This systematic review and meta-analysis examines the mortality rates and key causes of excess mortality among people who use extramedical opioids.
Key Points
Question
What are the mortality rates and key causes of excess mortality among people who use extramedical opioids?
Findings
In this systematic review and meta-analysis of 124 studies, people using extramedical opioids had a higher mortality rate than those of the same age and sex in the general population. Excess mortality occurred across individuals with traumatic causes of death, infectious diseases, and noncommunicable diseases.
Meaning
The findings suggest that response to elevated mortality in people using extramedical opioids should include overdose prevention and incorporate interventions to prevent and treat infectious diseases and noncommunicable diseases.
Abstract
Importance
Extramedical opioid use has escalated in recent years. A better understanding of cause-specific mortality in this population is needed to inform comprehensive responses.
Objective
To estimate all-cause and cause-specific crude mortality rates (CMRs) and standardized mortality ratios (SMRs) among people using extramedical opioids, including age- and sex-specific estimates when possible.
Data Sources
For this systematic review and meta-analysis, MEDLINE, PsycINFO, and Embase were searched for studies published from January 1, 2009, to October 3, 2019, and an earlier systematic review on this topic published in 2011.
Study Selection
Cohort studies of people using extramedical opioids and reporting mortality outcomes were screened for inclusion independently by 2 team members.
Data Extraction and Synthesis
Data were extracted by a team member and checked by another team member. Study quality was assessed using a custom set of items that examined risk of bias and quality of reporting. Data were pooled using random-effects meta-analysis models. Heterogeneity was assessed using stratified meta-analyses and meta-regression.
Main Outcomes and Measures
Outcome measures were all-cause and cause-specific CMRs and SMRs among people using extramedical opioids compared with the general population of the same age and sex.
Results
Of 8683 identified studies, 124 were included in this analysis (100 primary studies and 24 studies providing additional data for primary studies). The pooled all-cause CMR, based on 99 cohorts of 1 262 592 people, was 1.6 per 100 person-years (95% CI, 1.4-1.8 per 100 person-years), with substantial heterogeneity (I2 = 99.7%). Heterogeneity was associated with the proportion of the study sample that injected opioids or was living with HIV infection or hepatitis C. The pooled all-cause SMR, based on 43 cohorts, was 10.0 (95% CI, 7.6-13.2). Excess mortality was observed across a range of causes, including overdose, injuries, and infectious and noncommunicable diseases.
Conclusions and Relevance
The findings suggest that people using extramedical opioids experience significant excess mortality, much of which is preventable. The range of causes for which excess mortality was observed highlights the multiplicity of risk exposures experienced by this population and the need for comprehensive responses to address these. Better data on cause-specific mortality in this population in several world regions appear to be needed.
Introduction
Extramedical opioid use includes the use of heroin and other illicitly manufactured opioids and the use of pharmaceutical opioids outside the bounds of a medical prescription.1 Extramedical opioid use is a significant public health problem,2 with use and related harms escalating across many high-income countries.3 In the United States, HIV and hepatitis C virus (HCV) infection outbreaks associated with opioid injecting have been observed,4,5 and fatal opioid overdoses have increased markedly, exceeding 47 000 deaths in 2018.6 Increased fatal opioid overdoses have also been observed in Canada, the United Kingdom, Australia, and Europe.7,8,9,10
Overdose is not the only risk of extramedical opioid use. In a previous systematic review,11 AIDS-related causes were at least as common as overdose deaths in 6 of 25 cohorts reporting both causes of death. Increasing rates of HCV-related deaths have been observed in cohorts of people with a history of opioid dependence.12 Other elevated causes of death include suicide and other injuries.11
Given the dynamic nature of extramedical opioid use and related deaths, it appeared to be timely to review data on mortality, particularly cause-specific mortality, among people who use extramedical opioids. We aimed to estimate all-cause and cause-specific crude mortality rates (CMRs), standardized mortality ratios (SMRs), and relative risks (RRs) with sex- and age-specific estimates when possible.
Methods
This systematic review and meta-analysis followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.13 The review protocol was registered with PROSPERO (CRD42018094623).
Search Strategy and Study Selection
A previous systematic review11 on this topic was used to identify studies published from 1980 to 2008. MEDLINE, Embase, and PsycINFO databases were searched using the OVID interface and platform to identify relevant articles published from January 1, 2009, through February 22, 2018. Searches were updated in October 2019. Search strings are provided in eAppendix 1 in the Supplement. No language restrictions were applied to the search, with the research team able to read English, Italian, French, and Chinese. Studies in languages other than these were read using Google Translate.
Study selection was completed using Covidence.14 Team members were trained in the requirements for inclusion of a study. Each retrieved citation was screened for inclusion based on title and abstract. All publications marked as excluded at this stage were reviewed by a second investigator (S.L. or L.T.T.), and if there was a difference in the assessment, the publication was included for the next stage of the review. Each study included after initial screening was reviewed in full by 2 people (S.L., L.T.T., T.S., and/or L.D.). Disagreements were resolved through discussion between the 2 reviewers and referral to a third party if needed. Reference lists of reviews identified by the search were screened to identify any additional publications.
Inclusion and Exclusion Criteria
We included cohort studies of people who used extramedical opioids and were recruited in any setting that reported data on CMRs and/or SMRs. This could include cohort studies of people who injected drugs if at least 90% of the cohort reported extramedical opioid use. Cohorts did not need to be opioid dependent or have opioid use disorder to be included. Cohorts of people prescribed opioids for pain management or who were exclusively people living with HIV or HCV infection were excluded, as were studies that reported case-fatality rates only. Full inclusion and exclusion criteria are described in eAppendix 2 in the Supplement.
Data Extraction
Data were extracted into a spreadsheet by 1 member of the research team and checked by another (L.T.T. or T.S.). Extracted variables included study information (eg, years of data collection) and sample information (eg, sex distribution) considered a priori to be potentially relevant to between-study heterogeneity. We extracted number of observed deaths, person-years of follow-up, and expected deaths to allow for calculation of CMRs and SMRs and extracted CMRs and SMRs as reported. Specific causes for which data were extracted were overdose, AIDS related, suicide, unintentional injuries, homicide, liver disease, cardiovascular disease, respiratory disease, and cancer. When data for a study were incomplete, authors were contacted by email for additional information.
