Key Points
Question
Does the measurement of spreading depolarizations provide clinically meaningful information that is independent of other assessments?
Findings
In this cohort study of 138 patients who underwent surgery for traumatic brain injury, the occurrence of repetitive spreading depolarizations (clusters) in 51 patients (37.0%) was associated with poor neurologic recovery during intensive care and was a factor independently associated with worse 6-month functional outcomes.
Meaning
Spreading depolarizations are a common mechanism of secondary injury that should be considered for monitoring and treatment targeting in traumatic brain injury.
This multicenter cohort study assesses the association between spreading depolarizations in the brains of individuals who have undergone surgery for traumatic brain injury and neurologic outcomes.
Abstract
Importance
Advances in treatment of traumatic brain injury are hindered by the inability to monitor pathological mechanisms in individual patients for targeted neuroprotective treatment. Spreading depolarizations, a mechanism of lesion development in animal models, are a novel candidate for clinical monitoring in patients with brain trauma who need surgery.
Objective
To test the null hypothesis that spreading depolarizations are not associated with worse neurologic outcomes.
Design, Setting, and Participants
This prospective, observational, multicenter cohort study was conducted from February 2009 to August 2013 in 5 level 1 trauma centers. Consecutive patients who required neurological surgery for treatment of acute brain trauma and for whom research consent could be obtained were enrolled; participants were excluded because of technical problems in data quality, patient withdrawal, or loss to follow-up. Primary statistical analysis took place from April to December 2018. Evaluators of outcome assessments were blinded to other measures.
Interventions
A 6-contact electrode strip was placed on the brain surface during surgery for continuous electrocorticography during intensive care.
Main Outcomes and Measures
Electrocorticography was scored for depolarizations, following international consensus procedures. Six-month outcomes were assessed by the Glasgow Outcome Scale–Extended score.
Results
A total of 157 patients were initially enrolled; 19 were subsequently excluded. The 138 remaining patients (104 men [75%]; median [interquartile range] age, 45 [29-64] years) underwent a median (interquartile range) of 75.5 (42.2-117.1) hours of electrocorticography. A total of 2837 spreading depolarizations occurred in 83 of 138 patients (60.1% incidence) who, compared with patients who did not have spreading depolarizations, had lower prehospital systolic blood pressure levels (mean [SD], 133 [31] mm Hg vs 146 [33] mm Hg; P = .03), more traumatic subarachnoid hemorrhage (depolarization incidences of 17 of 37 [46%], 18 of 32 [56%], 22 of 33 [67%], and 23 of 30 patients [77%] for Morris-Marshall Grades 0, 1, 2, and 3/4, respectively; P = .047), and worse radiographic pathology (in 38 of 73 patients [52%] and 42 of 60 patients [70%] for Rotterdam Scores 2-4 vs 5-6, respectively; P = .04). Of patients with depolarizations, 32 of 83 (39%) had only sporadic events that induced cortical spreading depression of spontaneous electrical activity, whereas 51 of 83 patients (61%) exhibited temporal clusters of depolarizations (≥3 in a 2-hour span). Nearly half of those with clusters (23 of 51 [45%]) also had depolarizations in an electrically silent area of the cortex (isoelectric spreading depolarization). Patients with clusters did not improve in motor neurologic examinations from presurgery to postelectrocorticography, while other patients did improve. In multivariate ordinal regression adjusting for baseline prognostic variables, the occurrence of depolarization clusters had an odds ratio of 2.29 (95% CI, 1.13-4.65; P = .02) for worse outcomes.
Conclusions and Relevance
In this cohort study of patients with acute brain trauma, spreading depolarizations were predominant but heterogeneous and independently associated with poor neurologic recovery. Monitoring the occurrence of spreading depolarizations may identify patients most likely to benefit from targeted management strategies.
Introduction
The failure of dozens of clinical trials to improve outcomes from moderate-severe traumatic brain injury (TBI) has led to a broad consensus that new approaches are needed.1 Current efforts recognize that TBI is not a single disease entity and therefore aim to develop more refined TBI classifications.2,3 The goal is to implement a precision or personalized medicine approach by identifying variations of pathological anatomy and physiology to target treatments to patients whose conditions are likely to be responsive.4 Such precision targeting of treatments to diagnosed pathological mechanisms was notably lacking in prior failed trials, which relied heavily on neuroprotection studies in animal models and largely neglected clinical investigation of disease processes. Thus, the last decade has seen increasing use of multimodal intracranial monitoring for patients in traumatic coma to characterize secondary injury mechanisms and provide neuroprotection through personalized optimization of brain physiology.5,6
A major breakthrough in clinical studies has been identification of a direct neuronal measure of secondary injury, the pathological phenomenon of spreading depolarizations.7,8 Spreading depolarizations are a class of pathological waves that are triggered by ischemia, trauma, or other noxious stimuli and propagate slowly (1 to 9 mm/min) through cerebral gray matter. They are characterized by a near-complete, sustained collapse of electrochemical membrane gradients in neurons and astrocytes en masse in affected tissue, with consequent loss of electrical signaling (also called spreading depression of electroencephalography), intracellular calcium ion (Ca2+) loading, cytotoxic edema, and release of neurotransmitters. They are thought to be the major source of acute excitotoxic injury9,10 through N-methyl-d-aspartate receptor signaling11,12 and are a causal, requisite mechanism of infarction in animal models of stroke.13 After their clinical discovery in 2002,14 we developed an international consortium to test the null hypothesis that spreading depolarizations are not associated with neurologic outcomes. Interim studies have described many of the pathophysiologic correlates of depolarizations in most patients with severe acute brain injury from trauma and stroke,8 including their occurrence as the terminal event in brain death.15,16 In this article, we report the main findings from a surgical TBI cohort study with respect to the initial null hypothesis and the association of depolarizations with key clinical variables.
Methods
Patients
We undertook a prospective, observational study (ClinicalTrials.gov identifier: NCT00803036) at 5 neurosurgical centers (the University of Cincinnati, Cincinnati, Ohio; Virginia Commonwealth University, Richmond; University of Pittsburgh, Pittsburgh, Pennsylvania; University of Miami, Miami, Florida; and King’s College Hospital, London, United Kingdom). Research was approved by ethical boards and conducted in accordance with the Declaration of Helsinki. Written informed research consent was obtained for all patients. Inclusion criteria were the clinical need for neurosurgery to treat severe TBI, surgery less than 7 days after injury, and an age of 18 years or older. Patients judged to have nonsurvivable injury were excluded. Multiple traumas, penetrating head injuries, or additional preexisting diseases were not exclusion criteria.
