Loss of central vision critical to everyday activities such as reading, face-recognition and driving due to damage in the central retina (the macula) is the leading cause of irreversible blindness amongst adults in the developed world. This condition, termed age-related macular degeneration (AMD), is a complex, chronic degenerative disease driven by a combination of genetic and lifestyle risk factors. Early signs of retinal changes in people as young as 30–40 years have been reported, although these individuals appear to be asymptomatic. However, by the age of 65, the disease is present in ~3% of individuals, which increases dramatically to affect 1/3 of individuals by the eighth decade of life. Early to intermediate AMD is estimated to affect ~150 million individuals globally, with another 10 million individuals suffering from end-stage, sight-threatening forms. These terminal stages are broadly grouped into dry (geographic atrophy, GA) or wet (choroidal neovascular, CNV) AMD (Sarks et al., 1988; Bird et al., 2014), with similar frequencies reported in patients. Recent advances in identifying genetic risk factors, including our discoveries in this field, indicate an initial shared pathology before progressing to aforementioned late-stage phenotypes. Currently, GA patients have no effective treatment, which may in part be due to the lack of good in vivo models for GA studies. Here, we summarize our new findings that describe an altogether new mouse model with GA-like features which shows progressive outer retinal pathology (Ibbett et al., 2019) that can be used to gain novel insights into GA and potentially as a tool for drug development.
GA is characterized by irreversible damage to a monolayer of cells beneath the neuroretina, termed the retinal pigment epithelium (RPE), and the loss of overlying photoreceptors (Figure 1A). Initial RPE pathology including pigment abnormalities, disorganized/shortened apical RPE microvilli and formation of confluent or large drusen under RPE cells in the macula, supports the widely held view of presumed RPE dysfunction as a key player in GA (Sarks et al., 1988; Bird et al., 2014). This is followed by atrophy of rod and subsequently cone macula photoreceptors, which results in loss of central vision (Figure 1B). However, it must be noted that this sequence of tissue damage does not necessarily follow the same course in all patients. For instance, photoreceptor loss is reported in some cases without any obvious morphological changes to the underlying RPE and its supportive Bruch’s membrane (BrM) (Bird et al., 2014). Disease can also progress at different rates in different patients. Moreover, bilateral GA in the same patient does not always advance at the same rate. Collectively, these observations reflect the complexity of GA, and given the associated genetic and lifestyle risks involved, highlight the difficulties in understanding how disease is triggered and progresses in different individuals. A further hindrance to studying GA is the lack of good in vivo models, which has meant that present understanding is still largely based on descriptive features rather than on molecular definitions of the disease. These factors have resulted in the current unacceptable situation where GA patients have no effective treatment. In contrast, the CNV form of AMD is somewhat better understood, and is characterized by the development of choroidal vessels, breaks in the RPE/BrM, leakage of exudates from immature vessels causing retinal detachment and formation of fibrotic tissue in the macula. Compared to GA, which can occur over many years, neovascular AMD can progress rapidly to cause blindness in a matter of weeks or months. Fortunately, most CNV patients can be treated with regular intravitreal injections using off-label or bespoke vascular endothelial growth factor (VEGF) inhibitors, which target VEGF-driven choroidal angiogenic processes. However, initial visual gains that can be impressive in some patients can be lost over time, with recent evidence showing damage to the RPE after prolonged VEGF inhibition, suggesting a de facto switch to a GA phenotype in at least some CNV patients.
Figure 1.
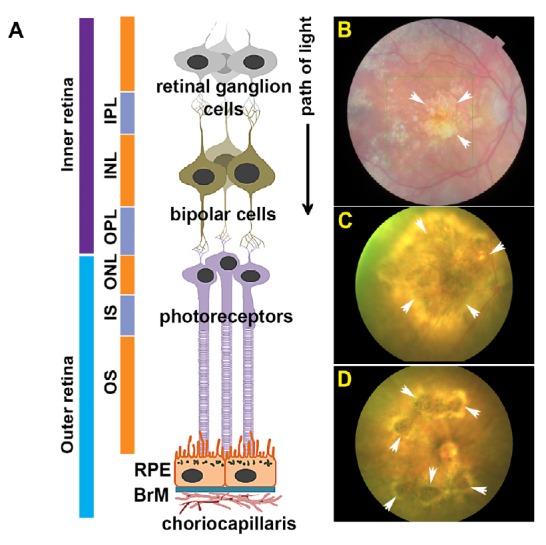
Anatomy of the retina and funduscopy showing geographic atrophy (GA) alongside laser-induced GA-like lesions in mouse eyes.
