Abstract
Dexmedetomidine is a selective α2-adrenoceptor agonist that is used because of its sedative, anxiolytic, and analgesic effects. Dexketoprofen, which is used as an analgesic, is a nonselective nonsteroidal anti-inflammatory drug (NSAID). The use of dexmedetomidine and dexketoprofen as adjuvants to local anesthetics for the peripheral nerve is gradually increasing. In this study, we aimed to investigate the effects of different doses of dexmedetomidine and dexketoprofen on conduction block of rat sciatic nerve. The isolated sciatic nerve from adult rats was transferred to a nerve chamber. The compound action potentials (CAPs) were recorded from stimulated nerve with electrophysiological methods. Dexmedetomidine (n = 8) and dexketoprofen (n = 8) were administered in the chamber with cumulative concentrations of 10–9 to 10–5 M, and the CAPs were recorded for 5 and 10 minutes. The CAP parameters were calculated. Both dexmedetomidine and dexketoprofen significantly depressed all CAP parameters in a dose-dependent manner compared with the control group, i.e., the group in which rats did not receive treatment. CAP parameters showed there was no significant difference in nerve conduction inhibition between dexmedetomidine and dexketoprofen. Higher doses of dexmedetomidine suppressed the conduction in the fast-conducting fibers; however, dexketoprofen was found to suppress the conduction in the slow-conducting fibers in a time-dependent manner and suppress the conduction in the medium- and slow-conducting fibers in a dose-dependent manner. These findings suggest that dexmedetomidine and dexketoprofen exhibit better anesthetic effects on peripheral nerve through different ways of action. The experimental procedures were approved by the Necmettin Erbakan University on January 30, 2013 (approval No. 2013-024).
Keywords: compound action potentials, dexketoprofen, dexmedetomidine, maximum depolarization, nerve chamber, nerve fibers, sciatic nerve
Chinese Library Classification No. R453; R364; R741
Introduction
The α2-adrenoceptors that are found in the central nervous system, peripheral nerve and autonomic ganglia are the target receptors of many drugs (Kosugi et al., 2010). Agonists of α2-adrenoceptor inhibit the conduction of nerve action potentials and can therefore contribute to an increase in the effects of local anesthetic agents (Kosugi et al., 2010). Dexmedetomidine, an α2 agonist for clinical anesthesia, is known to have various effects, such as anesthesia, analgesia, sedation, and vasoconstriction (Peng and Zhang, 2015). Additionally, dexmedetomidine has a potent anti-inflammatory effect (Li et al., 2017).
The use of dexketoprofen, a nonselective nonsteroidal anti-inflammatory drug (NSAID), is gradually increasing (Moore and Barden 2008; Miranda et al., 2012; Kara et al., 2014). Dexketoprofen is a preferred analgesic due to its faster onset of action and because it has fewer side effects than other NSAIDs (Moore et al., 2015; Hanna and Moon, 2018).
Rat sciatic nerve contains both sensory and motor nerve fibers, including nerve fibers with fast, medium and slow conduction velocities. The first appears as the activity of the fastest conducting fibers when a current pulse of the threshold stimulus intensity is applied to nerve. It is accepted that all the fibers are stimulated when a supramaximal stimulus is applied to the nerve (Katsuki et al., 2006). The compound action potentials (CAPs) recorded from supramaximal stimulus-induced rat sciatic nerve carry information about the activity of all the fibers forming the nerve. A change in the shape of the CAP may occur due to changes in the conduction velocity of the fibers (Gracias et al., 2011; Sousa et al., 2015).
The purpose of this study was to investigate the effects of different doses of dexmedetomidine and dexketoprofen on the conduction block of rat sciatic nerve.
Materials and Methods
Animals
The experimental procedures were approved by the Necmettin Erbakan University on January 30, 2013 (approval No. 2013-024) and conducted in accordance with the Guide for the Care and Use of Laboratory Animals (Konya/Turkey). Male Wistar albino rats (n = 24), aged 4–6 months and weighing 350 ± 50 g, were used in our study. Rats were provided with food and water ad libitum and were housed in a thermoneutral environment (22 ± 2°C) and relative humidity under a 12-hour light/dark cycle. Rats were randomly divided into three groups: a control group (n = 8), a dexmedetomidine group (n = 8) and a dexketoprofen group (n = 8).
Isolated rat sciatic nerve and experimental procedures
Rats were anesthetized with intraperitoneal pentobarbital (Pental Sodium 0.5 g injectable flacon, İ. E ULUGAY, Istanbul, Turkey) at 30 mg/kg. The sciatic nerve from the right hind limb of each rat was carefully isolated with a glass hook, and 5–6 cm of the nerve was removed. Nerve tissues were immediately transferred into the nerve chamber, which is a three-compartment system (nerve chamber, stimulating and recording Ag-AgCl electrodes) (Grass Model SIU5, Sequim, Washington, USA). The nerve chamber was superfused with a fresh Krebs solution (119 mM NaCl, 20 mM NaHCO3, 4.8 mM KCI, 1.8 mM CaCl2, 1.2 mM KHPO4, 1.2 mM MgSO4, and 10 mM glucose, gassed with a mixture 95% O2 and 5% CO2). The ambient temperature was maintained at 37°C with a pH of 7.4.
