 10.1016/j.str.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Hassaan Y, Handoussa H, El-Khatib AH, Linscheid MW, El Sayed N, Ayoub N. Evaluation of plant phenolic metabolites as a source of Alzheimer’s drug leads. Biomed Res Int. 2014;2014:1–10. doi: 10.1155/2014/843263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou Y, Xie G, Miao F, Ding L, Mou Y, Wang L, Su G, Chen G, Yang J, Wu C. Pterostilbene attenuates lipopolysaccharide-induced learning and memory impairment possibly via inhibiting microglia activation and protecting neuronal injury in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:92–102. doi: 10.1016/j.pnpbp.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Hu J, Lin T, Gao Y, Xu J, Jiang C, Wang G, Bu G, Xu H, Chen H, Zhang YW. The resveratrol trimer miyabenol C inhibits beta-secretase activity and beta-amyloid generation. PLoS One. 2015;10:1–13. doi: 10.1371/journal.pone.0115973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang TC, Lu KT, Wo YY, Wu YJ, Yang YL. Resveratrol protects rats from Abeta-induced neurotoxicity by the reduction of iNOS expression and lipid peroxidation. PLoS One. 2011;6:e29102. doi: 10.1371/journal.pone.0029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ittner LM, Gotz J. Amyloid-beta and tau--a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci. 2011;12:65–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- 19.Jeong HY, Kim JY, Lee HK, Ha do T, Song KS, Bae K, Seong YH. Leaf and stem of Vitis amurensis and its active components protect against amyloid beta protein (25-35)-induced neurotoxicity. Arch Pharm Res. 2010;33:1655–1664. doi: 10.1007/s12272-010-1015-6. [DOI] [PubMed] [Google Scholar]
- 20.Karuppagounder SS, Pinto JT, Xu H, Chen HL, Beal MF, Gibson GE. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem Int. 2009;54:111–118. doi: 10.1016/j.neuint.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan H, Sureda A, Belwal T, Cetinkaya S, Suntar I, Tejada S, Devkota HP, Ullah H, Aschner M. Polyphenols in the treatment of autoimmune diseases. Autoimmun Rev. 2019;18:647–657. doi: 10.1016/j.autrev.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Q, Chen L, Liu X, Li X, Cao Y, Bai Y, Qi F. Pterostilbene inhibits amyloid-beta-induced neuroinflammation in a microglia cell line by inactivating the NLRP3/caspase-1 inflammasome pathway. J Cell Biochem. 2018;119:7053–7062. doi: 10.1002/jcb.27023. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Jiang X, Hao J, Zhang Y, Huang R. Tea polyphenols: application in the control of oral microorganism infectious diseases. Arch Oral Biol. 2019;102:74–82. doi: 10.1016/j.archoralbio.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Qiang X, Yang X, Luo L, Xiao G, Cao Z, Tan Z, Deng Y. Pterostilbene-O-acetamidoalkylbenzylamines derivatives as novel dual inhibitors of cholinesterase with anti-beta-amyloid aggregation and antioxidant properties for the treatment of Alzheimer’s disease. Bioorg Med Chem Lett. 2016;26:2035–2039. doi: 10.1016/j.bmcl.2016.02.079. [DOI] [PubMed] [Google Scholar]
- 25.Mietelska-Porowska A, Wasik U, Goras M, Filipek A, Niewiadomska G. Tau protein modifications and interactions: their role in function and dysfunction. Int J Mol Sci. 2014;15:4671–4713. doi: 10.3390/ijms15034671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moussa C, Hebron M, Huang X, Ahn J, Rissman RA, Aisen PS, Turner RS. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J Neuroinflammation. 2017;14:1–10. doi: 10.1186/s12974-016-0779-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patterson C. World Alzheimer Report 2018. The state of the art of dementia research: New frontiers. Alzheimer's Disease International Edition. 2018:1–48. [Google Scholar]
- 28.Petrovski G, Gurusamy N, Das DK. Resveratrol in cardiovascular health and disease. Ann N Y Acad Sci. 2011;1215:22–33. doi: 10.1111/j.1749-6632.2010.05843.x. [DOI] [PubMed] [Google Scholar]
- 29.Pinho BR, Ferreres F, Valentao P, Andrade PB. Nature as a source of metabolites with cholinesterase-inhibitory activity: an approach to Alzheimer’s disease treatment. J Pharm Pharmacol. 2013;65:1681–1700. doi: 10.1111/jphp.12081. [DOI] [PubMed] [Google Scholar]
- 30.Porquet D, Casadesus G, Bayod S, Vicente A, Canudas AM, Vilaplana J, Pelegri C, Sanfeliu C, Camins A, Pallas M, del Valle J. Dietary resveratrol prevents Alzheimer’s markers and increases life span in SAMP8. Age (Dordr) 2013;35:1851–1865. doi: 10.1007/s11357-012-9489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quideau S, Deffieux D, Douat-Casassus C, Pouysegu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed Engl. 2011;50:586–621. doi: 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- 32.Richard T, Papastamoulis Y, Waffo-Teguo P, Monti JP. 3D NMR structure of a complex between the amyloid beta peptide (1-40) and the polyphenol epsilon-viniferin glucoside: implications in Alzheimer’s disease. Biochim Biophys Acta. 2013;1830:5068–5074. doi: 10.1016/j.bbagen.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 33.Richard T, Poupard P, Nassra M, Papastamoulis Y, Iglesias ML, Krisa S, Waffo-Teguo P, Merillon JM, Monti JP. Protective effect of epsilon-viniferin on beta-amyloid peptide aggregation investigated by electrospray ionization mass spectrometry. Bioorg Med Chem. 2011;19:3152–3155. doi: 10.1016/j.bmc.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Riviere C, Pawlus AD, Merillon JM. Natural stilbenoids: distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat Prod Rep. 2012;29:1317–1333. doi: 10.1039/c2np20049j. [DOI] [PubMed] [Google Scholar]
