Key Points
Question
To what extent does insurance play a role in the risk of later stage at breast cancer diagnosis among racial/ethnic minorities?
Findings
This cross-sectional study of 177 075 women from the SEER database found that nearly half the upstaging at breast cancer diagnosis seen in racial/ethnic minorities is mediated by insurance coverage.
Meaning
The findings suggest that insurance and access to care play an important role in disparities of stage of breast cancer diagnosis.
Abstract
Importance
Compared with non-Hispanic white women, racial/ethnic minority women receive a diagnosis of breast cancer at a more advanced stage and have higher morbidity and mortality with breast cancer diagnosis. Access to care with adequate insurance may be associated with earlier diagnosis, expedited treatment, and improved prognosis.
Objective
To examine the extent to which insurance is associated with access to timely breast cancer diagnosis and breast cancer stage differences among a large, diverse population of US patients with breast cancer.
Design, Setting, and Participants
This retrospective, cross-sectional population-based study used data from the Surveillance, Epidemiology, and End Results Program on 177 075 women aged 40 to 64 years who received a diagnosis of stage I to III breast cancer between January 1, 2010, and December 31, 2016. Statistical analysis was performed from August 1, 2017, to October 1, 2019.
Main Outcomes and Measures
The primary outcome was the risk of having a more advanced stage of breast cancer at diagnosis (ie, stage III vs stages I and II). Mediation analyses were conducted to determine associations of race/ethnicity and proportion of observed differences mediated by health insurance status with earlier stage of diagnosis.
Results
A total of 177 075 women (mean [SD] age, 53.5 [6.8] years; 148 124 insured and 28 951 uninsured or receiving Medicaid) were included in the study. A higher proportion of women either receiving Medicaid or who were uninsured received a diagnosis of locally advanced breast cancer (stage III) compared with women with health insurance (20% vs 11%). In multivariable models, non-Hispanic black (odds ratio [OR], 1.46 [95% CI, 1.40-1.53]), American Indian or Alaskan Native (OR, 1.31 [95% CI, 1.07-1.61]) and Hispanic (OR, 1.35 [95% CI, 1.30-1.42]) women had higher odds of receiving a diagnosis of locally advanced disease (stage III) compared with non-Hispanic white women. When adjusting for health insurance and other socioeconomic factors, associations between race/ethnicity and risk of locally advanced breast cancer were attenuated (non-Hispanic black: OR, 1.29 [95% CI, 1.23-1.35]; American Indian or Alaskan Native: OR, 1.11 [95% CI, 0.91-1.35]; Hispanic: OR, 1.17 [95% CI, 1.12-1.22]). Nearly half (45%-47%) of racial differences in the risk of locally advanced disease were mediated by health insurance.
Conclusions and Relevance
This study’s findings suggest that nearly half of the observed racial/ethnic disparities in higher stage at breast cancer diagnosis are mediated by health insurance coverage.
This cross-sectional study uses data from the Surveillance, Epidemiology, and End Results Program to examine the extent to which insurance is associated with access to timely breast cancer diagnosis and breast cancer stage differences among a large, diverse population of US patients with breast cancer.
Introduction
Today the 5-year survival rate is nearly 100% for stages 0 and I breast cancer, 93% for stage II, and 72% for stage III.1 Despite these positive trends, not all patients have benefited equally.1,2 Compared with non-Hispanic white (NHW) women, racial/ethnic minorities are more likely to receive a diagnosis of later-stage cancer, resulting in higher mortality, higher morbidity from intensive treatment, and poorer overall quality of life.3,4,5,6,7,8 Minority populations experience worse prognosis, more intense treatment, and inferior quality of life in survivorship.9
Racial/ethnic disparities in breast cancer outcomes are both a biological and social problem.10 Both factors must be addressed to achieve cancer health equity.11 We hypothesize that insurance coverage in patients with breast cancer plays a significant role in persistent disparities, given that studies show an association between insurance status and cancer outcomes.12,13,14 Inadequate health insurance is associated with disparities in cancer survival.14 Regular health insurance coverage is a modifiable factor that can significantly address disparities in outcomes among cancer survivors.
To our knowledge, this is the first study to use statistical mediation methods and a large cancer registry database to quantify the extent to which adequate health insurance is a factor in stage of breast cancer diagnosis among a diverse population of women in the United States.
Methods
Selection and Description of Participants
We conducted a retrospective, population-based cross-sectional study between January 1, 2010, and December 31, 2016, using data collected from the Surveillance, Epidemiology, and End Results (SEER) Program registries, including Atlanta, Georgia; Connecticut; Detroit, Michigan; Hawaii; Iowa; New Mexico; San Francisco-Oakland, California; Seattle-Puget Sound, Washington; Utah; Los Angeles, California; San Jose-Monterey, California; rural Georgia; the Alaska Native Tumor Registry; greater California; greater Georgia; Kentucky; Louisiana; and New Jersey.15 The SEER Program, funded by the National Cancer Institute, includes population-based cancer incidence data, such as demographic and clinical information including American Joint Committee on Cancer (AJCC) Stage, hormone receptor (HR), and ERBB2 only (formerly HER2) status, for approximately 28% of the United States population. Data from this period coincide with the availability of routinely collected information on health insurance status for patients who received a diagnosis of a primary cancer. The University of Illinois at Chicago Institutional Review Board reviewed this study and waived approval and informed consent, determining that it did not involve human participants research.
