Keywords: abnormal renal function, cognitive dysfunction, cystatin C, ischemic stroke, Mini-Mental State Examination, neural regeneration, neuroprotective effect, normal renal function
Abstract
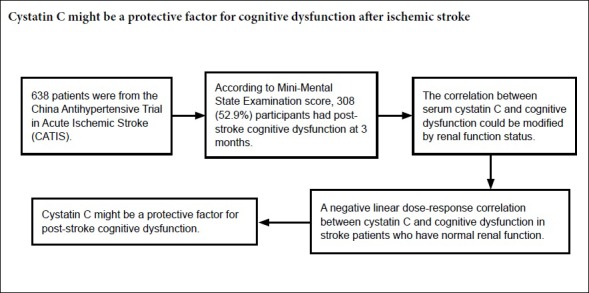
Stroke is the leading cause of death and long-term disability worldwide, and cognitive impairment and dementia are major complications of ischemic stroke. Cystatin C (CysC) has been found to be a neuroprotective factor in animal studies. However, the relationship between CysC levels and cognitive dysfunction in previous studies has revealed different results. This prospective observational study investigated the correlation between serum CysC levels and post-stroke cognitive dysfunction at 3 months. Data from 638 patients were obtained from the China Antihypertensive Trial in Acute Ischemic Stroke (CATIS). Cognitive dysfunction was assessed using the Mini-Mental State Examination (MMSE) at 3 months after stroke. According to the MMSE score, 308 patients (52.9%) had post-stroke cognitive dysfunction. After adjusting for potential confounding factors, the odds ratio (95% CI) of post-stroke cognitive dysfunction for the highest quartile of serum CysC levels was 0.54 (0.30–0.98), compared with the lowest quartile. The correlation between serum CysC and cognitive dysfunction was modified by renal function status. We observed a negative linear dose-response correlation between CysC and cognitive dysfunction in patients with normal renal function (Plinearity = 0.044), but not in those with abnormal renal function. Elevated serum CysC levels were correlated with a low risk of 3-month cognitive dysfunction in patients with acute ischemic stroke, especially in those with normal renal function. The current results suggest that CysC is a protective factor for post-stroke cognitive dysfunction, and could be used to treat post-stroke cognitive dysfunction. The CATIS study was approved by the Institutional Review Boards at Soochow University from China (approval No. 2012-02) on December 30, 2012, and was registered at ClinicalTrials.gov (identifier No. NCT01840072) on April 25, 2013.
Chinese Library Classification No. R449; R741; R446
Introduction
Cognitive dysfunction is one of the most common complications of stroke (Tatemichi et al., 1994; Patel et al., 2002; Zhang et al., 2018; He et al., 2019), and can lead to disability, affect the ability to carry out activities of daily life, and impair social functioning (Barker-Collo et al., 2010; Cumming et al., 2013). The development of post-stroke cognitive dysfunction may involve many factors (Guo et al., 2015). Although some factors, including age, brain infarcts, stroke severity, education levels, and medical history, are known risk factors for post-stroke cognitive dysfunction (Desmond et al., 2000; Henon et al., 2001; Vermeer et al., 2003), some unknown potential factors still need to be studied to effectively predict the risk of cognitive dysfunction after stroke.
Cystatin C (CysC) is an inhibitor of cysteine protease, which is produced by almost all human cells and secreted into the blood (Coll et al., 2000; Fliser and Ritz, 2001). Some animal studies have demonstrated that CysC protects against neuronal death (Olsson et al., 2004; Tizon et al., 2010), and exogenous CysC has been found to play a protective role in ischemic brain injury by reducing infarct volume in a mouse focal ischemia/reperfusion injury model (Yang et al., 2015). There have been inconsistent reports about the associations between CysC levels and cognitive dysfunction in the general population (Wada et al., 2010; Slinin et al., 2015; Zhang et al., 2016). Furthermore, the association between serum CysC levels and post-stroke cognitive dysfunction has not been studied. We therefore investigated the association between serum CysC levels and subsequent cognitive dysfunction after stroke onset in a sample of patients from the China Antihypertensive Trial in Acute Ischemic Stroke (CATIS) study.
Subjects and Methods
Study design and patients with ischemic stroke
This prospective observational study was embedded within the CATIS study, a multicenter, single-blind, randomized clinical trial in China (He et al., 2014; Bu et al., 2016). In this pre-planned ancillary study (Bu et al., 2016), 582 participants were included in the current analysis (Figure 1 and Table 1).
Figure 1.
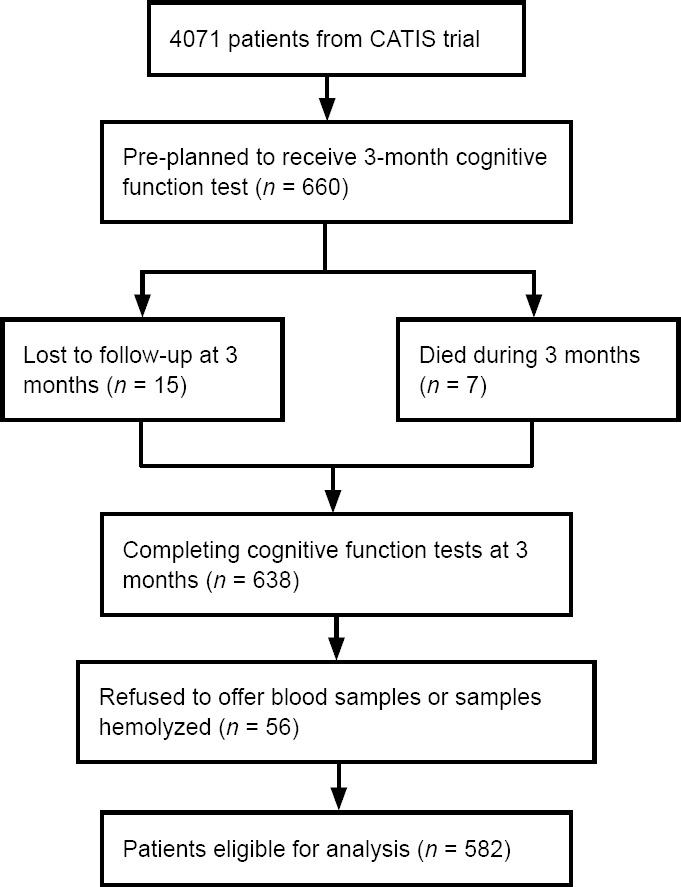
Study participant flow chart.