Study Procedures
At the conclusion of surgery, a linear electrocorticography strip (platinum, 6-contact, 10-mm electrode spacing [Wyler; Ad-Tech]) was placed on the cerebral cortex near a focus of primary injury (Figure 1A).8,14,17 Patients were transferred to intensive care units after surgery, and continuous neuromonitoring was initiated. Clinicians were blinded to electrocorticography results, and no treatment decisions were based on the recordings. Electrocorticography was terminated when invasive neuromonitoring was no longer clinically required or after a maximum of 7 days.
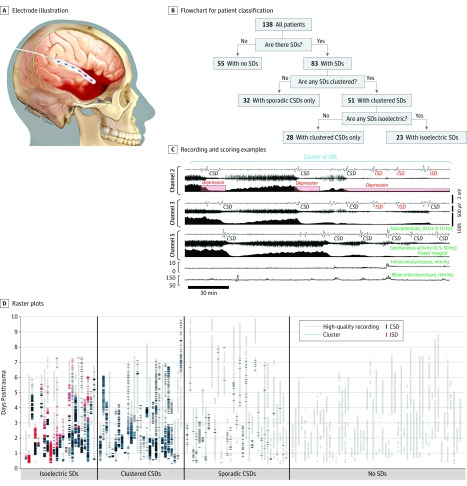
Figure 1. Scoring, Classification, and Timing of Spreading Depolarizations After Acute Brain Trauma.
A, Illustration of a 6-contact electrode strip, placed on the brain surface after a decompressive hemicraniectomy. The strip was placed in the frontal lobe in 97 of 133 patients (72.9%), temporal lobe in 27 of 133 (20.3%), and parietal lobe in 11 of 133 (8.3%). B, Flowchart showing patient classifications based on spreading depolarization (SD) types and patterns. Three classification steps yield 4 patient categories. C, Methods to measure and classify SDs are illustrated by recordings from 3 bipolar channels. Each channel is filtered separately to display slow potentials (top traces; 0.01-0.10 Hz) and spontaneous activity (middle traces; 0.5-50 Hz). The power integral of the spontaneous activity aids in scoring of depression periods, as shown for channel 2 (red boxes). Six SDs were observed in this period, and all met the criteria of clustered events. Designations as cortical spreading depression (CSD) or isoelectric SD (ISD) subtypes are shown. Intracranial and arterial pressures were in normal ranges. Scale bars for channel 3 (right) apply to all channels. Total duration of displayed traces was 3 hours and 20 minutes. D, Raster plots show electrocorticography data from individual patients, grouped according to the categories in panel B. Gray bars show periods of high-quality recordings, during which SDs could be evaluated. Black ticks show the times of individual CSDs and red ticks show isoelectric SDs. Blue bars highlight clusters. Patients with clustered CSDs had an initial pattern of sporadic events before cluster development in 18 of 28 cases (64%). By contrast, patients with isoelectric SDs had an initial pattern of sporadic events in only 6 of 23 cases (26%) and were more likely to show clustering from the start (P = .01 by Fisher exact test). In these patients, isoelectric SDs were usually preceded by an initial pattern of CSDs (21 of 23 [91%]). The final isoelectric SDs were sporadic events in 18 of 23 patients (78%), and recordings ended during an isoelectric SD cluster in the remaining 5 cases. In 23 of 23 patients, the final events recorded were isoelectric SDs rather than CSDs, demonstrating that spontaneous electrical activity did not recover within the period of monitoring.
Patients were managed according to local standard care. Midazolam was the preferred agent for sedation in patients requiring ventilation, but propofol was allowed for short-term sedation after surgery, when longer-term sedation was withdrawn, or as a bolus to reduce intracranial pressure. Analgesia was provided with fentanyl, its analogs, and morphine, and phenytoin or levetiracetam was given for seizure prophylaxis for 7 days.
Seven hospital-admission variables were collected prospectively as covariates for outcome prognostication, as defined by the International Mission for Prognosis and Clinical Trial Design (IMPACT) study (Table 1).18,19 Neuroimaging was performed according to local standard care. Preoperative head computed tomography studies were scored for pathologies and lesion types by a central neuroradiologist (A.V.) who was blinded to all other study data.20 Neurologic outcome was assessed at 6 months according to the Glasgow Outcome Scale–Extended (GOS-E) by a telephone interview or clinic visit. Assessors were blinded to electrocorticography results.
Table 1. Prognostic Variables and Anatomic Pathology.
| Hospital Admission Variable | Patients, No. (%) |
|---|---|
| Prognostic score, median (IQR) | 0.20 (−0.58 to 1.04)a |
| Age, median (IQR), y | 45 (29-64) |
| Glasgow Coma Scale motor score | |
| No response | 27 (19.6) |
| Extension | 7 (5.1) |
| Flexion | 8 (5.8) |
| Withdraws | 18 (13.0) |
| Localizes | 39 (28.3) |
| Obeys | 38 (27.5) |
| Unknown | 1 (0.7) |
| Pupils reacting | |
| Both | 104 (75.4) |
| 1 | 14 (10.1) |
| Neither | 18 (13.0) |
| Unknown | 2 (1.4) |
| Marshall computed tomography category | |
| 2 | 7 (5.1) |
| 3 | 3 (2.2) |
| 4 | 1 (0.7) |
| 5 | 120 (87.0) |
| 6 | 2 (1.4) |
| Traumatic subarachnoid hemorrhage | 101 (73.2) |
| Hypotension | |
| Yes | 24 (17.4) |
| No | 105 (76.1) |
| Unknown | 9 (6.5) |
| Hypoxia | |
| Yes | 39 (28.3) |
| No | 88 (6.3.8) |
| Unknown | 11 (8.0) |
| Presurgical Head Computed Tomography Imaging b | |
| Rotterdam score | |
| 1 | 0 |
| 2 | 4 (3.0) |
| 3 | 21 (15.8) |
| 4 | 48 (36.1) |
| 5 | 39 (29.3) |
| 6 | 21 (15.8) |
| Subdural hematoma | |
| Yes | 73 (54.9) |
| Thickness, median (IQR), mm | 10 (6-14) |
| Cerebral contusion | |
| None | 55 (41.4) |
| ≤25 cm3 | 63 (47.4) |
| >25 cm3 | 15 (11.3) |
| Intracerebral hemorrhage | 22 (16.5) |
| Epidural hematoma | 9 (6.8) |
| Depressed skull fracture | 23 (17.3) |
| Basilar skull fracture | 45 (33.8) |
| Basal cisterns | |
| Open | 22 (16.5) |
| Compressed | 74 (55.6) |
| Absent | 37 (27.8) |
| Midline shift, mm | |
| ≤5 | 38 (28.6) |
| 5-10 | 53 (39.8) |
| >10 | 42 (31.6) |
Abbreviation: IQR, interquartile range.