During data extraction, inconsistencies were identified in how overdose deaths were defined. Some studies15,16 reported overdose mortality rates without explaining how overdose was defined. When definitions were reported (typically using International Classification of Diseases, Ninth Revision [ICD-9] or International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10] codes), the most restrictive definitions included only opioid poisoning (n = 6), whereas others included any drug poisoning (n = 27). Less restrictive definitions included deaths caused by mental and behavioral disorders due to substance use (n = 29). Pooled estimates were therefore calculated separately for 3 definitions of overdose death: opioid poisoning, poisoning from any drug (including alcohol if it was not possible to disaggregate by drug), and poisoning from any drug and from psychoactive substance use for mental and behavioral disorders. Differences were also observed in how liver diseases were coded. We calculated pooled estimates for the following definitions: viral hepatitis (n = 7), digestive diseases (including codes 520-579 in ICD-9 or chapter XI of ICD-10 [n = 11]), and liver related (which typically included both the previous categories plus liver cancer [n = 20]).
Study Quality, Including Risk of Bias
Because risk-of-bias tools for observational epidemiological studies are still evolving,17 we developed a review-specific tool with reference to 2 recent publications17,18 on assessing risk of bias in observational studies of exposures. The tool assessed each study on 2 risk-of-bias domains and 3 quality-of-reporting domains (eAppendix 3 in the Supplement). Risk-of-bias domains were sample representativeness and outcome measurement. Studies were rated as being at higher or lower risk of bias on each of these domains. Quality-of-reporting domains were completeness of reporting of cohort characteristics, completeness of outcome data, and reporting of definitions used for cause-specific deaths. Studies were assessed as having higher- or lower-quality reporting on each of these domains.
Distribution of Causes of Death
To examine the distribution of causes of death across cohorts, we identified the subset of cohorts in which a cause was specified for all observed deaths (including that cause for x deaths was undetermined as opposed to not reported) and grouped deaths into the following categories: poisoning or substance dependence, infectious diseases, noncommunicable diseases, trauma, and undetermined. In keeping with classifications used in the included studies, deaths attributed to viral hepatitis were included in infectious diseases, but deaths attributed to liver disease were included in noncommunicable diseases despite likely being sequelae of viral hepatitis. The weighted mean of the proportion of deaths in the cohorts in each category was calculated along with 95% CIs based on a t distribution. Data on certain subordinate causes within a category were commonly reported, including AIDS-related deaths within infectious diseases, cancer and liver disease within noncommunicable diseases, and suicide within trauma. Weighted means of the proportion of deaths attributable to these causes within the cohorts were also calculated.
Statistical Analysis
The CMRs were calculated as deaths per 100 person-years and the SMRs as observed deaths over expected deaths. We derived 95% CIs for each metric using standard formulas (eAppendix 4 in the Supplement).
The SMRs represent the ratio of mortality risk among those exposed to the risk and the entire population, including those exposed to the risk. The RRs give the ratio of mortality risk between those exposed to the risk and those not exposed to the risk. With a low-prevalence exposure, such as extramedical opioid use, SMRs and RRs should be similar. We estimated RRs from SMRs by adjusting the SMR by the proportion of the general population that is exposed to the risk.19 Data on general population risk exposure were obtained from the Global Health Data Exchange.20
We explored heterogeneity through stratification and meta-regression and used random-effects models for pooling data because we expected that there would be variation among the samples selected by the studies. For pooled analyses, we used DerSimonian and Laird random-effects meta-analyses in Stata statistical software, version 14.2 (StataCorp). Study weights incorporated both within- and between-study error. Heterogeneity was quantified using the I2 statistic. We considered I2 of 25% or less to indicate low heterogeneity, 25% to 50% as moderate heterogeneity, and greater than 50% as substantial heterogeneity.21
Stratification variables for exploring heterogeneity included sex, age group, year of follow-up completion (with 1994 selected as the cut point because of the introduction of highly active antiretroviral therapy for HIV infection in that year), injecting drug use, opioid dependence or use disorder, recruitment setting (drug treatment or harm-reduction settings compared with all other settings), and geographic region as defined by the Global Burden of Disease study. Depending on how data were presented, age groups were defined as younger than 30 years compared with 30 years or older or younger than 35 years compared with 35 years or older (hereafter referred to as younger and older patients). All-cause and cause-specific CMRs comparing men and women and younger and older people were estimated.
Each meta-regression included a single moderator variable, which could be a feature of the study sample (eg, prevalence of HIV infection) or a feature of the study design or conduct (eg, recruitment setting). Variables were only included in meta-regressions if 5 or more data points were available. A 2-sided P < .05 was considered to indicate an explanatory moderator variable that influenced heterogeneity in the CMR or SMR.
Results
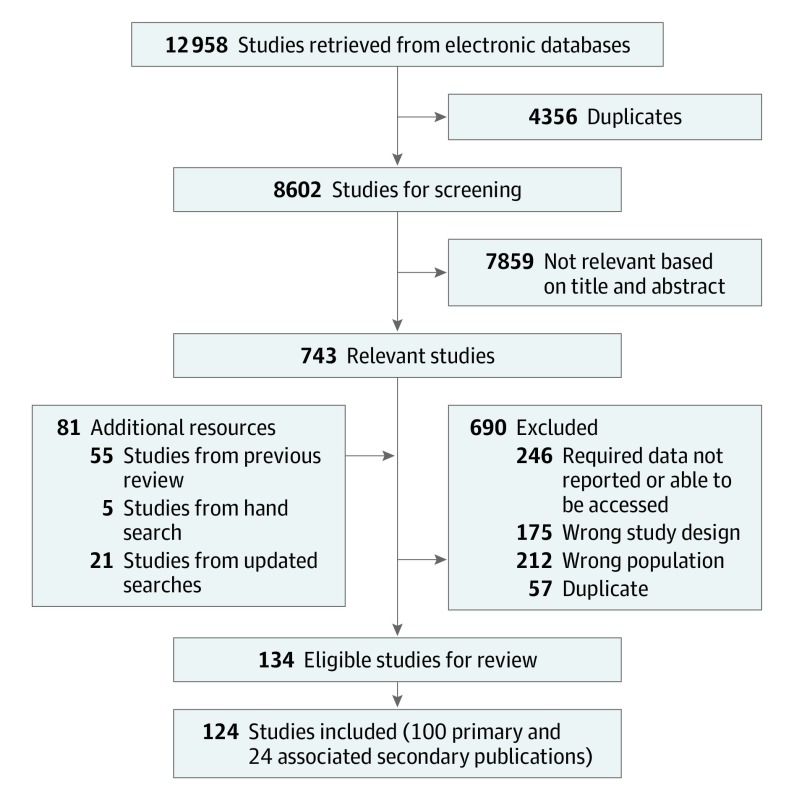
The PRISMA study flow diagram is shown in Figure 1. We included 124 of 8683 identified publications, including 100 primary publications and 24 secondary publications that provided additional data for these primary publications. Cohorts were recruited from 28 countries, including 5 low- and middle-income countries (9 studies). Cohort size ranged from 100 to 306 786 people, and person-years of follow-up ranged from 129 to 687 673. Characteristics of included studies are provided in eAppendix 5 in the Supplement.