CATIS: China Antihypertensive Trial in Acute Ischemic Stroke.
Table 1.
Design, setting, and participants information in CATIS study and the present study
| Variables | Information |
|---|---|
| CATIS study | |
| Participants and participating hospitals | A multicenter, single-blind, blinded end-points randomized clinical trial in 26 hospitals across China (n = 4071) |
| Brief introduction | In the CATIS study, 4071 patients with an adjudicated diagnosis of ischemic stroke by computed tomography or magnetic resonance imaging of the brain within 48 hours after symptom onset were recruited from August 2009 to May 2013. |
| Inclusion criteria | 1. Age ≥ 22 years 2. Having ischemic stroke confirmed by computed tomography or magnetic resonance imaging of the brain within 48 h of symptom onset 3. Having an elevated systolic blood pressure between 140 and 220 mmHg |
| Exclusion criteria | 1. Having a systolic blood pressure ≥ 220 mmHg or diastolic blood pressure ≥ 120 mmHg 2. Having severe heart failure, acute myocardial infarction, unstable angina, atrial fibrillation, aortic dissection, cerebrovascular stenosis, resistant hypertension, and deep coma 3. Having been treated with intravenous thrombolytic therapy |
| Pre-planned ancillary study based on CATIS | |
| Patients selection | CATIS trial participants were systemically selected prior to randomization from seven participating hospitals for cognitive function assessment at their 3-month follow-up visit. Eighty to 100 patients were recruited consecutively from each participating hospitals and the recruitment was completed by November 2012. A total of 660 patients were included in the pre-planned ancillary study. |
| Loss to follow-up | At the 3-month visit, 15 patients were lost to follow-up and 7 patients were deceased. A total of 638 participants who completed the cognitive function tests at 3 months were included in the present analysis. |
| Failed to test cystatin C | A total of 56 patients refused to offer blood samples and some collected samples were hemolyzed in storage or transport, a total of 582 participants were finally included in the present analysis. |
CATIS: China Antihypertensive Trial in Acute Ischemic Stroke (He et al., 2014; Bu et al., 2016).
The Institutional Review Boards at Soochow University from China (approval No. 2012-02) approved the present study on December 30, 2012, as well as ethical committees of the participating hospitals. Informed consent was obtained from all patients’ legal guardians when the patients were enrolled in the CATIS study. The CATIS trial was registered at ClinicalTrials.gov (identifier No. NCT01840072) on April 25, 2013.
Data acquisition
We acquired the baseline data on demographic characteristics at the time of enrollment, as well as medication history and clinical features (Brott et al., 1989; Pickering et al., 2005). Blood samples were collected, and serum CysC levels were tested by laboratory technicians blinded to the clinical data and outcomes of the study participants. The detection method is listed in Table 2 (Grubb et al., 2010).
Table 2.
Details of data collection and outcome assessment
| Variables | Details of method |
|---|---|
| Baseline data | |
| Stroke severity | Stroke severity was assessed using the National Institutes of Health Stroke Scale by trained neurologists at baseline |
| Ischemic stroke subtypes | Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification criterion was used to classify the ischemic stroke subtypes according to the symptoms and imaging data of the patients 1. Large-artery atherosclerosis (Thrombotic) 2. Cardiac embolism (Embolic) 3. Small-vessel occlusion (Lacunar) |
| Education | Educational attainment was assessed as 0–17 magnitude from the uneducated to higher education. |
| Blood pressure | Three blood pressure measurements were obtained at baseline while the patient was in the supine position using a standard mercury sphygmomanometer according to European Society of Cardiology guidelines. |
| Routine laboratory determinations | Routine laboratory determinations (fasting plasma glucose, blood lipids, etc.) were tested for all enrolled patients in each participating hospital at admission |
| Renal function | Assessment of renal function was based on estimated glomerular filtration rate calculated using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation with adjusted coefficient of 1.1 for the Chinese population. According to the Kidney Disease: Improving Global Outcomes, we defined normal renal function as estimated glomerular filtration rate ≥ 90 mL/min per 1.73 m2 and abnormal renal function as estimated glomerular filtration rate < 90 mL/min per 1.73 m2. |
| Cystatin C test | |
| Blood sample collection | Blood samples were collected after at last 8 h of fasting within 24 h of hospital admission. All serum samples were separated and frozen at −80°C in the Central Laboratory of School of Public Health in Soochow University, China until laboratory testing. |
| Cystatin C assay kit | Serum Cystatin C levels were determined with a Cystatin C assay kit by latex enhanced immunoturbidimetric method (Sichuan Maker Biotechnology Co., Ltd., China), and the Cystatin C calibrator was the primary reference material ERM-DA471/IFCC for cystatin C assays |
| Quality control | The range of Cystatin C measurement was from 0.13 to 7.80 mg/L. Intra- and inter-assay coefficients of variation were less than 3.9% and 4.8%, respectively. |
| Outcome assessment | |
| Study outcome | The study outcome was cognitive impairment at 3 months after stroke onset assessed by trained neurologists using the Mini-Mental State Examination. The MMSE contains 20 items that test cognitive performance in domains including orientation, registration, attention and calculation, recall, language, and visual construction. MMSE has been translated into Chinese and validated as a screening tool for cognitive impairment and dementia in the Chinese population. In this analysis, a score of < 27 on the Mini-Mental State Examination indicated cognitive impairment |
Outcome assessment
The primary outcome was cognitive dysfunction (Zhong et al., 2018) at 3 months after stroke onset, which was assessed using the 20-item Mini-Mental State Examination (MMSE) (Folstein et al., 1975), score < 27 (Pendlebury et al., 2010; Webb et al., 2014; Delavaran et al., 2017)) by trained neurologists. The MMSE has been translated into Chinese, and this evaluation tool has been validated as a screening tool for cognitive function and dementia in China.