(A) Schematic diagram showing different cell layers of the inner and outer retina in cross-section. Retinal ganglion cells (RGC), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), inner segments (IS), outer segments (OS), retinal pigment epithelium (RPE) and Bruch’s membrane (BrM). Adapted from Lynn et al. (2017). (B) Representative colour fundus photograph (CFP) from an age-related macular degeneration patient carrying a variant form of complement factor I (Gly119Arg). Notice prominent GA lesion in the macula (arrows). (C, D) Representative CFP demonstrating recapitulation of focal GA-like lesions in mouse eyes (arrows) 4 weeks after laser treatment.
Assessment of AMD patients at Southampton are carried out using multi-modal, non-invasive retinal scans including colour fundus photography (CFP), microperimetry, optical coherence tomography (OCT), fundus autofluorescence imaging, fluorescein angiography (FA) and most recently, optical coherent angiography. These tools are continuously evolving such that their versatility, design and sensitivities are being improved by manufacturers to provide powerful new devices for better patient assessment and monitoring. Despite recent advances in understanding its genetics alongside these technological developments, the current approach to AMD is still largely reactive. To better understand its pathology and transition to a more preventative strategy, investigators including ourselves have adopted a multi-layered approach by combining in vitro and in vivo models as well as studies in human donor eyes that translationally link together, providing potential insights into a downstream clinical pathway. Each of these aforementioned approaches have limitations on their own, but can also offer distinct advantages. For instance, studies in mouse models offer the possibility of elucidating how disease develops in living retinas in a manner that is difficult to recapitulate in a cell model, or indeed by analyzing fixed post-mortem AMD eyes alone. Studies using human donor eyes however can be hugely informative, since these are bona-fide AMD tissues rather than surrogate models of the disease. Fixed tissues though provide only a snapshot of GA with their value hinged on the state of preservation, which is depended on the manner and timeliness of fixation following enucleation (Williams et al., 2016). Unfortunately, the lack of easily accessible high-quality donor AMD tissues in the UK has proved to be an impediment to research. With this in mind, we recently established a dedicated eye biobank at Southampton. Rather than being left to research charities or local support groups to fund such important but costly programs, initiatives of this kind should ideally be supported through central funding. Larger/national tissue repositories, similar to those established for brain banks, would enable investigators to access GA tissues from different disease stages and better link together novel findings from in vitro and mouse studies.
As previously highlighted, an underlying reason for our limited understanding of GA is the lack of good in vivo models that reproduce salient features of the disease. This contrasts with advances in treating CNV, where a laser-induced CNV mouse model which was described two decades ago (Tobe et al., 1998) benefited studies into neovascular pathology, and was also used to test the initial effectiveness of VEGF inhibitors. Although a plethora of in vivo models were developed to study retinal degeneration over the past few decades (Pennesi et al., 2012), no single model reproduces the full AMD spectrum, with each animal recapitulating some features of AMD, or ‘AMD-like’ features. Notwithstanding that fact that rodents do not actually possess an anatomical macula equivalent to humans, these animals are nevertheless particularly amenable to studies (Volland et al., 2015) due to their (1) broad similarities to human eyes, (2) rapid onset/development of retinopathy within a short period, (3) the capacity to create transgenic knock-in/knock-out animals with relative ease, as well as (4) the potential for a high degree of experimental manipulation. However, questions related to the relevance of ‘actual AMD’ persists with such AMD-like models, underpinned by the development of phenotypes inconsistent with the sequence and type of pathology reported in donor AMD tissues. Murine models that specifically recapitulate GA-like features have been grouped into genetically engineered mice, immunologically manipulated mice and senescence-accelerated animals that spontaneously develop desirable pathology (Ramkumar et al., 2010). Some attractive models used for GA studies consist of these combinations such as the aged, human apolipoprotein E4 targeted replacement mice fed a high fat cholesterol-enriched diet. These mice were used effectively to demonstrate the importance of the Alzheimer’s-beta (Aβ)-driven pathology in the senescent retina with Aβ immunized animals rescued from developing visual defects (Pennesi et al., 2012). Nonetheless, several issues persist with mouse models used for investigations into AMD in general, some of which affect GA studies in particular. Genetically modified and combination models may have to be aged for long periods (sometimes for several months or years) before sought-after GA-like features develop and therefore involve high maintenance costs. Disease penetrance may also be relatively low and/or be unreliable. Hence, investigators are obliged to start with large cohorts so litters with the desired phenotype are generated to undertake studies with sufficient power. Of note, an issue that specifically affects GA studies is that disease often develops as a retina-wide pathology rather than as a focal GA-like lesion.
