Key Points
Question
Does nebulized normal saline have an association with physiologic measures of respiratory status in patients with acute viral bronchiolitis?
Findings
This systematic review and meta-analysis found that patients with bronchiolitis treated with nebulized normal saline showed significant improvement in respiratory rate and respiratory score after therapy, although oxygen saturation remained unchanged. In addition, when compared with patients treated with other placebos, those treated with nebulized normal saline showed greater improvements in posttreatment respiratory scores.
Meaning
Nebulized normal saline may not be an inert placebo for patients with bronchiolitis and should be further studied to establish the clinical significance of any potential treatment outcome.
Abstract
Importance
In therapeutic trials for acute viral bronchiolitis, consistent clinical improvement in groups that received nebulized normal saline (NS) as placebo raises the question of whether nebulized NS acts as a treatment rather than a placebo.
Objective
To measure the short-term association of nebulized NS with physiologic measures of respiratory status in children with bronchiolitis by analyzing the changes in these measures between the use of nebulized NS and the use of other placebos and the changes before and after nebulized NS treatment.
Data Sources
MEDLINE and Scopus were searched through March 2019, as were bibliographies of included studies and relevant systematic reviews, for randomized clinical trials evaluating nebulized therapies in bronchiolitis.
Study Selection
Randomized clinical trials comparing children 2 years or younger with bronchiolitis who were treated with nebulized NS were included. Studies enrolling a treatment group receiving an alternative placebo were included for comparison of NS with other placebos.
Data Extraction and Synthesis
Data abstraction was performed per PRISMA guidelines. Fixed- and random-effects, variance-weighted meta-analytic models were used.
Main Outcomes and Measures
Pooled estimates of the association with respiratory scores, respiratory rates, and oxygen saturation within 60 minutes of treatment were generated for nebulized NS vs another placebo and for change before and after receiving nebulized NS.
Results
A total of 29 studies including 1583 patients were included. Standardized mean differences in respiratory scores for nebulized NS vs other placebo (3 studies) favored nebulized NS by −0.9 points (95% CI, −1.2 to −0.6 points) at 60 minutes after treatment (P < .001). There were no differences in respiratory rate or oxygen saturation comparing nebulized NS with other placebo. The standardized mean difference in respiratory score (25 studies) after nebulized NS was −0.7 (95% CI, −0.7 to −0.6; I2 = 62%). The weighted mean difference in respiratory scores using a consistent scale (13 studies) after nebulized NS was −1.6 points (95% CI, −1.9 to −1.3 points; I2 = 72%). The weighted mean difference in respiratory rate (17 studies) after nebulized NS was −5.5 breaths per minute (95% CI, −6.3 to −4.6 breaths per minute; I2 = 24%). The weighted mean difference in oxygen saturation (23 studies) after nebulized NS was −0.4% (95% CI, −0.6% to −0.2%; I2 = 79%).
Conclusions and Relevance
Nebulized NS may be an active treatment for acute viral bronchiolitis. Further evaluation should occur to establish whether it is a true placebo.
This systematic review and meta-analysis studies change in physiologic measures of respiratory status with the use of nebulized normal saline vs placebo in children with bronchiolitis and examines change before and after treatment with nebulized normal saline.
Introduction
Acute viral bronchiolitis, one of the most common childhood illnesses, has no established, evidence-based nebulized therapy, to our knowledge.1,2,3,4,5,6,7,8 In most randomized clinical trials, nebulized normal saline (NS) is used as the placebo, which is logical given that NS is also used as the vehicle for nebulization of the active treatment. However, consistent improvement above expected rates in patients receiving placebo has been noted across bronchodilator trials.9 The waxing and waning nature of bronchiolitis may explain these findings, but given the strong trend toward improvement, it is also possible that nebulized NS is acting as an effective treatment.
Nebulized NS has historically been used as a placebo in other conditions as well, typically in studies examining bronchodilator medications and sputum expectorants.10 A small amount of evidence exists to suggest that nebulized NS may be an active treatment for adults with chronic obstructive pulmonary disease. In a randomized clinical trial of 40 patients with chronic obstructive pulmonary disease, investigators used a technique of “inefficient” nebulization to blind participants to treatment allocation and demonstrated significant improvements in dyspnea scores favoring NS nebulized through the effective nebulizer vs the inefficient nebulizer.10 Randomized clinical trials comparing nebulized NS with inhaled terbutaline and with inhaled furosemide among patients with dyspnea have also found similar improvement in breathlessness scores among groups receiving active treatment and those receiving NS.11,12 However, in another very small trial of laboratory-induced dyspnea in 5 healthy volunteers that was specifically designed to reduce expectation of dyspnea relief, only 1 in 5 patients experienced relief, which investigators ascribed to the placebo effect.12
The concept of the placebo effect has recently undergone reconsideration and refinement. The 1955 article by Beecher13 that first established the idea of the approximately equal to 30% placebo response rate has been critically reassessed, suggesting that the reported findings were influenced by the natural fluctuations of the diseases studied.14,15 Furthermore, 2 large meta-analyses of randomized clinical trials comparing placebo vs no treatment failed to detect significant placebo effects in many types of studies. In particular, the more objective the outcome assessment, the less likely that there was any measurable placebo effect detected.15,16 Less is known about the effect of placebos on infants, in whom any improvement is necessarily determined by external assessment rather than self-report.
In this context, we sought to examine the hypothesis that nebulized NS may be an active treatment rather than a placebo for patients with acute viral bronchiolitis. We undertook a systematic review and meta-analysis of randomized clinical trials in acute viral bronchiolitis using nebulized NS with 2 objectives: to compare nebulized NS with other forms of placebo (eg, nonnebulized placebo or sham nebulization) and to estimate the association of nebulized NS with short-term physiologic measures of respiratory status in randomized clinical trials in which it was used as the placebo.
Methods
Eligibility Criteria
Studies were considered for inclusion if they reported results of a clinical trial of patients 2 years or younger with a primary diagnosis of acute viral bronchiolitis who were treated with nebulized NS. For trials comparing nebulized NS with another placebo, at least 1 treatment group receiving only the alternative form of placebo was additionally required for inclusion. Only published studies were considered for this review.