Statistical analysis
The statistical analysis is shown in Table 3.
Table 3.
Statistical analysis
| Variables | Details of statistical analysis |
|---|---|
| Baseline characteristics | Baseline characteristics were compared between the four groups using the chi-square test, variance or Kruskal-Wallis tests when appropriate. |
| Logistic regression analysis | Multiple-adjusted logistic regression analysis was used to estimate the risk of cognitive impairment by calculating odds ratio (OR) and 95% confidence interval (CI). Model 1 adjusted for age, sex, baseline National Institutes of Health Stroke Scale scores, education, current smoking, alcohol drinking, systolic blood pressure, estimated glomerular filtration rate, body mass index, time from onset to randomization, ischemic stroke subtype, and family history of stroke. Model 2 included the factors in model 1 as well as the use of antihypertensive treatment and hypoglycemic treatment. Model 3 included the factors in model 2 as well as medical history of hypertension, medical history of diabetes mellitus, medical history of hyperlipidemia, and medical history of coronary heart disease. Potential covariates for cognitive impairment were selected based on prior knowledge. |
| Effect modification by renal function | We tested the statistical significance of cystatin C quartiles × renal function status on the cognitive impairment in multivariable logistic model by the likelihood ratio test. We further evaluated the pattern and magnitude of associations between serum cystatin C and cognitive impairment using a logistic regression model with restricted cubic splines among the patients without or with normal renal function, with four knots (at the 5th, 35th, 65th, and 95th percentiles). |
| Statistical software | Statistical analysis was conducted using SAS statistical software (version 9.4, Cary, NC, USA). |
All P values were two-tailed, and a significance level of 0.05 was used.
Results
Baseline characteristics of patients with acute ischemic stroke
We compared the characteristics between patients enrolled and those excluded at baseline (Table 4). The patients included in the present study did not have significantly different characteristics from those excluded at baseline. Among the patients in our study (n = 582, mean age 60.5 ± 10.4 years), the median serum CysC concentration was 0.77 mg/L (interquartile range, 0.65–0.91 mg/L). A total of 112 (19.2%) patients had abnormal renal function. The characteristics based on the serum CysC quartiles at baseline are presented in Table 5.
Table 4.
Baseline characteristics of acute ischemic stroke patients
| Characteristics | Excluded (n = 3489) | Enrolled (n = 582) | P-value |
|---|---|---|---|
| Demographic | |||
| Age (yr) | 62.8±11.0 | 60.5±10.4 | < 0.001 |
| Male sex | 2199 (63.0) | 405 (69.6) | 0.002 |
| Education (yr) | 6.8±4.2 | 7.7±4.1 | < 0.001 |
| Current cigarette smoking | 1265 (36.3) | 220 (37.8) | 0.474 |
| Current alcohol drinking | 1057 (30.3) | 196 (33.7) | 0.102 |
| Clinical features | |||
| Time from onset to randomization (h) | 10.0 (4.5–24.0) | 10.0 (5.0–24.0) | 0.992 |
| Baseline systolic blood pressure (mmHg) | 165.9±16.9 | 167.3±16.6 | 0.083 |
| Baseline diastolic blood pressure (mmHg) | 96.4±11.2 | 98.3±10.0 | < 0.001 |
| Body mass index (kg/m2) | 25.0±3.1 | 24.9±3.1 | 0.553 |
| Baseline National Institute of Health Stroke Scale score | 4.0 (2.0–8.0) | 4.0 (3.0–7.0) | 0.427 |
| Medical history | |||
| History of hypertension | 2761 (79.1) | 448 (77.0) | 0.238 |
| History of hyperlipidemia | 235 (6.7) | 42 (7.2) | 0.67 |
| History of diabetes mellitus | 622 (17.8) | 97 (16.7) | 0.497 |
| History of coronary heart disease | 383 (11.0) | 61 (10.5) | 0.722 |
| Family history of stroke | 657 (18.8) | 96 (16.5) | 0.179 |
| Receiving immediate blood pressure reduction | 1756 (50.3) | 282 (48.5) | 0.402 |
Continuous variables are expressed as the mean ± standard deviation, or as the median (interquartile range). Categorical variables are expressed as the frequency (percent). Baseline characteristics were compared between the four groups using the chi-square test, variance or Kruskal-Wallis tests, where appropriate.
Table 5.