The CAP recordings were obtained from the sciatic nerve using a suction method, as previously described (Wijesinghe et al., 1991). Stimulations were applied to the proximal ends of the nerve trunk. Square wave pulses were delivered as supramaximal stimulations with a frequency of 1 Hz and duration of 200 µs by a stimulator (Grass Model S88, Sequim, Washington, USA) through a stimulation isolation unit (Grass Model SIU5, Sequim, Washington, USA). CAP recordings were obtained from the distal end of the nerve trunk (tibial branch) using a suction electrode. During the experiments, the testing equipment was kept in a Faraday’s cage to avoid external noise. Preamplified (Grass Model CP511, Sequim, WA, USA) CAP signals were digitized at a 50-kHz sampling rate by an Analog/Digital converter (Advantech Model PCL1710, Cincinnati, OH, USA), acquired with the BiosigW data acquisition software (Bios, New Orleans, LA, USA), and saved for future analysis on a hard drive.
Dexmedetomidine and dexketoprofen were added to the nerve chamber at cumulative doses of 10–9 M, 10–8 M, 10–7 M, 10–6 M, and 10–5 M with a volume of 0.1 mL. To investigate the acute effects of dexmedetomidine and dexketoprofen, the CAPs were recorded for 5 and 10 minutes after each dose was administered. Dexmedetomidine (Precedex 200 µg/2 mL, Meditera, Izmir, Turkey), dexketoprofen (Arveles 50 mg/2 mL ampule, UFSA İlaç, Istanbul, Turkey), pentobarbital (Pental Sodium 0.5 g injectable flacon, İ. E ULUGAY, Istanbul, Turkey). Lower concentrations were obtained from the stock solutions that were made with distilled water. All chemicals were supplied by Sigma-Aldrich (Steinheim, Germany).
Analysis procedure
Several parameters were calculated to evaluate the effects of 10–9 M to 10–5 M doses of dexmedetomidine and dexketoprofen at 5 and 10 minutes after dosing. The analysis provided information regarding the electrophysiological changes in the sciatic nerve as a result of drug administration. Evaluation showed that on an example of a CAP recording (Figure 1), MD (maximum depolarization) and AUC (area under the curve) are proportional to the number of stimulated nerve fibers in that nerve. Therefore, these values were calculated and are expressed as a percentage value relative to the control. MD was calculated with the changes in the rising phases of the CAP. MD was related to the fiber diameter distribution in the nerve fiber bundles and provided information about Na+ channel availability. Thus, these values were calculated and are expressed as percentage values relative to the control. The latency periods were calculated, with L1 measuring the time between the start of CAP and stimulation time and L2 measuring the time between the start of CAP and MD. These periods were used in the following equations (Eq {1} and Eq {2}) to calculate the conduction velocity of the fast-conducting nerve fibers (VCAP) and the conduction velocity of the slow- and medium-conducting nerve fibers (VMD), respectively (Dalkilic and Pehlivan, 2002). The distance was taken as the optimum value (Δx) of 50 mm between the stimulation electrode and the recording electrode.
Figure 1.
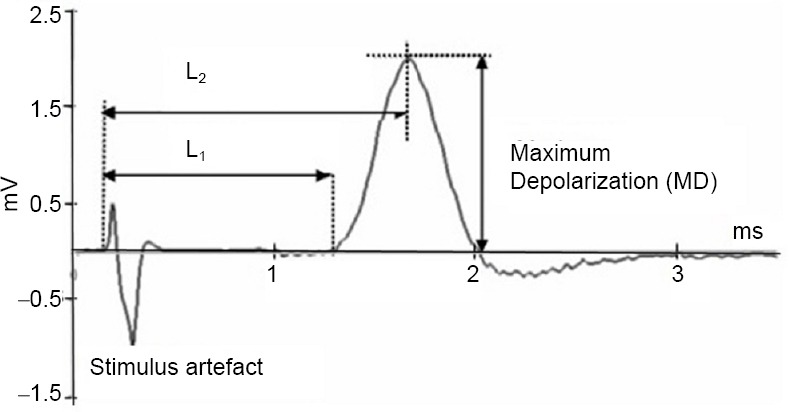
Demonstration of the measurement parameters on a sample recording of the compound action potentials.
VCAP = Δx/ΔtL1 {1}
VMD = Δx/ΔtL2 {2}
Statistical analysis
SPSS 18.0 software package was used for the statistical analysis of the recorded data. Two-way analysis of variance was used to calculate the difference in parameters between experimental groups. Paired samples t-test with Bonferroni correction was used for the repeated measures to evaluate the difference between groups. A level of P < 0.05 was considered statistically significant. All experimental parameters are expressed as the mean ± SEM (standard error of mean).
Results
CAP records
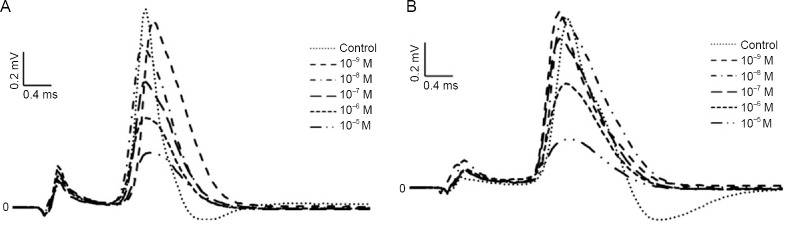
Both dexmedetomidine and dexketoprofen significantly depressed all CAP parameters in a dose-dependent (Tables 1 and 2) and reversible manner compared to those of the control group. CAP records for both drugs returned to baseline after 15 minutes. An example of CAP records from each drug group recorded from the rat sciatic nerve and using previously calculated parameters that best reflected the difference between the groups are presented in Figure 2, with the same temporal axis as the stimulus artifacts.