Unknown values (n = 23) were imputed for computation of the prognostic score.
Percentages are based on 133 patients for whom presurgical scans were obtained.
Electrocorticography
Electrocorticography was continuously monitored, and intracranial pressure, arterial pressure, and brain tissue oxygen signals were recorded by the same data acquisition systems when possible. Electrocorticography was scored centrally (by J.A.H.) for spreading depolarizations in offline analysis performed in LabChart (ADInstruments) according to established consensus procedures (Figure 1C).8 In brief, depolarizations were identified by (1) a slow-potential change of 0.5-mV to 5.0-mV peak-to-peak amplitude in the near-direct current (infraslow; ~ 0.01-0.10 Hz) frequency band, (2) simultaneous onset of depressed spontaneous activity in the 0.5-Hz to 50-Hz band, when spontaneous activity was present in the baseline, and (3) spread of both slow-potential change and high-frequency depression between at least 2 electrodes. Depolarizations that invaded spontaneously active tissue and thus induced amplitude depression in all affected channels were classified as cortical spreading depressions (CSDs). However, if at least 1 channel participating in the wave was already electrically silent, the depolarization was classified as an isoelectric spreading depolarization (isoelectric SDs; Figure 1C). Durations of activity depression induced by depolarizations were measured as previously described (Figure 1C).8 Based on scoring of individual events, several summary measures of depolarization burden were calculated for each patient (Table 2). (Seizures and interictal activity were also scored but will be described in a separate report.)
Table 2. Electrocorticography Recording and Spreading Depolarization Characteristicsa.
| Factor | Patient Category, Median (IQR) | P Value | Total, No. (%) | |||
|---|---|---|---|---|---|---|
| No SDs | Sporadic CSDs Only | Clustered CSDs Only | ≥1 Isoelectric SDs | |||
| Patients, No. (%) | 55 (39.9) | 32 (23.2) | 28 (20.3) | 23 (16.7) | NA | 138 |
| Recording duration, h | 58.7 (32.8-96.6) | 78.6 (45.3-105.5) | 88.7 (57.8-133.0) | 91.2 (47.3-127.1) | .08 | 10 916 |
| CSDs, No. | NA | 3.0 (2.0-8.3) | 38.0 (14.0-54.8) | 40.0 (17.0-67.5) | <.001 | 2402 |
| Isoelectric SDs, No. | NA | NA | NA | 9.0 (4.0-32.0) | NA | 435 |
| Continuous measures | ||||||
| Overall SDs/db | NA | 1.3 (0.6-2.7) | 11.7 (4.9-20.9) | 16.0 (12.8-24.9) | <.001 | NA |
| Total time depressed, %c | NA | 0.6 (0.3-1.1) | 6.2 (2.9-9.9) | 16.6 (11.3-19.7) | <.001 | NA |
| Peak No. of SDs/dd | NA | 3.1 (2.1-6.3) | 27.8 (12.4-40.4) | 22.3 (14.8-40.5) | <.001 | NA |
| Peak total depression duration/d, mine | NA | 17.7 (11.9-41.4) | 183.9 (100.8-373.2) | 525.7 (263.1-842.5) | <.001 | NA |
| Maximum single depression duration per single event or episode, minf | NA | 6.6 (5.3-12.3) | 15.9 (11.5-20.7) | 158.5 (69.7-386.7) | <.001 | NA |
Abbreviations: CSD, cortical spreading depression; IQR, interquartile range; NA, not applicable; SD, spreading depolarization/spreading depression.
Medians and IQRs are reported for 4 patient groups classified according to the presence, type, and temporal pattern of spreading depolarizations. Across the full duration of electrocorticography, the total percentage depressed (by recording time) and the overall SD rates were highly correlated (r = 0.95; P < .001 by Spearman rank-order correlation; eFigure 2 in the Supplement). Both measures were similar for patients with clustered CSDs and those with isoelectric SDs but were significantly less for patients with only sporadic CSDs (by Kruskal-Wallis and Dunn tests). The same results were found for measures of peak total depression duration per day and peak SDs per day; both were similar for patients with clustered CSDs and isoelectric SDs but less for those with sporadic CSDs (by Kruskal-Wallis and Dunn tests). The only measure that strongly differed between patients with clustered CSDs and isoelectric SDs was the maximal single depression duration (by Kruskal-Wallis and Dunn tests; eFigure 2 in the Supplement). Thus, the 3 patient categories can be distinguished based on continuous measures of depolarization burden, although clustered CSD and isoelectric SD groups are similar by most measures.
The total number of SDs divided by the total valid recording duration for the patient. The entire recording is used.
The longest depression duration of any affected channel is measured for each SD and summed over all SDs. The sum is divided by the total valid recording duration for the patient and multiplied by 100. The entire recording is used.
The total numbers of SDs in each 24-hour period are normalized to the valid recording durations for the corresponding 24-hour periods to give the number of SDs per day. A minimum of 8 valid recording hours is required. The maximal value for all 24-hour periods is the peak SDs per day.
The longest depression duration of any affected channel is measured for each SD and summed over all SDs for each 24-hour period. The summed durations are normalized to the valid recording durations in each 24-hour period to give the total depression duration per day. A minimum of 8 valid recording hours is required. The maximal value of all 24-hour periods is the peak total depression duration per day.
The longest single depression period induced by an SD, which may be prolonged as a continuous depression maintained by subsequent SDs. The value is calculated per episode or event.
Statistical Methods
The associations of depolarizations with clinical outcomes were addressed by ordinal regression analysis, assuming a proportional odds model. The model was fitted with depolarizations as a categorical variable and the GOS-E score as the dependent variable. Because of low numbers of individuals so categorized, GOS-E scores 7 and 8 were combined. Model comparisons were based on a likelihood ratio test. Covariates were included in the model to control for baseline prognosis and were chosen a priori based on their prior validation in the large, international IMPACT study19,21 as the most robust independent factors associated with outcomes. The core IMPACT model of age, motor score, and pupils was used. We also calculated the linear predictor value from the full 7-variable IMPACT model to use as a single covariate that reflects a summary prognostic score.22 In this model, prognostic scores of −2.2, 0.0, and 2.2 correspond to 10%, 50%, and 90% probabilities of poor outcome, respectively (GOS-E scores 1-4).19 A few covariate values (23 of 966 values [2.4%]) were missing and were imputed using their expected values conditional on the observed covariates, depolarization category, and GOS-E scores.
