Keywords: cognitive dysfunction, corpus callosum, differentiation/DNA binding factor 2, hypoxia/ischemia, myelin basic protein, myelin sheath, remyelination, rosmarinic acid
Abstract
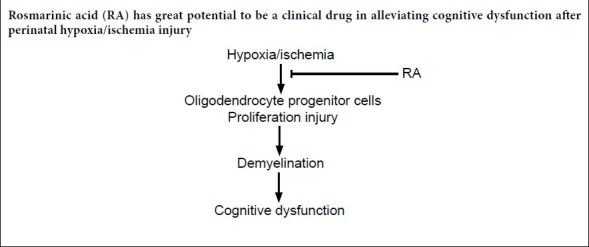
Rosmarinic acid, a common ester extracted from Rosemary, Perilla frutescens, and Salvia miltiorrhiza Bunge, has been shown to have protective effects against various diseases. This is an investigation into whether rosmarinic acid can also affect the changes of white matter fibers and cognitive deficits caused by hypoxic injury. The right common carotid artery of 3-day-old rats was ligated for 2 hours. The rats were then prewarmed in a plastic container with holes in the lid, which was placed in 37°C water bath for 30 minutes. Afterwards, the rats were exposed to an atmosphere with 8% O2 and 92% N2 for 30 minutes to establish the perinatal hypoxia/ischemia injury models. The rat models were intraperitoneally injected with rosmarinic acid 20 mg/kg for 5 consecutive days. At 22 days after birth, rosmarinic acid was found to improve motor, anxiety, learning and spatial memory impairments induced by hypoxia/ischemia injury. Furthermore, rosmarinic acid promoted the proliferation of oligodendrocyte progenitor cells in the subventricular zone. After hypoxia/ischemia injury, rosmarinic acid reversed to some extent the downregulation of myelin basic protein and the loss of myelin sheath in the corpus callosum of white matter structure. Rosmarinic acid partially slowed down the expression of oligodendrocyte marker Olig2 and myelin basic protein and the increase of oligodendrocyte apoptosis marker inhibitors of DNA binding 2. These data indicate that rosmarinic acid ameliorated the cognitive dysfunction after perinatal hypoxia/ischemia injury by improving remyelination in corpus callosum. This study was approved by the Animal Experimental Ethics Committee of Xuzhou Medical University, China (approval No. 20161636721) on September 16, 2017.
Chinese Library Classification No. R453; R741; R842.1
Introduction
Hypoxia/ischemia (H/I) injury, a common type of brain injury, causes long-term neurological disabilities in the brains of newborns and a high mortality rate, approximately 23% of all neonatal deaths worldwide (Jaworska et al., 2017; Ziemka-Nalecz et al., 2017). Following H/I injury, the surviving infants will exhibit symptoms such as cerebral palsy, cognitive and intellectual impairments and other behavior problems (van Handel et al., 2007; Jaworska et al., 2017). Unfortunately, the precise mechanism underlying the pathogenesis of behavior disorders following H/I injury remains unknown. Until now, the only commonly used treatment after H/I has been hypothermia, which stabilizes brain metabolism, but does not prevent brain injury, neither providing complete brain protection nor the facilitating repair to the injured brain (Tang et al., 2016; Jaworska et al., 2017). The importance of investigating potential therapies that attenuate brain damage and improve cognitive function impairments induced by H/I injury is driving this crucial research.
White matter, 50% of the central nervous system, plays a vital role in learning and other brain functions, coordinating communication between different brain regions (Fields et al., 2008; Davies et al., 2019). H/I injury in premature infants can lead to periventricular leukomalacia, which is predominately related to incomplete vascular development and impairment in the regulation of cerebral blood flow to the cerebral white matter (Volpe et al., 2001). The corpus callosum, the largest white matter structure in the brain, is especially affected. Periventricular leukomalacia causes injury to oligodendrocytes (a myelin-forming cell of the central nervous system) in the developing white matter and results in hypomyelination. This is closely associated with cognitive disabilities (Haynes et al., 2003). H/I causes rapid and severe damage to the oligodendrocytes via several pathways, including that of oxidative stress (Mifsud et al., 2014). Neighboring microglia also promote apoptosis and toxicity of oligodendrocytes after H/I injury through the production of cytokines tumor necrosis factor-α (Noda et al., 2000) and interleukin-1β (Takahashi et al., 2003). The pathology of oligodendrocytes further induces demyelination and dysmyelination, which profoundly influence the axonal normal function, transport, structure, metabolism, and survival (Merrill and Scolding, 1999; McTigue and Tripathi, 2008; Matute and Ransom, 2012). Apart from periventricular leukomalacia, the greatest risk is damage to pre-myelination oligodendrocyte progenitor cells when infants’ cerebral white matter is developing (Back et al., 2001). There are numerous adverse effects related with hypomyelination, including impaired axon conduction, motor deficits and sensory or cognitive function, depending on the location of the affected axons (Guardia Clausi et al., 2010).
A natural compound, rosmarinic acid (RA) is a common ester, which can be extracted from many traditional Chinese medicine herbs, i.e., Rosemary, Perilla frutescens, and Salvia miltiorrhiza Bunge (Amoah et al., 2016). RA was reported to have a protective effect, alleviating working, spatial, and recognition memory deficits after ischemic injury in mice via suppression of neuronal loss, increasing the expression of synaptophysin and brain-derived neurotrophic factor (Fonteles et al., 2016). Various benefits of RA were found in protecting different diseases, including temporal lobe epilepsy (Khamse et al., 2015), chronic ethanol-induced learning and memory deficits (Hasanein et al., 2017) and cerebral ischemia/reperfusion injury (Zhang et al., 2017). However, few studies focused on RA’s effect on H/I injury induced changes of white matter fiber and cognitive deficits. In this study, a perinatal H/I rat model was used to identify whether RA had any protective effect on white matter fiber injury and cognitive dysfunction caused by H/I injury.
