Abstract
Interstitial fluid movement in the brain parenchyma has been suggested to contribute to sustaining the metabolism in brain parenchyma and maintaining the function of neurons and glial cells. The pulsatile hydrostatic pressure gradient may be one of the driving forces of this bulk flow. However, osmotic pressure-related factors have not been studied until now. In this prospective observational study, to elucidate the relationship between osmolality (mOsm/kg) in the serum and that in the cerebrospinal fluid (CSF), we simultaneously measured the serum and CSF osmolality of 179 subjects with suspected neurological conditions. Serum osmolality was 283.6 ± 6.5 mOsm/kg and CSF osmolality was 289.5 ± 6.6 mOsm/kg. Because the specific gravity of serum and CSF is known to be 1.024–1.028 and 1.004–1.007, respectively, the estimated average of osmolarity (mOsm/L) in the serum and CSF covered exactly the same range (i.e., 290.5–291.5 mOsm/L). There was strong correlation between CSF osmolality and serum osmolality, but the difference in osmolality between serum and CSF was not correlated with serum osmolality, serum electrolyte levels, protein levels, or quotient of albumin. In conclusion, CSF osmolarity was suggested to be equal to serum osmolarity. Osmolarity is not one of the driving forces of this bulk flow. Other factors such as hydrostatic pressure gradient should be used to explain the mechanism of bulk flow in the brain parenchyma. This study was approved by the Institutional Review Board of the Tohoku University Hospital (approval No. IRB No. 2015-1-257) on July 29, 2015.
Keywords: brain parenchyma, bulk flow, cerebrospinal fluid, hydrostatic pressure, interstitial fluid, osmolarity, osmotic pressure
Chinese Library Classification No. R446; R741
Introduction
The concept of fluid dynamics in the central nervous system (CNS) has dramatically changed in the last decades. The fluid circulation within the CNS was once believed to occur simply from the arteries to the veins, and to be totally independent from circulation of cerebrospinal fluid (CSF) (Bering, 1955; Whedon and Glassey, 2009; Veening and Barendregt, 2010). In other words, circulation of CSF was believed to be completely independent from the fluid circulation inside the CNS. Recently, a new concept known as “glymphatic system” has been proposed and experimentally confirmed (Iliff et al., 2013; Nedergaard, 2013; Hladky and Barrand, 2014, 2016). In the glymphatic hypothesis, interstitial fluid in the CNS is discharged into the perivascular spaces, which consist of a continuum of CSF around the CNS. This drainage system is suggested to discharge dissolved toxic metabolites and carbon dioxide from the brain parenchyma into the CSF around the CNS (Eugene and Masiak, 2015; Akaishi et al., 2019). At present, one of the primary driving forces producing such slow bulk flow in the brain parenchyma is expected to be the hydrostatic pressure gradient from the arteries to the brain parenchyma (Papisov et al., 2013; Hladky and Barrand, 2014). However, when we think of the factors producing bulk flow within the parenchyma, difference in some unidentified factors from the cerebral arteries to the CSF surrounding the CNS will be needed. Osmotic pressure-based fluid movement may be one of the factors regulating such bulk flow of interstitial fluid (Stohrer et al., 2000; Hladky and Barrand, 2014). Rosenberg et al. (1980) showed that intravenous administration of 1.5–3.0 g/kg mannitol to the animals led to inflow of fluid into gray matter from the ventricles. Even in human subjects, some previous reports suggested that drastic change of the osmotic pressure in the blood or in the CSF may trigger neurological symptoms (Martin, 2004; Giuliani and Peri, 2014; Akaishi et al., 2018a, b). It is likely that an abnormal osmolarity difference between the serum and the CSF may cause neuronal and glial dysfunctions by disturbing the bulk flow within the parenchyma.
In this prospective observational study, we simultaneously collected data associated with the osmotic pressure from both serum and the CSF from a large number of human subjects to determine whether the osmotic pressures in serum and CSF contribute to producing the supposed bulk flow in the central nervous system. Also, we tried to identify the possible factors that produce such an osmolality gradient, if any, between serum and CSF.