Figure 1. PRISMA Flow Diagram of Studies of Mortality Among People Using Extramedical Opioids.
Risk of Bias and Study Quality
A total of 48 included cohorts (45.3%) were rated as being at higher risk of bias on the basis of cohort representativeness (eg, were recruited from a single site), and 23 (21.7%) were at higher risk of bias on the basis of outcome measurement (eg, mortality data derived from clinical records rather than death registries). Only 10 cohorts (9.4%) did not report age and sex data to characterize the cohort sample. A total of 29 cohorts (27.4%) reported incomplete mortality data (ie, missing numerator or denominator), and 34 (41.0%) of the 83 cohorts that reported cause-specific mortality did not report the definitions used to categorize deaths. A summary table and individual study assessments are provided in eAppendix 6 in the Supplement.
All-Cause Mortality
The pooled all-cause CMR, based on 99 cohorts, was 1.59 per 100 person-years (95% CI, 1.40-1.80 per 100 person-years), with substantial heterogeneity (I2 = 99.7%) (Table 1). Forest plots for this and all following pooled analyses are provided in eAppendix 7 in the Supplement. The highest CMRs were observed in South Asia (7.62 per 100 person-years; 95% CI, 4.84-12.00 per 100 person-years; 2 cohorts, both from Bangladesh) and the lowest in Australasia (0.80 per 100 person-years; 95% CI, 0.67-0.96 per 100 person-years; 7 cohorts, all from Australia) (Table 1).
Table 1. Pooled All-Cause Crude Mortality Rates Among People Using Extramedical Opioids by Sex, Age Group, Drug Use Characteristics, and Region.
| Variable | Mortality | |||||
|---|---|---|---|---|---|---|
| Crude Rate | Standardized Ratio | |||||
| No. of Cohorts | Pooled Crude Mortality Rate per 100 Person-Years (95% CI) | I2, % | No. of Cohorts | Pooled Standardized Mortality Ratio (95% CI) | I2, % | |
| All-cause mortality | 99a | 1.59 (1.40-1.80) | 99.7 | 43 | 10.03 (7.64-13.17) | 99.9 |
| Sex | ||||||
| Male | 47 | 1.85 (1.51-2.27) | 99.7 | 31 | 8.56 (6.70-10.93) | 99.9 |
| Female | 43 | 1.28 (1.03-1.58) | 99.4 | 30 | 13.40 (8.90-20.16) | 99.9 |
| Ageb | ||||||
| Younger | 18 | 1.25 (0.91-1.71) | 99.5 | 5 | 10.96 (4.59-26.16) | 99.8 |
| Older | 18 | 2.09 (1.51-2.91) | 99.9 | 5 | 5.24 (4.53-6.05) | 94.6 |
| Follow-up completed | ||||||
| By end of 1994 | 7 | 1.85 (0.97-3.55) | 99.8 | 4 | 9.33 (4.98-17.46) | 99.4 |
| Continued or commenced in 1995 or later | 90 | 1.57 (1.39-1.78) | 99.6 | 39 | 10.10 (7.56-13.50) | 99.9 |
| Cohorts | ||||||
| Injectingc | 19 | 2.71 (2.14-3.42) | 95.2 | 10 | 16.37 (10.92-24.55) | 98.8 |
| Dependentd | 67 | 1.54 (1.33-1.78) | 99.7 | 36 | 10.31 (7.63-13.93) | 99.9 |
| Recruitment setting | ||||||
| Drug treatment or harm reduction setting | 57 | 1.58 (1.37-1.82) | 99.5 | 29 | 10.25 (7.78-13.51) | 99.8 |
| Other settingse | 40 | 1.60 (1.31-1.96) | 99.7 | 14 | 9.42 (7.74-11.46) | 99.5 |
| GBD regionf | ||||||
| Australasia | 7 | 0.80 (0.67-0.96) | 91.3 | 3 | 8.63 (6.49-11.47) | 93.2 |
| East Asia | 6 | 1.80 (1.37-2.35) | 96.7 | 4 | 9.89 (6.15-15.92) | 98.2 |
| Southeast Asia | 3 | 4.53 (3.11-6.62) | 69.3 | 1 | 13.40 (11.40-15.30) | NA |
| South Asia | 2 | 7.62 (4.84-12.00) | 60.9 | NI | NA | NA |
| North Africa and Middle East | 2 | 3.40 (0.97-11.87) | 95.2 | NI | NA | NA |
| Central Europe | 3 | 1.17 (0.61-2.23) | 96.2 | 1 | 12.06 (9.60-15.00) | NA |
| Western Europe | 54 | 1.56 (1.32-1.84) | 99.5 | 27 | 11.87 (9.15-15.40) | 99.8 |
| North America | 20 | 1.61 (1.36-1.91) | 99.5 | 7 | 5.02 (4.21-5.98) | 99.2 |
Abbreviations: GBD, Global Burden of Disease; NA, not applicable; NI, not identified.
Studies presented age-specific data using various age groups; this analysis includes studies in which the age groups could be summarized as younger than 30 years vs 30 years or older or younger than 35 years vs 35 years or older.
Includes only cohorts defined by opioid injecting.