Table 1.
Effects of different concentrations of dexmedetomidine on CAP parameters at 5 and 10 minutes
| Concentration (M) | AUC change (%) | MD change (%) | Maximum derivative change (%) | VCAP (m/s) | ||||
|---|---|---|---|---|---|---|---|---|
| 5 min | 10 min | 5 min | 10 min | 5 min | 10 min | 5 min | 10 min | |
| Control | 100.00±0.00 | 100.00±0.00 | 100.00±0.00 | 100.00±0.00 | 100.00±0.00 | 100.00±0.00 | 70.28±4.86 | 70.28±4.86 |
| 10–9 | 103.44±28.29 | 103.94±13.31 | 100.74±12.16 | 100.22±9.55 | 101.34±14.44 | 95.64±10.86 | 64.64±4.60* | 66.00±6.26 |
| 10–8 | 95.82±25.17 | 85.42±15.33* | 89.54±20.49 | 84.82±14.02* | 91.79±23.10 | 78.07±18.15 | 68.98±7.23 | 67.93±5.97 |
| 10–7 | 80.14±27.39 | 74.90±26.48* | 77.00±19.24* | 72.46±27.71* | 74.61±21.05* | 64.54±28.74* | 66.93±4.63* | 62.84±4.48* |
| 10–6 | 62.87±25.80* | 55.54±26.86* | 57.25±24.65* | 53.75±27.17* | 53.78±25.69* | 48.20±28.18* | 66.89±7.11 | 69.25±3.12 |
| 10–5 | 57.58±27.35* | 48.80±24.44*$ | 52.73±28.67* | 49.10±29.25* | 49.52±29.59* | 43.61±29.28* | 66.57±7.10 | 64.91±6.37* |
| Concentration (M) | VMD (m/s) | L1 (ms) | L2 (ms) | |||||
| 5 min | 10 min | 5 min | 10 min | 5 min | 10 min | |||
| Control | 52.79±5.50 | 52.79±5.50 | 0.57±0.04 | 0.57±0.04 | 0.76±0.07 | 0.76±0.07 | ||
| 10–9 | 48.02±2.98 | 48.78±4.14* | 0.62±0.04 | 0.61±0.06 | 0.84±0.05 | 0.83±0.07 | ||
| 10–8 | 51.28±6.01 | 48.87±4.59*$ | 0.58±0.06 | 0.59±0.05 | 0.79±0.09 | 0.83±0.08$ | ||
| 10–7 | 48.21±2.87* | 45.64±2.49*$ | 0.60±0.04 | 0.64±0.04* | 0.83±0.05 | 0.87±0.05 | ||
| 10–6 | 47.26±3.38* | 47.51±1.73* | 0.60±0.06 | 0.58±0.03 | 0.85±0.06 | 0.84±0.03* | ||
| 10–5 | 46.67±5.30* | 45.80±5.60* | 0.61±0.06 | 0.62±0.06 | 0.87±0.11* | 0.89±0.11* | ||
Data are expressed as the mean ± SEM. Sample size: 6, experiment repeats: 3. Two-way analysis of variance was used to compare parameters between experimental groups. *P < 0.05 represents the significance compared to the control, and $P < 0.05 represents the significance compared to that at 5 minutes in the same drug concentration group. AUC: Area under the curve; CAP: compound action potential; L1: latency period 1, the time between the start of CAP and stimulation time; L2: latency period 2, the time between the start of CAP and maximum depolarization; MD: maximum depolarization; VCAP: conduction velocity of the fast-conducting nerve fibers; VMD: conduction velocity of the slow- and medium-conducting nerve fibers.
Table 2.
Effects of different concentrations of dexketoprofen on CAP parameters at 5 and 10 minutes
| Concentration (M) | AUC change (%) | MD change (%) | Maximum derivative change (%) | VCAP (m/s) | ||||
|---|---|---|---|---|---|---|---|---|
| 5 min | 10 min | 5 min | 10 min | 5 min | 10 min | 5 min | 10 min | |
| Control | 100.00±0.00 | 100.00±0.00 | 100.00±0.00 | 100.00±0.00 | 100.00±0.00 | 100.00±0.00 | 73.75±2.53 | 73.75±2.53 |
| 10–9 | 102.34±16.51 | 134.04±8.55* | 102.40±13.87 | 101.24±12.37 | 100.33±16.03 | 95.78±24.77 | 67.66±3.84* | 67.66±6.75* |
| 10–8 | 109.74±5.32* | 119.78±16.32*$ | 95.38±17.33 | 87.91±18.97 | 89.72±26.03 | 83.54±28.35 | 69.42±4.89* | 72.03±4.39* |
| 10–7 | 90.97±15.36 | 97.76±31.90 | 80.09±17.20* | 69.68±23.28*$ | 74.67±22.22* | 65.55±27.77* | 68.36±2.36* | 70.59±3.49*$ |
| 10–6 | 72.24±26.37* | 78.58±42.95 | 61.06±23.81* | 53.33±29.62*$ | 55.68±26.93* | 47.35±31.63* | 68.92±4.14* | 67.59±3.20* |
| 10–5 | 61.94±33.87* | 74.02±38.63$ | 49.04±27.77* | 48.70±25.67* | 43.03±28.22* | 43.35±26.90* | 68.09±4.23* | 69.30±3.71* |
| Concentration (M) | VMD (m/s) | L1 (ms) | L2 (ms) | |||||
| 5 min | 10 min | 5 min | 10 min | 5 min | 10 min | |||
| Control | 53.11±1.53 | 53.11±1.53 | 0.54±0.02 | 0.54±0.02 | 0.78±0.06 | 0.78±0.06 | ||