Subjects and Methods
Subjects
In this prospective observational study, patients with suspected miscellaneous neurological disorders, who underwent blood testing and a lumbar puncture in Tohoku University Hospital (Japan) between November 2015 and March 2017 were asked to be enrolled. Therefore, 179 consecutive inpatients agreed to participate in this study. The eventual definite diagnoses of the enrolled subjects included polyneuropathy (n = 28) as well as motor neuron diseases consisting of amyotrophic lateral sclerosis (n = 22), multiple sclerosis (n = 14), myelitis or myelopathy (n = 13), spinocerebellar degeneration (n = 8), Parkinson’s disease (n = 6), multiple system atrophy (n = 6), neuromyelitis optica spectrum disorders (n = 5), epilepsy (n = 4), sarcoidosis (n = 4), spastic paraplegia (n = 4), Creutzfeldt-Jakob disease (n = 3), psychosomatic disorder (n = 3), and other miscellaneous conditions (n = 59). Among the 179 subjects, 102 were male and 77 were female. The age during the sample collection was 55.5 ± 17.5 years (mean ± standard deviation). None of the included patients suffered from diseases that are known to cause osmotic pressure abnormality, such as the syndrome of inappropriate antidiuretic hormone secretion (SIADH). This study was approved by the Institutional Review Board of the Tohoku University Hospital (approval No. IRB No. 2015-1-257) on July 29, 2015 and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all enrolled patients.
Studied variables and sample measurement
The following variables were comprehensively collected from the enrolled subjects: age, diagnosis, serum and CSF osmolality [mOsm/kg], serum sodium ion level, serum potassium ion level, serum and CSF chloride ion levels, serum and CSF total protein levels, serum and CSF albumin levels, serum and CSF immunoglobulin G (IgG) levels, serum and CSF glucose levels, and cell count in the CSF. Based on these measured variables, the albumin quotient (QAlb), quotient of IgG (QIgG), and the IgG index were calculated. The IgG index was calculated based on the following equation (Tibbling et al., 1977; Blennow et al., 1994):
IgG-index = 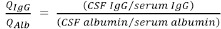
The difference in osmolality between serum and CSF (Δ-osmolality) was calculated in each subject by the following equation:
Δ-osmolality [mOsm/kg] = CSF osmolality – serum osmolality
Serum samples were prepared by centrifugation at 2000–2500 × g for 5 minutes, and the following measurements of serum substances were swiftly performed after centrifugation. Serum and CSF osmolality was measured by freezing point depression method using OSMO STATIONTM OM-6060 (Arkray, Kyoto, Japan). Serum and CSF sample measurements were performed within 120 minutes. Because the specific gravity for each acquired sample was not measured, data from previous reports were utilized to estimate serum and CSF osmolarity [mOsm/L] based on the acquired osmolality [mOsm/kg]. The range of specific gravity in serum was regarded to be 1.024–1.028 and that in the CSF was regarded to be 1.004–1.007 (Araki et al., 2012; Lee et al., 2015).
Statistical analysis
Osmotic pressure-related variables between serum and CSF were compared using a paired t-test. The correlation coefficient between each pair of studied variables was calculated using the Pearson’s correlation coefficient. Multiple regression analysis was also performed to assess the relationship between each fraction of osmolality and the achieved Δ-osmolality within the enrolled subjects.
Because multiple comparisons were simultaneously performed, a P-value < 0.01 was regarded as statistically significant. Based on the same reason, 99% confidence intervals (CI) of the estimated correlation coefficients in the studied pairs were calculated. Statistical analyses were conducted using either SPSS Statistics Base 22 software (IBM, Armonk, NY, USA) or MATLAB R2015a (MathWorks, Natick, MA, USA).
Results
Measurements of blood and CSF data
Measurements of laboratory serum and CSF data are shown in Table 1. Osmolality levels and chloride ion levels were significantly higher in the CSF than those in the serum. As described later, CSF osmolality was slightly greater than serum osmolality in most of the studied subjects with the difference of 0–12 mOsm/kg. The types of neurological disorders of the enrolled subjects did not significantly affect the value of serum osmolality, CSF osmolality, or Δ-osmolality. The grouped scatter plots of serum osmolality, CSF osmolality, and Δ-osmolality among the enrolled subjects are shown in Figure 1. As described above, if we regard the estimated range of specific gravity in serum to be 1.024–1.028 and that in CSF to be 1.004–1.007, the estimated average of the osmolarity [mOsm/L] in serum will be 290.4–291.5 and that in CSF will be 290.7–291.5, both of which are almost totally overlapped.