Characteristics of participants according to serum Cystatin C
| Cystatin C (mg/L) | |||||
|---|---|---|---|---|---|
| Total (n = 582) | < 0.65 (n = 136) | 0.65–0.77 (n = 143) | 0.77–0.91 (n = 152) | ≥ 0.91 (n = 151) | |
| Demographic | |||||
| Age (yr) | 60.5±10.4 | 57.4±10.2 | 59.1±9.7 | 60.4±10.1 | 64.9±10.0 |
| Sex (male) | 405 (69.6) | 87 (64.0) | 105 (73.4) | 102 (67.1) | 111 (73.5) |
| Education | 7.7±4.1 | 8.5±4.1 | 8.1±3.8 | 7.0±4.0 | 7.2±4.2 |
| Current cigarette smoking | 220 (37.8) | 55 (40.4) | 47 (32.9) | 59 (38.8) | 59 (39.1) |
| Current alcohol drinking | 196 (33.7) | 54 (39.7) | 55 (38.5) | 50 (32.9) | 37 (24.5) |
| Clinical features | |||||
| Time from onset to randomization | 10.0 (5.0, 24.0) | 9.0 (4.5, 24.0) | 10.0 (5.0, 22.5) | 12.0 (4.0, 24.0) | 10.5 (5.8, 24.0) |
| Baseline systolic blood pressure (mmHg) | 167.3±16.6 | 165.6±18.4 | 167.4±16.3 | 167.1±15.2 | 168.7±16.5 |
| Baseline diastolic blood pressure (mmHg) | 98.3±10.0 | 98.6±10.5 | 99.4±8.8 | 97.9±10.5 | 97.3±10.3 |
| Body-mass index (kg/m2) | 24.9±3.1 | 24.6±2.5 | 25.1±3.2 | 25.3±3.4 | 24.4±3.0 |
| Baseline National Institute of Health Stroke Scale score | 4.0 (2.0, 7.0) | 4.0 (2.0, 7.0) | 4.0 (3.0, 7.0) | 4.0 (2.0, 7.0) | 4.0 (3.0, 7.0) |
| Estimated glomerular filtration rate (mL/min per 1.73 m2) | 105.5 (94.9, 113.6) | 112.2 (105.3, 119.9) | 108.8 (99.7, 115.7) | 103.8 (95.8, 110.5) | 94.2 (74.7, 104.8) |
| Medical history | |||||
| History of hypertension | 448 (77.0) | 97 (71.3) | 111 (77.6) | 116 (76.3) | 124 (82.1) |
| History of hyperlipidemia | 42 (7.2) | 11 (8.1) | 12 (8.4) | 9 (5.9) | 10 (6.6) |
| History of diabetes mellitus | 97 (16.7) | 29 (21.3) | 22 (15.4) | 20 (13.2) | 26 (17.2) |
| History of coronary heart disease | 61 (10.5) | 17 (12.5) | 11 (7.7) | 14 (9.2) | 19 (12.6) |
| Family history of stroke | 96 (16.5) | 30 (22.1) | 24 (16.8) | 24 (15.8) | 18 (11.9) |
| Ischemic stroke subtype | |||||
| Thrombotic | 371 (63.8) | 91 (66.9) | 88 (61.5) | 103 (67.8) | 89 (58.9) |
| Embolic | 23 (4.0) | 2 (1.5) | 4 (2.8) | 6 (4.0) | 11 (7.3) |
| Lacunar | 197 (33.9) | 45 (33.1) | 53 (37.1) | 46 (30.3) | 53 (35.1) |
| Abnormal renal function† | 112 (19.2) | 6 (4.4) | 17 (11.9) | 25 (16.5) | 64 (42.4) |
| Treatment | |||||
| Receiving immediate blood pressure reduction | 282 (48.5) | 68 (50.0) | 75 (52.5) | 67 (44.1) | 72 (47.7) |
| Use of hypoglycemic treatment | 97 (18.0) | 22 (16.7) | 25 (18.5) | 18 (13.5) | 32 (22.9) |
Continuous variables are expressed as mean ± standard deviation, or as median (interquartile range). Categorical variables are expressed as frequency (percent). † Abnormal renal function: estimated glomerular filtration rate < 90 mL/min per 1.73 m2 (Levey et al., 2011).
The association between serum CysC and cognitive dysfunction
The median (interquartile range) MMSE score was 26 (22–29) at the 3-month follow up. According to MMSE categories, 308 (52.9%) participants had cognitive dysfunction. After adjustment for potential confounders in models one, two, and three, the odds ratios of cognitive dysfunction associated with the highest quartile of serum CysC levels were 0.56 (95% confidence interval (CI), 0.32, 0.98; Ptrend = 0.044), 0.52 (95% CI, 0.29, 0.93; Ptrend = 0.029), and 0.54 (95% CI, 0.30, 0.98; Ptrend = 0.041), respectively.
The association between serum CysC levels and cognitive dysfunction was modified by renal function status (Pinteraction = 0.040). Furthermore, serum CysC was only associated with cognitive dysfunction in patients with normal renal function, and not in those with abnormal renal function (Table 6). In the normal renal function group, after adjustment for potential confounders in models 1–3, the odds ratios of cognitive dysfunction associated with the highest quartile of serum CysC levels were 0.48 (95% CI, 0.25, 0.90; Ptrend = 0.022), 0.42 (95% CI, 0.22, 0.81; Ptrend = 0.010), and 0.45 (95% CI, 0.23, 0.88; Ptrend = 0.020), respectively.
Table 6.