For data that were not normally distributed, values are reported as median (interquartile range), and Kruskal-Wallis analysis of variance and Spearman rank-order correlation were used. All P values less than .05 were considered significant. Data were collected from February 2009 to August 2013, and primary statistical analysis took place from April to December 2018. Analyses were conducted using GraphPad version 7.04 (Prism) and SPSS Statistics version 26 (IBM).
Results
Study Participants
A total of 157 patients were enrolled. Of these, 17 were excluded because of poor technical quality of electrocorticography, 1 patient withdrew consent, and 1 was lost to follow-up. The final cohort thus consisted of 138 patients (104 men [75%]). Table 1 reports prognostic variables at hospital admission and anatomical pathology on preoperative head computed tomography studies, which were the admission studies in 86 of 138 participants (62.3%). Most patients had cerebral contusions (78 of 133 [58.6%]) and/or subdural hematomas (73 of 133 [54.9%]), while a minority had intracerebral hemorrhage (22 of 133 [16.5%]) or epidural hematomas (9 of 133 [6.8%]), and most had signs of mass effect (midline shift >5 mm in 95 of 133 patients [71.4%] and compressed or absent cisterns in 111 of 133 patients [83.5%]). Causes of TBI, indications for neurosurgery, and surgical procedures are summarized in the eTable in the Supplement.
Measures of Depolarization Activity
Electrocorticography started a median (interquartile range) of 13.5 (8.5-24.5) hours after TBI, and the duration of valid recordings was 75.5 (42.2–117.1) hours per patient (Figure 1D). In this time, a total of 2837 spreading depolarizations were recorded in 83 of 138 patients (a 60.1% incidence). No depolarizations were recorded in the other patients (55 of 138 patients [39.9%]). Figure 1D shows the time course of recordings and depolarizations. Most depolarizations (2402 [84.6%]) induced typical depressions (CSDs) of baseline spontaneous electrocorticographic activity in all affected recording channels. The remaining depolarizations (435 [15.4%]) occurred in at least 1 channel that was already electrically silent and thus were classified as isoelectric depolarizations (isoelectric SDs). These occurred in 23 patients (a 16.7% incidence), with or without additional CSDs. In 18 of the 23 affected participants, the baseline isoelectricity of isoelectric SDs developed as a result of prior depolarizations that induced prolonged or sustained depressions of spontaneous electrical activity, as shown in Figure 1C. Subsequent depolarizations, classified as isoelectric SDs, were thus observed only as spreading slow-potential changes, without the possibility for further activity depression. In the other individuals, the onset of isoelectricity was not recorded (2 individuals) or the cause could not be clearly determined (3 individuals).
Patterns of depolarization recurrence were highly variable, ranging from sporadic, infrequent events to those that occurred repetitively at short intervals (Figure 1D). To distinguish these patterns, based on analysis of interdepolarization intervals (eFigure 1 in the Supplement), we defined a temporal cluster as the occurrence of 3 or more depolarizations within 120 minutes. Most depolarizations (2129 of 2837 [75.0%]) occurred in such clusters. Cluster patterns were observed in 51 of 138 patients (37.0%), and the other 32 patients (23.2%) had only sporadic events. Interestingly, isoelectric SDs only occurred in patients with cluster SDs (23 of 51 [45%]) and never in patients with only sporadic depolarizations (0 of 32; P < .001; Figure 1B). In fact, isoelectric SDs always began in a patient recording either within a cluster (in 19 of 23 patients) or after a cluster (4 of 23 patients) and never as an initial sporadic event (Figure 1D). Furthermore, nearly all isoelectric SDs (424 of 435 [97.4%]) occurred within a cluster of depolarizations (eg, Figure 1C), compared with only 697 of 2402 CSDs (71.0%; χ2; P < .001). These results thus revealed a nested ordinal scale to categorize patients based on increasing severity or burden of depolarization patterns and subtypes (Figure 1B): (1) no depolarizations, (2) only sporadic CSDs, (3) clusters of CSDs only, or (4) isoelectric SDs. These categories were also distinguished based on more detailed scoring of continuous measures (Table 2; eFigure 2 in the Supplement), although patients with clustered CSDs and isoelectric SDs were similar by most measures.
Risk Factors for Depolarizations
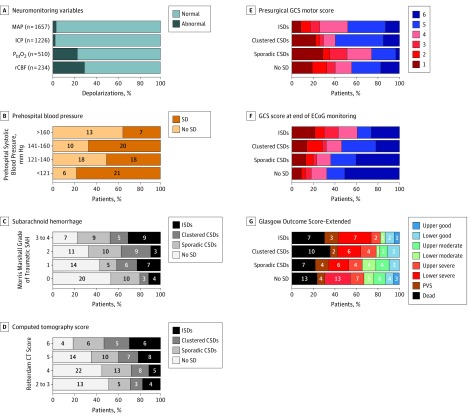
Various clinical, physiologic, and anatomic variables were examined for associations with depolarizations. Figure 2A shows that nearly all depolarizations occurred when patients had normal blood pressure levels (1602 of 1657 events [96.7%]) and intracranial pressure was less than 20 mm Hg (1196 of 1226 events [97.6%]). However, patients were hypoxic (<20 mm Hg) for 117 of 510 depolarizations (22.9%), and cerebral blood flow measurements were at ischemic levels (<18 mL/100 mg/min) for 69 of 234 depolarizations (29.5%).
Figure 2. Risk Factors and Outcomes.