| 10–9 | 49.25±2.75 | 47.98±5.39 | 0.59±0.03 | 0.59±0.06 | 0.81±0.04 | 0.84±0.09 | ||
| 10–8 | 49.15±5.9 | 49.52±5.48 | 0.58±0.04* | 0.55±0.03 | 0.82±0.111 | 0.82±0,10 | ||
| 10–7 | 48.58±2.33* | 49.26±2.81* | 0.58±0.02* | 0.56±0.03*$ | 0.82±0.04 | 0.81±0.05 | ||
| 10–6 | 48.13±1.97* | 46,19±2.43* | 0.58±0.03* | 0.59±0.03* | 0.83±0.03 | 0.87±0.04* | ||
| 10–5 | 46.75±2.21* | 46.75±2.16* | 0.58±0.03* | 0.058±0.03* | 0.86±0.04* | 0.86±0.04* | ||
Data are expressed as the mean ± SEM. Sample size: 6, experiment repeats: 3. Two-way analysis of variance was used to compare parameters between experimental groups. *P < 0.05 represents the significance compared to the control, and $P < 0.05 represents the significance compared to that at 5 minutes in the same drug concentration group. AUC: Area under the curve; CAP: compound action potential; L1: latency period 1, the time between the start of CAP and stimulation time; L2: latency period 2, the time between the start of CAP and maximum depolarization; MD: maximum depolarization; VCAP: conduction velocity of the fast-conducting nerve fibers; VMD: Conduction velocity of the slow- and medium-conducting nerve fibers.
Figure 2.
The dose-dependent effects of dexmedetomidine and dexketoprofen on the compound action potentials of the rat sciatic nerve recorded at 10 minutes.
(A) Dexmedetomidine; (B) dexketoprofen.
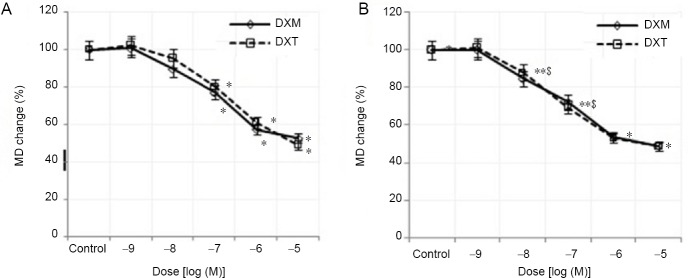
MD values
MD values were compared between dexmedetomidine and dexketoprofen at 5 and 10 minutes are shown in Figure 3. The CAP peak values recorded for the dexmedetomidine group at the 10–5 M dose were 52.73 ± 28.67% at 5 minutes and 49.10 ± 29.25% at 10 minutes, which were both less than those recorded for the control group. In the dexketoprofen group treated with the same dose, these values were measured as 49.04 ± 27.77% and 48.70 ± 25.67% at 5 and 10 minutes, respectively. There was no significant difference in MD values that were calculated from the CAP records between the two drug groups (P > 0.05).
Figure 3.
Percent changes in the dose-dependent MD of dexmedetomidine and dexketoprofen at 5 and 10 minutes.
(A) At 5 minutes, (B) at 10 minutes. Sample size: 6, experiment repeats: 3, two-way analysis of variance was used to compare parameters between experimental groups. *P < 0.05 represents the significance compared to the control, and $P < 0.05 represents the significance compared to that of 5 minutes). DXM: Dexmedetomidine; DXT: dexketoprofen; MD: maximum depolarization.
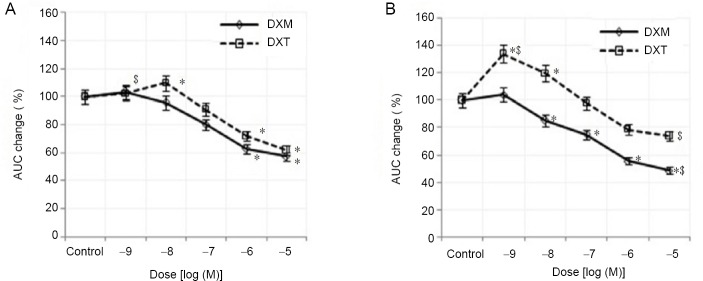
AUC values
At the highest dose of dexmedetomidine and dexketoprofen (10–5 M), the AUC values that were calculated from the CAP records were decreased at 5 and 10 minutes when compared to those of the control group (Figure 4). Compared to those of the controls, the AUC values of the dexmedetomidine group (10–5 M) at 10 minutes were more greatly reduced than those of the dexketoprofen group, but the difference was not statistically significant (P > 0.05). For the 10–6 and 10–5 M doses, a statistically significant decrease in the AUC values was found at 5 and 10 minutes for the dexmedetomidine group, but only at 5 minutes for the dexketoprofen group, compared with those of the control group (P < 0.05). The inhibitory concentration 50 (IC50) values were calculated with semilogarithmic sigmoidal dose-response curves using the decrease in AUC value (%) and potency, pD2 (–log IC50), and were found to be 7.62 ± 0.75 for dexmedetomidine and 6.96 ± 0.74 for dexketoprofen (Table 3). There were no statistically significant differences in potency (pD2) and efficacy (Emax) values between dexmedetomidine and dexketoprofen (P > 0.05).