Includes only cohorts defined by opioid dependence or opioid use disorder.
Other settings include acute care (eg, emergency departments), prisons, and community settings.
Regions are defined per the GBD project. No studies were found for the following GBD regions: Latin America and the Caribbean, sub-Saharan Africa, Oceania, Central Asia, or Eastern Europe.
In cohorts of people who injected opioids, the pooled all-cause CMR was 2.71 per 100 person-years (95% CI, 2.14-3.42 per 100 person-years) (Table 1). The prevalence of injecting drug use, HIV infection, and HCV infection within study samples were all important sources of heterogeneity and associated with higher CMRs (Table 2). The CMRs were higher among men compared with women (CMR ratio, 1.38; 95% CI, 1.30-1.47) and among younger people compared with older people (CMR ratio, 1.98; 95% CI, 1.59-2.47) (Table 3).
Table 2. Meta-Regression of Potential Sources of Heterogeneity in the Pooled All-Cause Crude Mortality Rate and Standardized Mortality Ratio.
| Characteristic | Mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| Crude Rate | Standardized Ratio | |||||||
| No. of Studies | Coefficient (SE) | Adjusted R2, % | P Value | No. of Studies | Coefficient (SE) | Adjusted R2, % | P Value | |
| Cohort characteristics at baseline | ||||||||
| Percentage drug injectors | 43 | 4.297 (1.818) | 22.67 | .001 | 16 | 7.592 (4.725) | 43.41 | .006 |
| Percentage male | 76 | 2.316 (1.745) | –0.26 | .27 | 30 | 2.305 (2.908) | –1.89 | .51 |
| Mean or median age | 42 | 1.000 (0.022) | –2.65 | .99 | 19 | 0.953 (0.027) | 10.97 | .10 |
| Percentage HIV positive | 23 | 13.569 (12.041) | 27.68 | .008 | 10 | 107.159 (270.897) | 21.99 | .10 |
| Percentage HCV positive | 19 | 4.134 (2.478) | 19.73 | .03 | 6 | 12.924 (19.898) | 27.35 | .17 |
| Percentage with history of homelessness | 8 | 11.081 (20.865) | 7.24 | .25 | 3 | 0 (0) | 91.52 | .16 |
| History of incarceration | 16 | 0.736 (0.399) | –4.11 | .58 | 6 | 0.674 (0.514) | –19.27 | .63 |
| Study characteristic | ||||||||
| Year of follow-up completion | ||||||||
| Completed by end of 1994 | 7 | Reference | Reference | Reference | 4 | Reference | Reference | Reference |
| Continued or commenced since 1995 | 90 | 0.851 (0.266) | –0.91 | .61 | 39 | 1.058 (0.384) | –2.55 | .88 |
| Sample size | 95 | 1.000 (0) | –0.19 | .39 | 41 | 1.000 (0) | 7.74 | .05 |
| Person-years of follow-up | 96 | 1.000 (0) | 0.66 | .25 | 39 | 1.000 (0) | 6.43 | .07 |
| Recruitment setting | ||||||||
| Drug treatment or harm reduction setting | 57 | Reference | Reference | Reference | 29 | Reference | Reference | Reference |
| Other settingsa | 40 | –0.023 (0.167) | –1.25 | .89 | 14 | 0.927 (0.209) | –2.27 | .74 |
Abbreviation: HCV, hepatitis C virus.
Other settings include acute care (eg, emergency departments), prisons, and community settings.
Table 3. Pooled All-Cause and Cause-Specific CMRs Among People Using Extramedical Opioids, Pooled CMR Ratios Comparing Men and Women and Older and Younger People, and Pooled All-Cause and Cause-Specific SMRs.
| Cause | CMR | CMR Ratio | SMR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men vs Women | Older vs Younger | |||||||||||
| No. of Cohorts | Pooled CMR (95% CI) | I2, % | No. of Cohorts | Pooled CMR Ratio (95% CI) | I2, % | No. of Cohorts | Pooled CMR Ratio (95% CI) | I2, % | No. of Cohorts | Pooled SMR (95%CI) | I2, % | |
| All-cause | 99 | 1.59 (1.40-1.80) | 99.7 | 45 | 1.38 (1.30-1.47) | 84.0 | 10 | 1.98 (1.59-2.47) | 97.5 | 43 | 10.03 (7.64-13.17) | 99.9 |
| Drug related | 56 | 0.52 (0.46-0.59) | 98.3 | 15 | 1.44 (1.27-1.64) | 71.4 | 8 | 1.19 (0.94-1.50) | 87.6 | 12 | 58.43 (38.09-89.64) | 99.7 |
| Poisoning | ||||||||||||
| Opioid | 6 | 0.56 (0.34-0.94) | 99.3 | 0 | NI | NI | 1 | 2.84 (2.50-3.23) | NA | 1 | 43.50 (41.40-45.80) | NA |
| Drug | 30 | 0.44 (0.36-0.53) | 97.7 | 7 | 1.70 (1.34-2.16) | 31.3 | 4 | 0.94 (0.48-1.84) | 85.5 | 6 | 63.33 (31.31-128.08) | 98.4 |
| Poisoning and disorders due to psychoactive substance use | 28 | 0.50 (0.43-0.59) | 98.3 | 9 | 1.39 (1.20-1.61) | 80.3 | 4 | 1.31 (1.02-1.69) | 91.1 | 6 | 60.42 (31.81-114.76) | 99.8 |
| Suicide | 36 | 0.12 (0.10-0.16) | 96.1 | 10 | 1.78 (1.42-2.24) | 30.3 | 4 | 1.57 (1.14-2.17) | 63.0 | 10 | 7.93 (5.69-11.04) | 97.1 |
| Unintentional injury | 29 | 0.14 (0.10-0.18) | 97.4 | 7 | 1.82 (1.61-2.07) | 0 | 1 | 0.99 (0.65-1.51) | NA | 8 | 6.85 (4.41-10.64) | 98.2 |
| Violence | 19 | 0.03 (0.02-0.03) | 70.8 | 4 | 1.68 (0.76-3.72) | 66.8 | 3 | 1.15 (0.65-2.05) | 64.9 | 8 | 9.75 (6.60-14.39) | 81.8 |