Odds ratios (ORs) and 95% confidence interval (CIs) for the risk of cognitive impairment according to cystatin C quartiles
| Subjects* | Variable | Cystatin C (mg/L) | ||||
|---|---|---|---|---|---|---|
| < 0.65 | 0.65–0.77 | 0.77–0.91 | ≥ 0.91 | Ptrend | ||
| Totle population | MMSE score < 27 [n (%)]† | 72 (52.9) | 75 (52.5) | 81 (53.3) | 80 (53.0) | |
| Model 1 | 1 | 0.80 (0.48–1.34) | 0.83 (0.49–1.38) | 0.56 (0.32–0.98) | 0.044 | |
| Model 2 | 1 | 0.73 (0.43–1.23) | 0.78 (0.46–1.33) | 0.52 (0.29–0.93) | 0.029 | |
| Model 3 | 1 | 0.73 (0.43–1.25) | 0.79 (0.46–1.35) | 0.54 (0.30–0.98) | 0.041 | |
| eGFR < 90 mL/min per 1.73 m2 | MMSE score < 27 [n (%)] | 3 (50) | 8 (47.1) | 15 (60) | 39 (60.9) | |
| Model 1 | 1 | 0.13 (0.01–2.31) | 0.28 (0.02–4.1) | 0.43 (0.04–4.89) | 0.498 | |
| Model 2 | 1 | 0.13 (0.01–2.33) | 0.25 (0.02–3.95) | 0.42 (0.03–5.37) | 0.507 | |
| Model 3 | 1 | 0.15 (0.01–2.8) | 0.3 (0.02–4.82) | 0.51 (0.04–6.51) | 0.602 | |
| eGFR ≥ 90 mL/min per 1.73 m2 | MMSE score < 27 [n (%)] | 69 (53.1) | 67 (53.2) | 66 (52) | 41 (47.1) | |
| Model 1 | 1 | 0.90 (0.53–1.53) | 0.88 (0.51–1.51) | 0.48 (0.25–0.90) | 0.022 | |
| Model 2 | 1 | 0.82 (0.48–1.43) | 0.85 (0.48–1.49) | 0.42 (0.22–0.81) | 0.01 | |
| Model 3 | 1 | 0.84 (0.48–1.47) | 0.86 (0.48–1.53) | 0.45 (0.23–0.88) | 0.02 | |
*The total population and subgroup analysis. †MMSE score < 27 indicates cognitive impairment. Model 1: Adjusted for age, sex, baseline National Institute of Health Stroke Scale scores, education, current smoking, alcohol drinking, systolic blood pressure, eGFR, body mass index, time from onset to randomization, ischemic stroke subtype, and family history of stroke; Model 2: adjusted for Model 1 and further adjusted for use of antihypertensive treatment and hypoglycemic treatment; Model 3: adjusted for Model 2 and further adjusted for medical history (hypertension, diabetes mellitus, hyperlipidemia, and coronary heart disease). eGFR: Estimated glomerular filtration rate; MMSE: Mini-Mental State Examination.
The correlation between serum CysC levels and cognitive dysfunction was tested by logistic regression model with restricted cubic splines according to the renal function status. As shown in Figure 2A, there was no significant association between serum CysC and cognitive dysfunction in patients with abnormal renal function (Pnonlinearity = 0.622; Plinearity = 0.364). In contrast, the risk of post-stroke cognitive dysfunction at 3 months in patients with normal renal function decreased with an increase of serum CysC levels (Pnonlinearity = 0.249; Plinearity = 0.044, Figure 2B). Moreover, different trends were observed in these two subgroups stratified by renal function status.
Figure 2.
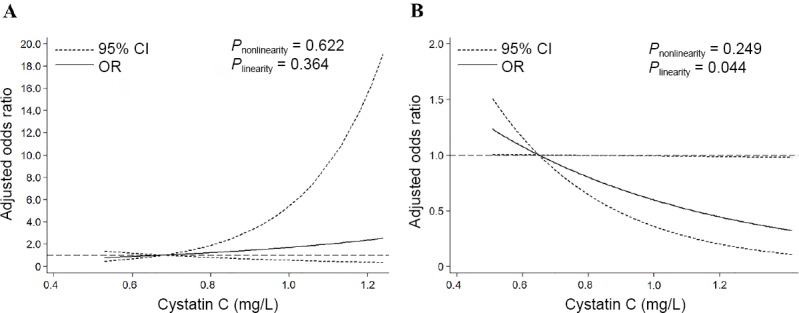
Association of cystatin C at baseline with odds ratios (ORs) of cognitive impairment.
We use restricted cubic spline regression to predict the odds ratios and 95% confidence intervals (CIs) with knots placed at the 5th, 35th, 65th, and 95th percentiles of baseline cystatin C levels adjusting for variables in model 3, as shown in Table 3. (A) Patients with estimated glomerular filtration rate < 90 mL/min per 1.73 m2; (B) patients with estimated glomerular filtration rate ≥ 90 mL/min per 1.73 m2.
Discussion
In this pre-planned ancillary study of the CATIS study, high CysC levels at baseline were independently correlated with a decreased risk of cognitive dysfunction 3 months after acute ischemic stroke. In the secondary analysis, we further found that renal function status modified this association. In addition, there was a negative linear dose-response correlation between CysC and cognitive dysfunction in patients with normal renal function, but not in those with abnormal renal function. These results suggest that serum CysC might be a protective factor for cognitive dysfunction after stroke, and that this protective effect is modified by renal function status.
Previous studies have reported that age, brain infarcts, stroke severity, education level, fasting plasma glucose levels and medical history influence cognitive dysfunction (Desmond et al., 2000; Henon et al., 2001; Vermeer et al., 2003). Few studies have reported there to be a correlation between serum CysC levels and cognitive dysfunction (Wada et al., 2010; Slinin et al., 2015; Zhang et al., 2016). A cross-sectional study showed that an increased level of serum CysC was independently correlated with elevated risk of cognitive dysfunction in 375 patients with type 2 diabetes (Zhang et al., 2016). In another cross-sectional study, Wada et al. (2010) found a correlation between CysC and cognitive dysfunction, as well as cerebral small vessel disease, in 604 community-based elderly people in Japan. In Wada et al.’s study, participants with higher CysC levels seemed to have lower MMSE scores, but this finding was not statistically significant. Slinin et al. (2015) reported that CysC levels and cognitive dysfunction presented a U-shaped association in older women after ten years, but this finding was not statistically significant after adjusting for potential confounding factors. However, there have been no reports on the correlation between serum CysC levels at baseline and subsequent cognitive dysfunction after stroke onset. Based on a subset in the CATIS trial, we investigated the correlation between serum CysC levels at baseline and subsequent cognitive dysfunction after stroke onset. As age, stroke severity, education level, and medical history are established influencing factors for cognitive dysfunction (Desmond et al., 2000; Henon et al., 2001), we adjusted for these confounding factors. We also adjusted for glomerular filtration rate because renal function status has also been associated with structural changes of the brain and cognitive dysfunction in the elderly (Sink et al., 2015). We found a significant correlation in all three models, whereby an increased serum CysC was associated with a decreased risk of cognitive dysfunction. Furthermore, serum CysC in the highest quartile was associated with a decreased risk of over 50% of subsequent cognitive dysfunction, as measured by the MMSE score.