Table 1.
Laboratory blood and CSF data
| Pairs (n) | Venous blood | CSF | P-value | |
|---|---|---|---|---|
| Osmolality (mOsm/kg) | 179 | 283.6±6.5 | 289.5±6.6 | < 0.0001 |
| Chloride ion (mEq/L) | 157 | 104.2±3.0 | 126.5±3.2 | < 0.0001 |
| Total protein (g/dL) | 170 | 6.78±0.56 | 0.05±0.03 | < 0.0001 |
| Albumin (g/dL) | 168 | 4.04±0.49 | 0.03±0.02 | < 0.0001 |
| IgG (mg/dL) | 162 | 1123±335 | 4.2±2.8 | < 0.0001 |
| Glucose (mg/dL) | 173 | 101.7±30.1 | 61.1±15.0 | < 0.0001 |
Data are expressed as the mean ± SD. The shown P-values are the results of the paired t-test. CSF: Cerebrospinal fluid; IgG: immunoglobulin G; SD: standard deviation.
Figure 1.
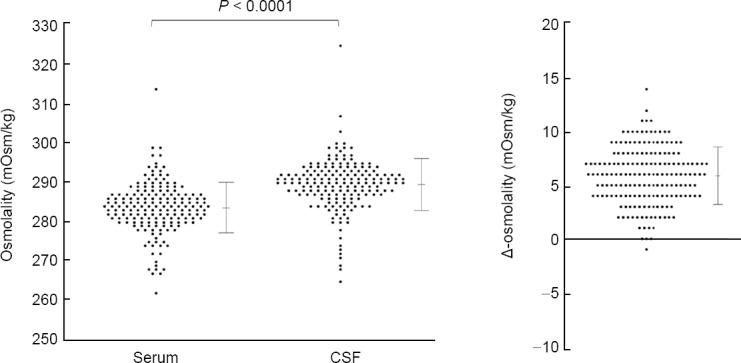
Difference in osmolality between serum and CSF.
The osmolality (mOsm/kg) was significantly higher in CSF than in serum (P < 0.0001; n = 179, paired t-test). Almost all of the subjects showed positive values for Δ-osmolality. CSF: Cerebrospinal fluid; Δ-Osm: difference in osmolality between serum and CSF.
Correlation coefficients between the osmolality and other laboratory data
To assess the relationship between the value of Δ-osmolality and each of the osmotic pressure-related variables, we evaluated the correlation coefficients between the measured osmolalities (i.e., serum osmolality, CSF osmolality, Δ-osmolality) and other laboratory data. The correlation coefficient matrix is shown in Table 2. The osmolality of CSF showed a very strong positive correlation with serum osmolality, and also showed a strong positive correlation with serum sodium ion level (P < 0.0001 for both). Meanwhile, CSF osmolality and Δ-osmolality did not correlate with QAlb, QIgG, IgG-index or CSF cell count (P ≥ 0.01 for all). Lastly, a multiple regression analysis was performed using Δ-osmolality as the objective variable. Sex, age, serum and CSF protein levels, serum and CSF chloride levels, and serum and CSF glucose levels were used as the explanatory variables. None of the above-described explanatory variables were significantly correlated with the value of Δ-osmolality (P ≥ 0.10 for all variables).
Table 2.