Information Sources and Search Strategy
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline. We searched MEDLINE from 1946 to March 2019 and Scopus from 1976 to March 2019. Search terms are shown in eFigure 1 in the Supplement. Bibliographies of qualifying studies and relevant systematic reviews were also hand searched for additional candidate studies. A review protocol was not registered prior to study completion.
Study Selection
Studies identified from the initial search results underwent title and abstract review conducted by 2 independent reviewers (S.A.H. and S.L.R.). Any discrepancies regarding inclusion were resolved by consensus. Studies were then excluded through full-text review if they failed to provide information on short-term physiologic measures of respiratory status, which were defined as composite respiratory score, respiratory rate, and/or oxygen saturation values within the first hour after treatment. Further exclusion criteria were inclusion of children older than 2 years and receipt of another nebulized medication in the NS group within 1 hour of study entry. For studies with incomplete data and those excluded owing to lack of reporting of short-term physiologic outcomes in which the study protocol suggested these data were collected, corresponding authors were contacted via email to determine whether these additional data were available. Foreign language studies were translated into English.
Data Collection Process
Data abstraction was performed independently by 2 study investigators (S.A.H. and S.L.R.) using a standardized data extraction sheet, cross-checked for agreement, and referred to a third investigator (A.M.G.) for any disagreements. Data elements extracted included study setting and geographical location, types of interventions, mean or median age of patients, illness severity criteria for study entry, respiratory scores, respiratory rates, and oxygen saturation. For all outcome measures, the mean and SD of baseline and posttreatment values were collected when available and calculated or imputed from presented data when not directly available using methods provided in the Cochrane Handbook.17 Sixty-minute posttherapy measures were used preferentially; 20- to 30-minute posttreatment measures were used when 60-minute measures were unavailable.
Quality Assessment and Risk of Bias
The revised Cochrane risk of bias tool for randomized clinical trials was used to screen for overall risk of bias within each included study at the level of the outcomes investigated in our meta-analysis. Two investigators (S.A.H. and S.L.R.) evaluated each study using this tool; discrepancies were referred to the third study author (A.M.G.) for resolution.
Summary Measures and Data Synthesis
For studies evaluating nebulized NS vs another placebo, the study-weighted mean differences in outcomes between treatment groups were calculated. For studies providing data on treatment with nebulized NS over time, the study-weighted mean differences in outcomes within the first 60 minutes of the study vs study baseline were calculated. Standardized mean differences were used when combining respiratory scores for the full study cohort because study-specific scores use different scales; however, the scale was preserved in subgroup analysis of studies using a consistent score. The point estimates and 95% CIs were computed with inverse-variance–weighted fixed- and random-effects models using Comprehensive Meta-Analysis 2.0. Heterogeneity was assessed using the I2 statistic.
Additional Analyses
Preplanned subgroup analyses were conducted based on whether the study population was outpatients vs inpatients and in studies using the same respiratory scoring tool when more than 5 studies met this criterion. Sensitivity analyses were performed by removing 1 study sequentially, removing outliers as defined by failure of the individual study CI to overlap either end of the CIs generated for the point estimates, and by removing studies at high risk of bias based on the revised Cochrane risk of bias tool for randomized trials.18
Results
Study Selection
A total of 1084 citations were identified in the initial database search. Bibliography review of included studies and relevant meta-analyses resulted in an additional 26 candidate studies. After duplicate removal, 874 studies were screened, with 118 potentially meeting inclusion criteria after title and abstract screening. Eighty-nine studies were excluded by full-text review. The most frequent reason for trial exclusion in full-text review was that the study did not report data on outcomes of interest. Twenty-nine studies met inclusion criteria based on reported outcomes.19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47 Additional data were requested by email from 5 authors, and 3 responded with unpublished data for 4 included studies.26,27,46,47 Study selection is depicted in eFigure 1 in the Supplement. Three studies enrolling a group receiving nonnebulized placebo were identified.26,27,32
Study Characteristics
The Table provides detailed information on the included studies. These 29 trials were performed in numerous countries and evaluate a range of active therapies, including nebulized epinephrine, albuterol, hypertonic saline, terbutaline, ipratropium, and furosemide. Fifteen studies were conducted in the inpatient setting,20,22,23,25,28,29,30,33,37,38,40,41,44,45,46 and 14 were conducted in the outpatient setting.19,21,24,26,27,31,32,34,35,36,39,42,43,47 In total, there were 1477 patients who received nebulized NS and 106 patients who received a nonnebulized placebo. The Respiratory Distress Assessment Index (RDAI)48 was the most commonly used scoring system in 14 trials.20,21,23,24,28,33,34,36,38,39,40,44,46,47 About half of the trials specified a minimum level of severity of disease for study entry.21,25,29,30,32,33,34,35,36,37,39,40,41,43,45,47
Table. Characteristics of Included Studies.