In order to overcome some of these challenges, we recently developed the ‘Southampton AMD model’, which recapitulate important GA-like features (Ibbett et al., 2019). Eyes of adult C57BL/6J mice that are widely used in laboratories were lasered at multiple spots, which coalesced after 1 week to produce a well-defined focal lesion in the retina. Mice were longitudinally assessed by CFP, OCT and ERGs, with tissues analyzed at specific time points by light microscopy of semi-thin sections, confocal-immunofluorescence and by transmission electron microscopy (TEM). The gene expression profile between lasered and adjacent non-lasered regions of the retina was quantified by mRNA analysis. This model showed focal GA-like lesions by CFP in the central mouse retina which persisted for 3 months (Figure 1C and D). One week after laser treatment, OCT scans showed an obliterated outer nuclear layer, photoreceptor inner and outer segments as well as loss of demarcation between the RPE and BrM. These features persisted in the following weeks with hyper-reflective pathology and progressive involvement of the inner nuclear and plexiform layers. Moreover, these changes were associated with diminished retinal thickness within the first few weeks after laser treatment, which became stable approximately a month later. Hyper-reflective OCT in GA retinas as well as reduced retinal thickness during initial stages are also reported in patients. Assessment of tissues in the form of toluidine blue-stained semi-thin sections followed by ultrathin sections by TEM revealed the complete absence of photoreceptors in lesions associated with a collapsed inner retina where the inner nuclear layer was observed lying apposed to an intact RPE monolayer. The lesion margins, which formed wedge-shaped triangles when viewed in cross-section, contained tapering outer plexiform and nuclear layers alongside photoreceptor inner and outer segments similar to GA histopathology described in seminal studies by Sarks and colleagues (Sarks et al., 1988). Ultrastructural images revealed an RPE monolayer with hypo and hyper-pigmentation, sometimes in adjacent cells, shortened and disorganized apical microvilli as well as overlying debris which were discernible as leftover photoreceptor outer segments within lesions. Apart from occasional atrophic RPE cells, there were no discernible breaks in the RPE monolayer. Release of pigmented granules were observed in lasered spots in a manner described in GA tissues (Sarks et al., 1988), which could originate from atrophic RPE. Importantly, electron micrographs revealed an intact BrM in lesioned regions. There was also no evidence of new vessel formation as assessed by confocal immunofluorescence, nor any evidence of VEGF upregulation by qPCR analysis. FA of lasered retinae showed no signs of dye leakage. Collectively, these results preclude any choroidal involvement observed in CNV patients and in the laser CNV mouse model. One of the most exciting features of our model is the presence of a focal GA-like lesion with diminishing damage radiating from the lesion, which is consistent with GA histopathology (Sarks et al., 1988). These graded zones in lasered mice corresponded to an inflamed retina with GFAP (Müller cell activation) and the FcγR1 microglial maker upregulation (recruitment/activation of myeloid cells), which is consistent with GA. Scotopic ERGs of lasered mice revealed decreased A and B-wave amplitudes and increased B-wave implicit times similar to GA patients. mRNA analysis of mouse lesions revealed upregulated complement/complement regulatory pathways, glial activation as well as activated inflammatory pathways reported in GA retinas. Its acute nature, however, preclude studies into FAF and drusen-related pathology, but is nonetheless a good model to investigate other important early pathology. The Southampton AMD model also overcomes many of the constraints associated with in vivo models currently used for GA studies and is furthermore an excellent tool for drug discovery.