Figure 4.
Percent changes in the dose-dependent AUC of dexmedetomidine and dexketoprofen at 5 and 10 minutes.
(A) At 5 minutes; (B) at 10 minutes. Sample size: 6, experiment repeats: 3. Two-way analysis of variance was used to compare the parameters between experimental groups. *P < 0.05 represents the significance compared to the control, and $P < 0.05 represents the significance compared to that at 5 minutes. AUC: Area under the curve; DXM: dexmedetomidine; DXT: dexketoprofen.
Table 3.
pD2 and Emax values for dexmedetomidine and dexketoprofen at 10 minutes
| pD2 (–log[drug]) | Emax (efficacy, % decrease of AUC) | |
|---|---|---|
| Dexmedetomidine | 7.62±0.75 | 53.7±14.7 |
| Dexketoprofen | 6.96±0.74 | 60.3±14.6 |
Data are expressed as the mean ± SEM. AUC: Area under the curve; Emax: efficacy, maximum response of drug; pD2: potency of the drug.
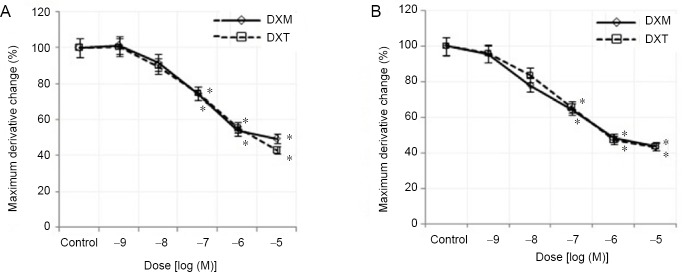
Maximum derivative, conduction velocity values
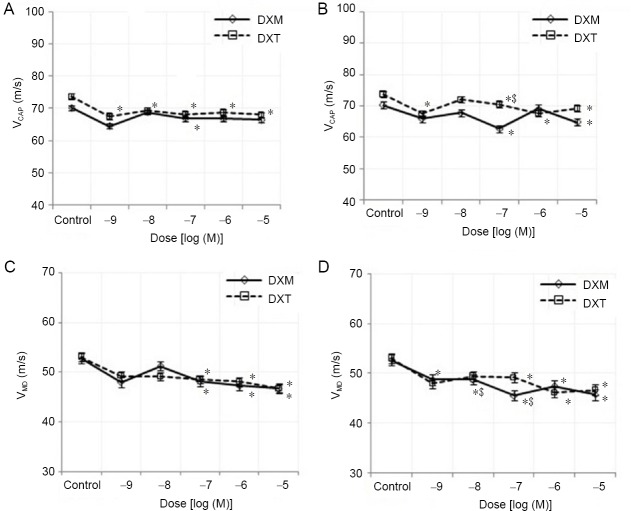
Treatment with three highest doses of both drugs (10–7, 10–6 and 10–5 M) caused a statistically significant (P < 0.05) decrease in the maximum derivative of CAP at 5 and 10 minutes compared to those of the control group (Figure 5). In addition, the reduction in conduction velocity value was significantly different (P > 0.05) between the two drugs. In contrast, the conduction velocity of the fast-conducting fibers, the medium- and slow-conducting fibers was decreased for each drug at all doses and times (Figure 6) compared to those of the controls.
Figure 5.
Percent changes in the dose-dependent maximum derivative of dexmedetomidine and dexketoprofen at 5 and 10 minutes.
(A) At 5 minutes; (B) at 10 minutes. Sample size: 6, experiment repeats: 3. Two-way analysis of variance was used to compare parameters between experimental groups. *P < 0.05 represents the significance compared to the control, and $P < 0.05 represents the significance compared to that at 5 minutes. DXM: Dexmedetomidine; DXT: dexketoprofen.
Figure 6.
Percent changes in the dose-dependent VCAP and VMD of dexmedetomidine and dexketoprofen at 5 and 10 minutes.
(A) VCAP at 5 minutes; (B) VCAP at 10 minutes; (C) VMD at 5 minutes; (D) VMD at 10 minutes. Sample size: 6, experiment repeats: 3. Two-way analysis of variance was used to investigate the difference in parameters between experimental groups. *P < 0.05 represents the significance compared to the control, and $P < 0.05 represents the significance compared to that at 5 minutes. DXM: Dexmedetomidine; DXT: dexketoprofen; VCAP: conduction velocity of the fast-conducting nerve fibers; VMD: conduction velocity of the slow- and medium-conducting nerve fibers.
Discussion
A recent study reported that dexmedetomidine is used as an analgesic adjuvant in peripheral nerve block with an inflammatory reaction (Bagry et al., 2008; Grosu and Lavand’homme, 2015). Peripheral nerve block is a very popular technique that is used in combination with general anesthesia for postoperative analgesia in major surgery (Li et al., 2017). A series of experimental animal studies have shown the anti-inflammatory effects of peripheral nerve blocks after major surgery (Combettes et al., 2010; He et al., 2015).