Materials and Methods
Animals
The protocol was approved by the Animal Experimental Ethics Committee of Xuzhou Medical University, China (approval No. 20161636721) on September 16, 2017. All surgical interventions and postoperative animal care were performed according to the National Institutes of Health (USA) guidelines for care and use of laboratory animals (NIH Publication No. 85-23, revised 1996). Twenty-four specific-pathogen-free Sprague-Dawley rats aged three days were purchased from the Experimental Animal Center of Xuzhou Medical University (SYXK (Su) 2016-0028). Their mother was maintained in standard cages with independent ventilation system at 25 ± 1°C on a 12-hour light/dark cycle. All animals were allowed free access to food and water. The newborn rats were randomly assigned to a control group, H/I group, H/I + RA group (treatment with RA), or H/I + vehicle group (treatment with normal saline) (n = 6 per group).
Induction of H/I injury and RA administration
The perinatal H/I surgery was conducted in postnatal day 3 Sprague-Dawley rats as described previously (Iwai et al., 2007). Briefly, rats were anesthetized with 2% isoflurane (Jinan Qiqi Pharmaceutical Technology Co., Ltd., Jinan, China) through a ventilator (Shanghai Qiansheng Biological Technology Co., Ltd.) until they lost their righting reflex. Once totally anesthetized, the right common carotid artery was identified and separated from the vagus nerve then ligated using 5-0 silk. After a 2-hour recovery, rats were then warmed in a plastic container, with holes on the lid, which was placed in a 37°C water bath for 30 minutes. Afterwards, the rats were exposed to an atmosphere of 8% O2 and 92% N2 for 30 minutes, and then returned to their biological mother’s cages. For control rats, only the common carotid artery was exposed without ligation or hypoxia.
On the next day after surgery, the control and H/I groups received no treatment. RA was dissolved in normal physiological saline, and normal saline was used as vehicle. H/I + RA group received 0.2 M RA (20 mg/kg daily, intraperitoneally; Refenson Biotechnology Co., Ltd., Chengdu, China) for 5 consecutive days. H/I + vehicle group just received 0.9 mL normal saline intraperitoneally. Approximately 22 days after birth, the four groups of rats were subjected to behavior tests.
Open field test
On postnatal day 22, the locomotive activity of the rats was evaluated by an open-field test. The open field (50 cm × 50 cm) was surrounded by a wall (30 cm high). The rats were placed into the center of the field to acclimatize to the environment for 30 minutes for 2 consecutive days (Charles et al., 2018). For the trials, the rat behavior was monitored and recorded for 5 minutes by an auto-tracking system (SmarTrack, Smartech, Madison, WI, USA). The behavior items analyzed included distance travelled (m), speed (cm/s), break time, time in corner, and grooming time.
Morris water maze test
Morris water maze test was used to evaluate the spatial learning and memory behavior of rats at postnatal day 22 as described previously (Wu et al., 2015, 2017). Briefly, we randomly chose one of four assigned release points and released the rat into the water facing the wall of the pool to find the platform hidden below the water. If the rat could not find the platform successfully in 60 seconds, the experimenter would gently guide the rat to the platform and let them stay there for 15 seconds. Each animal was trained for five trials per day and for 4 consecutive days. On the day after completion of the acquisition phase, the platform was removed and the rat was placed in the quadrant furthest from where the platform had been located. The rats were allowed to swim in the pool for 120 seconds and their behaviors were recorded by ANY-maze video tracking system (Stoelting, Wood Dale, IL, USA) with a CCD camera. The target quadrant retention time as a percentage of the total time, swimming distance, and number of crossings were analyzed.
BrdU labeling and immunohistochemistry staining
To evaluate the newborn cells in subventricular zone, BrdU (100 mg/kg; Sigma (Shanghai) Yingxin Laboratory Equipment Co., Ltd., Shanghai, China) was intraperitoneally injected once daily for 2 weeks from the postnatal day 30. After the behavior test and bromodeoxyuridine (BrdU) injections, six rats from each group were deeply anesthetized by 10% chloral hydrate and transcardially perfused with normal saline followed by 4% paraformaldehyde. Their brains were removed and fixed in 4% paraformaldehyde overnight at 4°C. After dehydration through 10%, 20% and 30% sucrose gradient, the brain was coronally cut into 20-μm thick sections on a freezing microtome. Immunohistochemical staining was performed to evaluate the expression of BrdU, Olig2, and DAPI in subventricular zone and myelin basic protein (MBP) in the corpus callosum. Sections were blocked by 10% donkey serum and then incubated by rabbit anti-BrdU (1:100; Sigma-Aldrich, Louis, MO, USA) and mouse anti-Olig2 (1:200; Abcam, Cambridge, MA, USA) at 4°C overnight. To detect the MBP expression of injured white matter in the corpus callosum, mouse anti-MBP (1:200; Covance Antibody Services, Princeton, NJ, USA) was used to stain the white matter at 4°C overnight. Next day, the corresponding species of secondary antibodies (DYLIGHT488 donkey anti-rabbit, 1:200; all from Amersham Biosciences Ltd. Healthcare, San Diego, CA, USA) were added at room temperature for 2 hours. The nucleus was stained by DAPI for five minutes. After washing three times with PBS, images were captured by a fluorescence microscope (Olympus, Osaka, Japan).
Luxol fast blue staining
The protocol of Luxol fast blue staining was in accordance with a previous study (Liu et al., 2017). After their behavior test, two rats of each group were deeply anesthetized and brains were immediately removed for processing. The removed brain tissue was paraffin-embedded and cut into 4-μm-thick coronal sections. After de-paraffinization with xylene, the sections in slides needed to be rehydrated with ethanol gradient from 95% to 70%. Afterwards, 1% Luxol fast blue dye (Sigma-Aldrich) was added and the slides incubated overnight in a 60°C Thermostat box. The sections were rinsed with 95% ethanol and double distilled water (ddH2O) followed by incubation with 0.05% lithium carbonate solution (Sigma-Aldrich) for 5 seconds. After two washes with 70% ethanol and ddH2O, the sections were dehydrated with 95% ethanol to 100% ethanol, and cleared with xylene. Images were captured by bright-field and fluorescence microscope.