| Source | Country | Study Descriptiona | Score Used | Severity of Illness Included | Baseline Score, Mean (SD) | Patients Receiving Placebo, No. |
|---|---|---|---|---|---|---|
| Outpatient Studies | ||||||
| Schuh et al,19 1990 | Canada | Albuterol vs normal saline | Other (each 0-3)b | No minimum severity | 1.6 (0.1), 1.8 (0.1) | 19 |
| Klassen et al,21 1991 | Canada | Albuterol vs normal saline | RDAI (0-17) | RDAI score 4-15 | 8.5 (2.9) | 41 |
| Schweich et al,24 1992 | United States | Albuterol vs normal saline | RDAI (0-17) | No minimum severity | 7.9 (2.2) | 12 |
| Gadomski et al,27 1994c | United States | Albuterol vs normal saline vs oral albuterol vs oral placebo | Study specific (0-27) | No minimum severity | 10 (5) | 18d |
| Gadomski et al,26 1994c | Egypt | Albuterol vs normal saline vs oral albuterol vs oral placebo | Study specific (0-27) | No minimum severity | 14.2 (6.2) | 32d |
| Van Bever et al,31 1995 | Belgium | Furosemide vs normal saline | Tal (0-12) | No minimum severity | 5.1 (1.5) | 14 |
| Can et al,32 1998 | Turkey | Albuterol vs normal saline vs mist tent | Other (0-20) | Score ≥5 | 11.3 (3.6) | 52d |
| Hariprakash et al,34 2003 | United Kingdom | Epinephrine vs normal saline | RDAI (0-17) | RDAI score ≥3 | 7.6 (4) | 36 |
| Khashabi et al,35 2005 | Iran | Epinephrine vs salbutamol vs normal saline | Other (0-26) | Score 9-18 | 10.6 (3.8) | 24 |
| Ralston et al,36 2005 | United States | Epinephrine vs albuterol vs normal saline | RDAI (0-17) | RDAI score ≥4 | 8 (2) | 25 |
| Plint et al, 200939 | Canada | Epinephrine and oral dexamethasone vs epinephrine and oral placebo vs normal saline and oral dexamethasone vs normal saline and oral placebo | RDAI (0-17) | RDAI score 4-15 | 8 (3) | 201 |
| Anil et al,42 2010 | Turkey | Epinephrine in normal saline vs epinephrine in 3% saline vs albuterol in normal saline vs albuterol in 3% saline vs normal saline | Wang (0-12) | Wang score 1-9 | 3.6 (1) | 37 |
| Ipek et al,43 2011 | Turkey | Nebulized albuterol in normal saline vs albuterol in 3% saline vs 3% saline vs normal saline | Wang (0-12) | Wang score 4-8 | 4.7 (1) | 30 |
| Angoulvant et al,47 2017c | France | 3% Hypertonic saline vs normal saline | RDAI (0-17) | Moderate to severe | 7.7 (3.4) | 378 |
| Inpatient Studies | ||||||
| Lines et al,20 1990 | Australia | Salbutamol vs normal saline | RDAI (0-17) | No minimum severity | 7.7 (2) | 23 |
| Ho et al,22 1991 | Australia | Salbutamol vs normal saline | Noneb | No minimum severity | NA | 8 |
| Lines et al,23 1992 | Australia | Ipratropium bromide vs normal saline | RDAI (0-17)b | No minimum severity | NR | 14 |
| Kristjánsson et al,25 1993 | Sweden and Norway | Racemic adrenaline vs normal saline | Other (0-10) | Score ≥4 | 4.5 (1) | 14 |
| Chowdhury et al,29 1995 | Saudi Arabia | Salbutamol vs ipratropium bromide vs combined salbutamol and ipratropium bromide vs normal saline | Modified RDAI (0-20) | Moderate | 10 (2.4) | 22 |
| Reijonen et al,28 1995 | Finland | Racemic epinephrine followed by normal saline vs albuterol followed by normal saline vs normal saline followed by racemic epinephrine vs normal saline followed by albuterol | RDAI (0-17) | No minimum severity | 9.2 (3.3) | 49 |
| Chevallier et al,30 1995 | France | Salbutamol vs normal saline | Study specific (each 0-3)b | Moderate | 2.4 (0.5), 2.1 (0.7) | 17 |
| Abul-Ainine et al,33 2002 | United Kingdom | Adrenaline vs normal saline | RDAI (0-17) | Moderately severe | 11.2 (3.3) | 19 |
| Karadag et al,37 2008 | Turkey | Salbutamol vs ipratropium bromide vs normal saline | Wang (0-12) | Wang score ≥6 | 8.8 (1.3) | 23 |
| Bar et al,38 2008 | Israel | Furosemide vs normal saline | RDAI (0-17) | No minimum severity | 8.9 (5) | 16 |
| Tinsa et al,40 2009 | Tunisia | Terbutaline vs normal saline | RDAI (0-17) | RDAI score 4-15 | 7.4 (2.4) | 19 |
| Scarlett et al,44 2012 | United States | Albuterol vs normal saline | RDAI (0-17) | No minimum severity | 3.1 (2.3) | 10 |
| Skjerven et al,41 2013 | Norway | Racemic adrenaline (on demand) vs racemic adrenaline (fixed schedule) vs normal saline (on demand) vs normal saline (fixed schedule) | Other (0-10) | Score ≥4 | 4.9 (1) | 201 |
| Tinsa et al,45 2014 | Tunisia | 5% Hypertonic saline vs mixed 5% hypertonic saline with standard epinephrine 0.1% vs normal saline | Wang (0-12) | Wang score ≥3 | 4.3 (1.5) | 26 |
| Silver et al,46 2015c | United States | 3% Hypertonic saline vs normal saline | RDAI (0-17) | No minimum severity | 3.5 (2.1) | 97 |
Abbreviations: NA, not applicable; NR, not reported; RDAI, respiratory distress assessment index.
All medications were nebulized unless otherwise specified.
Excluded from score analysis owing to a 2-component score structure with baseline scores for each score component (Schuh et al19 and Chevallier et al30), no score used (Ho et al22), or no baseline score reported (Lines et al23).
Analysis includes unpublished data provided by authors.
Risk of Bias
Most studies were categorized as having some concern for bias, although 8 studies were found to be at high risk for bias20,23,25,29,31,32,41,44 (eFigure 2 in the Supplement).
Nebulized NS vs Other Placebo
Three studies compared nebulized NS with a nonnebulized placebo, including 2 using an oral placebo and 1 using a mist tent.26,27,32 Standardized mean differences in scores for the NS group (n = 102) vs other placebo group (n = 106) favored NS by −0.9 points (95% CI, −1.2 to −0.6 points) at 60 minutes after therapy (P < .001). The study protocols in the 2 studies comparing NS with oral placebo were identical because they were performed by the same team of investigators in 2 different countries; thus, we subanalyzed these data with the respiratory score scale preserved.26,27 Weighted mean differences in scores for the NS group (n = 50) vs oral placebo group (n = 54) favored NS by −1.6 points (95% CI, −0.8 to −0.03 points) on a study-specific respiratory score (scale, 0-20) at 60 minutes after therapy (P = .04). There were no differences in respiratory rate or oxygen saturation between the group receiving nebulized NS and the group receiving the other placebos.