In our study, the effects of the α2 agonist dexmedetomidine and the NSAID dexketoprofen, both of which are used for pain control and have an anti-inflammatory effect, on peripheral nerve conduction were examined. Results from this study showed that dexmedetomidine and dexketoprofen caused a dose-dependent suppression of all CAP parameters. The suppressed CAPs returned to baseline 15 minutes after washing. In our study, the effects of dexmedetomidine and dexketoprofen on the sciatic nerve were investigated only in vitro. If further studies are performed in vivo, the effects of dexmedetomidine and dexketoprofen on all systems can be examined or their effects on other peripheral or central nerves can be examined to provide more information about use of dexmedetomidine and dexketoprofen in pain management.
The peak values (MDs) of CAP curves decrease with an increase in dexmedetomidine dose, and as a result, AUC was decreased. The changes of MDs of CAP curves were significant at high doses (10–6 and 10–5) were significant. Oda et al. (2007) reported that dexmedetomidine inhibited voltage-dependent Na+ channels. Additionally, in a study using a patch-clamp method on cardiac cells, dexmedetomidine blocked the persistent sodium current induced by veratridine (Stoetzer et al., 2016). Another study reported that in frog sciatic nerve, high doses of dexmedetomidine (5 × 10–4 M) reduced the value of MD, but this effect might not be related to adrenoceptors (Kosugi et al., 2010). In our study, reduction of MD may be the result of inhibition of sodium channels that are active in the rising phases of CAPs.
The suppression of fast-conducting fibers causes an increase in L1 values, while the suppression of slow-conducting fibers causes an increase in L2 values (Dalkilic and Pehlivan, 2002). In the dexmedetomidine group, both latency periods increased, and consequent conduction velocity decreased compared to those of the controls. At the doses of 10–6 and 10–5 M, there were significant differences in L2 parameters between two drug groups and the control group (P < 0.05). In this study, dexmedetomidine showed dose-dependent activity on slow-conducting fibers. At high doses (10–7, 10–6 and 10–5 M) of dexmedetomidine, a significant reduction in the maximum derivative was observed when compared to the controls. The maximum derivative is related to the maximum rate of change in the rising phase of the CAP over time and gives information about Na+ channels (Katsuki et al., 2006). Additionally, fast-conducting fibers make a greater contribution during the CAP rising phase. For this reason, the changes in the maximum derivatives can be interpreted as changes in the fast-conducting fibers (Dalkilic et al., 2009). Therefore, dexmedetomidine at high doses suppresses the fast-conducting fibers in the rat sciatic nerve. These results show similarities to previous studies. Butterworth and Strichartz (1993) reported that clonidine, an α2 agonist that is similar to dexmedetomidine, inhibited CAP amplitude, and this inhibition was reported to act on Aα and C fibers. According to the changes in the maximum derivative that we observed in our study, dexmedetomidine at high doses produced suppression in the fast-conducting fibers, and therefore, type A fibers could be affected.
Dexmedetomidine is used in combination with a local anesthetic to increase the amount of time that peripheral nerve conduction is blocked (Brummett et al., 2011). A meta-analysis of neuraxial adjuvant anesthesia and analgesia indicates that dexmedetomidine is a good adjuvant for local anesthesia, causing the extension of the duration of postoperative pain relief and lowering pain intensity (Wu et al., 2014). Another meta-analysis regarding the faciliatory effects of perineural dexmedetomidine reported that dexmedetomidine speeds up the sensory and motor nerve blockage, and sensory nerve blockage time was significantly extended compared to that of a placebo (Abdallah and Brull, 2013). This effect is attributed in the literature to the delayed absorption of the local anesthetic due to the local vasoconstriction caused by dexmedetomidine (Yabuki et al., 2014). The results of our in vitro study suggest that dexmedetomidine directly inhibits neuronal conduction.
According to the AUC analysis in our study, dexmedetomidine and dexketoprofen have similar effects on the CAP parameters recorded from rat sciatic nerve, but dexketoprofen primarily suppresses slow-fiber conduction. At low doses (10–9 and 10–8 M) of dexketoprofen, the change of AUC (%) was significantly increased at 10 minutes compared to those of the controls. This increase is explained as follows. The constant CAP amplitude (peak value, MD) during the expansion falling phase causes an increase in the AUC. However, a significant inhibition (55.90 ± 30.57%) was observed at the lowest dose (10–9 M) compared to that at the highest dose (10–5 M), resulting in the dose-dependent suppression of the conduction velocity for all fiber types distributed in the nerve bundle. Mazario et al. (1999), in a study involving rats, found that dexketoprofen inhibited single motor unit (SMU) records, which showed electrical and mechanical stimulation even at a very low dose (25 nmol/kg).
Most dexketoprofen studies to date have investigated the analgesic potency of dexketoprofen, its use in combination with other drugs, the usefulness of preemptive or postoperative administration, its use in the management of chronic pain, and its effectiveness for reducing opioid drug consumption (Yucel et al., 2013; Kelsaka et al., 2014; Kaye et al., 2018), as well as the effects on the SMU records generated by electrical or mechanical stimulation (Mazario et al., 2001; Gaitan and Herrero, 2002). However, most of the studies were conducted in vivo, and our study was the first to report the effect of dexketoprofen on rat sciatic nerve in vitro.
In this study on sciatic nerve, we observed no significant difference between dexmedetomidine and dexketoprofen in terms of inhibiting nerve conduction. However, we report that dexmedetomidine at higher doses suppresses the conduction of fast-conducting fibers, whereas dexketoprofen has a time-dependent effect on slow-conducting fibers and a dose-dependent effect on medium- and fast-conducting fibers.