Electron microscopy
The ultrastructure of white matter in the corpus callosum visualized by electron microscopy after H/I injury has improved our understanding of the biogenesis and structure of myelin in healthy and pathological conditions (Möbius W et al., 2016). The standard protocols are referred as follows: Six rats from each group were lightly anesthetized in an anesthesia coma and the left ventricle was perfused with 2.5% glutaraldehyde buffer. A small block of white matter (1 × 1 × 1 mm3, < 1 mm2 posterior to bregma and > 1 mm2 from midline) was removed immediately and post-fixed with 3% glutaraldehyde at 4°C. After thorough rinsing, it was immersed in 1% osmic acid for 3 hours. The sample was dehydrated with acetone and embedded in epoxy resin. The resin-embedded samples were cut into 60 nm ultrathin sections with a microtome. The sections were stained by uranyl acetate and lead citrate. The images were captured using a transmission electron microscope (Oubo Tong Optical Technology Co., Ltd., Beijing, China).
Western blot assay
Rats were lightly anesthetized and sacrificed immediately to remove the brain quickly. The right corpus callosum was collected for western blot assay to test whether MBP and Olig2 were involved in the effect of RA. The following antibodies (their final dilution and source) were used for assay: rabbit anti-MBP (a marker of oligodendrocytes (Meffre et al., 2015); 1:1000, ab254026, Covance Antibody Services), goat anti-Olig2 (a marker of oligodendrocytes (Bojrab et al., 2017); 1:1000, ab109186, Abcam), rabbit anti-inhibitors of DNA binding 2 (Id2) antibody (Id2, a marker of oligodendrocyte apoptosis (Huang et al., 2015); 1:500, ab52093, Abcam), and rabbit anti-actin (1:500, ab179467 Abcam). The brain corpus callosum was lysed by the electrophoresis sample buffer containing Tris-HCl (62.5 mM), 2% w/v sodium dodecyl sulfate, 10% glycerol, dithiothreitol (50 mM) and 0.1% w/v bromophenol blue. The insoluble material was removed by centrifugation and the supernatant was collected. The supernatant was boiled for 10 minutes then cooled in ice for half an hour. Proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis in 10% acrylamide-bisacrylamide gels and then removed to nitrocellulose membranes, which were filled with blocking solution containing 5% skim milk powder and 0.1% Tween-20 in Tris-buffered saline for 1 hour. After three washes with Tris-buffered saline, the primary antibodies were incubated overnight at 4°C. After three washes with Tris-buffered saline, the blots were incubated with peroxidase-conjugated secondary antibody (rabbit anti-mouse; 1:2000; ab6728; Shanghai Sur Biotech Co., Ltd., Shanghai, China) for 1 hour at room temperature. After three washes with Tris-buffered saline, a chemiluminescence detection kit (Kanglang Biological Technology Co., Ltd., Shanghai, China) and X-ray film (Yuduo Biotechnology Co., Ltd., Shanghai, China) were used to detect the membrane bands. ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used to analyze the membrane signals.
Statistical analysis
All data are expressed as the mean ± SD. One-way analysis of variance was used to validate behavior and immunostaining related data. The two factors are H/I injury and RA injection. Post hoc Tukey’s tests were applied when needed. All statistical analyses were performed with Graphpad Prism 5 software (GraphPad Software, San Diego, CA, USA). A value of P < 0.05 was considered statistically significant.
Results
RA ameliorates locomotor activity deficits induced by H/I injury
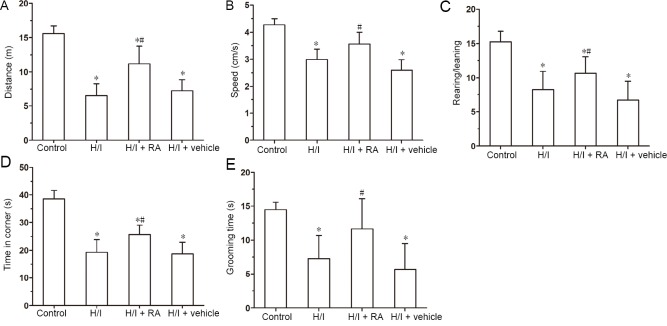
Spontaneous motor activity and anxiety behavior were tested by an open field test. As shown in Figure 1, the motor distance, speed, the ratio of rearing/leaning, time in the corner, and grooming time were significantly decreased in the H/I group compared with the control group (P < 0.05). This implies that H/I injury induces spontaneous locomotor activities deficits and anxiety behavior compared with the control group. Treatment with RA significantly improved the motor distance, speed, the ratio of rearing/leaning, time in the corner and grooming time compared with the H/I group (P < 0.05). The changes in several parameters between H/I + vehicle group and H/I group were similar (P > 0.05).
Figure 1.
Effects of RA on exercise performance and anxiety behavior in neonatal rats with H/I injury in open field trials 22 days after birth.
Distance (A), speed (B), rearing/leaning (C), time in corner (D) and grooming time (E) were evaluated among the four groups of rats. Data are presented as the mean ± SD (n = 6 per group). *P < 0.05, vs. control group; #P < 0.05, vs. H/I group (one-way analysis of variance followed by post hoc Tukey’s test). H/I: Hypoxia/ischemia; RA: rosmarinic acid.
RA improves spatial learning and memory functions after H/I injury
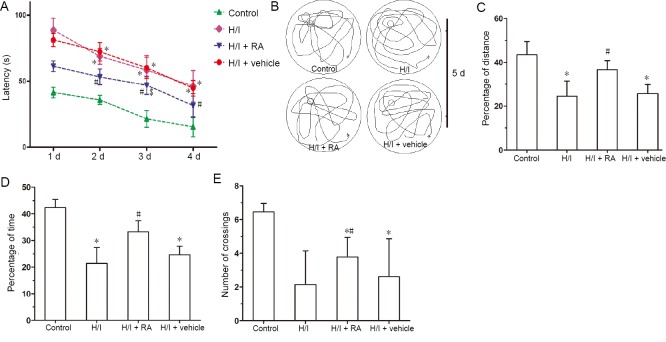
The abilities of spatial learning and memory functions were measured by the Morris water maze test. The task was completed after 3 trials daily, over 4 days, with an inter-trial interval of 1 hour. In the four-day probe trial, the escape latencies in four groups all decreased with increasing number of training days. The latencies to reach the platform were longer in the H/I group than in the control group (Figure 2A). Swimming latencies were shorter in the H/I + RA group than in the H/I group. The latencies were similar between the HI + vehicle group and the H/I group. No significant changes in swimming latencies were detectable between the H/I + RA and control groups at 1, 2, and 4 days (P > 0.05).