Renal dysfunction normally gives rise to higher CysC serum levels. However, CysC is an indicator rather than an independent risk factor for renal dysfunction because it can be completely reabsorbed and broken down in the renal tubules due to the characteristics of freely filtering by the glomerulus (Coll et al., 2000). Hence, we hypothesized that CysC can have a range of normal values in clinical practice after ischemic stroke, and a lower level is not necessarily better. Namely, higher CysC levels reflect renal dysfunction, which may lead to subsequent post-stroke cognitive dysfunction, but lower CysC levels can also induce post-stroke cognitive dysfunction due to the neuroprotective feature of CysC. Indeed, we found that renal function status modified the correlation between serum CysC and cognitive dysfunction. Moreover, serum CysC was negatively associated with cognitive dysfunction in patients with normal renal function. However, the correlation between CysC levels and cognitive dysfunction was not statistically significant in patients with abnormal renal function. Our findings do not contradict the detrimental effects of renal dysfunction on cognitive function (Ben Assayag et al., 2017) and may have two important clinical implications. First, CysC level should be measured at hospital admission to predict for post-stroke cognitive dysfunction and adopt corresponding treatments. Second, CysC could be considered as a target drug therapy for post-stroke cognitive dysfunction, especially for those with normal renal function. However, further large-scale prospective cohort studies are warranted to replicate our observations, and intervention trials are needed to validate our hypothesis. Our study also suggests that the range of normal CysC values should be studied in both healthy people and patients with ischemic stroke.
Several potential pathophysiological pathways could explain the mechanisms by which the increased CysC levels were associated with a decreased risk of post-stroke cognitive dysfunction. CysC may be an endogenous neuroprotectant. It may exert its neuroprotective effect by preserving lysosomal integrity, which could induce ischemic tolerance and might be of great value for stroke treatment (Fang et al., 2017). In addition, the intracellular transport of CysC, as well as CysC secretion, can be modulated by neuroglobin to prevent neurons from dying due to oxidative stress (Wakasugi et al., 2004). Several animal studies have reported that CysC itself is a neuroprotective factor. For example, in CysC gene knockout mice, larger brain infarcts were found following focal ischemia, which suggests that endogenous CysC is protective in focal ischemia, probably through its inhibitory effect on the cathepsins during ischemia (Olsson et al., 2004). Furthermore, exogenous CysC can also play a protective role in reducing infarct volume after ischemic brain injury (Yang et al., 2015).
Our study has several strengths. First, this observational study recruited data from individuals who participated in the CATIS study, which had precise quality controls in data acquisition and outcome appraisal. Second, the relevant covariates were controlled in the present study, which provides a more valid, appropriate, and rigorous evaluation of the correlation between serum CysC levels and cognitive dysfunction after ischemic stroke. Several limitations of our study need to be considered. First, a selection bias might exist, as the participants were from a random sample of the CATIS trial. However, we found that the patients in our study had similar baseline characteristics to patients of the China National Stroke Registry (Luo et al., 2014). Thus, the selection bias was likely to be minimal. Second, there might be some residual confounding factors that we could not fully eliminate, although several known confounding factors were controlled in the multi-variable correction models. Third, it was difficult to carry out complicated tests of cognitive status in the acute phase of ischemic stroke. Therefore, we could not test cognitive function at baseline using the MMSE and control it as a potential confounding factor. However, the National Institutes of Health Stroke Scale has a subset cognitive dysfunction evaluation, which has almost the same diagnostic value as the MMSE (area under the ROC curve (AUC) values of 0.78 and 0.84, respectively) (Cumming et al., 2010). Moreover, cognitive function at baseline was also largely correlated with the National Institutes of Health Stroke Scale score and other characteristics at admission that were adjusted for, which suggests that this potential confounding influence was likely to be minimal. Fourth, post-stroke cognitive function was only assessed using the MMSE. Therefore, further studies using other cognitive tests are needed to validate our findings in patients with ischemic stroke.
Elevated serum CysC levels in the acute stage of ischemic stroke were correlated with a decreased risk of 3-month cognitive dysfunction, especially in those with normal renal function. CysC may therefore be a protective factor for cognitive dysfunction after ischemic stroke. In addition, CysC levels may have a range of normal values in clinical practice. Further prospective studies are needed in other samples to replicate our findings.
Acknowledgments:
We thank the study participants and their relatives and the clinical staff at all participating hospitals for their support and contribution to this project.
Footnotes
Conflicts of interest: None declared.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81673263 (to YHZ), Ministry of Science and Technology of China, No. 2016YFC1307300 (to YHZ), and a Project of the Priority Academic Program Development of Jiangsu Higher Education Institutions, China (to YHZ). All authors declared that the financial supports did not affect the paper’s views and statistical analysis of the objective results of the research data and their reports.
Institutional review board statement: This prospective observational study was embedded within the CATIS study, and CATIS study was approved by the institutional review boards at Soochow University from China (approval No. 2012-02) on December 30, 2012, and was registered at ClinicalTrials.gov (identifier No. NCT01840072) on April 25, 2013.
Informed consent statement: Informed consent has been obtained from the patients’ legal guardians when the patients were enrolled in the CATIS study. In CATIS study, the authors certify that they have obtained all appropriate patient consent forms. In the forms, the legal guardians have given their consent for the patients’ images and other clinical information to be reported in the journal. The patients’ legal guardians understand that the patients’ names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement: This study followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) Statement.