Footnotes
Conflicts of interest: The authors have no conflict of interest to declare.
Financial support: This study was supported by the Scientific Committee Foundation (No. 13102007) of Selçuk University, Konya, Turkey (to HB).
Institutional review board statement: The experimental procedures were approved by the Necmettin Erbakan University on January 30, 2013 (approval No. 2013-024).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Alonzo D Cook, Brigham Young Unversity, USA; Lorenzo Di Cesare Mannelli, University of Florence, Italy.
Funding: This study was supported by Scientific Committee Foundation (No. 13102007) of Selçuk University, Konya, Turkey (to HB).
P-reviewers: Cook AD, Di Cesare Mannelli L; C-Editor: Zhao M; S-Editor: Li CH; L-Editor: Song LP; T-Editor: Jia Y
References
- 1.Abdallah FW, Brull R. Facilitatory effects of perineural dexmedetomidine on neuraxial and peripheral nerve block: a systematic review and meta-analysis. Br J Anaesth. 2013;110:915–925. doi: 10.1093/bja/aet066. [DOI] [PubMed] [Google Scholar]
- 2.Bagry H, de la Cuadra Fontaine JC, Asenjo JF, Bracco D, Carli F. Effect of a continuous peripheral nerve block on the inflammatory response in knee arthroplasty. Reg Anesth Pain Med. 2008;33:17–23. doi: 10.1016/j.rapm.2007.06.398. [DOI] [PubMed] [Google Scholar]
- 3.Brummett CM, Hong EK, Janda AM, Amodeo FS, Lydic R. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization-activated cation current. Anesthesiology. 2011;115:836–843. doi: 10.1097/ALN.0b013e318221fcc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butterworth JF, Strichartz GR. The alpha 2-adrenergic agonists clonidine and guanfacine produce tonic and phasic block of conduction in rat sciatic nerve fibers. Anesth Analg. 1993;76:295–301. [PubMed] [Google Scholar]
- 5.Combettes E, Benhamou D, Mazoit JX, Beloeil H. Comparison of a bupivacaine peripheral nerve block and systemic ketoprofen on peripheral inflammation and hyperalgesia in rats. Eur J Anaesthesiol. 2010;27:642–647. doi: 10.1097/eja.0b013e3283366590. [DOI] [PubMed] [Google Scholar]
- 6.Dalkiliç N, Pehlivan F. Comparison of fiber diameter distributions deduced by modeling compound action potentials recorded by extracellular and suction techniques. Int J Neurosci. 2002;112:913–930. doi: 10.1080/00207450290025923. [DOI] [PubMed] [Google Scholar]
- 7.Dalkilic N, Tuncer S, Bariskaner H, Kiziltan E. Effect of tramadol on the rat sciatic nerve conduction: a numerical analysis and conduction velocity distribution study. Yakugaku Zasshi. 2009;129:485–493. doi: 10.1248/yakushi.129.485. [DOI] [PubMed] [Google Scholar]
- 8.Gaitan G, Herrero JF. Subeffective doses of dexketoprofen trometamol enhance the potency and duration of fentanyl antinociception. Br J Pharmacol. 2002;135:393–398. doi: 10.1038/sj.bjp.0704491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gracias NG, Cummins TR, Kelley MR, Basile DP, Iqbal T, Vasko MR. Vasodilatation in the rat dorsal hindpaw induced by activation of sensory neurons is reduced by paclitaxel. Neurotoxicology. 2011;32:140–149. doi: 10.1016/j.neuro.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grosu I, Lavand’homme P. Continuous regional anesthesia and inflammation: a new target. Minerva Anestesiol. 2015;81:1001–1009. [PubMed] [Google Scholar]
- 11.Hanna M, Moon JY. A review of dexketoprofen trometamol in acute pain. Curr Med Res Opin. 2018;24:1–14. doi: 10.1080/03007995.2018.1457016. [DOI] [PubMed] [Google Scholar]
- 12.He Y, Li Z, Zuo YX. Nerve blockage attenuates postoperative inflammation in hippocampus of young rat model with surgical trauma. Mediators Inflamm. 2015;2015:460125. doi: 10.1155/2015/460125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kara I, Apiliogullari S, Bagcı Taylan S, Bariskaner H, Celik JB. The effects of dexketoprofen on duration of analgesia to a thermal stimulus when compared with a systemic control in a rat sciatic nerve block with levobupivacaine. Fundam Clin Pharmacol. 2014;28:205–210. doi: 10.1111/fcp.12010. [DOI] [PubMed] [Google Scholar]
- 14.Katsuki R, Fujita T, Koga A, Liu T, Nakatsuka T, Nakashima M, Kumamoto E. Tramadol, but not its major metabolite (mono-O-demethyl tramadol) depresses compound action potentials in frog sciatic nerves. Br J Pharmacol. 2006;149:319–327. doi: 10.1038/sj.bjp.0706868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaye AD, Cornett EM, Hart B, Patil S, Pham A, Spalitta M, Mancuso KF. Novel pharmacological nonopioid therapies in chronic pain. Curr Pain Headache Rep. 2018;22:31. doi: 10.1007/s11916-018-0674-8. [DOI] [PubMed] [Google Scholar]