Figure 2.
Effects of RA on learning and spatial memory functions in rats with H/I injury evaluated by a Morris water maze test.
(A) Latency to the hidden platform in 4 consecutive training days. (B) Traces of swimming track on day 5 of Morris Water Maze testing. According to the swimming traces, percentage of distance (C), percentage of time (D), number of crossings (E) were analyzed on day 5 of Morris water maze test. Data are presented as the mean ± SD (n = 6 per group). *P < 0.05, vs. control group; #P < 0.05, vs. H/I group (one-way analysis of variance followed by post hoc Tukey’s tests). H/I: Hypoxia/ischemia; RA: rosmarinic acid.
After 4 days of training, during which the platform was removed from the pool (Figure 2B–E), the swim traces, percentage of distance, percentage of time, and number of crossings were recorded over 120 seconds and analyzed. The swimming trace (Figure 2B) results showed that control rats preferred staying at the zone where the platform was once located, indicating a good spatial reference memory. However, the H/I group showed no significant preference on day 5. After the treatment with RA, slightly more traces around where the platform had been located reappeared compared to the H/I + vehicle treatment group, which showed little preference as in the H/I group. The percentage of distance, percentage of time spent in the target quadrant and the number of crossings were all significantly lower in the H/I group compared with the control group (Figure 2C–E). The target quadrant retention time as a percentage of the total time and the number of crossing over the platform area significantly improved in the H/I + RA group compared with the H/I group. The effects were similar between the H/I + vehicle and H/I groups. The above parameters significantly decreased in the H/I group compared with the control group. The percentage of distance and percentage of time were similar between the H/I + RA and control groups (P > 0.05). However, the number of platform crossings was still less in the H/I, H/I + RA, and H/I + vehicle groups than in the control group (P < 0.05).
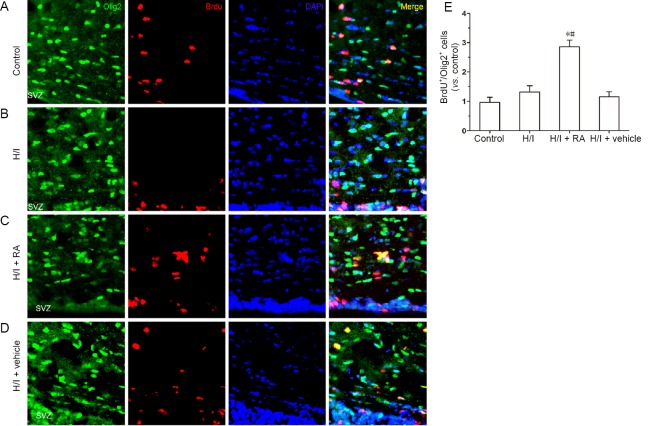
RA increases the number of BrdU+/Olig2+ cells in subventricular zone after H/I injury
A previous study has shown that pathological conditions stimulated neurogenesis in the subventricular zone and newly mature neural cells moved directly toward the injury site to replace the dead cells (Yamashita et al., 2006). The same occurred in response to H/I injury (Ruan et al., 2014). BrdU immunostaining was employed in each group to label proliferative neural stem cells in the subventricular zone. Olig2, a basic helix-loop-helix transcription factor, is widely distributed in progenitor cells and is recognized as an oligodendrocyte lineage marker for phenotypic identification. The expression of Olig2 changed significantly after various injuries via different mechanisms (Buffo et al., 2007). Thus, Olig2 acts as a marker of neurogenesis while BrdU acts as a proliferation indicator. As illustrated in Figure 3, the number of BrdU cells in the H/I group was slightly higher compared with the control group. However, the number of BrdU+/Olig2+ cells showed no significant change between the H/I and control groups (P > 0.05). RA treatment after H/I injury significantly improved the number of BrdU+/Olig2+ cells in the subventricular zone (P < 0.05). The number of BrdU+/Olig2+ cells was greater in the H/I + RA group than in the control group (P < 0.05). No significant changes were found between the H/I + vehicle and H/I groups.
Figure 3.
RA promoted the proliferation of oligodendrocyte progenitor cells, detected by immunofluorescence staining, in the subventricular zone of H/I injured rats 50 days after birth.
(A–D) BrdU+/Olig2+ cells in the subventricular zone. DAPI (blue) was used to stain nuclei. Representative images in the control group (A), H/I group (B), H/I + RA group (C) and H/I + vehicle group (D). (E) Quantification of Olig2+/BrdU+ cells. BrdU expression was slightly higher in the H/I group than in the control group but there was no significant change in the number of double positive cells (BrdU+/Olig2+). RA treatment after H/I injury significantly increased the number of BrdU+/Olig2+ in the subventricular zone. Data are presented as the mean ± SD (n = 6 per group). *P < 0.05, vs. control group; #P < 0.05, vs. H/I group (one-way analysis of variance followed by post hoc Tukey’s tests). H/I: Hypoxia/ischemia; RA: rosmarinic acid.