| AIDS related | 36 | 0.19 (0.12-0.28) | 99.3 | 7 | 1.35 (0.70-2.60) | 97.1 | 1 | 2.31 (0.47-11.44) | NA | 5 | 18.50 (8.15-41.99) | 99.1 |
| Liver | 33 | 0.14 (0.08-0.27) | 99.8 | 6 | 1.69 (1.38-2.07) | 8.5 | 3 | 8.00 (6.45-9.92) | 0 | 11 | 8.60 (6.13-12.07) | 96.4 |
| Viral hepatitis | 7 | 0.13 (0.01-1.10) | 99.9 | 3 | 1.42 (0.80-2.54) | 65.5 | 0 | NI | NI | 4 | 35.94 (16.06-80.42) | 98.3 |
| Digestive diseases | 13 | 0.06 (0.04-0.10) | 97.7 | 2 | 1.43 (0.77-2.68) | 66.5 | 2 | 7.94 (6.38-9.87) | 0 | 7 | 7.00 (4.45-11.00) | 96.2 |
| Liver related | 20 | 0.16 (0.08-0.35) | 99.8 | 5 | 1.63 (1.29-2.07) | 20.5 | 3 | 8.64 (6.79-11.00) | 0 | 6 | 6.58 (3.62-11.95) | 98.6 |
| Cardiovascular | 30 | 0.14 (0.10-0.19) | 99.1 | 7 | 0.95 (0.87-1.03) | 1.7 | 3 | 7.82 (4.06-15.08) | 83.2 | 6 | 4.45 (2.97-6.66) | 97.8 |
| Cancer | 31 | 0.12 (0.08-0.18) | 99.3 | 6 | 1.03 (0.79-1.34) | 55.0 | 3 | 11.51 (4.71-28.13) | 86.9 | 8 | 2.69 (1.84-3.92) | 97.8 |
| Respiratory | 24 | 0.08 (0.06-0.12) | 98.1 | 3 | 0.65 (0.58-0.73) | 0 | 2 | 14.09 (3.05-65.13) | 92.8 | 5 | 10.59 (7.79-14.38) | 61.8 |
Abbreviations: CMR, crude mortality rate; NA, not applicable; NI, no information; SMR, standardized mortality ratio.
The pooled all-cause SMR, based on 43 cohorts, was 10.03 (95% CI, 7.64-13.17), with substantial heterogeneity (I2 = 99.9%); among cohorts of people injecting opioids, the pooled all-cause SMR was 16.37 (95% CI, 10.92-24.55) (Table 1). The highest pooled SMR was observed in Southeast Asia (13.40; 95% CI, 11.40-15.30) and the lowest in North America (5.02; 95% CI, 4.21-5.98). Excess mortality was more pronounced among women compared with men and in younger people compared with older people (Table 3). Only the proportion of the cohort that injected drugs had evidence of an association with greater excess mortality (Table 2).
Drug-Related Deaths
There were 56 cohorts presenting data on drug-related deaths (Table 3). Across the 3 definitions for which data were extracted, the pooled CMR was 0.52 per 100 person-years (95% CI, 0.46-0.59 per 100 person-years). Men had significantly higher drug-related mortality rates than women, as did older people compared with younger people (Table 3). The number of drug-related death was substantially elevated compared with the general population (SMR, 58.43; 95% CI, 38.09-89.64) (Table 3 and eAppendix 7 and eAppendix 8 in the Supplement).
Traumatic Deaths: Suicide, Unintentional Injuries, and Homicide
Suicide and unintentional injury deaths occurred at similar rates (pooled CMR for suicides and unintentional injuries, 0.12 per 100 person-years [95% CI, 0.10-0.16 per 100 person-years] and 0.14 per 100 person-years [95% CI, 0.10-0.18], respectively). Suicide deaths occurred at almost 8 times the expected rate (pooled SMR, 7.93; 95% CI, 5.69-11.04) and unintentional injuries at 7 times the expected rate (pooled SMR, 6.85; 95% CI, 4.41-10.64) (Table 3). Death from interpersonal violence was a relatively infrequent cause of death (pooled CMR, 0.03 per 100 person-years; 95% CI, 0.02-0.03 per 100 person-years) but occurred at more than 9 times the expected rate (pooled SMR, 9.75; 95% CI, 6.60-14.39) (Table 3).
AIDS-Related Deaths
The pooled CMR for AIDS-related deaths was 0.19 per 100 person-years (95% CI, 0.12-0.28 per 100 person-years), and the pooled SMR was 18.50 (95% CI, 8.15-41.99) (Table 3). Excess mortality due to AIDS was particularly pronounced among women (pooled SMR, 53.98; 95% CI, 21.62-134.73) (eAppendix 8 in the Supplement).
Liver-Related Deaths
The overall CMR was 0.14 per 100 person-years (95% CI, 0.08-0.27 per 100 person-years), and liver-related deaths were more common among men compared with women (pooled CMR ratio, 1.69; 95% CI, 1.38-2.07) and older people compared with younger people (pooled CMR ratio, 8.00; 95% CI, 6.45-9.92) (Table 3). Liver-related deaths occurred at 8 times the expected rate (pooled SMR, 8.60; 95% CI, 6.13-12.07) (Table 3).
Other Disease-Related Deaths: Cardiovascular Disease, Cancer, and Respiratory Disease
Pooled CMRs were similar for cardiovascular disease (pooled CMR, 0.14 per 100 person-years; 95% CI, 0.10-0.19 per 100 person-years), cancer (pooled CMR, 0.12 per 100 person-years; 95% CI, 0.08-0.18 per 100 person-years), and respiratory disease, including chronic respiratory disease and acute infections (pooled CMR, 0.08 per 100 person-years; 95% CI, 0.06-0.12 per 100 person-years) (Table 3). The SMRs were elevated for all these causes, particularly respiratory disease (pooled SMR, 10.59; 95% CI, 7.79-14.38) (Table 3).
Relative Risks
The RRs of all-cause and cause-specific death were similar to the SMRs and are listed in eAppendix 9 in the Supplement.