Biostatistics statement: The statistical methods of this study were reviewed by Yong-Hong Zhang.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: The data that support the fndings of this study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81673263 (to YHZ), Ministry of Science and Technology of China, No. 2016YFC1307300 (to YHZ), and a Project of the Priority Academic Program Development of Jiangsu Higher Education Institutions, China (to YHZ).
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Cason N, Yu J, Song LP; T-Editor: Jia Y
References
- 1.Barker-Collo S, Feigin VL, Parag V, Lawes CM, Senior H. Auckland Stroke Outcomes Study. Part 2: Cognition and functional outcomes 5 years poststroke. Neurology. 2010;75:1608–1616. doi: 10.1212/WNL.0b013e3181fb44c8. [DOI] [PubMed] [Google Scholar]
- 2.Ben Assayag E, Eldor R, Korczyn AD, Kliper E, Shenhar-Tsarfaty S, Tene O, Molad J, Shapira I, Berliner S, Volfson V, Shopin L, Strauss Y, Hallevi H, Bornstein NM, Auriel E. Type 2 diabetes mellitus and impaired renal function are associated with brain alterations and poststroke cognitive decline. Stroke. 2017;48:2368–2374. doi: 10.1161/STROKEAHA.117.017709. [DOI] [PubMed] [Google Scholar]
- 3.Brott T, Adams HP, Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 4.Bu X, Zhang Y, Bazzano LA, Xu T, Guo L, Wang X, Zhang J, Cui Y, Li D, Zhang F, Ju Z, Xu T, Chen CS, Chen J, He J. Effects of early blood pressure reduction on cognitive function in patients with acute ischemic stroke. Int J Stroke. 2016;11:1009–1019. doi: 10.1177/1747493016660094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coll E, Botey A, Alvarez L, Poch E, Quintó L, Saurina A, Vera M, Piera C, Darnell A. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36:29–34. doi: 10.1053/ajkd.2000.8237. [DOI] [PubMed] [Google Scholar]
- 6.Cumming TB, Marshall RS, Lazar RM. Stroke, cognitive deficits, and rehabilitation: still an incomplete picture. Int J Stroke. 2013;8:38–45. doi: 10.1111/j.1747-4949.2012.00972.x. [DOI] [PubMed] [Google Scholar]
- 7.Cumming TB, Blomstrand C, Bernhardt J, Linden T. The NIH stroke scale can establish cognitive function after stroke. Cerebrovasc Dis. 2010;30:7–14. doi: 10.1159/000313438. [DOI] [PubMed] [Google Scholar]
- 8.Delavaran H, Jonsson AC, Lovkvist H, Iwarsson S, Elmståhl S, Norrving B, Lindgren A. Cognitive function in stroke survivors: A 10-year follow-up study. Acta Neurol Scand. 2017;136:187–194. doi: 10.1111/ane.12709. [DOI] [PubMed] [Google Scholar]
- 9.Desmond DW, Moroney JT, Paik MC, Sano M, Mohr JP, Aboumatar S, Tseng CL, Chan S, Williams JB, Remien RH, Hauser WA, Stern Y. Frequency and clinical determinants of dementia after ischemic stroke. Neurology. 2000;54:1124–1131. doi: 10.1212/wnl.54.5.1124. [DOI] [PubMed] [Google Scholar]
- 10.Fang Z, Deng J, Wu Z, Dong B, Wang S, Chen X, Nie H, Dong H, Xiong L. Cystatin C is a crucial endogenous protective determinant against stroke. Stroke. 2017;48:436–444. doi: 10.1161/STROKEAHA.116.014975. [DOI] [PubMed] [Google Scholar]
- 11.Fliser D, Ritz E. Serum cystatin C concentration as a marker of renal dysfunction in the elderly. Am J Kidney Dis. 2001;37:79–83. doi: 10.1053/ajkd.2001.20628. [DOI] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Grubb A, Blirup-Jensen S, Lindstrom V, Schmidt C, Althaus H, Zegers I IFCC Working Group on Standardisation of Cystatin C (WG-SCC) First certified reference material for cystatin C in human serum RM-DA471/IFCC. Clin Chem Lab Med. (2010;48:1619–1621. doi: 10.1515/CCLM.2010.318. [DOI] [PubMed] [Google Scholar]
- 14.Guo ZW, Qin XY, Zhang GH. Expression of repulsive guidance molecule A in the hippocampus of rat models of cerebral ischemia-reperfusion injury during treadmill exercise. Zhongguo Zuzhi Gongcheng Yanjiu. 2015;19:685–690. [Google Scholar]
- 15.He HW, Zhang YL, Yu BQ, Ye G, You W, So KF, Li X. Soluble Nogo receptor 1 fusion protein protects neural progenitor cells in rats with ischemic stroke. Neural Regen Res. 2019;14:1755–1764. doi: 10.4103/1673-5374.257531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He J, Zhang Y, Xu T, Zhao Q, Wang D, Chen CS, Tong W, Liu C, Xu T, Ju Z, Peng Y, Peng H, Li Q, Geng D, Zhang J, Li D, Zhang F, Guo L, Sun Y, Wang X, et al. Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke: the CATIS randomized clinical trial. JAMA. 2014;311:479–489. doi: 10.1001/jama.2013.282543. [DOI] [PubMed] [Google Scholar]
- 17.Henon H, Durieu I, Guerouaou D, Lebert F, Pasquier F, Leys D. Poststroke dementia: incidence and relationship to prestroke cognitive decline. Neurology. 2001;57:1216–1222. doi: 10.1212/wnl.57.7.1216. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 19.Luo Y, Wang X, Matsushita K, Wang C, Zhao X, Hu B, Liu L, Li H, Liu G, Jia Q, Wang Y, Wang Y. Associations between estimated glomerular filtration rate and stroke outcomes in diabetic versus nondiabetic patients. Stroke. 2014;45:2887–2893. doi: 10.1161/STROKEAHA.114.005380. [DOI] [PubMed] [Google Scholar]