Correlation coefficient matrix between osmolality and other laboratory tests
| Serum osmolality | CSF osmolality | Δ-Osmolality | |
|---|---|---|---|
| Serum osmolality | – | 0.917*** | −0.144 |
| (0.880–0.943) | (−0.327–0.049) | ||
| CSF osmolality | – | – | 0.262** |
| (0.074–0.432) | |||
| Age | 0.244* | 0.183 | −0.148 |
| (0.049–0.421) | (−0.015–0.367) | (−0.335–0.051) | |
| Serum Na+ | 0.648*** | 0.709*** | 0.096 |
| (0.501–0.758) | (0.582–0.803) | (−0.124–0.307) | |
| Serum K+ | 0.151 | 0.017 | −0.295* |
| (−0.099–0.383) | (−0.230–0.262) | (−0.504– −0.052) | |
| Serum Cl– | 0.421*** | 0.527*** | 0.187 |
| (0.196–0.604) | (0.323–0.684) | (−0.061–0.413) | |
| CSF Cl– | 0.713*** | 0.786*** | 0.122 |
| (0.570–0.813) | (0.673–0.863) | (−0.122–0.351) | |
| Serum TP | 0.237 | 0.194 | −0.105 |
| (−0.020–0.465) | (−0.065–0.429) | (−0.351–0.155) | |
| CSF TP | −0.229 | −0.322** | −0.199 |
| (−0.437–0.002) | (−0.514– −0.098) | (−0.411–0.034) | |
| QAlb | −0.102 | −0.192 | −0.175 |
| (−0.351–0.160) | (−0.429–0.070) | (−0.415–0.087) | |
| CSF-CC | −0.001 | −0.069 | −0.142 |
| (−0.225–0.223) | (−0.288–0.157) | (−0.355–0.084) | |
| IgG-index | −0.139 | −0.231 | −0.099 |
| (−0.413–0.158) | (−0.489–0.064) | (−0.379–0.198) |
A list of Pearson’s correlation coefficients (r) with the 99% confidence intervals. Δ-Osmolality is the difference of osmolality between CSF and venous blood. Δ(TP-Alb) is the difference between the serum total protein level and the serum albumin level. CSF: Cerebrospinal fluid; CSF-CC: cell count in the cerebrospinal fluid; IgG: immunoglobulin G; QAlb: quotient of albumin; QIgG: quotient of immunoglobulin G; TP: total protein. *P < 0.01, **P < 0.001, ***P < 0.0001.
Discussion
In this study, to further elucidate the physiological mechanisms of bulk flow in the CNS, we focused on the osmotic pressure in the blood and the CSF. In each subject, CSF osmolality (mOsm/kg) was slightly higher than serum osmolality. The observed osmolality difference was independent of age, sex, and type of neurological conditions. Moreover, based on the assumption that the specific gravity of serum is 1.024–1.028 and that of the CSF is 1.004–1.007, the deduced serum and CSF osmolarity was estimated to be 290–292 mOsm/L. This study showed that CSF osmolality was strongly correlated with serum sodium ion levels, but it did not correlate with protein levels or QAlb, a supposed marker of blood-brain barrier (BBB) (Chen, 2011; Akaishi et al., 2015). These results suggest that the level of CSF osmolality is not regulated by CSF protein level or the permeability of BBB, but it is regulated simply by serum electrolyte level and the relatively free movement of water molecules across the blood-CSF barrier.
The interstitial fluid of the brain parenchyma is believed to be mostly produced by slow bulk flow from the capillary side to the CSF side in the concept of glymphatic hypothesis (Nedergaard, 2013; Hladky and Barrand, 2014, 2016; Nakada and Kwee, 2019). The achieved results showed that the calculated osmolarity [mOsm/L] was exactly the same between serum and CSF, implying that osmotic pressure is not one of the driving forces to produce the bulk flow within the brain parenchyma. This result further supports the previous studies reporting that the primary driving force of the bulk flow in the brain parenchyma may be the hydrostatic pressure gradient (Iliff et al., 2013; Smith et al., 2017; Mestre et al., 2018).