A, Bar graphs show the proportion of depolarizations that occurred when variables were in normal (light blue) and abnormal (dark blue) ranges. The number of depolarizations evaluated for each variable is shown at left. Mean values of continuously recorded variables were measured from 60-second epochs within 5 minutes prior to each depolarization onset. Regional cerebral blood flow was monitored in 25 patients with thermal diffusion probes (Hemedex Inc) placed alongside the subdural electrode strip.23 Intracranial pressure was monitored in 109 patients and brain tissue oxygen in 60 patients. B, Proportions of patients with and without depolarizations according to ranges of prehospital systolic blood pressures. In contrast to prehospital values, systolic blood pressure levels at hospital admission did not differ between patients with and without depolarizations (mean [SD], 143.6 [32.4] mm Hg vs 145.4 [27.6] mm Hg; P = .73). C, Distribution of patients across depolarization categories according to the Morris-Marshall Grade scoring of traumatic subarachnoid hemorrhage (SAH) on presurgical computed tomography head studies. Morris-Marshall Grades are no subarachnoid hemorrhage (0); subarachnoid hemorrhage in only 1 location (1); subarachnoid hemorrhage fills structure at 1 location or any 2 sites, filling neither (2); subarachnoid hemorrhage at 2 sites, including a filled tentorium (3); and subarachnoid hemorrhage at 3 or more sites in any quantity (4). D, Distribution of patients across depolarization categories according to the Rotterdam Computed Tomography (CT) Sum Score, based on assessment of basal cisterns, midline shift, epidural mass, and intraventricular or subarachnoid blood; higher numbers represent more severe pathology. E and F, Distribution of Glasgow Coma Scale (GCS) motor scores, according to depolarization category. Motor scores obtained in presurgical examinations (E) are compared with those obtained several days later, at the end of electrocorticography (F). A score of 4 (withdrawal to painful stimuli) is often used as a threshold to indicate the need for invasive neuromonitoring, whereas those able to localize pain (score 5) or follow commands (score 6) are generally not monitored. F, Status at the end of electrocorticography (ECoG) monitoring. G, Distribution of Glasgow Outcome Scale–Extended scores at 6 months, according to depolarization category. Raw patient numbers are shown for each category in panels B, C, and D. CSD indicates cortical spreading depression; ICP, intracranial pressure; ISD, isoelectric spreading depolarization; MAP, mean arterial pressure; PbtO2, partial pressure of brain tissue oxygen; PVS, persistent vegetative state; rCBF, regional cerebral blood flow; SD, spreading depolarization.
There were no significant associations of depolarizations (incidence, burden, or category) with the IMPACT prognostic variables (Table 1) of age, Glasgow Coma Scale motor score, pupils, presence of traumatic subarachnoid hemorrhage, hypoxia, or prognostic score. However, depolarizations were more likely to occur in patients judged to have early (prehospital) hypotension (19 of 22 patients [86%] vs 60 of 107 patients [56.1%]; P = .008). Examination of the first systolic blood pressures recorded after trauma but before hospital admission shows that higher systolic blood pressures were associated with lower depolarization risk across a wide range (60-200 mm Hg; Figure 2B). Accordingly, patients with depolarizations had significantly lower prehospital systolic blood pressures (mean [SD], 132.7 [31.3] mm Hg vs 146.3 [33.3] mm Hg; P = .03) yet rarely met clinical criteria for hypotension (<90 mm Hg). Depolarizations were not associated with other hospital admission variables of blood pressure, airway status, hemoglobin, core temperature, or arterial partial pressure of carbon dioxide, partial pressure of oxygen, or pH. Plasma glucose levels at admission did not differ between patients with and without depolarizations but were significantly higher among patients with depolarization in worse severity categories (median [IQR]: sporadic CSDs, 133 [108-172] mg/dL; clustered CSDs, 154 [134-191] mg/dL, isoelectric SDs, 181 [147-228] mg/dL; Kruskal-Wallis, P = .02; eFigure 3 in the Supplement; to convert to millimoles per liter, multiply by 0.0555).
Of the various anatomic pathologies, only the Morris-Marshall grade24 of traumatic subarachnoid hemorrhage was significantly associated with depolarization risk. Depolarization incidence for Morris-Marshall traumatic subarachnoid hemorrhage grades 0, 1, 2, and 3 or 4 increased significantly from 17 of 37 (46%) to 18 of 32 (56%), 22 of 33 (67%), and 23 of 30 patients (77%), respectively (P = .047; Figure 2C). There was also a nonsignificant association with the Rotterdam score,25 a composite measure for outcome prognostication based on head computed tomography pathology (Figure 2D). Patients with scores of 2 or 3 had 48% depolarization incidence (12 of 25 patients), but this increased to 54% (26 of 48), 64% (25 of 39), and 81% (17 of 21) for higher scores (P = .10). The trend is significant when scores 2 to 4 and 5 to 6 are grouped (38 of 73 [52%] and 42 of 60 [70%]; P = .04). A logistic regression model based on the Morris-Marshall grade, Rotterdam Score, and occurrence of early hypotension showed which patients were likely to have depolarizations, with a positive predictive value of 81%, a negative predictive value of 54%, and an area under the curve of 0.74.
Neurologic Examinations and Outcome
Glasgow Coma Scale motor scores prior to neurosurgery did not differ per subsequent depolarization activity. However, motor scores improved significantly by the end of electrocorticography monitoring for patients with no depolarizations or only sporadic CSDs, with 37 of 55 patients (67%) and 19 of 30 patients (63%) able to localize pain, respectively, compared with 23 of 52 (44%; P = .02) and 8 of 31 (26%; P = .005) before surgery (both comparisons by Fisher exact test; Figure 2E). By contrast, patients with clustered CSDs or isoelectric SDs did not significantly improve, with 15 of 28 (54%) and 9 of 23 (39%) localizing after electrocorticography, compared with 16 of 27 (59%) and 11 of 23 (48%) before surgery (Figure 2E).
At 6 months posttrauma, 99 of 138 patients (71.7%) had a poor outcome, defined as death, vegetative state, or a severe disability by the GOS-E score, which was higher than expected by the IMPACT prognostic score (77 of 138 [55.8%]). There were no significant differences in outcomes between study centers (GOS-E median [IQR] scores at 5 centers: 4 [3-5], 3.5 [1-5.5], 3 [2.0-5.3], 2.0 [1.0-4.0], 3.0 [1.0-5.0]; P = .18 by Kruskal-Wallis test). Outcome distributions are shown by depolarization category in Figure 2F. When we dichotomized good and poor outcomes between patients with moderate and severe disability levels, we found that 18 of 55 patients with no depolarizations (33%) had good outcomes. Eleven of 32 patients (34%) with sporadic CSDs only, 6 of 28 patients with clustered CSDs (21%), and 4 of 23 patients with isoelectric SD (17%) had good outcomes. In ordinal regression analysis, the odds ratio for patients with depolarizations to have a worse GOS-E score than those without depolarizations was 1.39 (95% CI, 0.76-2.54; P = .29). When depolarization categories were considered separately, the odds ratios were 1.02 (95% CI, 0.48-2.20; P = .95) for sporadic CSDs, 1.64 (95% CI, 0.73-3.69; P = .23) for clustered CSDs, and 1.76 (95% CI, 0.74-4.20; P = .20) for isoelectric SDs.
In a multivariate model using the core IMPACT variables of age, motor score, and pupils as covariates, the odds ratios for worse outcomes were 1.17 (95% CI, 0.52-2.60; P = .70) for sporadic CSDs, 2.43 (95% CI; 1.03-5.68; P = .04) for clustered CSDs, and 2.13 (95% CI, 0.87-5.23; P = .10) for isoelectric SDs. When patients with cluster SDs and isoelectric SDs were combined in a single category, they had an odds ratio of 2.29 (95% CI, 1.13-4.65; P = .02) for worse outcomes (Table 3). Results were similar using the prognostic score as the covariate: odds ratios were 1.03 (95% CI, 0.48-2.22; P = .94) for sporadic CSDs and 1.74 (95% CI, 0.88-3.44; P = .11) for cluster SDs and isoelectric SDs combined.