Women ages 40 to 64 years with a diagnosis of AJCC stage I to III first primary invasive breast cancer were included in our study. Women of unknown race/ethnicity (n = 1454) or health insurance status (n = 2921) and those with breast cancer diagnosed by autopsy, per death certificate only, or nonmicroscopically confirmed were all excluded. A total of 177 075 women were included in our final analytic cohort.
Data Collection
We collected demographic information on women at the time of breast cancer diagnosis. Race/ethnicity was coded as NHW, non-Hispanic black (NHB), American Indian or Alaskan Native, Asian or Pacific Islander, and Hispanic (all races). We collected information on adjudicated AJCC stage (I, II, or III), HR (estrogen or progesterone receptor positive, estrogen receptor negative and progesterone receptor positive, and estrogen receptor positive and progesterone receptor negative) and ERBB2 status. Breast cancer subtypes were coded as ERBB2 positive and HR positive, ERBB2 positive and HR negative, ERBB2 negative and HR positive, triple negative, and unknown. Data on selected sociodemographic variables considered to be a priori race outcome or mediator outcome confounders were collected based on county-level attributes ascertained from the 2010-2014 American Community Survey for women diagnosed between 2010 and 2014 and from the 2012-2016 survey for women diagnosed in 2015 and 2016.16 These data included median household income, percentage with less than high school education, percentage living at less than 150% of the federal poverty level, percentage of households with language isolation, and percentage living in urban areas.
Through the National Breast and Cervical Cancer Early Detection Program, uninsured women with a diagnosis of breast cancer can receive Medicaid coverage for their cancer treatment in several states. Because duration of Medicaid coverage was not available, we combined uninsured women and those with Medicaid coverage into 1 group.
Statistical Analysis
Statistical analysis was performed from August 1, 2017, to October 1, 2019. Our approach to conducting these mediation analyses follows the product method approach proposed by Baron and Kenny17 and later described by VanderWeele.18,19 This method estimates the presence of mediation, direct effects, and indirect effects through a series of regression analyses. First, we conducted univariate (equation 1) and multivariable (equation 2) analyses regressing the outcome (ie, locally advanced stage III breast cancer) on the exposure (ie, race/ethnicity) and a priori measured confounders. In our models below, a represents the level of the exposure, m represents fixing the mediator (M) at a constant level, and c are the measured confounders adjusted for in the model:
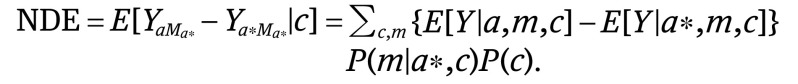 |
We then conducted univariate (equation 3) and multivariate (equation 4) analyses separately, regressing the mediator of interest (ie, health insurance status) on race/ethnicity and a priori measured confounders. Using these estimates, we calculated the natural direct effects (NDE) (equation 2), natural indirect effects (NIE) (equation 4), and total effects (TE) (equation 5).19 The direct effect describes the exposure-outcome association that does not include the mediator (θ) and the indirect effect describes the part of the exposure-outcome association that incorporates the mediator (1 − θ). Although our approach accounted for the possibility of interaction between socioeconomic status (SES) measures and race/ethnicity, we did not find a significant interaction and excluded this from the final model:
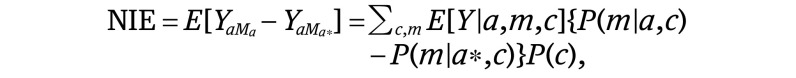 |
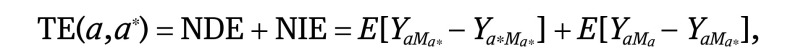 |
To estimate the extent to which the exposure-outcome association is affected by the mediator, we conducted a proportion-mediated calculation (equation 6) using the PARAMED module in Stata, release 14 (Stata Corp).20,21 We used the calculated coefficients from the logistic regression model to characterize the outcome variables and the calculated coefficients from the linear regression model to characterize the mediator variables. Sensitivity analyses were performed examining the mediator as insured or uninsured, not including patients who had Medicaid coverage at diagnosis and/or during treatment. All analyses were conducted using Stata, release 14.21 All P values were from 2-sided tests and results were deemed statistically significant at P < .05.