Association of Nebulized NS With Respiratory Scores
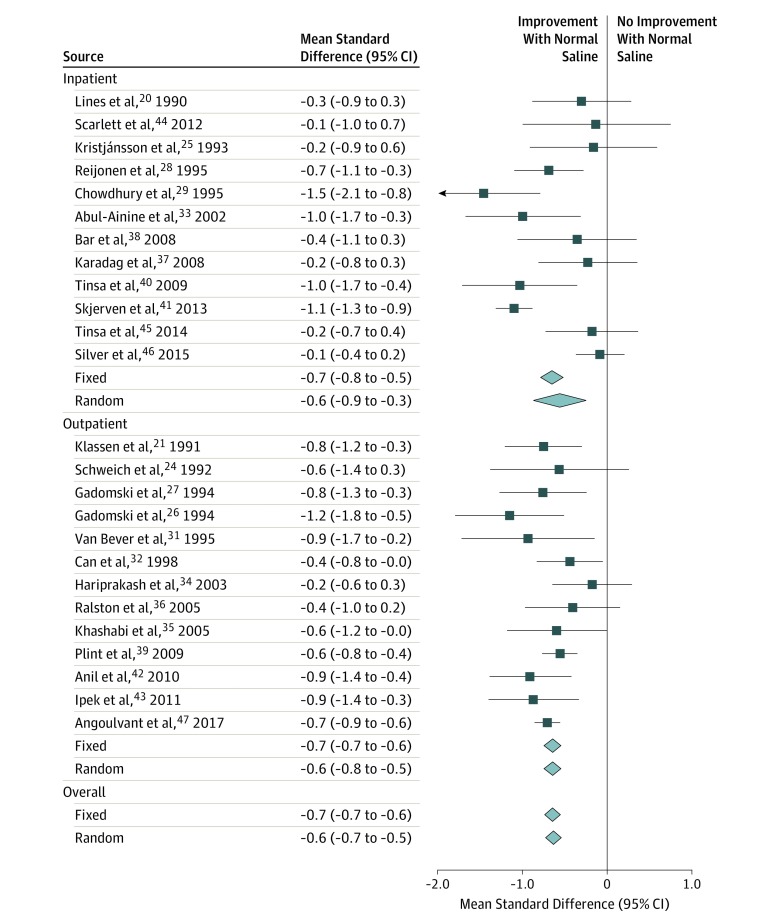
A total of 25 studies reported composite respiratory scores over time for patients receiving nebulized NS.20,21,24,25,26,27,28,29,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47 The standardized mean difference in scores within 60 minutes after nebulized NS decreased by −0.7 (95% CI, −0.7 to −0.6; I2 = 62%; P < .001) (Figure 1). Random-effects modeling and subgroup analysis by study setting did not substantively alter the point estimates; however, heterogeneity appeared to be associated with inconsistency in the inpatient subgroup (outpatient I2 = 4%; inpatient I2 = 79%).
Figure 1. Summary of Differences in Respiratory Score After Treatment With Nebulized Normal Saline.
Association of Nebulized NS With RDAI Score
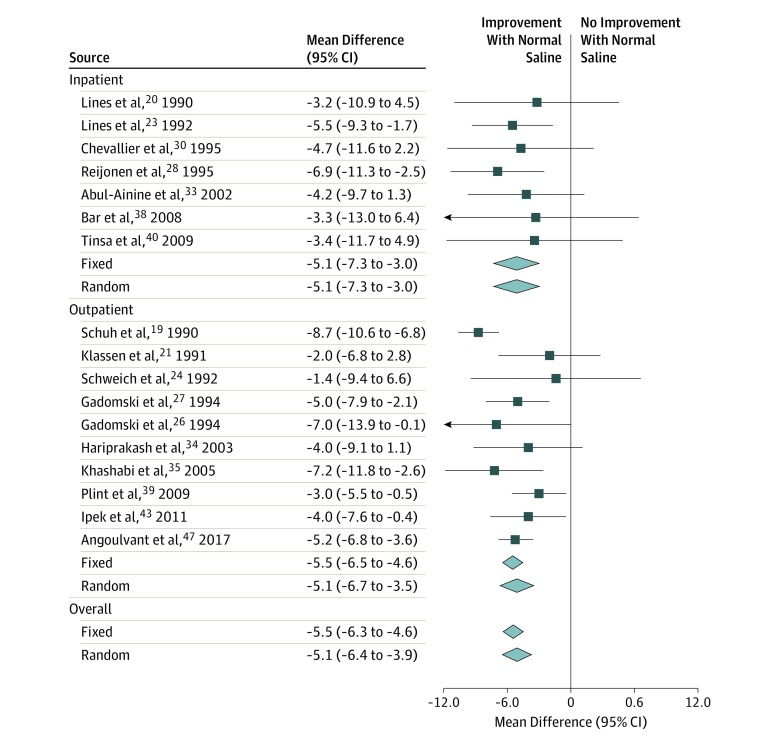
The RDAI (scale, 0-17 points) was the only score used in enough studies to perform subgroup analysis preserving scale. A total of 13 studies reported RDAI data20,21,24,28,33,34,36,38,39,40,44,46,47; 1 study used this scoring system, but baseline scores could not be ascertained, and thus the score was not analyzed for this study.23 The weighted mean difference in scores within 60 minutes after nebulized NS decreased by −1.6 points (95% CI, −1.9 to −1.3 points; I2 = 72%; P < .001) (Figure 2). Subgroup analysis demonstrated a −2.0-point (95% CI, −2.3 to −1.6 points) decrease in the outpatient subgroup vs a −0.9-point (95% CI, −1.4 to −0.4 points) decrease in the inpatient subgroup. Random-effects modeling did not substantively alter the point estimates, whereas heterogeneity appeared to be associated with inconsistency in the inpatient subgroup (outpatient I2 = 42%; inpatient I2 = 72%).
Figure 2. Summary of Differences in Respiratory Score After Nebulized Normal Saline for All Studies Reporting Respiratory Distress Assessment Index Scores.