There are some limitations to this study. First, the data were collected from human subjects with suspected neurological disorders, but not from healthy controls. Second, the specific gravity of each acquired sample was not measured in this study. Thus, serum and CSF osmolarity [mOsm/l] is exactly the same, we need to collect samples from subjects without neurological disorders, such as pre-operative patients receiving lumbar anesthesia. Third, serum osmolality (mOsm/kg) and other serum laboratory data were measured using venous blood, but not arterial blood. The electrolyte levels seem to be similar between the venous blood and the arterial blood, but this has not been validated by clinical studies. Thus, strictly speaking, we need to simultaneously collect arterial blood and CSF, together with the data of the specific gravity. Serum and CSF osmolarity (mOsm/L) is truly the same. Osmotic pressure gradient does not contribute to producing the fluid movement in the brain parenchyma. The last limitation is that serum osmolality could have been affected by the sample processing, such as centrifugation. To solve this problem, whole blood without any sample processing should be used to evaluate the osmolality in the blood. However, because the plasma level of fibrinogen is usually less than 10% of the total plasma proteins, so the effect of sample processing on serum osmolality seems to be minimal and negligible.
To conclude, CSF osmolality (mOsm/kg) is higher than serum osmolality, but the estimated osmolarities (mOsm/L) of the serum and the CSF were exactly the same. Thus, osmotic pressure would not be one of the factors to produce the bulk flow in the brain parenchyma. Rather, hydrostatic pressure gradient from the arterial side to the venous or CSF side would be the primary driving force to realize fluid movement within the brain parenchyma.
Additional file: Open peer review report 1 (84.7KB, pdf) .
Acknowledgments:
We thank Mr. Takuya Takeda, Ms. Yuko Abe, and Prof. Mitsuo Kaku (Infection Control and Laboratory Diagnostics, Tohoku University Hospital, Japan) for measuring the data and offering professional advice regarding this study.
Footnotes
Conflicts of interest: The authors declare no conflict of interest for this study.
Financial support: The authors receive no funding from any organizations related to this study.
Institutional review board statement: This study was approved by the Institutional Review Board of the Tohoku University Hospital (approval No. IRB No. 2015-1-257) on July 29, 2015.
Declaration of patient consent: The authors certify that they have obtained all appropriate patient consent forms. In the forms, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement: This study followed the Strengthening the Reporting of OBervational studies in Epidemiology (STROBE) statement.
Biostatistics statement: The statistical methods of this study were reviewed by the statisticians of Tohoku University Hospital in Japan.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Christophe Pellegrino, Aix-Marseille University, France.
P-reviewer: Pellegrino C; C-Editor: Zhao M; S-Editor: Li CH; L-Editor: Song LP; T-Editor: Jia Y
References
- 1.Akaishi T, Takahashi T, Nakashima I, Aoki M. Abnormal osmolality gap exists in distal symmetric polyneuropathy. Tohoku J Exp Med. 2018a;246:59–64. doi: 10.1620/tjem.246.59. [DOI] [PubMed] [Google Scholar]
- 2.Akaishi T, Takahashi T, Himori N, Takeshita T, Nakazawa T, Aoki M, Nakashima I. Chloride imbalance is involved in the pathogenesis of optic neuritis in neuromyelitis optica. J Neuroimmunol. 2018b;320:98–100. doi: 10.1016/j.jneuroim.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Akaishi T, Narikawa K, Suzuki Y, Mitsuzawa S, Tsukita K, Kuroda H, Nakashima I, Fujihara K, Aoki M. Importance of the quotient of albumin, quotient of immunoglobulin G and Reibergram in inflammatory neurological disorders with diseasewith disease-specific patterns of blood-brain barrier permeability. Neurol Clin Neurosci. 2015;3:94–100. [Google Scholar]