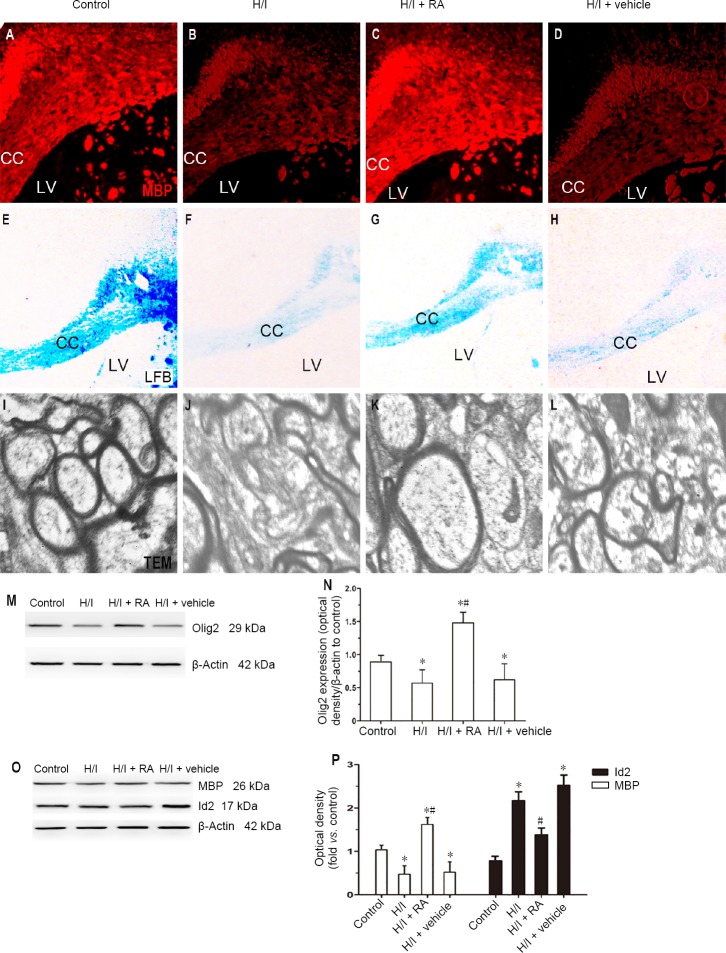
RA ameliorates abnormal myelinations in H/I rats
MBP immunostaining has been applied to evaluate the myelin protein component and has been shown to be sensitive to the loss of myelin (Ludwin and Sternberger, 1984). To further evaluate the effect of RA in the development of oligodendrocyte, we detected the expression of MBP in all groups treated with RA or none. As shown in Figure 4A–D, H/I injury downregulated the MBP immunopositivity in the corpus callosum compared with the control group (P < 0.05). There was significantly less downregulation of MBP in the H/I + RA group compared with the H/I group (P < 0.05). The expression of MBP was similar between the H/I + vehicle and H/I groups.
Figure 4.
Effect of RA on the expression of myelin-associated protein and myelin morphology in H/I injured rats at 50 days after birth.
(A–D) Immunopositivity of MBP in the CC was stained among four groups. (E–H) LFB was used to detect the status of myelination in the CC. (I–L) TEM was used to observe the morphological changes of myelin sheaths in the CC. H/I injury down-regulated the expression of MBP in the CC. The myelinated nerve fibers of the control group were stained blue in the CC, whereas blue staining was rarely observed in the CC of H/I injured rats. In contrast, H/I damage resulted in a decrease in the bulk density of myelinated axons in the CC and a decrease in axonal diameter. RA treatment significantly reduced the effects of H/I injury, partially improving the packing density of the myelinated axons and improving their diameters. (M, N) Western blot assay of the Olig2 expression in the CC. (O, P) Quantification of western blot assays of the MBP and Id2 expression in the CC. Data are presented as the mean ± SD (n = 6 per group). *P < 0.05, vs. control group; #P < 0.05, vs. H/I group (one-way analysis of variance followed by post hoc Tukey’s tests). CC: Corpus callosum; H/I: hypoxia/ischemia; Id2: DNA binding 2; LFB: luxol fast blue staining: LV: lateral ventricle; MBP: myelin basic protein; RA: rosmarinic acid; TEM: transmission electron microscopy.
Luxol fast blue staining was used to evaluate the morphological and biological changes of myelin in the corpus callosum. As illustrated in Figure 4E–H, the myelinated neural fibers of the control group were stained blue in the corpus callosum, whereas blue stained fibers were rarely seen in the corpus callosum of rats with H/I injury. RA treatment partially improved the blue staining over that in the H/I group. The amount of blue staining was similar between the H/I + vehicle and H/I groups.
Electron microscopy was used to detect myelin sheaths and tracts in the corpus callosum directly. As shown in Figure 4I–L, the packing density of myelinated axons decreased and the diameters of axons were smaller in the corpus callosum from the H/I group compared with those from the control group. The packing density of myelinated axons and the diameter of axons improved in the H/I + RA group compared with the H/I group. The morphology of axons was similar between the H/I + vehicle and H/I groups.
The protein levels in the corpus callosum of rats in the control, H/I, H/I + RA, and H/I + vehicle groups were examined by western bolt assay. As illustrated in Figure 4M and N, the expression level of Olig2 was less in the H/I group than in the control group (P < 0.05). The Olig2 expression levels significantly increased in the corpus callosum in the H/I + RA group from those in the H/I group (P < 0.05). As predicted, the H/I + vehicle group showed similar expressions of Olig2 in the corpus callosum compared with the H/I group. The expression levels of MBP and Id2 were also detected by western blot assay. As illustrated in Figure 4O and P, the expression level of MBP was significantly lower in the H/I group compared with the control group (P < 0.05), however it was partially reversed by administration with RA (P < 0.05). MBP expression decreased in the H/I group compared with the control group (P < 0.05). The expression levels of Olig2 and MBP were both higher in the H/I + RA group compared with the control group (P < 0.05).
The inhibitors of DNA binding 2 (Id2) proteins modulate the proliferation of oligodendrocyte precursor cells out of the cell-cycle to mature cells in the corpus callosum (Chen et al., 2007). As shown in Figure 4O and P, Id2 expression in the corpus callosum significantly increased in the H/I group compared with the control group (P < 0.05). However, the above-mentioned increasing trend of Id2 expression in the H/I group was significantly lower in the H/I + RA group (P < 0.05). Id2 expression in the corpus callosum was higher in all H/I groups than in the control group (P < 0.05).
Discussion
Perinatal H/I injury occurs in a critical period of brain growth and development but has not yet been studied thoroughly (Gong and Liu, 2019). A previous study focused not only on the neural regeneration but also on the self-renewing precursors in the subventricular zone, which subsequently produce neuroblasts (Felling et al., 2006). Our study shows that RA provides protection from cognitive impairments induced by H/I injury by increasing the proliferating oligodendrocyte progenitor cells in the subventricular zone and remyelination in the corpus callosum. Both the open-field test and the Morris water maze test showed that RA administration reduced the cognitive impairment induced by H/I injury. Moreover, further results indicated that the induction of oligodendrogenesis in the subventricular zone and enhancement of axonal remyelination in the corpus callosum resulted from RA treatment after H/I injury. As one of the predominant cell types, oligodendrocytes are vulnerable to H/I injury (Chen et al., 2015), therefore, RA treatment may provide a new potential approach to therapy for perinatal H/I injury-induced cognitive impairments.