Association of Nebulized NS With Respiratory Rate
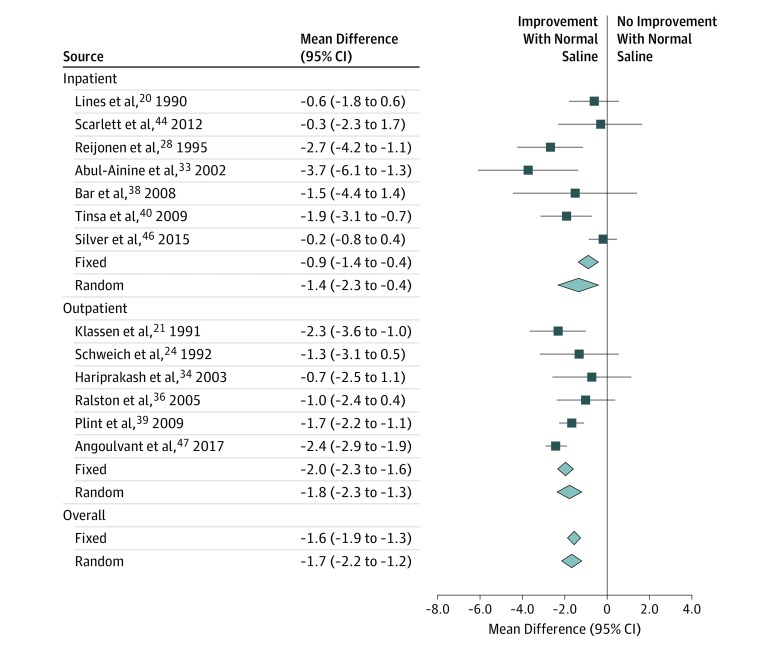
In the 17 studies providing data on respiratory rates,19,20,21,23,24,26,27,28,30,33,34,35,38,39,40,43,47 the weighted mean difference within 60 minutes after nebulized NS decreased by −5.5 breaths per minute (95% CI, −6.3 to −4.6 breaths per minute; I2 = 24%; P < .001) (Figure 3). Fixed-effects vs random-effects modeling and subgroup analyses by study setting did not substantively alter the point estimates, and heterogeneity was low in the overall analysis.
Figure 3. Summary of Differences in Respiratory Rate After Treatment With Nebulized Normal Saline.
Association of Nebulized NS With Oxygen Saturation
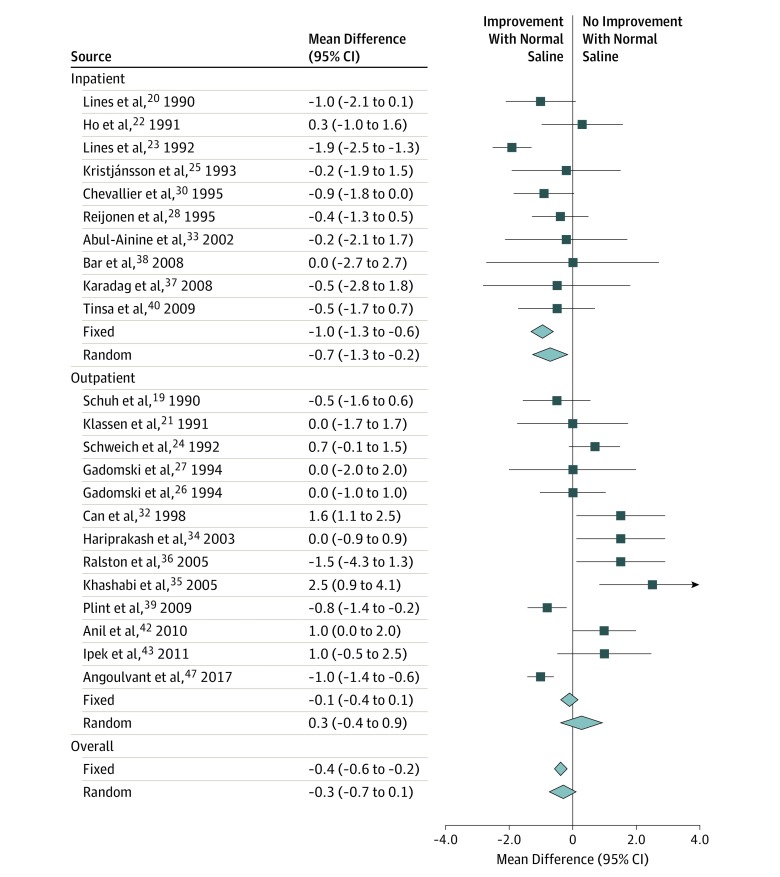
In the 23 studies providing data on oxygen saturation,19,20,21,22,23,24,25,26,27,28,30,32,33,34,35,36,37,38,39,40,42,43,47 the weighted mean difference within 60 minutes after nebulized NS decreased by −0.4% (95% CI, −0.6% to −0.2%; I2 = 79%; P < .001) (Figure 4). Random-effects modeling substantively altered the overall point estimate toward nonsignificance (mean difference, −0.3%; P = .10). Subgroup analysis also produced a nonsignificant point estimate in the outpatient subgroup (mean difference, 0.3%; P = .38). Furthermore, heterogeneity was high in the outpatient subgroup (I2 = 83%) vs the inpatient subgroup (I2 = 49%).
Figure 4. Summary of Differences in Oxygen Saturation After Treatment With Nebulized Normal Saline.
Sensitivity Analyses
Sensitivity analyses removing 1 study did not alter the overall point estimates for any of the analyses. For the RDAI, sensitivity analysis removing outliers46 reduced heterogeneity to acceptable levels (I2 = 42%) while producing a similar point estimate (mean difference, −1.9; 95% CI, −2.2 to −1.6). For respiratory rate, sensitivity analysis removing outliers19 did not substantively alter findings. For oxygen saturation, sensitivity analysis removing outliers23,32,35,47 produced an overall point estimate of −0.2%, which was not statistically significant (P = .10), with acceptable heterogeneity (I2 = 24%).
