Abstract
This pharmacoepidemiology study uses claims data to characterize angiotensin receptor blocker (ARB) prescription trends to evaluate whether recalls of ARBs prompted by discovery of potentially carcinogenic impurities shifted utilization of ARBs individually and as a drug class.
Angiotensin receptor blockers (ARBs) are one of the most common prescription drug classes, used by 5.8% of adults in the United States in 2011-2012 for treatment of hypertension, heart failure, and diabetic nephropathy.1 Discovery of potentially carcinogenic nitrosamine impurities (N-nitrosodimethylamine, N-nitroso-N-diethylamine, and N-nitroso-N-methyl-4-aminobutyric acid ) in generic ARBs resulted in recalls of multiple lots of valsartan, losartan, and irbesartan from different manufacturers. As of August 8, 2019, the US Food and Drug Administration (FDA) reported recalls of 139 valsartan, 57 losartan, and 16 irbesartan national drug codes (NDCs).2 National drug codes are unique product identifiers that have 3 segments that encode the labeler (eg, manufacturer or repackager), the specific drug, and the packaging information (eg, bottle size). Manufacturers typically produce many lots of the same NDC. Although the FDA noted that the potential cancer risk posed by exposure to ARB products with nitrosamine impurities is very small and advised patients to continue taking their current medications until consulting their physician,3 recalls have led to concern and confusion among patients, physicians, and pharmacists.4 We assessed prescription dispensing patterns for ARBs to evaluate whether recalls shifted the utilization of ARBs, individually and as a class. Furthermore, we evaluated dispensing patterns for recalled NDCs to estimate the size of the patient population with potential exposure to impurities.
Methods
We analyzed ARB utilization using prescription dispensing claims from Optum Clinformatics Data Mart, which includes deidentified data from employer-based commercial insurance programs representing 14 million individuals from 50 states. We calculated utilization based on the total number of all generic ARB prescriptions dispensed (losartan, valsartan, irbesartan, candesartan, eprosartan, telmisartan, and olmesartan) between January 2018 and March 2019. We excluded brand prescriptions, as they accounted for less than 1% of total ARB use. We estimated utilization of recalled products based on the total number of prescriptions dispensed for NDCs reported in the FDA recall list.2 We further characterized use of ARBs as a class proportional to utilization of all antihypertensive medications (including angiotensin-converting enzyme inhibitors, β-blockers, calcium channel blockers, and diuretics).
Results
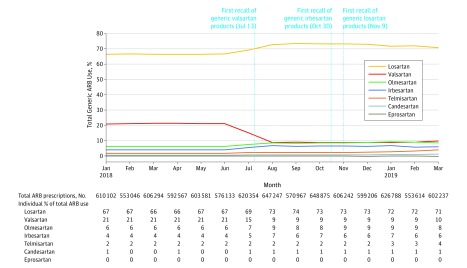
We analyzed 9 million generic ARB prescriptions filled by 1.7 million unique patients. Generic valsartan use as a proportion of overall ARB use decreased from 21% in June 2018 (prior to the first recall) to 10% by March 2019. Utilization of generic losartan increased from 67% to 73% between June and October 2018 but decreased to 71% in March 2019 following recalls of losartan, which began in November 2018. Utilization increased for olmesartan (from 6% to 8%) and irbesartan (from 4% to 6%) between June 2018 and March 2019 (Figure 1). Utilization of ARBs as a class slightly increased, comprising 15.4% of total antihypertensive prescriptions dispensed in January 2018 and 16.9% in March 2019.
Figure 1. Overall Generic ARB Utilization Trends Between January 2018 and March 2019.
The vertical lines indicate calendar dates of the first product recalls of valsartan, irbesartan, and losartan. Total numbers of prescriptions filled for all generic angiotensin receptor blockers (ARBs) every month are reported in the first row beneath the x-axis; percentages of total generic ARB use accounted for by each individual ARB are also shown.
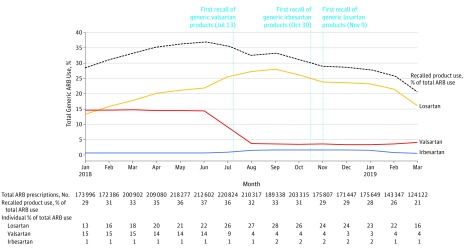
We observed a higher proportion of NDCs affected by recalls for valsartan (98 of 289 total NDCs [33.9%]) than for losartan (40 of 342 [11.7%]) or irbesartan (14 of 121 [11.6%]). In June 2018, generic valsartan, losartan, and irbesartan NDCs affected by recalls accounted for 37% of overall generic ARB use, decreasing to 21% in March 2019 (Figure 2).
Figure 2. Utilization Trends for Generic ARB Products Affected by Recalls Between January 2018 and March 2019.
The dashed line indicates utilization of products affected by recalls as a proportion of total generic angiotensin receptor blocker (ARB) utilization. The vertical lines indicate calendar dates of the first product recalls of valsartan, irbesartan, and losartan. Total numbers of prescriptions filled for all products affected by recalls every month are reported in the first row beneath the x-axis; percentages of total generic ARB use accounted for by recalled valsartan, irbesartan, and losartan products are also shown.
Discussion
National drug codes affected by recalls represented 37% of total ARB use prior to recalls. A substantial decrease in utilization of valsartan but not losartan or irbesartan was noted, which may be because fewer NDCs for losartan and irbesartan were affected by recalls compared with valsartan. The total volume of generic ARB prescriptions did not decrease, suggesting shifts within the class of ARBs, which may have prevented major disruptions in ARB treatment. Limitations include that the prescription-level analysis did not evaluate patient-level treatment discontinuation or switching, and it is unknown whether the observed patterns are generalizable to patients with other types of prescription drug insurance.
Section Editor: Jody W. Zylke, MD, Deputy Editor.
References
- 1.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831. doi: 10.1001/jama.2015.13766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.FDA updates and press announcements on angiotensin ii receptor blocker (ARB) recalls (valsartan, losartan, and irbesartan). US Food and Drug Administration website. https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-angiotensin-ii-receptor-blocker-arb-recalls-valsartan-losartan. Accessed August 9, 2019.
- 3.FDA statement on the FDA’s ongoing investigation into valsartan and ARB class impurities and the agency’s steps to address the root causes of the safety issues. US Food and Drug Administration website. https://www.fda.gov/news-events/press-announcements/fda-statement-fdas-ongoing-investigation-valsartan-and-arb-class-impurities-and-agencys-steps. Published January 25, 2019. Accessed May 1, 2019.
- 4.Byrd JB, Chertow GM, Bhalla V. Hypertension hot potato—anatomy of the angiotensin-receptor blocker recalls. N Engl J Med. 2019;380(17):1589-1591. doi: 10.1056/NEJMp1901657 [DOI] [PMC free article] [PubMed] [Google Scholar]