Distribution of Causes of Death
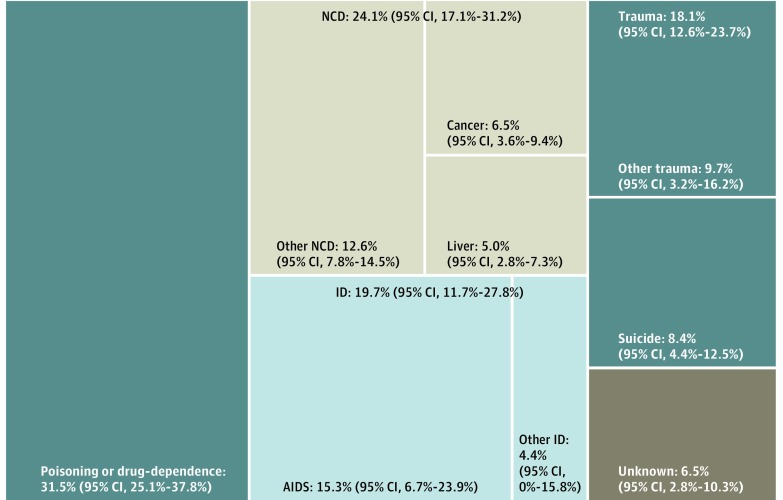
A total of 19 cohorts could be included in the analysis of distribution of causes of death, mostly originating from Western Europe (eAppendix 10 in the Supplement gives the included cohorts). Poisoning- and substance-related deaths were the most common cause, accounting for 31.5% of deaths (95% CI, 25.1%-37.8%) (Figure 2). Noncommunicable diseases accounted for 24.1% of deaths (95% CI, 17.1%-31.2%). Infectious diseases accounted for 19.7% of deaths (95% CI, 11.7%-27.8%) and trauma for 18.1% (95% CI, 12.6%-23.7%).
Figure 2. Distribution of Causes of Death in 19 Cohorts of People Using Extramedical Opioids.
ID indicates infectious diseases; NCD, noncommunicable diseases.
Discussion
People who used extramedical opioids had an elevated risk of mortality across major causes of deaths, including noncommunicable diseases, overdose, infectious diseases, and injuries. Variation in mortality among cohorts was associated with the prevalence of injecting drug use, HIV infection, and HCV infection within study cohorts. Most cohorts were recruited from multiple sites or used population-based registries covering a broad geographic area, and mortality was typically ascertained using official death registries, contributing to confidence in the findings. Compared with a previous systematic review11, our findings suggest a significant burden of mortality due to noncommunicable diseases in this population, and our study provided sex- and age-specific estimates of cause-specific excess mortality.
Implications
Addressing this burden requires a range of strategies to address different risk exposures. Opioid agonist treatment is associated with significantly reduced mortality across a range of causes, including drug-related deaths, suicides, and injuries, but is often not accessible for many people who could benefit from treatment, even in high-income countries.24,25,26 In addition to an association with reduced overdose and other mortality, opioid agonist treatment is associated with reduced incidence of HIV and HCV infections27,28 and contact with the legal system,29 generating broad public health benefits.
Increased access to naloxone in the community is also needed to enable short-term management of overdoses. Take-home naloxone programs are effective in reducing mortality among program participants,30 and emerging evidence suggests that widespread naloxone distribution may affect population overdose mortality.31
Excess mortality due to HIV infection and viral hepatitis suggests the need to increase access to treatment for HIV and HCV infections. People who use and inject drugs have poor access to HIV antiretroviral therapies in many countries, largely because of sociostructural barriers, such as policies or practitioner preference to avoid treatment initiation in people who use drugs; stigma; and discrimination.32,33 Hepatitis C virus infection is endemic among people who inject drugs.34 New highly effective, curative treatments for HCV infection should address this burden, but access to treatment is likely to remain an issue in many countries.35
Smoking is highly prevalent among people who use extramedical opioids,36 reflected in our study by the excess mortality from cardiovascular disease, respiratory disease, and cancer. Smoking cessation programs have been trialed in opioid agonist treatment settings, with nicotine-replacement therapies being superior to placebo and adjunctive behavioral therapies having no additional effect on abstinence at follow-up.37 However, absolute rates of sustained smoking cessation are low.38 There is a need to improve access to and effectiveness of smoking cessation interventions in this population.38
In terms of structural factors potentially associated with all-cause mortality, in meta-regression analyses, neither homelessness nor past incarceration appeared to be important variables. However, only a few studies have reported on homelessness, and extreme excess mortality across all causes has frequently been observed in people who are unsheltered,39 with unnatural deaths particularly increased compared with housed populations.40 In light of the current overdose crisis, there is a need for evidence on the role of housing in overdose incidence and outcomes. Studies that have reported on incarceration are rare, and incarceration is often defined in terms of lifetime exposure. Given that release from incarceration is associated with increased overdose mortality risk,41 better characterization of recent incarceration is essential for better understanding its effect on mortality in this population.
Limitations
A key limitation of this study was lack of information on how causes of death were defined. Of the 83 cohorts with cause-specific mortality rates, 34 did not report how specific causes were defined. Of those with definitions, significant variation was found among studies in defining drug- and opioid-related deaths. Consistency in defining drug- and opioid-related deaths is critical to ensuring accurate monitoring and assessing progress toward reducing drug-related deaths across and within countries. Liver-related deaths were another broad area in which inconsistencies were identified. Clarification and increasing consistency of the codes included in this category would assist in enabling monitoring of the public health effects of HCV antiviral therapies.
A previous systematic review11 on this topic identified few data from low- and middle-income countries. There were only minor increases in data from low- and middle-income countries in this review, and there remain several world regions (eg, Latin America, the Caribbean, and sub-Saharan Africa) with no relevant data, to our knowledge.
Despite a comprehensive search strategy of reports in any language, it is possible that we did not identify some cohorts. There were limited age- and sex-specific CMRs and SMRs for several key causes of death, which is a concern given changes in dominant causes of death across the lifespan for people using extramedical opioids.42 We did not seek to determine mortality rates associated with engagement in opioid agonist treatment because this work has recently been completed. That review reported that opioid agonist treatment with methadone or buprenorphine was highly protective against death, although there were periods of elevated mortality risk during methadone induction and after treatment cessation.43 We were unable to explore heterogeneity in cause-specific deaths associated with country or region of origin because of small numbers of studies for most causes.