Table 3. Outcome Prognostication by Multivariate Ordinal Regression.
| Factor | Proportional Odds Ratio (95% CI) | P Value |
|---|---|---|
| Age | 1.05 (1.03-1.07) | <.001 |
| Motor score | 0.94 (0.79-1.12) | .50 |
| Pupils reacting | ||
| Both | 1 [Reference] | |
| 1 | 1.89 (0.66-5.43) | .24 |
| Neither | 3.67 (1.31-10.31) | .01 |
| Depolarizations | ||
| No spreading depolarizations | 1 [Reference] | |
| Sporadic cortical spreading depressions | 1.17 (0.52-2.60) | .70 |
| Clusters or isoelectric spreading depolarizations | 2.29 (1.13-4.65) | .02 |
Discussion
In this large prospective study, 60% of patients with severe TBI requiring cranial surgery developed spreading depolarizations during intensive care. We identified a simple, clinically applicable, ordinal categorization of depolarization burden or severity based on subtypes and temporal patterning. Using these categories, we found that the occurrence of repetitive depolarizations (clusters) in 37% of patients was associated with a lack of motor improvement during intensive care and was a factor independently associated with worse 6-month functional outcomes. This study is the culmination of years of translational research since the discovery of spreading depolarizations in the human brain8,13 and validates this neuronal pathological mechanism as a novel, clinically relevant target for patient management in intensive care units.
A key result of this study is that isoelectric SDs develop as a consequence of temporally clustered depolarizations. We recorded direct monitoring proof that depolarizations induce the isoelectric state in 18 of 23 cases (eg, Figure 1C). The isoelectric state, with imposed depolarizations, is foundational in understanding lesion development in cerebral gray matter, since its discovery 4 decades ago yielded the very concepts of ischemic penumbra, so-called salvageable tissue, and neuroprotective intervention.26,27,28 Isoelectricity indicates critically impaired function, and recoverable depolarization waves indicate continued viability of the tissue, if not of all cells. These results show that this penumbral state can be diagnosed in patients with TBI and that it is induced as an active secondary injury process by spreading depolarizations. Such deterioration likely reflects the cumulative metabolic depletion29,30 and spreading ischemia7,23 that are both induced by depolarizations.
In the prior pilot study,22 we did not differentiate CSDs with respect to their sporadic vs clustered occurrence. Here, however, we found that this distinction is useful to identify patients with a more malignant disease course. Only clustered CSDs and not sporadic CSDs are similar to isoelectric SDs in summary measures of depolarization burden (Table 2) and associated with development of the isoelectric state. Furthermore, patients with no depolarizations and those with sporadic CSDs showed significant improvement in motor examinations through the course of intensive care and had similar rates of good 6-month outcomes (good outcomes: patients with no depolarizations, 18 of 55 [33%]; patients with sporadic CSDs: 11 of 32 [34%]), whereas patients with clustered CSDs and isoelectric SDs did not improve in motor examinations and had only good outcomes in 6 of 28 cases (21%) and 4 of 23 cases (17%), respectively. In our pilot study, isoelectric SDs occurring in 20 of 103 patients (19%) were identified as the worst prognostic category, with an odds ratio of 7.6 (95% CI, 2.6 to 21.8; P < .001) for worse outcomes. The present findings thus effectively double the percentage of patients (from 19% to 37%) with depolarizations that are likely to be harmful and might be considered for treatment targeting. The odds ratio of clustered CSDs (including isoelectric SDs) for worse outcome was 2.29 (95% CI, 1.13-4.65; P = .02), suggesting greater independent prognostic value than Glasgow Coma Scale motor score, and similar to pupillary reactivity. This association appears less extreme compared with patients with isoelectric SDs in the prior study, despite similar rates of poor outcome for these patients (19 of 23 [83%] vs 17 of 20 [85%]). This may reflect the higher overall rate of poor outcomes in the current study (99 of 138 [71.7%]) compared with our pilot study (56 of 103 [54.4%]).
We found that most established TBI prognostic factors had no value for assessment of the likely occurrence of depolarizations. An exception was that higher prehospital blood pressure was associated with decreased depolarization risk over a wide range of values. This result is consistent with the progressive reduction in mortality with higher prehospital blood pressures31 and provides further evidence against a threshold effect. It is possible that higher pressures mitigate focal ischemic conditions in this hyperacute period, forestalling subsequent pathological developments.17,32
Another depolarization risk factor we identified was the degree of subarachnoid hemorrhage. It has been shown that subarachnoid blood is sufficient to trigger depolarizations in the short term,33 and the amount of subarachnoid blood is a factor associated with early depolarizations after aneurysm rupture.34 After several days, the breakdown of erythrocytes may further provoke a secondary phase of depolarization clusters that contribute to development of new infarcts and neurologic deficits.35,36,37 Other individual pathoanatomic factors, such as subdural hematoma,13,38,39 may be important despite lack of statistical significance in this cohort. While in this study we characterize depolarizations as they occur in the context of real-world variations in neurological trauma treatment, it will be important in the future to determine how practice variations, such as surgical approach,40 may also affect depolarization risk.
Depolarizations are likely triggered by highly focal tissue instabilities32 that escape detection by clinical neuromonitoring modalities, often performed remote or contralateral to injury. Thus, even continuously monitored variables, such as intracranial pressure, brain tissue oxygenation, and cerebral blood flow, are in normal ranges when most depolarizations occur. A limitation of these neuromonitoring modalities is their measurement at a single location, such that measures of oxygenation and blood flow may not capture changes relevant to focal lesion progression.41 An advantage of electrocorticography is the broad sampling over 5 centimeters of the cortex, and the spreading nature of depolarizations permits detection of tissue instabilities, even when electrodes are somewhat remote from the focus.8,42
Limitations
A limitation was that electrocorticography amplifiers were susceptible to low-frequency noise, resulting in shorter valid recording periods. This raises the likely possibility that some patients had more severe depolarization burdens than those scored. Furthermore, the interrater reliability of scoring has not been tested on recordings from these amplifiers. We note, however, that bedside recording systems have greatly improved since the data collection period of this study, and interrater reliability for the newer systems is excellent even for novice users.42,43 Another limitation is that the present results have limited generalizability, since subdural electrodes can only be used by patients requiring surgery and are only used at select academic neurosurgical centers. Future efforts should aim to develop minimally invasive and noninvasive methods for depolarization monitoring to enable more widespread clinical application.44 This would further allow the study of larger cohorts compared with the present, limited sample size.