- 35.Riviere C, Richard T, Quentin L, Krisa S, Merillon JM, Monti JP. Inhibitory activity of stilbenes on Alzheimer’s beta-amyloid fibrils in vitro. Bioorg Med Chem. 2007;15:1160–1167. doi: 10.1016/j.bmc.2006.09.069. [DOI] [PubMed] [Google Scholar]
- 36.Rodríguez-García C, Sánchez-Quesada C, J Gaforio J. Dietary flavonoids as cancer chemopreventive agents: an updated review of human studies. Antioxidants (Basel) 2019 doi: 10.3390/antiox8050137. doi: 103390/antiox8050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuck F, Schmitt U, Reinhardt S, Freese C, Lee IS, Thines E, Efferth T, Endres K. Extract of Caragana sinica as a potential therapeutic option for increasing alpha-secretase gene expression. Phytomedicine. 2015;22:1027–1036. doi: 10.1016/j.phymed.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Seino S, Kimoto T, Yoshida H, Tanji K, Matsumiya T, Hayakari R, Seya K, Kawaguchi S, Tsuruga K, Tanaka H, Imaizumi T. Gnetin C, a resveratrol dimer, reduces amyloid-beta 1-42 (Abeta42) production and ameliorates Abeta42-lowered cell viability in cultured SH-SY5Y human neuroblastoma cells. Biomed Res. 2018;39:105–115. doi: 10.2220/biomedres.39.105. [DOI] [PubMed] [Google Scholar]
- 39.Shen T, Wang XN, Lou HX. Natural stilbenes: an overview. Nat Prod Rep. 2009;26:916–935. doi: 10.1039/b905960a. [DOI] [PubMed] [Google Scholar]
- 40.Silva P, Sureda A, Tur JA, Andreoletti P, Cherkaoui-Malki M, Latruffe N. How efficient is resveratrol as an antioxidant of the Mediterranean Diet, towards alterations during the aging process? Free Radic Res. 2019;1:1–311. doi: 10.1080/10715762.2019.1614176. [DOI] [PubMed] [Google Scholar]
- 41.Sirerol JA, Rodriguez ML, Mena S, Asensi MA, Estrela JM, Ortega AL. Role of natural stilbenes in the prevention of cancer. Oxid Med Cell Longev. 2016;2016:1–15. doi: 10.1155/2016/3128951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szkudelska K, Szkudelski T. Resveratrol, obesity and diabetes. Eur J Pharmacol. 2010;635:1–8. doi: 10.1016/j.ejphar.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 43.Szkudelski T, Szkudelska K. Resveratrol and diabetes: from animal to human studies. Biochim Biophys Acta. 2015;1852:1145–1154. doi: 10.1016/j.bbadis.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 44.Tellone E, Galtieri A, Russo A, Giardina B, Ficarra S. Resveratrol: a focus on several neurodegenerative diseases. Oxid Med Cell Longev. 2015;2015:1–14. doi: 10.1155/2015/392169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomasset SC, Berry DP, Garcea G, Marczylo T, Steward WP, Gescher AJ. Dietary polyphenolic phytochemicals--promising cancer chemopreventive agents in humans? A review of their clinical properties. Int J Cancer. 2007;120:451–458. doi: 10.1002/ijc.22419. [DOI] [PubMed] [Google Scholar]
- 46.Turner RS, Thomas RG, Craft S, van Dyck CH, Mintzer J, Reynolds BA, Brewer JB, Rissman RA, Raman R, Aisen PS. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015;85:1383–1391. doi: 10.1212/WNL.0000000000002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Varamini B, Sikalidis AK, Bradford KL. Resveratrol increases cerebral glycogen synthase kinase phosphorylation as well as protein levels of drebrin and transthyretin in mice: an exploratory study. Int J Food Sci Nutr. 2014;65:89–96. doi: 10.3109/09637486.2013.832171. [DOI] [PubMed] [Google Scholar]
- 48.Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, Wu Q, Simon JE, Janle EM, Lobo J, Ferruzzi MG, Davies P, Marambaud P. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem. 2010;285:9100–9113. doi: 10.1074/jbc.M109.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vion E, Page G, Bourdeaud E, Paccalin M, Guillard J, Rioux Bilan A. Trans epsilon-viniferin is an amyloid-beta disaggregating and anti-inflammatory drug in a mouse primary cellular model of Alzheimer’s disease. Mol Cell Neurosci. 2018;88:1–6. doi: 10.1016/j.mcn.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Wang P, Sang S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. Biofactors. 2018;44:16–25. doi: 10.1002/biof.1410. [DOI] [PubMed] [Google Scholar]
- 51.Zhao H, Niu Q, Li X, Liu T, Xu Y, Han H, Wang W, Fan N, Tian Q, Zhang H, Wang Z. Long-term resveratrol consumption protects ovariectomized rats chronically treated with D-galactose from developing memory decline without effects on the uterus. Brain Res. 2012;1467:67–80. doi: 10.1016/j.brainres.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 52.Zhao HF, Li N, Wang Q, Cheng XJ, Li XM, Liu TT. Resveratrol decreases the insoluble Abeta1-42 level in hippocampus and protects the integrity of the blood-brain barrier in AD rats. Neuroscience. 2015;310:641–649. doi: 10.1016/j.neuroscience.2015.10.006. [DOI] [PubMed] [Google Scholar]