This review related specifically to people using, injecting, and/or seeking treatment for their use of extramedical opioids, such as heroin. We did not exclude people with infrequent or nondisordered extramedical opioid use, which may have contributed to heterogeneity in our estimates. However, the CMR limited to opioid-dependent cohorts was similar to the overall CMR, suggesting that this definition did not substantially affect the results. Notwithstanding that in some settings there is considerable overlap between people using illicit opioids and people using extramedical pharmaceutical opioids, we do not consider that the results presented here apply to people who are prescribed opioids and not engaging in extramedical opioid use.
Conclusions
This study found that people who use extramedical opioids experienced a high burden of excess mortality across a range of causes. Combinations of evidence-based interventions to reduce mortality may have a greater effect than single interventions.44 Combinations of opioid agonist treatment, needle and syringe programs, and naloxone, as well as treatment for HIV and HCV infections, may be associated with reduced overdose, disease incidence, and mortality from multiple adverse health outcomes. There appears to be an urgent need to scale up combination interventions across myriad health issues to ensure that people who use opioids no longer face elevated mortality risks for health outcomes for which evidence-based interventions (such as nicotine-replacement therapy for smoking cessation) are easily available to the wider community.
eAppendix 1. Search Strings for Electronic Literature Searches
eAppendix 2. Study Inclusion and Exclusion Criteria
eAppendix 3. Risk of Bias and Quality of Reporting Assessment Tool
eAppendix 4. Data Derivation and Calculation Methods
eAppendix 5. Characteristics of Included Studies
eAppendix 6. Study Risk of Bias and Quality of Reporting Assessment
eAppendix 7. Forest Plots for Pooled Analyses
eAppendix 8. Sex-Specific Standardised Mortality Ratios
eAppendix 9. Mortality Relative Risks: All-Cause, GBD Regions and Cause-Specific
eAppendix 10. Studies Included in Analysis of Distribution of Causes of Death
eReferences
References
- 1.Larance B, Degenhardt L, Lintzeris N, Winstock A, Mattick R. Definitions related to the use of pharmaceutical opioids: extramedical use, diversion, non-adherence and aberrant medication-related behaviours. Drug Alcohol Rev. 2011;30(3):236-245. doi: 10.1111/j.1465-3362.2010.00283.x [DOI] [PubMed] [Google Scholar]
- 2.Degenhardt L, Charlson F, Ferrari A, et al. ; GBD 2016 Alcohol and Drug Use Collaborators . The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry. 2018;5(12):987-1012. doi: 10.1016/S2215-0366(18)30337-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.OECD Addressing Problematic Opioid Use in OECD Countries. Paris: OECD Health Policy Studies; 2019. [Google Scholar]
- 4.Zibbell JE, Iqbal K, Patel RC, et al. ; Centers for Disease Control and Prevention (CDC) . Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. MMWR Morb Mortal Wkly Rep. 2015;64(17):453-458. [PMC free article] [PubMed] [Google Scholar]
- 5.Peters PJ, Pontones P, Hoover KW, et al. ; Indiana HIV Outbreak Investigation Team . HIV infection linked to injection use of oxymorphone in Indiana, 2014-2015. N Engl J Med. 2016;375(3):229-239. doi: 10.1056/NEJMoa1515195 [DOI] [PubMed] [Google Scholar]
- 6.Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths: United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427. doi: 10.15585/mmwr.mm675152e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roxburgh A, Hall WD, Dobbins T, et al. Trends in heroin and pharmaceutical opioid overdose deaths in Australia. Drug Alcohol Depend. 2017;179:291-298. doi: 10.1016/j.drugalcdep.2017.07.018 [DOI] [PubMed] [Google Scholar]
- 8.British Columbia Coroners Service Illicit Drug Overdose Deaths in BC. Vancouver, British Columbia: Office of the Chief Coroner; 2017. [Google Scholar]
- 9.Office for National Statistics Deaths Related to Drug Poisoning in England and Wales. London, United Kingdom: Office for National Statistics; 2017. [Google Scholar]
- 10.European Monitoring Centre on Drugs and Drug Addiction European Drug Report. Lisbon, Portugal: European Monitoring Centre on Drugs and Drug Addiction; 2018. [Google Scholar]
- 11.Degenhardt L, Bucello C, Mathers B, et al. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction. 2011;106(1):32-51. doi: 10.1111/j.1360-0443.2010.03140.x [DOI] [PubMed] [Google Scholar]
- 12.Larney S, Randall D, Gibson A, Degenhardt L. The contributions of viral hepatitis and alcohol to liver-related deaths in opioid-dependent people. Drug Alcohol Depend. 2013;131(3):252-257. doi: 10.1016/j.drugalcdep.2012.11.012 [DOI] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 14. https://www.covidence.org/home
- 15.Marzo JN, Rotily M, Meroueh F, et al. Maintenance therapy and 3-year outcome of opioid-dependent prisoners: a prospective study in France (2003-06). Addiction. 2009;104(7):1233-1240. doi: 10.1111/j.1360-0443.2009.02558.x [DOI] [PubMed] [Google Scholar]
- 16.Pavarin RM, Fioritti A, Sanchini S. Mortality trends among heroin users treated between 1975 and 2013 in northern Italy: results of a longitudinal study. J Subst Abuse Treat. 2017;77:166-173. doi: 10.1016/j.jsat.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 17.Bero L, Chartres N, Diong J, et al. The risk of bias in observational studies of exposures (ROBINS-E) tool: concerns arising from application to observational studies of exposures. Syst Rev. 2018;7(1):242. doi: 10.1186/s13643-018-0915-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, Taylor K, Allman-Farinelli M, et al. A Systematic Review: Tools for Assessing Methodological Quality of Human Observational Studies. Canberra, Australia: National Health and Medical Research Council; 2019. [Google Scholar]
- 19.Jones ME, Swerdlow AJ. Bias in the standardized mortality ratio when using general population rates to estimate expected number of deaths. Am J Epidemiol. 1998;148(10):1012-1017. doi: 10.1093/oxfordjournals.aje.a009567 [DOI] [PubMed] [Google Scholar]