- 4.Akaishi T, Onishi E, Abe M, Toyama H, Ishizawa K, Kumagai M, Kubo R, Nakashima I, Aoki M, Yamauchi M, Ishii T. The human central nervous system discharges carbon dioxide and lactic acid into the cerebrospinal fluid. Fluids Barriers CNS. 2019;16:8. doi: 10.1186/s12987-019-0128-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Araki J, Jona M, Eto H, Aoi N, Kato H, Suga H, Doi K, Yatomi Y, Yoshimura K. Optimized preparation method of platelet-concentrated plasma and noncoagulating platelet-derived factor concentrates: maximization of platelet concentration and removal of fibrinogen. Tissue Eng Part C Methods. 2012;18:176–185. doi: 10.1089/ten.tec.2011.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bering EA., Jr Choroid plexus and arterial pulsation of cerebrospinal fluid; demonstration of the choroid plexuses as a cerebrospinal fluid pump. AMA Arch Neurol Psychiatry. 1955;73:165–172. doi: 10.1001/archneurpsyc.1955.02330080043012. [DOI] [PubMed] [Google Scholar]
- 7.Blennow K, Fredman P, Wallin A, Gottfries CG, Frey H, Pirttila T, Skoog I, Wikkelso C, Svennerholm L. Formulas for the quantitation of intrathecal IgG production. Their validity in the presence of blood-brain barrier damage and their utility in multiple sclerosis. J Neurol Sci. 1994;121:90–96. doi: 10.1016/0022-510x(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 8.Chen RL. Is it appropriate to use albumin CSF/plasma ratio to assess blood brain barrier permeability? Neurobiol Aging. 2011;32:1338–1339. doi: 10.1016/j.neurobiolaging.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Eugene AR, Masiak J. The neuroprotective aspects of sleep. MEDtube Sci. 2015;3:35–40. [PMC free article] [PubMed] [Google Scholar]
- 10.Giuliani C, Peri A. Effects of hyponatremia on the brain. J Clin Med. 2014;3:1163–1177. doi: 10.3390/jcm3041163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hladky SB, Barrand MA. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS. 2014;11:26. doi: 10.1186/2045-8118-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hladky SB, Barrand MA. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS. 2016;13:19. doi: 10.1186/s12987-016-0040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, Deane R, Nedergaard M. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci. 2013;33:18190–18199. doi: 10.1523/JNEUROSCI.1592-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee H, Xie L, Yu M, Kang H, Feng T, Deane R, Logan J, Nedergaard M, Benveniste H. The effect of body posture on brain glymphatic transport. J Neurosci. 2015;35:11034–11044. doi: 10.1523/JNEUROSCI.1625-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin RJ. Central pontine and extrapontine myelinolysis: the osmotic demyelination syndromes. J Neurol Neurosurg Psychiatry. 2004;75(Suppl 3):iii22–28. doi: 10.1136/jnnp.2004.045906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mestre H, Tithof J, Du T, Song W, Peng W, Sweeney AM, Olveda G, Thomas JH, Nedergaard M, Kelley DH. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun. 2018;9:4878. doi: 10.1038/s41467-018-07318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakada T, Kwee IL. Fluid dynamics inside the brain barrier: current concept of interstitial flow, glymphatic flow, and cerebrospinal fluid circulation in the brain. Neuroscientist. 2019;25:155–166. doi: 10.1177/1073858418775027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nedergaard M. Neuroscience. Garbage truck of the brain. Science. 2013;340:1529–1530. doi: 10.1126/science.1240514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papisov MI, Belov VV, Gannon KS. Physiology of the intrathecal bolus: the leptomeningeal route for macromolecule and particle delivery to CNS. Mol Pharm. 2013;10:1522–1532. doi: 10.1021/mp300474m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg GA, Kyner WT, Estrada E. Bulk flow of brain interstitial fluid under normal and hyperosmolar conditions. Am J Physiol. 1980;238:F42–49. doi: 10.1152/ajprenal.1980.238.1.F42. [DOI] [PubMed] [Google Scholar]
- 21.Smith AJ, Yao X, Dix JA, Jin BJ, Verkman AS. Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife. 2017;6 doi: 10.7554/eLife.27679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stohrer M, Boucher Y, Stangassinger M, Jain RK. Oncotic pressure in solid tumors is elevated. Cancer Res. 2000;60:4251–4255. [PubMed] [Google Scholar]
- 23.Tibbling G, Link H, Ohman S. Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand J Clin Lab Invest. 1977;37:385–390. doi: 10.1080/00365517709091496. [DOI] [PubMed] [Google Scholar]
- 24.Veening JG, Barendregt HP. The regulation of brain states by neuroactive substances distributed via the cerebrospinal fluid; a review. Cerebrospinal Fluid Res. 2010;7:1. doi: 10.1186/1743-8454-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whedon JM, Glassey D. Cerebrospinal fluid stasis and its clinical significance. Altern Ther Health Med. 2009;15:54–60. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