RA is a naturally hydroxylated compound and its therapeutic role has been investigated in several diseases, including alcohol abuse (Wang et al., 2012), Parkinson’s disease (Wang et al., 2012), amyotrophic lateral sclerosis (Seo et al., 2015), and H/I injury (Zhang et al., 2017). RA exerts neuroprotective effects on hippocampal neurons through the Akt/JNK3/caspase-3 signaling pathway after H/I injury (Zhang et al., 2017). It is unlikely that neuronal genesis alone would be sufficient to restore the brain injury induced by H/I injury. A previous study has shown that by decreasing iron-induced damage myelination increased and oligodendrocyte maturation accelerated in the corpus callosum, providing a novel therapeutic tool for the recovery after H/I injury (Guardia Clausi et al., 2010). Metformin provided a significant amelioration of cognitive impairment after H/I injury by improving remyelination in the corpus callosum (Qi et al., 2017). However, little study has focused on the underlying mechanism of RA treatment after H/I injury. The combined research on metformin and RA suggests there could be some similar, unknown mechanism underlying their effects on the recovery of H/I injury. RA enhanced neural plasticity by modulating glutamatergic signaling pathways via increasing the expression of GluR-2 proteins to prevent cell death (Hwang et al., 2016) The effects of RA in the prevention of oxidative stress, intracellular Ca2+ overload and c-fos expression were also found in models of neuronal death in vitro (Fallarini et al., 2009). Furthermore, in sodium taurocholate-induced acute pancreatitis rats, pretreatment with RA appears to reduce the releasing of inflammatory cytokines by inhibiting the activation of nuclear factor-κB (Fan et al., 2015). It is possible that several potential signaling pathways may contribute to the improvement of cognitive function after perinatal H/I injury shown in our study. Traditional Chinese herb medicine is one of the oldest and most widely used systems of medicine in the world (Zhang et al., 2013), and the mechanisms underlying the therapies for various disease need to be explored further with modern scientific techniques. The protective effect of RA on H/I injury-induced cognitive dysfunction may be a step forward in clarifying the mechanism underlying Chinese herb medicine and benefit more patients around the world.
White matter damage often occurs after H/I injury and leads to demyelination that, depending on the site of injury, strongly impairs motor, sensory and/or cognitive function (Crawford et al., 2009). Therefore, remyelination can be recognized as an effective therapy to recover the conduction properties of axons, thus benefitting their neurological function. Various investigations have focused on how to improve the remyelination effect in the injured brain. Oligodendrocyte progenitor cells are largely resident in cerebral white matter and once injured in their immature period would result in hypomyelination (Rice et al., 1981). RA treatment improves the oligodendrocyte progenitor cells differentiation and maturation in the subventricular zone. They then restore the myelin sheaths in the corpus callosum, a positive sign of recovery from H/I injury. MBP is a component of myelin and sensitive to the loss of myelin. The inhibitors of DNA binding 2 (Id2) proteins modulate the proliferation of oligodendrocyte precursor cells out of the cell-cycle to mature cells in the corpus callosum. As an inhibitor of Oligo2, Id2 regulates the activity of the MBP gene through inhibition of its promoter by inactivating Olig2 in vitro. In this study, we confirmed that RA reduced the decreased expression of MBP and inhibited the increased expression of Id2 induced by perinatal H/I injury in the brain. Both MBP and Id2 are involved in this protective effect of RA. Apart from MBP and Id2 signals, many signaling pathways, such as Wnt/β-catenin and Akt/mTOR pathways, participate in the differentiation of oligodendrocyte progenitor cells, and the ERK/MAPK pathway directly regulates the growth of the myelin sheath during myelination (Gaesser and Fyffe-Maricich, 2016). The communication among the multiple pathways is complex. Therefore the molecular mechanism underlying the effect of RA on the remyelination after H/I injury needs to further exploration.
In conclusion, RA ameliorated the cognitive dysfunctions (locomotor activity, anxiety behavior, learning and spatial memory deficits) induced by perinatal H/I injury. This protection may be effected by improving the differentiation and maturation of oligodendrocyte progenitor cells and restoring myelin sheaths in the corpus callosum. Different signaling pathways are involved in the protection of RA after injury. The clinical feasibility and significant benefits of RA treatment of cognitive impairments after perinatal H/I injury make us hope RA will be used soon to benefit more patients.
Additional file: Open peer review report 1 (70.7KB, pdf) .
Acknowledgments:
We are very grateful to Professor Hong-Yan Dong, Ting-Ting Li, Hong-Hua Yuan, Hong-Li Yu, Fu-Xing Dong, Zhi-Wei Liu, Qing-Li Huang, and Meng Meng from Xuzhou Medical University for their support and help.
Footnotes
Conflicts of interest: None declared.
Financial support: This work was supported by the Natural Science Foundation of Jiangsu Province of China, No. BK20171180 (to XRW). The funding body played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Institutional review board statement: The protocol was approved by the Animal Experimental Ethics Committee of Xuzhou Medical University, China (approval No. 20161636721) on September 16, 2017.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Jing Wang, Boston Children’s Hospital, USA.
Funding: This work was supported by the Natural Science Foundation of Jiangsu Province of China, No. BK20171180 (to XRW).