- 20.Global Burden of Disease Collaborative Network Global Burden of Disease Study 2017 (GBD 2017) Results. Seattle, WA: Institute for Health Metrics and Evaluation; 2018. [Google Scholar]
- 21.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Review of Interventions. Version 5.1.0. London, United Kingdom: The Cochrane Collaboration; 2011. [Google Scholar]
- 22.Apelt SM, Scherbaum N, Gölz J, Backmund M, Soyka M. Safety, effectiveness and tolerance of buprenorphine-naloxone in the treatment of opioid dependence: results from a nationwide non-interventional study in routine care. Pharmacopsychiatry. 2013;46(3):94-107. doi: 10.1055/s-0032-1330033 [DOI] [PubMed] [Google Scholar]
- 23.Smyth BP, Fagan J, Kernan K. Outcome of heroin-dependent adolescents presenting for opiate substitution treatment. J Subst Abuse Treat. 2012;42(1):35-44. doi: 10.1016/j.jsat.2011.07.007 [DOI] [PubMed] [Google Scholar]
- 24.Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health. 2015;105(8):e55-e63. doi: 10.2105/AJPH.2015.302664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abraham AJ, Adams GB, Bradford AC, Bradford WD. County-level access to opioid use disorder medications in medicare Part D (2010-2015). Health Serv Res. 2019;54(2):390-398. doi: 10.1111/1475-6773.13113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larney S, Peacock A, Leung J, et al. Global, regional, and country-level coverage of interventions to prevent and manage HIV and hepatitis C among people who inject drugs: a systematic review. Lancet Glob Health. 2017;5(12):e1208-e1220. doi: 10.1016/S2214-109X(17)30373-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Platt L, Minozzi S, Reed J, et al. Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane Review and meta-analysis. Addiction. 2018;113(3):545-563. doi: 10.1111/add.14012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacArthur GJ, Minozzi S, Martin N, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ. 2012;345:e5945. doi: 10.1136/bmj.e5945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gisev N, Bharat C, Larney S, et al. The effect of entry and retention in opioid substitution therapy on contact with the criminal justice system among opioid dependent people. Lancet Public Health. 2019;4:e334-e42. doi: 10.1016/S2468-2667(19)30060-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonald R, Strang J. Are take-home naloxone programmes effective? systematic review utilizing application of the Bradford Hill criteria. Addiction. 2016;111(7):1177-1187. doi: 10.1111/add.13326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bird SM, McAuley A. Scotland’s National Naloxone Programme. Lancet. 2019;393(10169):316-318. doi: 10.1016/S0140-6736(18)33065-4 [DOI] [PubMed] [Google Scholar]
- 32.UNAIDS Health, Rights and Drugs: Harm Reduction, Decriminalization and Zero Discrimination for People Who Use Drugs. Geneva, Switzerland: UNAIDS; 2019. [Google Scholar]
- 33.Parashar S, Collins AB, Montaner JSG, Hogg RS, Milloy MJ. Reducing rates of preventable HIV/AIDS-associated mortality among people living with HIV who inject drugs. Curr Opin HIV AIDS. 2016;11(5):507-513. doi: 10.1097/COH.0000000000000297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health. 2017;5(12):e1192-e1207. doi: 10.1016/S2214-109X(17)30375-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Day E, Hellard M, Treloar C, et al. ; International Network on Hepatitis in Substance Users (INHSU) . Hepatitis C elimination among people who inject drugs: challenges and recommendations for action within a health systems framework. Liver Int. 2019;39(1):20-30. doi: 10.1111/liv.13949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guydish J, Passalacqua E, Pagano A, et al. An international systematic review of smoking prevalence in addiction treatment. Addiction. 2016;111(2):220-230. doi: 10.1111/add.13099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yee A, Hoong MC, Joyce YC, Loh HS. Smoking cessation among methadone-maintained patients: a meta-analysis. Subst Use Misuse. 2018;53(2):276-285. doi: 10.1080/10826084.2017.1342661 [DOI] [PubMed] [Google Scholar]
- 38.de Dios MA, Cano MA, Vaughan EL, McNeel MM, Childress S, Niaura R. Nicotine maintenance for smokers in methadone treatment: a new direction. Addict Res Theory. 2019;27:269-276. doi: 10.1080/16066359.2018.1504032 [DOI] [Google Scholar]
- 39.Aldridge RW, Story A, Hwang SW, et al. Morbidity and mortality in homeless individuals, prisoners, sex workers, and individuals with substance use disorders in high-income countries: a systematic review and meta-analysis. Lancet. 2018;391(10117):241-250. doi: 10.1016/S0140-6736(17)31869-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slockers MT, Nusselder WJ, Rietjens J, van Beeck EF. Unnatural death: a major but largely preventable cause-of-death among homeless people? Eur J Public Health. 2018;28(2):248-252. doi: 10.1093/eurpub/cky002 [DOI] [PubMed] [Google Scholar]
- 41.Merrall ELC, Kariminia A, Binswanger IA, et al. Meta-analysis of drug-related deaths soon after release from prison. Addiction. 2010;105(9):1545-1554. doi: 10.1111/j.1360-0443.2010.02990.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Degenhardt L, Larney S, Randall D, Burns L, Hall W. Causes of death in a cohort treated for opioid dependence between 1985 and 2005. Addiction. 2014;109(1):90-99. doi: 10.1111/add.12337 [DOI] [PubMed] [Google Scholar]
- 43.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Degenhardt L, Grebely J, Stone J, et al. Global patterns of opioid use and dependence: harms to populations, interventions, and future action. Lancet. 2019;394(10208):1560-1579. doi: 10.1016/S0140-6736(19)32229-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Search Strings for Electronic Literature Searches
eAppendix 2. Study Inclusion and Exclusion Criteria
eAppendix 3. Risk of Bias and Quality of Reporting Assessment Tool
eAppendix 4. Data Derivation and Calculation Methods
eAppendix 5. Characteristics of Included Studies
eAppendix 6. Study Risk of Bias and Quality of Reporting Assessment
eAppendix 7. Forest Plots for Pooled Analyses
eAppendix 8. Sex-Specific Standardised Mortality Ratios
eAppendix 9. Mortality Relative Risks: All-Cause, GBD Regions and Cause-Specific
eAppendix 10. Studies Included in Analysis of Distribution of Causes of Death
eReferences