- 16.Kelsaka E, Güldoğuş F, Cetinoğlu E. Effect of intravenous dexketoprofen use on postoperative analgesic consumption in patients with lumbar disc surgery. Agri. 2014;26:82–86. doi: 10.5505/agri.2014.47550. [DOI] [PubMed] [Google Scholar]
- 17.Kosugi T, Mizuta K, Fujita T, Nakashima M, Kumamoto E. High concentrations of dexmedetomidine inhibit compound action potentials in frog sciatic nerves without alpha(2) adrenoceptor activation. Br J Pharmacol. 2010;160:1662–1676. doi: 10.1111/j.1476-5381.2010.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Wang H, Dong B, Ma J, Wu X. Adding dexmedetomidine to ropivacaine for femoral nerve block inhibits local inflammatory response. Minevra Anestesiol. 2017;83:590–597. doi: 10.23736/S0375-9393.17.11430-6. [DOI] [PubMed] [Google Scholar]
- 19.Mazario J, Gaitan G, Herrero JF. Cyclooxygenase-1 vs. cyclooxygenase-2 inhibitors in the induction of antinociception in rodent withdrawal reflexes. Neuropharmacology. 2001;40:937–946. doi: 10.1016/s0028-3908(01)00020-x. [DOI] [PubMed] [Google Scholar]
- 20.Mazario J, Roza C, Herrero JF. The NSAID dexketoprofen trometamol is as potent as mu-opioids in the depression of wind-up and spinal cord nociceptive reflexes in normal rats. Brain Res. 1999;816:512–517. doi: 10.1016/s0006-8993(98)01203-7. [DOI] [PubMed] [Google Scholar]
- 21.Miranda HF, Romero MA, Puig MM. Antinociceptive and anti-exudative synergism between dexketoprofen and tramadol in a model of inflammatory pain in mice. Fundam Clin Pharmacol. 2012;26:373–382. doi: 10.1111/j.1472-8206.2010.00922.x. [DOI] [PubMed] [Google Scholar]
- 22.Moore RA, Barden J. Systematic review of dexketoprofen in acute and chronic pain. BMC Clin Pharmacol. 2008;8:11. doi: 10.1186/1472-6904-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore RA, Gay-Escoda C, Figueiredo R, Toth-Bagi Z, Dietrich T, Milleri S, Torres-Lagares D, Hill CM, García-García A, Coulthard P, Wojtowicz A, Matenko D, Peñarrocha-Diago M, Cuadripani S, Pizà-Vallespir B, Guerrero-Bayón C, Bertolotti M, Contini MP, Scartoni S, Nizzardo A, et al. Dexketoprofen/tramadol: randomised double-blind trial and confirmation of empirical theory of combination analgesics in acute pain. J Headache Pain. 2015;16:541. doi: 10.1186/s10194-015-0541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oda A, Iida H, Tanahashi S, Osawa Y, Yamaguchi S, Dohi S. Effects of alpha2-adrenoceptor agonists on tetrodotoxin-resistant Na+ channels in rat dorsal root ganglion neurons. Eur J Anaesthesiol. 2007;24:934–941. doi: 10.1017/S0265021507000543. [DOI] [PubMed] [Google Scholar]
- 25.Peng W, Zhang T. Dexmedetomidine decreases the emergence agitation in infant patients undergoing cleft palate repair surgery after general anesthesia. BMC Anesthesiol. 2015;15:145. doi: 10.1186/s12871-015-0124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sousa DG, Sousa SD, Silva RE, Silva-Alves KS, Ferreira-da-Silva FW, Kerntopf MR, Menezes IR, Leal-cardoso JH, Barbosa R. Essential oil of Lippia alba and its main constituent citral block the excitability of rat sciatic nerves. Braz J Med Biol Res. 2015;48:697–702. doi: 10.1590/1414-431X20154710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoetzer C, Reuter S, Doll T, Foadi N, Wegner F, Leffler A. Inhibition of the cardiac Na+ channel α-subunit Nav1. 5 by propofol and dexmedetomidine. Naunyn Schmiedebergs Arch Pharmacol. 2016;389:315–325. doi: 10.1007/s00210-015-1195-1. [DOI] [PubMed] [Google Scholar]
- 28.Wijesinghe RS1, Gielen FL, Wikswo JP Jr A model for compound action potentials and currents in a nerve bundle III: A comparison of the conduction velocity distributions calculated from compound action currents and potentials. Ann Biomed Eng. 1991;19:97–121. doi: 10.1007/BF02368462. [DOI] [PubMed] [Google Scholar]
- 29.Wu HH, Wang HT, Jin JJ, Cui GB, Zhou KC, Chen Y, Chen GZ, Dong YL, Wang W. Does dexmedetomidine as a neuraxial adjuvant facilitate better anesthesia and analgesia. A systematic review and meta-analysis? PLoS One. 2014;9:e93114. doi: 10.1371/journal.pone.0093114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yabuki A, Higuchi H, Yoshitomi T, Tomoyasu Y, Ishii-Maruhama M, Maeda S, Miyawaki T. Locally injected dexmedetomidine induces vasoconstriction via peripheral alpha-2A adrenoceptor subtype in guinea pigs. Reg Anesth Pain Med. 2014;39:133–136. doi: 10.1097/AAP.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 31.Yucel E, Kol IO, Duger C, Kaygusuz K, Gursoy S, Mimaroglu C. Ilioinguinal-iliohypogastric nerve block with intravenous dexketoprofen improves postoperative analgesia in abdominal hysterectomies. Braz J Anesthesiol. 2013;63:334–339. doi: 10.1016/j.bjane.2012.07.003. [DOI] [PubMed] [Google Scholar]