P-Reviewer: Wang J; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Dawes EA, de Souza M, Qiu Y, Song LP; T-Editor: Jia Y
References
- 1.Amoah SK, Sandjo LP, Kratz JM, Biavatti MW. Rosmarinic acid--pharmaceutical and clinical aspects. Planta Med. 2016;82:388–406. doi: 10.1055/s-0035-1568274. [DOI] [PubMed] [Google Scholar]
- 2.Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21:1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bojrab D, 2nd, Zhang B, Jiang H, Zhang L, Cohen DS, Luo X, Hu Z. Expression of oligodendrocyte marker during peripheral-central transitional zone formation of the postnatal mouse cochlear nerve. Otolaryngol Head Neck Surg. 2017;157:488–492. doi: 10.1177/0194599817718806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buffo A. Fate determinant expression in the lesioned brain: Olig2 induction and its implications for neuronal repair. Neurodegener Dis. 2007;4:328–332. doi: 10.1159/000101890. [DOI] [PubMed] [Google Scholar]
- 5.Charles KA, Naudet F, Bouali-Benazzouz R, Landry M, De Deurwaerdere P, Fossat P, Benazzouz A. Alteration of nociceptive integration in the spinal cord of a rat model of Parkinson’s disease. Mov Disord. 2018;33:1010–1015. doi: 10.1002/mds.27377. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Sun M, Zhang X, Miao Z, Chua BH, Hamdy RC, Zhang QG, Liu CF, Xu X. Increased oligodendrogenesis by humanin promotes axonal remyelination and neurological recovery in hypoxic/ischemic brains. Hippocampus. 2015;25:62–71. doi: 10.1002/hipo.22350. [DOI] [PubMed] [Google Scholar]
- 7.Chen XS, Zhou DS, Yao ZX. The inhibitor of DNA binding 2 is mainly expressed in oligodendrocyte lineage cells in adult rat brain. Neurosci Lett. 2007;428:93–98. doi: 10.1016/j.neulet.2007.09.044. [DOI] [PubMed] [Google Scholar]
- 8.Crawford DK, Mangiardi M, Xia X, López-Valdés HE, Tiwari-Woodruff SK. Functional recovery of callosal axons following demyelination: a critical window. Neuroscience. 2009;164:1407–1421. doi: 10.1016/j.neuroscience.2009.09.069. [DOI] [PubMed] [Google Scholar]
- 9.Davies A, Wassink G, Bennet L, Gunn AJ, Davidson JO. Can we further optimize therapeutic hypothermia for hypoxic-ischemic encephalopathy? Neural Regen Res. 2019;14:1678–1683. doi: 10.4103/1673-5374.257512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fallarini S, Miglio G, Paoletti T, Minassi A, Amoruso A, Bardelli C, Brunelleschi S, Lombardi G. Clovamide and rosmarinic acid induce neuroprotective effects in in vitro models of neuronal death. Br J Pharmacol. 2009;157:1072–1084. doi: 10.1111/j.1476-5381.2009.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan YT, Yin GJ, Xiao WQ, Qiu L, Yu G, Hu YL, Xing M, Wu DQ, Cang XF, Wan R, Wang XP, Hu GY. Rosmarinic acid attenuates sodium taurocholate-induced acute pancreatitis in rats by inhibiting nuclear factor-kappaB activation. Am J Chin Med. 2015;43:1117–1135. doi: 10.1142/S0192415X15500640. [DOI] [PubMed] [Google Scholar]
- 12.Felling RJ, Snyder MJ, Romanko MJ, Rothstein RP, Ziegler AN, Yang Z, Givogri MI, Bongarzone ER, Levison SW. Neural stem/progenitor cells participate in the regenerative response to perinatal hypoxia/ischemia. J Neurosci. 2006;26:4359–4369. doi: 10.1523/JNEUROSCI.1898-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fields RD. White matter matters. Sci Am. 2008;298:42–49. [PubMed] [Google Scholar]
- 14.Fonteles AA, de Souza CM, de Sousa Neves JC, Menezes AP, Santos do Carmo MR, Fernandes FD, de Araujo PR, de Andrade GM. Rosmarinic acid prevents against memory deficits in ischemic mice. Behav Brain Res. 2016;297:91–103. doi: 10.1016/j.bbr.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 15.Gaesser JM, Fyffe-Maricich SL. Intracellular signaling pathway regulation of myelination and remyelination in the CNS. Exp Neurol. 2016;283:501–511. doi: 10.1016/j.expneurol.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong J, Liu M. Human induced pluripotent stem cell transplantation for hypoxic-ischemic encephalopathy in neonatal mice. Zhongguo Zuzhi Gongcheng Yanjiu. 2019;23:5322–5327. [Google Scholar]
- 17.Guardia Clausi M, Pasquini LA, Soto EF, Pasquini JM. Apotransferrin-induced recovery after hypoxic/ischaemic injury on myelination. ASN Neuro. 2010;2:e00048. doi: 10.1042/AN20100020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasanein P, Seifi R, Hajinezhad MR, Emamjomeh A. Rosmarinic acid protects against chronic ethanol-induced learning and memory deficits in rats. Nutr Neurosci. 2017;20:547–554. doi: 10.1080/1028415X.2016.1203125. [DOI] [PubMed] [Google Scholar]
- 19.Haynes RL, Folkerth RD, Keefe RJ, Sung I, Swzeda LI, Rosenberg PA, Volpe JJ, Kinney HC. Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J Neuropathol Exp Neurol. 2003;62:441–450. doi: 10.1093/jnen/62.5.441. [DOI] [PubMed] [Google Scholar]
- 20.Huang S, Tang C, Sun S, Cao W, Qi W, Xu J, Huang J, Lu W, Liu Q, Gong B, Zhang Y, Jiang J. Protective effect of electroacupuncture on neural myelin sheaths is mediated via promotion of oligodendrocyte proliferation and inhibition of oligodendrocyte death after compressed spinal cord injury. Mol Neurobiol. 2015;52:1870–1881. doi: 10.1007/s12035-014-9022-0. [DOI] [PubMed] [Google Scholar]
- 21.Hwang ES, Kim HB, Choi GY, Lee S, Lee SO, Kim S, Park JH. Acute rosmarinic acid treatment enhances long-term potentiation, BDNF and GluR-2 protein expression, and cell survival rate against scopolamine challenge in rat organotypic hippocampal slice cultures. Biochem Biophys Res Commun. 2016;475:44–50. doi: 10.1016/j.bbrc.2016.04.153. [DOI] [PubMed] [Google Scholar]
- 22.Iwai M, Cao G, Yin W, Stetler RA, Liu J, Chen J. Erythropoietin promotes neuronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke. 2007;38:2795–2803. doi: 10.1161/STROKEAHA.107.483008. [DOI] [PubMed] [Google Scholar]