Amongst the few other notable mouse models that recapitulate focal GA-like lesions is the sodium iodide injection (Bhutto et al., 2018) and the subretinal Aβ-injection mouse models developed by ourselves and others (Bruban et al., 2009; Liu et al., 2015; Ratnayaka and Lynn, 2016). These, alongside the development of the Southampton AMD mouse model and initiatives such as the establishment of eye biobanks, as well as new clinical trials at Southampton, mean that better progress in understanding this irreversible blinding disease can now be anticipated.
This work was supported by the Awards to JAR from the NC3R (NC/L0001152/1), Macular Society UK, National Eye Research Centre, Alzheimer’s Research UK (ARUK) South Coast Network, Fight for Sight, Retina UK and the Gift of Sight Appeal.
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Bhutto IA, Ogura S, Baldeosingh R, McLeod DS, Lutty GA, Edwards MM. An acute injury model for the phenotypic characteristics of geographic atrophy. Invest Ophthalmol Vis Sci. 2018;59:143–151. doi: 10.1167/iovs.18-24245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bird AC, Phillips RL, Hageman GS. Geographic atrophy: a histopathological assessment. JAMA Ophthalmol. 2014;132:338–345. doi: 10.1001/jamaophthalmol.2013.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruban J, Glotin AL, Dinet V, Chalour N, Sennlaub F, Jonet L, An N, Faussat AM, Mascarelli F. Amyloid-beta(1-42) alters structure and function of retinal pigmented epithelial cells. Aging Cell. 2009;8:162–177. doi: 10.1111/j.1474-9726.2009.00456.x. [DOI] [PubMed] [Google Scholar]
- 4.Ibbett P, Goverdhan SV, Pipi E, Chouhan JK, Keeling E, Angus EM, Scott JA, Gatherer M, Page A, Teeling JL, Lotery AJ, Arjuna Ratnayaka J. A lasered mouse model of retinal degeneration displays progressive outer retinal pathology providing insights into early geographic atrophy. Sci Rep. 2019;9:7475. doi: 10.1038/s41598-019-43906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C, Cao L, Yang S, Xu L, Liu P, Wang F, Xu D. Subretinal injection of amyloid-beta peptide accelerates RPE cell senescence and retinal degeneration. Int J Mol Med. 2015;35:169–176. doi: 10.3892/ijmm.2014.1993. [DOI] [PubMed] [Google Scholar]
- 6.Lynn SA, Keeling E, Munday R, Gabha G, Griffiths H, Lotery AJ, Ratnayaka JA. The complexities underlying age-related macular degeneration: could amyloid beta play an important role? Neural Regen Res. 2017;12:538–548. doi: 10.4103/1673-5374.205083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pennesi ME, Neuringer M, Courtney RJ. Animal models of age related macular degeneration. Mol Aspects Med. 2012;33:487–509. doi: 10.1016/j.mam.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramkumar HL, Zhang J, Chan CC. Retinal ultrastructure of murine models of dry age-related macular degeneration (AMD) Prog Retin Eye Res. 2010;29:169–190. doi: 10.1016/j.preteyeres.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ratnayaka JA, Lynn S. 1st ed. Croatia: Intech Publishing; 2016. Alzheimer’s-related amyloid beta peptide aggregates in the ageing retina: implications for sight loss and dementia. [Google Scholar]
- 10.Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye (Lond) 1988;2:552–577. doi: 10.1038/eye.1988.106. [DOI] [PubMed] [Google Scholar]
- 11.Tobe T, Ortega S, Luna JD, Ozaki H, Okamoto N, Derevjanik NL, Vinores SA, Basilico C, Campochiaro PA. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am J Pathol. 1998;153:1641–1646. doi: 10.1016/S0002-9440(10)65753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volland S, Esteve-Rudd J, Hoo J, Yee C, Williams DS. A comparison of some organizational characteristics of the mouse central retina and the human macula. PloS One. 2015;10:e0125631. doi: 10.1371/journal.pone.0125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams AM, Stamer WD, Allingham RR. Increasing the availability and quality of donor eyes for research. JAMA Ophthalmol. 2016;134:351–352. doi: 10.1001/jamaophthalmol.2015.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]