Sensitivity analysis removing studies at high risk of bias in the respiratory score analysis20,25,29,31,32,41,44 did not substantively alter the point estimate, although it reduced heterogeneity (I2 = 47%). Removal of studies at high risk of bias in the RDAI analysis20,44 did not substantively alter the point estimate or measures of heterogeneity. Removal of studies at high risk of bias from the respiratory rate analysis20,23 did not substantively alter the point estimate or measures of heterogeneity. Removing studies at high risk of bias from the oxygen saturation analysis20,23,25,32 did not substantively alter the point estimates, although it did produce a modest decrease in heterogeneity (I2 = 60%).
Discussion
In this systematic review of randomized clinical trials using nebulized NS as a placebo, we encountered direct and indirect evidence that NS may be an active treatment rather than a placebo. The direct evidence comes from 3 studies involving more than 200 patients, in which nebulized NS was evaluated vs other placebos and significantly improved respiratory scores. The indirect evidence comes from significantly improved assessments of respiratory scores and respiratory rates before and after nebulized NS in several randomized clinical trials. Although the quality of this evidence is not strong owing to the small number of studies in the first analysis and the absence of a comparator group in the second, it is consistent across a wide variety of trials and is concordant. Finally, there was an equivocal association with oxygen saturation noted in the meta-analysis, with high heterogeneity in the broad sample; subgroup analysis and sensitivity analysis resolving this heterogeneity suggest that nebulized NS has no association with oxygen saturation.
There are several potential explanations for our findings. It is possible that nebulized NS is truly not a placebo; that is, it provides a small beneficial effect on young children with acute viral bronchiolitis through improvement in hydration of airway surfaces and mucous clearance and decreased upper airway resistance. It is also possible that the clinical course of bronchiolitis waxes and wanes independent of nebulized NS or other therapies being evaluated. Patients may be given antipyretics or nasal suction or simply settle down after the initial evaluation phase, thus appearing improved. Several studies have noted that respiratory rate and respiratory score are correlated with a patient’s state (eg, awake, asleep, or crying).25,26,27,33 It is not possible to isolate the relative effect of additional symptomatic therapies or patient state vs that of nebulized NS on the outcomes evaluated in this data set. A third possibility is that the primary method of evaluation (respiratory score) is a biased tool that is susceptible to psychological effects in the evaluator and that these repeated measures tend to regress toward the mean. Modern thinking about the placebo effect has become more nuanced, and it appears that any potentially subjective symptom measurement tool (such as pain or depression scales) is more likely to demonstrate a placebo effect than objective outcomes.15 This phenomenon could be associated with the findings on the respiratory scoring tools in our study as well.
We cannot draw firm conclusions regarding treatment from our results owing to the wide range of potential explanations described and the inherent bias associated with pretreatment and posttreatment evaluation. Our data should not affect the current consensus that the active therapies evaluated in acute viral bronchiolitis do not alter the disease course. Although the presence of a treatment effect from a medication given in an ancillary manner to both treatment groups in a randomized clinical trial (either as the placebo therapy or the vehicle for active therapy) may complicate study power analyses, there is no compelling reason to believe that it would interfere with the treatment effect from other active medications. At a minimum, our data should highlight the need for future randomized clinical trials of this disease to account for the fact that short-term physiologic measures will strongly trend toward improvement. Thus, power analyses will need to account for decreased sensitivity to changes in these measures.
Limitations
This meta-analysis has some limitations, including the heterogeneity of the included trials, with varying maximum ages, populations, and criteria for severity of illness for study entry. Study heterogeneity was excessive for some of our aggregate analyses, although we managed to resolve it to acceptable levels with subgroup and sensitivity analyses without changing the overall conclusions. It is highly likely that severity of illness would have a significant association with our study outcomes because patients with mild disease may have a small margin for improvement given their relatively normal vital signs and low distress scores at baseline. In addition, the clinical significance of the short-term physiologic outcomes that we propose from treatment with nebulized NS is unclear. Although reductions in respiratory score and respiratory rate may signal short-term clinical improvement, we did not evaluate other relevant clinical outcomes more reflective of overall disease course and severity, such as hospitalization rates or hospital length of stay. Finally, we made no effort to include unpublished abstracts; thus, it is possible that missing data could alter our conclusions, although this possibility is unlikely given the numbers of patients and studies included.
Conclusions
This meta-analysis suggests that nebulized NS could be an active treatment for acute viral bronchiolitis rather than an inert placebo. Further evaluation of nebulized NS vs sham nebulization and/or oral placebo should occur to establish whether or not nebulized NS is a true placebo. Future study designs should factor in the potential treatment effect of nebulized NS on short-term outcomes.