- 20.Olsson T, Nygren J, Hakansson K, Lundblad C, Grubb A, Smith ML, Wieloch T. Gene deletion of cystatin C aggravates brain damage following focal ischemia but mitigates the neuronal injury after global ischemia in the mouse. Neuroscience. 2004;128:65–71. doi: 10.1016/j.neuroscience.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 21.Patel MD, Coshall C, Rudd AG, Wolfe CD. Cognitive impairment after stroke: clinical determinants and its associations with long-term stroke outcomes. J Am Geriatr Soc. 2002;50:700–706. doi: 10.1046/j.1532-5415.2002.50165.x. [DOI] [PubMed] [Google Scholar]
- 22.Pendlebury ST, Cuthbertson FC, Welch SJ, Mehta Z, Rothwell PM. Underestimation of cognitive impairment by Mini-Mental State Examination versus the Montreal Cognitive Assessment in patients with transient ischemic attack and stroke: a population-based study. Stroke. 2010;41:1290–1293. doi: 10.1161/STROKEAHA.110.579888. [DOI] [PubMed] [Google Scholar]
- 23.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves JW, Hill MN, Jones DH, Kurtz T, Sheps SG, Roccella EJ Council on High Blood Pressure Research Professional and Public Education Subcommittee. American Heart Association. Recommendations for blood pressure measurement in humans: an AHA scientific statement from the Council on High Blood Pressure Research Professional and Public Education Subcommittee. J Clin Hypertens (Greenwich) 2005;7:102–109. doi: 10.1111/j.1524-6175.2005.04377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sink KM, Divers J, Whitlow CT, Palmer ND, Smith SC, Xu J, Hugenschmidt CE, Wagner BC, Williamson JD, Bowden DW, Maldjian JA, Freedman BI. Cerebral structural changes in diabetic kidney disease: African American-Diabetes Heart Study MIND. Diabetes Care. 2015;38:206–212. doi: 10.2337/dc14-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slinin Y, Peters KW, Ishani A, Yaffe K, Fink HA, Stone KL, Steffes M, Ensrud KE Study of Osteoporotic Fractures. Cystatin C and cognitive impairment 10 years later in older women. J Gerontol A Biol Sci Med Sci. 2015;70:771–778. doi: 10.1093/gerona/glu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tatemichi TK, Desmond DW, Stern Y, Paik M, Sano M, Bagiella E. Cognitive impairment after stroke: frequency, patterns, and relationship to functional abilities. J Neurol Neurosurg Psychiatry. 1994;57:202–207. doi: 10.1136/jnnp.57.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tizon B, Sahoo S, Yu H, Gauthier S, Kumar AR, Mohan P, Figliola M, Pawlik M, Grubb A, Uchiyama Y, Bandyopadhyay U, Cuervo AM, Nixon RA, Levy E. Induction of autophagy by cystatin C: a mechanism that protects murine primary cortical neurons and neuronal cell lines. PLoS One. 2010;5:e9819. doi: 10.1371/journal.pone.0009819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 29.Wada M, Nagasawa H, Kawanami T, Kurita K, Daimon M, Kubota I, Kayama T, Kato T. Cystatin C as an index of cerebral small vessel disease: results of a cross-sectional study in community-based Japanese elderly. Eur J Neurol. 2010;17:383–390. doi: 10.1111/j.1468-1331.2009.02809.x. [DOI] [PubMed] [Google Scholar]
- 30.Wakasugi K, Nakano T, Morishima I. Association of human neuroglobin with cystatin C, a cysteine proteinase inhibitor. Biochemistry. 2004;43:5119–5125. doi: 10.1021/bi0495782. [DOI] [PubMed] [Google Scholar]
- 31.Webb AJ, Pendlebury ST, Li L, Simoni M, Lovett N, Mehta Z, Rothwell PM. Validation of the Montreal cognitive assessment versus mini-mental state examination against hypertension and hypertensive arteriopathy after transient ischemic attack or minor stroke. Stroke. 2014;45:3337–3342. doi: 10.1161/STROKEAHA.114.006309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang B, Zhu J, Miao Z, Zhou B, Ge W, Zhao H, Xu X. Cystatin C is an independent risk factor and therapeutic target for acute ischemic stroke. Neurotox Res. 2015;28:1–7. doi: 10.1007/s12640-015-9522-3. [DOI] [PubMed] [Google Scholar]
- 33.Zhang JB, Geng N, Li ZG, Qiao HJ, Sun HR, Li F. Biomarkers of renal function in type 2 diabetic patients with cognitive impairment. Neurosci Lett. 2016;610:19–23. doi: 10.1016/j.neulet.2015.10.059. [DOI] [PubMed] [Google Scholar]
- 34.Zhang XQ, Li L, Huo JT, Cheng M, Li LH. Effects of repetitive transcranial magnetic stimulation on cognitive function and cholinergic activity in the rat hippocampus after vascular dementia. Neural Regen Res. 2018;13:1384–1389. doi: 10.4103/1673-5374.235251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong C, Bu X, Xu T, Guo L, Wang X, Zhang J, Cui Y, Li D, Zhang J, Ju Z, Chen CS, Chen J, Zhang Y, He J. Serum matrix metalloproteinase-9 and cognitive impairment sfter scute ischemic stroke. J Am Heart Assoc. 2018;7:e007776. doi: 10.1161/JAHA.117.007776. [DOI] [PMC free article] [PubMed] [Google Scholar]