Conclusions
In summary, we found that spreading depolarizations occur as sporadic, possibly benign events in one-quarter of patients and in a more adverse course of repetitive, clustered events in one-third of patients. The adverse course was associated with a lack of motor improvement during intensive care and higher risk of poor outcome independent of traditional prognostic factors, such as age, Glasgow Coma Scale motor score, and pupillary reactivity. Taken together with a large body of prior literature,8,13 we conclude that depolarizations are not an epiphenomenon or marker of injury severity but rather are an independent measure and mechanism of secondary brain injury relevant to patient outcomes. Electrocorticography should be pursued as a clinical neuromonitoring method and spreading depolarizations should be investigated for targeted neuroprotective treatment in precision management of patients with brain trauma.45
eFigure 1. Definition of spreading depolarization clustering.
eFigure 2. Continuous measures of spreading depolarizations.
eFigure 3. Plasma glucose at hospital admission.
eTable. Surgical procedures and indications.
References
- 1.Maas AI, Menon DK, Lingsma HF, Pineda JA, Sandel ME, Manley GT. Re-orientation of clinical research in traumatic brain injury: report of an international workshop on comparative effectiveness research. J Neurotrauma. 2012;29(1):32-46. doi: 10.1089/neu.2010.1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maas AI, Menon DK, Steyerberg EW, et al. ; CENTER-TBI Participants and Investigators . Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI): a prospective longitudinal observational study. Neurosurgery. 2015;76(1):67-80. doi: 10.1227/NEU.0000000000000575 [DOI] [PubMed] [Google Scholar]
- 3.Yue JK, Vassar MJ, Lingsma HF, et al. ; TRACK-TBI Investigators . Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury. J Neurotrauma. 2013;30(22):1831-1844. doi: 10.1089/neu.2013.2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maas AIR, Menon DK, Adelson PD, et al. ; InTBIR Participants and Investigators . Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16(12):987-1048. doi: 10.1016/S1474-4422(17)30371-X [DOI] [PubMed] [Google Scholar]
- 5.Stocchetti N, Carbonara M, Citerio G, et al. Severe traumatic brain injury: targeted management in the intensive care unit. Lancet Neurol. 2017;16(6):452-464. doi: 10.1016/S1474-4422(17)30118-7 [DOI] [PubMed] [Google Scholar]
- 6.Okonkwo DO, Shutter LA, Moore C, et al. Brain oxygen optimization in severe traumatic brain injury phase-II: a phase II randomized trial. Crit Care Med. 2017;45(11):1907-1914. doi: 10.1097/CCM.0000000000002619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17(4):439-447. doi: 10.1038/nm.2333 [DOI] [PubMed] [Google Scholar]
- 8.Dreier JP, Fabricius M, Ayata C, et al. Recording, analysis, and interpretation of spreading depolarizations in neurointensive care: review and recommendations of the COSBID research group. J Cereb Blood Flow Metab. 2017;37(5):1595-1625. doi: 10.1177/0271678X16654496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinzman JM, DiNapoli VA, Mahoney EJ, Gerhardt GA, Hartings JA. Spreading depolarizations mediate excitotoxicity in the development of acute cortical lesions. Exp Neurol. 2015;267:243-253. doi: 10.1016/j.expneurol.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 10.Hinzman JM, Wilson JA, Mazzeo AT, Bullock MR, Hartings JA. Excitotoxicity and metabolic crisis are associated with spreading depolarizations in severe traumatic brain injury patients. J Neurotrauma. 2016;33(19):1775-1783. doi: 10.1089/neu.2015.4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reinhart KM, Shuttleworth CW. Ketamine reduces deleterious consequences of spreading depolarizations. Exp Neurol. 2018;305:121-128. doi: 10.1016/j.expneurol.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aiba I, Shuttleworth CW. Sustained NMDA receptor activation by spreading depolarizations can initiate excitotoxic injury in metabolically compromised neurons. J Physiol. 2012;590(22):5877-5893. doi: 10.1113/jphysiol.2012.234476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartings JA, Shuttleworth CW, Kirov SA, et al. The continuum of spreading depolarizations in acute cortical lesion development: examining Leão’s legacy. J Cereb Blood Flow Metab. 2017;37(5):1571-1594. doi: 10.1177/0271678X16654495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strong AJ, Fabricius M, Boutelle MG, et al. Spreading and synchronous depressions of cortical activity in acutely injured human brain. Stroke. 2002;33(12):2738-2743. doi: 10.1161/01.STR.0000043073.69602.09 [DOI] [PubMed] [Google Scholar]
- 15.Dreier JP, Major S, Foreman B, et al. Terminal spreading depolarization and electrical silence in death of human cerebral cortex. Ann Neurol. 2018;83(2):295-310. doi: 10.1002/ana.25147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlson AP, Shuttleworth CW, Major S, Lemale CL, Dreier JP, Hartings JA. Terminal spreading depolarizations causing electrocortical silencing prior to clinical brain death: case report. J Neurosurg. 2018;1-7. doi: 10.3171/2018.7.JNS181478 [DOI] [PubMed] [Google Scholar]
- 17.Hartings JA, Strong AJ, Fabricius M, et al. ; Co-Operative Study of Brain Injury Depolarizations . Spreading depolarizations and late secondary insults after traumatic brain injury. J Neurotrauma. 2009;26(11):1857-1866. doi: 10.1089/neu.2009.0961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray GD, Butcher I, McHugh GS, et al. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):329-337. doi: 10.1089/neu.2006.0035 [DOI] [PubMed] [Google Scholar]
- 19.Hukkelhoven CW, Steyerberg EW, Habbema JD, et al. Predicting outcome after traumatic brain injury: development and validation of a prognostic score based on admission characteristics. J Neurotrauma. 2005;22(10):1025-1039. doi: 10.1089/neu.2005.22.1025 [DOI] [PubMed] [Google Scholar]
- 20.Vande Vyvere T, Wilms G, Claes L, et al. ; Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) Investigators and Participants . Central versus local radiological reading of acute computed tomography characteristics in multi-center traumatic brain injury research. J Neurotrauma. 2019;36(7):1080-1092. doi: 10.1089/neu.2018.6061 [DOI] [PubMed] [Google Scholar]