- 23.Jaworska J, Ziemka-Nalecz M, Sypecka J, Zalewska T. The potential neuroprotective role of a histone deacetylase inhibitor, sodium butyrate, after neonatal hypoxia-ischemia. J Neuroinflammation. 2017;14:34. doi: 10.1186/s12974-017-0807-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khamse S, Sadr SS, Roghani M, Hasanzadeh G, Mohammadian M. Rosmarinic acid exerts a neuroprotective effect in the kainate rat model of temporal lobe epilepsy: Underlying mechanisms. Pharm Biol. 2015;53:1818–1825. doi: 10.3109/13880209.2015.1010738. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Yang R, Kong Q, Wang Y, Zhang B, Sun J, Yang Y, Zheng B, Yuan H, Shi J. Metabolomic changes in rat model of cauda equina injury. World Neurosurg. 2017;102:449–458. doi: 10.1016/j.wneu.2017.03.072. [DOI] [PubMed] [Google Scholar]
- 26.Ludwin SK, Sternberger NH. An immunohistochemical study of myelin proteins during remyelination in the central nervous system. Acta Neuropathol. 1984;63:240–248. doi: 10.1007/BF00685250. [DOI] [PubMed] [Google Scholar]
- 27.Matute C, Ransom BR. Roles of white matter in central nervous system pathophysiologies. ASN Neuro. 2012;4:e00079. doi: 10.1042/AN20110060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- 29.Meffre D, Massaad C, Grenier J. Lithium chloride stimulates PLP and MBP expression in oligodendrocytes via Wnt/beta-catenin and Akt/CREB pathways. Neuroscience. 2015;284:962–971. doi: 10.1016/j.neuroscience.2014.10.064. [DOI] [PubMed] [Google Scholar]
- 30.Merrill JE, Scolding NJ. Mechanisms of damage to myelin and oligodendrocytes and their relevance to disease. Neuropathol Appl Neurobiol. 1999;25:435–458. doi: 10.1046/j.1365-2990.1999.00200.x. [DOI] [PubMed] [Google Scholar]
- 31.Mifsud G, Zammit C, Muscat R, Di Giovanni G, Valentino M. Oligodendrocyte pathophysiology and treatment strategies in cerebral ischemia. CNS Neurosci Ther. 2014;20:603–612. doi: 10.1111/cns.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Möbius W, Nave KA, Werner HB. Electron microscopy of myelin: Structure preservation by high-pressure freezing. Brain Res. 2016;1641:92–100. doi: 10.1016/j.brainres.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 33.Noda M, Nakanishi H, Nabekura J, Akaike N. AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. J Neurosci. 2000;20:251–258. doi: 10.1523/JNEUROSCI.20-01-00251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qi B, Hu L, Zhu L, Shang L, Sheng L, Wang X, Liu N, Wen N, Yu X, Wang Q, Yang Y. Metformin attenuates cognitive impairments in hypoxia-ischemia neonatal rats via improving remyelination. Cell Mol Neurobiol. 2017;37:1269–1278. doi: 10.1007/s10571-016-0459-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 36.Ruan L, Lau BW, Wang J, Huang L, Zhuge Q, Wang B, Jin K, So KF. Neurogenesis in neurological and psychiatric diseases and brain injury: from bench to bedside. Prog Neurobiol. 2014;115:116–137. doi: 10.1016/j.pneurobio.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Seo JS, Choi J, Leem YH, Han PL. Rosmarinic acid alleviates neurological symptoms in the G93A-SOD1 transgenic mouse model of amyotrophic lateral sclerosis. Exp Neurobiol. 2015;24:341–350. doi: 10.5607/en.2015.24.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi JL, Giuliani F, Power C, Imai Y, Yong VW. Interleukin-1beta promotes oligodendrocyte death through glutamate excitotoxicity. Ann Neurol. 2003;53:588–595. doi: 10.1002/ana.10519. [DOI] [PubMed] [Google Scholar]
- 39.Tang S, Xu S, Lu X, Gullapalli RP, McKenna MC, Waddell J. Neuroprotective effects of acetyl-l-carnitine on neonatal hypoxia ischemia-induced brain injury in rats. Dev Neurosci. 2016;38:384–396. doi: 10.1159/000455041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Handel M, Swaab H, de Vries LS, Jongmans MJ. Long-term cognitive and behavioral consequences of neonatal encephalopathy following perinatal asphyxia: a review. Eur J Pediatr. 2007;166:645–654. doi: 10.1007/s00431-007-0437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50:553–562. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Xu H, Jiang H, Du X, Sun P, Xie J. Neurorescue effect of rosmarinic acid on 6-hydroxydopamine-lesioned nigral dopamine neurons in rat model of Parkinson’s disease. J Mol Neurosci. 2012;47:113–119. doi: 10.1007/s12031-011-9693-1. [DOI] [PubMed] [Google Scholar]
- 43.Wu M, He Y, Zhang J, Yang J, Qi J. Co-injection of Abeta1-40 and ApoE4 impaired spatial memory and hippocampal long-term potentiation in rats. Neurosci Lett. 2017;648:47–52. doi: 10.1016/j.neulet.2017.03.043. [DOI] [PubMed] [Google Scholar]
- 44.Wu MN, Zhou LW, Wang ZJ, Han WN, Zhang J, Liu XJ, Tong JQ, Qi JS. Colivelin ameliorates amyloid beta peptide-induced impairments in spatial memory, synaptic plasticity, and calcium homeostasis in rats. Hippocampus. 2015;25:363–372. doi: 10.1002/hipo.22378. [DOI] [PubMed] [Google Scholar]
- 45.Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Abe K, Okano H, Sawamoto K. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang M, Yan H, Li S, Yang J. Rosmarinic acid protects rat hippocampal neurons from cerebral ischemia/reperfusion injury via the Akt/JNK3/caspase-3 signaling pathway. Brain Res. 2017;1657:9–15. doi: 10.1016/j.brainres.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 47.Zhang S, Wang D, Wang X, Li S, Li J, Li H, Yan Z. Aqueous extract of Bai-Hu-Tang, a classical Chinese herb formula, prevents excessive immune response and liver injury induced by LPS in rabbits. J Ethnopharmacol. 2013;149:321–327. doi: 10.1016/j.jep.2013.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ziemka-Nalecz M, Jaworska J, Sypecka J, Polowy R, Filipkowski RK, Zalewska T. Sodium butyrate, a histone deacetylase inhibitor, exhibits neuroprotective/neurogenic effects in a rat model of neonatal hypoxia-ischemia. Mol Neurobiol. 2017;54:5300–5318. doi: 10.1007/s12035-016-0049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.