eFigure 1. Flow of Studies From Database Search Through Meta-Analysis Inclusion
eFigure 2. Risk of Bias by Domain as Assessed by Revised Cochrane Risk-of-Bias Tool for Randomized Trials
References
- 1.Hartling L, Bialy LM, Vandermeer B, et al. . Epinephrine for bronchiolitis. Cochrane Database Syst Rev. 2011;(6):CD003123. [DOI] [PubMed] [Google Scholar]
- 2.Enriquez A, Chu IW, Mellis C, Lin WY. Nebulised deoxyribonuclease for viral bronchiolitis in children younger than 24 months. Cochrane Database Syst Rev. 2012;11:CD008395. doi: 10.1002/14651858.CD008395.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandes RM, Bialy LM, Vandermeer B, et al. . Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2013;(6):CD004878. doi: 10.1002/14651858.CD001266.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gadomski AM, Scribani MB. Bronchodilators for bronchiolitis. Cochrane Database Syst Rev. 2014;(6):CD001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maguire C, Cantrill H, Hind D, Bradburn M, Everard ML. Hypertonic saline (HS) for acute bronchiolitis: systematic review and meta-analysis. BMC Pulm Med. 2015;15:148. doi: 10.1186/s12890-015-0140-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks CG, Harrison WN, Ralston SL. Association between hypertonic saline and hospital length of stay in acute viral bronchiolitis: a reanalysis of 2 meta-analyses. JAMA Pediatr. 2016;170(6):577-584. doi: 10.1001/jamapediatrics.2016.0079 [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Mendoza-Sassi RA, Wainwright C, Klassen TP. Nebulised hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database Syst Rev. 2017;12:CD006458. doi: 10.1002/14651858.CD006458.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison W, Angoulvant F, House S, Gajdos V, Ralston SL. Hypertonic saline in bronchiolitis and type I error: a trial sequential analysis. Pediatrics. 2018;142(3):e20181144. doi: 10.1542/peds.2018-1144 [DOI] [PubMed] [Google Scholar]
- 9.Seiden JA, Scarfone RJ. Bronchiolitis: an evidence-based approach to management. Clin Pediatr Emerg Med. 2009;10:75-81. doi: 10.1016/j.cpem.2009.03.006 [DOI] [Google Scholar]
- 10.Khan SY, O’Driscoll BR. Is nebulized saline a placebo in COPD? BMC Pulm Med. 2004;4:9. doi: 10.1186/1471-2466-4-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poole PJ, Brodie SM, Stewart JM, Black PN. The effects of nebulised isotonic saline and terbutaline on breathlessness in severe chronic obstructive pulmonary disease (COPD). Aust N Z J Med. 1998;28(3):322-326. doi: 10.1111/j.1445-5994.1998.tb01956.x [DOI] [PubMed] [Google Scholar]
- 12.O’Donnell CR, Lansing RW, Schwartzstein RM, Banzett R. The effect of aerosol saline on laboratory-induced dyspnea. Lung. 2017;195(1):37-42. doi: 10.1007/s00408-016-9971-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beecher HK. The powerful placebo. JAMA. 1955;159(17):1602-1606. doi: 10.1001/jama.1955.02960340022006 [DOI] [PubMed] [Google Scholar]
- 14.Kienle GS, Kiene H. The powerful placebo effect: fact or fiction? J Clin Epidemiol. 1997;50(12):1311-1318. doi: 10.1016/S0895-4356(97)00203-5 [DOI] [PubMed] [Google Scholar]
- 15.Hróbjartsson A, Gøtzsche PC. Is the placebo powerless? update of a systematic review with 52 new randomized trials comparing placebo with no treatment. J Intern Med. 2004;256(2):91-100. doi: 10.1111/j.1365-2796.2004.01355.x [DOI] [PubMed] [Google Scholar]
- 16.Hróbjartsson A, Gøtzsche PC. Is the placebo powerless? an analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344(21):1594-1602. doi: 10.1056/NEJM200105243442106 [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Green S, eds. Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration.. http://handbook-5-1.cochrane.org/. Updated March 2011. Accessed July 7, 2018.
- 18.Higgins JPT, Sterne JAC, Savovic J, et al. . A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst Rev. 2016;10(suppl 1):29-31. [Google Scholar]
- 19.Schuh S, Canny G, Reisman JJ, et al. . Nebulized albuterol in acute bronchiolitis. J Pediatr. 1990;117(4):633-637. doi: 10.1016/S0022-3476(05)80706-1 [DOI] [PubMed] [Google Scholar]
- 20.Lines DR, Kattampallil JS, Liston P. Efficacy of nebulised salbutamol in bronchiolitis. Pediatric Rev Commun. 1990;5:121-129. [Google Scholar]
- 21.Klassen TP, Rowe PC, Sutcliffe T, Ropp LJ, McDowell IW, Li MM. Randomized trial of salbutamol in acute bronchiolitis. J Pediatr. 1991;118(5):807-811. doi: 10.1016/S0022-3476(05)80051-4 [DOI] [PubMed] [Google Scholar]
- 22.Ho L, Collis G, Landau LI, Le Souef PN. Effect of salbutamol on oxygen saturation in bronchiolitis. Arch Dis Child. 1991;66(9):1061-1064. doi: 10.1136/adc.66.9.1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lines DR, Bates ML, Rechtman AR, Sammartino LP. Efficacy of nebulised ipratropium bromide in acute bronchiolitis. Pediatric Rev Commun. 1992;6:161-167. [Google Scholar]
- 24.Schweich PJ, Hurt TL, Walkley EI, Mullen N, Archibald LF. The use of nebulized albuterol in wheezing infants. Pediatr Emerg Care. 1992;8(4):184-188. doi: 10.1097/00006565-199208000-00003 [DOI] [PubMed] [Google Scholar]
- 25.Kristjánsson S, Lødrup Carlsen KC, Wennergren G, Strannegård IL, Carlsen KH. Nebulised racemic adrenaline in the treatment of acute bronchiolitis in infants and toddlers. Arch Dis Child. 1993;69(6):650-654. doi: 10.1136/adc.69.6.650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gadomski AM, Aref GH, el Din OB, el Sawy IH, Khallaf N, Black RE. Oral versus nebulized albuterol in the management of bronchiolitis in Egypt. J Pediatr. 1994;124(1):131-138. doi: 10.1016/S0022-3476(94)70269-1 [DOI] [PubMed] [Google Scholar]