- 21.Steyerberg EW, Mushkudiani N, Perel P, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5(8):e165. doi: 10.1371/journal.pmed.0050165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartings JA, Bullock MR, Okonkwo DO, et al. ; Co-Operative Study on Brain Injury Depolarisations . Spreading depolarisations and outcome after traumatic brain injury: a prospective observational study. Lancet Neurol. 2011;10(12):1058-1064. doi: 10.1016/S1474-4422(11)70243-5 [DOI] [PubMed] [Google Scholar]
- 23.Hinzman JM, Andaluz N, Shutter LA, et al. Inverse neurovascular coupling to cortical spreading depolarizations in severe brain trauma. Brain. 2014;137(pt 11):2960-2972. doi: 10.1093/brain/awu241 [DOI] [PubMed] [Google Scholar]
- 24.Morris GF, Marshall LF. A new, practical classification of traumatic subarachnoid hemorrhage. Acta Neurochirurgica Supplementum (Wein). 1997;71:382. doi: 10.1016/S0303-8467(97)81312-1 [DOI] [Google Scholar]
- 25.Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57(6):1173-1182. doi: 10.1227/01.NEU.0000186013.63046.6B [DOI] [PubMed] [Google Scholar]
- 26.Fabricius M, Fuhr S, Bhatia R, et al. Cortical spreading depression and peri-infarct depolarization in acutely injured human cerebral cortex. Brain. 2006;129(pt 3):778-790. doi: 10.1093/brain/awh716 [DOI] [PubMed] [Google Scholar]
- 27.Branston NM, Strong AJ, Symon L. Extracellular potassium activity, evoked potential and tissue blood flow. Relationships during progressive ischaemia in baboon cerebral cortex. J Neurol Sci. 1977;32(3):305-321. doi: 10.1016/0022-510X(77)90014-4 [DOI] [PubMed] [Google Scholar]
- 28.Astrup J, Siesjö BK, Symon L. Thresholds in cerebral ischemia—the ischemic penumbra. Stroke. 1981;12(6):723-725. doi: 10.1161/01.STR.12.6.723 [DOI] [PubMed] [Google Scholar]
- 29.Lauritzen M, Dreier JP, Fabricius M, Hartings JA, Graf R, Strong AJ. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab. 2011;31(1):17-35. doi: 10.1038/jcbfm.2010.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feuerstein D, Manning A, Hashemi P, et al. Dynamic metabolic response to multiple spreading depolarizations in patients with acute brain injury: an online microdialysis study. J Cereb Blood Flow Metab. 2010;30(7):1343-1355. doi: 10.1038/jcbfm.2010.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spaite DW, Hu C, Bobrow BJ, et al. Mortality and prehospital blood pressure in patients with major traumatic brain injury: implications for the hypotension threshold. JAMA Surg. 2017;152(4):360-368. doi: 10.1001/jamasurg.2016.4686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Bornstädt D, Houben T, Seidel JL, et al. Supply-demand mismatch transients in susceptible peri-infarct hot zones explain the origins of spreading injury depolarizations. Neuron. 2015;85(5):1117-1131. doi: 10.1016/j.neuron.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartings JA, York J, Carroll CP, et al. Subarachnoid blood acutely induces spreading depolarizations and early cortical infarction. Brain. 2017;140(10):2673-2690. doi: 10.1093/brain/awx214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eriksen N, Rostrup E, Fabricius M, et al. Early focal brain injury after subarachnoid hemorrhage correlates with spreading depolarizations. Neurology. 2019;92(4):e326-e341. doi: 10.1212/WNL.0000000000006814 [DOI] [PubMed] [Google Scholar]
- 35.Dreier JP, Woitzik J, Fabricius M, et al. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129(pt 12):3224-3237. doi: 10.1093/brain/awl297 [DOI] [PubMed] [Google Scholar]
- 36.Dreier JP, Ebert N, Priller J, et al. Products of hemolysis in the subarachnoid space inducing spreading ischemia in the cortex and focal necrosis in rats: a model for delayed ischemic neurological deficits after subarachnoid hemorrhage? J Neurosurg. 2000;93(4):658-666. doi: 10.3171/jns.2000.93.4.0658 [DOI] [PubMed] [Google Scholar]
- 37.Dreier JP, Major S, Manning A, et al. ; COSBID study group . Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain. 2009;132(Pt 7):1866-1881. doi: 10.1093/brain/awp102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krenzlin H, Jussen D, Plath M, et al. Occurrence of spontaneous cortical spreading depression is increased by blood constituents and impairs neurological recovery after subdural hematoma in rats. J Neurotrauma. 2019;36(2):395-402. doi: 10.1089/neu.2018.5657 [DOI] [PubMed] [Google Scholar]
- 39.Eriksen N, Pakkenberg B, Rostrup E, et al. Neurostereologic lesion volumes and spreading depolarizations in severe traumatic brain injury patients: a pilot study. Neurocrit Care. 2019;30(3):557-568. doi: 10.1007/s12028-019-00692-w [DOI] [PubMed] [Google Scholar]
- 40.Hartings JA, Vidgeon S, Strong AJ, et al. ; Co-Operative Studies on Brain Injury Depolarizations . Surgical management of traumatic brain injury: a comparative-effectiveness study of 2 centers. J Neurosurg. 2014;120(2):434-446. doi: 10.3171/2013.9.JNS13581 [DOI] [PubMed] [Google Scholar]
- 41.Hawryluk GW, Phan N, Ferguson AR, et al. Brain tissue oxygen tension and its response to physiological manipulations: influence of distance from injury site in a swine model of traumatic brain injury. J Neurosurg. 2016;125(5):1217-1228. doi: 10.3171/2015.7.JNS15809 [DOI] [PubMed] [Google Scholar]
- 42.Hartings JA. Spreading depolarization monitoring in neurocritical care of acute brain injury. Curr Opin Crit Care. 2017;23(2):94-102. doi: 10.1097/MCC.0000000000000395 [DOI] [PubMed] [Google Scholar]
- 43.Hartings JA, Li C, Hinzman JM, et al. Direct current electrocorticography for clinical neuromonitoring of spreading depolarizations. J Cereb Blood Flow Metab. 2017;37(5):1857-1870. doi: 10.1177/0271678X16653135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hartings JA, Ngwenya LB, Watanabe T, Foreman B. Commentary: detecting cortical spreading depolarization with full band scalp electroencephalography: an illusion? Front Syst Neurosci. 2018;12:19. doi: 10.3389/fnsys.2018.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Helbok R, Hartings JA, Schiefecker A, et al. What should a clinician do when spreading depolarizations are observed in a patient? Neurocrit Care. 2019. doi: 10.1007/s12028-019-00777-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Definition of spreading depolarization clustering.
eFigure 2. Continuous measures of spreading depolarizations.
eFigure 3. Plasma glucose at hospital admission.
eTable. Surgical procedures and indications.