- 27.Gadomski AM, Lichenstein R, Horton L, King J, Keane V, Permutt T. Efficacy of albuterol in the management of bronchiolitis. Pediatrics. 1994;93(6, pt 1):907-912. [PubMed] [Google Scholar]
- 28.Reijonen T, Korppi M, Pitkäkangas S, Tenhola S, Remes K. The clinical efficacy of nebulized racemic epinephrine and albuterol in acute bronchiolitis. Arch Pediatr Adolesc Med. 1995;149(6):686-692. doi: 10.1001/archpedi.1995.02170190096017 [DOI] [PubMed] [Google Scholar]
- 29.Chowdhury D, al Howasi M, Khalil M, al-Frayh AS, Chowdhury S, Ramia S. The role of bronchodilators in the management of bronchiolitis: a clinical trial. Ann Trop Paediatr. 1995;15(1):77-84. doi: 10.1080/02724936.1995.11747752 [DOI] [PubMed] [Google Scholar]
- 30.Chevallier B, Aegerter P, Parat S, Bidat E, Renaud C, Lagardère B. Controlled trial of nebulized salbutamol in children under 6 months of age with acute bronchiotis [in French]. Arch Pediatr. 1995;2(1):11-17. doi: 10.1016/0929-693X(96)89802-2 [DOI] [PubMed] [Google Scholar]
- 31.Van Bever HP, Desager KN, Pauwels JH, Wojciechowski M, Vermeire PA. Aerosolized furosemide in wheezy infants: a negative report. Pediatr Pulmonol. 1995;20(1):16-20. doi: 10.1002/ppul.1950200104 [DOI] [PubMed] [Google Scholar]
- 32.Can D, Inan G, Yendur G, Oral R, Günay I. Salbutamol or mist in acute bronchiolitis. Acta Paediatr Jpn. 1998;40(3):252-255. doi: 10.1111/j.1442-200X.1998.tb01922.x [DOI] [PubMed] [Google Scholar]
- 33.Abul-Ainine A, Luyt D. Short term effects of adrenaline in bronchiolitis: a randomised controlled trial. Arch Dis Child. 2002;86(4):276-279. doi: 10.1136/adc.86.4.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hariprakash S, Alexander J, Carroll W, et al. . Randomized controlled trial of nebulized adrenaline in acute bronchiolitis. Pediatr Allergy Immunol. 2003;14(2):134-139. doi: 10.1034/j.1399-3038.2003.00014.x [DOI] [PubMed] [Google Scholar]
- 35.Khashabi J, Salari S, Karamiyar M, Mussavi H. Comparison of the efficacy of nebulized l-epinephrine, salbutamol and normal saline in acute bronchiolitis: a randomized clinical trial. Med J Islam Repub Iran. 2005;19(2):119-125. [Google Scholar]
- 36.Ralston S, Hartenberger C, Anaya T, Qualls C, Kelly HW. Randomized, placebo-controlled trial of albuterol and epinephrine at equipotent beta-2 agonist doses in acute bronchiolitis. Pediatr Pulmonol. 2005;40(4):292-299. doi: 10.1002/ppul.20260 [DOI] [PubMed] [Google Scholar]
- 37.Karadag B, Ceran O, Guven G, et al. . Efficacy of salbutamol and ipratropium bromide in the management of acute bronchiolitis–a clinical trial. Respiration. 2008;76(3):283-287. doi: 10.1159/000111817 [DOI] [PubMed] [Google Scholar]
- 38.Bar A, Srugo I, Amirav I, Tzverling C, Naftali G, Kugelman A. Inhaled furosemide in hospitalized infants with viral bronchiolitis: a randomized, double-blind, placebo-controlled pilot study. Pediatr Pulmonol. 2008;43(3):261-267. doi: 10.1002/ppul.20765 [DOI] [PubMed] [Google Scholar]
- 39.Plint AC, Johnson DW, Patel H, et al. ; Pediatric Emergency Research Canada (PERC) . Epinephrine and dexamethasone in children with bronchiolitis. N Engl J Med. 2009;360(20):2079-2089. doi: 10.1056/NEJMoa0900544 [DOI] [PubMed] [Google Scholar]
- 40.Tinsa F, Ben Rhouma A, Ghaffari H, et al. . A randomized, controlled trial of nebulized terbutaline for the first acute bronchiolitis in infants less than 12-months-old. Tunis Med. 2009;87(3):200-203. [PubMed] [Google Scholar]
- 41.Skjerven HO, Hunderi JO, Brügmann-Pieper SK, et al. . Racemic adrenaline and inhalation strategies in acute bronchiolitis. N Engl J Med. 2013;368(24):2286-2293. doi: 10.1056/NEJMoa1301839 [DOI] [PubMed] [Google Scholar]
- 42.Anil AB, Anil M, Saglam AB, Cetin N, Bal A, Aksu N. High volume normal saline alone is as effective as nebulized salbutamol–normal saline, epinephrine–normal saline, and 3% saline in mild bronchiolitis. Pediatr Pulmonol. 2010;45(1):41-47. doi: 10.1002/ppul.21108 [DOI] [PubMed] [Google Scholar]
- 43.Ipek IO, Yalcin EU, Sezer RG, Bozaykut A. The efficacy of nebulized salbutamol, hypertonic saline and salbutamol/hypertonic saline combination in moderate bronchiolitis. Pulm Pharmacol Ther. 2011;24(6):633-637. doi: 10.1016/j.pupt.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 44.Scarlett EE, Walker S, Rovitelli A, Ren CL. Tidal breathing responses to albuterol and normal saline in infants with viral bronchiolitis. Pediatr Allergy Immunol Pulmonol. 2012;25(4):220-224. doi: 10.1089/ped.2012.0141 [DOI] [Google Scholar]
- 45.Tinsa F, Abdelkafi S, Bel Haj I, et al. . A randomized, controlled trial of nebulized 5% hypertonic saline and mixed 5% hypertonic saline with epinephrine in bronchiolitis. Tunis Med. 2014;92(11):674-677. [PubMed] [Google Scholar]
- 46.Silver AH, Esteban-Cruciani N, Azzarone G, et al. . 3% Hypertonic saline versus normal saline in inpatient bronchiolitis: a randomized controlled trial. Pediatrics. 2015;136(6):1036-1043. doi: 10.1542/peds.2015-1037 [DOI] [PubMed] [Google Scholar]
- 47.Angoulvant F, Bellêttre X, Milcent K, et al. ; Efficacy of 3% Hypertonic Saline in Acute Viral Bronchiolitis (GUERANDE) Study Group . Effect of nebulized hypertonic saline treatment in emergency departments on the hospitalization rate for acute bronchiolitis: a randomized clinical trial. JAMA Pediatr. 2017;171(8):e171333. doi: 10.1001/jamapediatrics.2017.1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lowell DI, Lister G, Von Koss H, McCarthy P. Wheezing in infants: the response to epinephrine. Pediatrics. 1987;79(6):939-945. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flow of Studies From Database Search Through Meta-Analysis Inclusion
eFigure 2. Risk of Bias by Domain as Assessed by Revised Cochrane Risk-of-Bias Tool for Randomized Trials