Results
Our study included a total of 177 075 women aged 40 to 64 years, with a diagnosis of incident, invasive stage I to III breast cancer between 2010 and 2016. Descriptive characteristics of women who had adequate health insurance coverage (n = 148 124) vs women who were uninsured or had Medicaid coverage (n = 28 951) at breast cancer diagnosis are reported in eTable 1 in the Supplement. Compared with women without insurance or with Medicaid, insured women were slightly older at diagnosis (mean [SD], 53.6 [6.8] vs 53.0 [6.8] years), less likely to receive a diagnosis of locally advanced stage III vs stages I or II breast cancer (11% vs 20%) and more likely to receive a diagnosis of luminal A (HR-positive, ERBB2-negative) breast cancer (69% vs 61%). Women also differed by insurance status on county-level socioeconomic attributes. Compared with women who were insured at the time of diagnosis, a higher proportion of women without insurance or with Medicaid coverage were not married (58% vs 29%), living in census tracts with the lowest quintiles of median income (27% vs 19%) and living in census tracts in the highest quintiles of percentage of adults with less than a high school education (31% vs 18%), percentage living at less than 150% of the federal poverty level (26% vs 17%), and percentage living in language isolation (26% vs 19%).
Characteristics of women also differed by race/ethnicity (Table 1). Higher proportions of NHB (17%), American Indian or Alaskan Native (15%), and Hispanic (16%) women were diagnosed at locally advanced stage III (vs stages I or II) compared with NHW (12%) and Asian or Pacific Islander (12%) women. Non-Hispanic white women (89%) had a higher proportion of insurance at the time of diagnosis compared with NHB (75%), American Indian or Alaskan Native (58%), Asian or Pacific Islander (83%), and Hispanic (67%) women.
Table 1. Descriptive Characteristics of Women Aged 40 to 64 Years With a Diagnosis of Incident, Invasive Stage I to III Breast Cancer in the Surveillance, Epidemiology, and End Results Program by Race/Ethnicity, 2010-2016.
| Characteristic at Breast Cancer Diagnosis | Women, No. (%) | ||||
|---|---|---|---|---|---|
| White (n = 113 079) | Black (n = 20 822) | American Indian or Alaskan Native (n = 1098) | Asian or Pacific Islander (n = 18 231) | Hispanic (n = 23 845) | |
| Age, mean (SD), y | 54.0 (6.7) | 53.3 (6.7) | 53.6 (6.8) | 52.6 (6.9) | 52.1 (6.9) |
| 40-44 | 11 763 (10.4) | 2632 (12.6) | 127 (11.6) | 2814 (15.4) | 3945 (16.5) |
| 45-49 | 19 591 (17.3) | 3906 (18.8) | 195 (17.8) | 3834 (21.0) | 5258 (22.1) |
| 50-54 | 24 674 (21.8) | 4679 (22.5) | 243 (22.1) | 3943 (21.6) | 5372 (22.5) |
| 55-59 | 26 681 (23.6) | 4899 (23.5) | 259 (23.6) | 3753 (20.6) | 4842 (20.3) |
| 60-64 | 30 370 (26.9) | 4706 (22.6) | 274 (25.0) | 3887 (21.3) | 4428 (18.6) |
| AJCC stage | |||||
| I | 60 961 (53.9) | 8620 (41.4) | 509 (46.4) | 8914 (48.9) | 10 381 (43.5) |
| II | 39 109 (34.6) | 8654 (41.6) | 427 (38.9) | 7156 (39.3) | 9648 (40.5) |
| III | 13 009 (11.5) | 3548 (17.0) | 162 (14.8) | 2161 (11.9) | 3816 (16.0) |
| Breast cancer subtype | |||||
| HR positive and ERBB2positive | 12 068 (10.7) | 2405 (11.6) | 148 (13.5) | 2352 (12.9) | 2996 (12.6) |
| HR negative and ERBB2 positive | 4723 (4.2) | 1230 (5.9) | 55 (5.0) | 1227 (6.7) | 1326 (5.6) |
| HR positive and ERBB2 negative | 79 575 (70.4) | 11 564 (55.5) | 725 (66.0) | 12 162 (66.7) | 15 186 (63.7) |
| Triple negativea | 10 893 (9.6) | 4407 (21.2) | 113 (10.3) | 1403 (7.7) | 2746 (11.5) |
| Unknown | 5820 (5.1) | 1216 (5.8) | 57 (5.2) | 1087 (6.0) | 1591 (6.7) |
| Marital status | |||||
| Not married | 34 262 (30.3) | 11 622 (55.8) | 461 (42.0) | 4613 (25.3) | 8616 (36.1) |
| Married | 74 333 (65.7) | 8148 (39.1) | 533 (48.5) | 12 898 (70.7) | 14 112 (59.2) |
| Health insurance status | |||||
| Insured | 100 996 (89.3) | 15 524 (74.6) | 634 (57.7) | 15 119 (82.9) | 15 851 (66.5) |
| Uninsured or Medicaid | 12 083 (10.7) | 5298 (25.4) | 464 (42.3) | 3112 (17.1) | 7994 (33.5) |
| ACS County Attributes | |||||
| Median income, quintileb | |||||
| First | 24 216 (21.4) | 7634 (36.7) | 328 (29.9) | 642 (3.5) | 3477 (14.6) |
| Second | 24 749 (21.9) | 5522 (26.5) | 172 (15.7) | 5028 (27.6) | 8721 (36.6) |
| Third | 18 394 (16.3) | 2949 (14.2) | 122 (11.1) | 1377 (7.6) | 3614 (15.2) |
| Fourth | 22 610 (20.0) | 2392 (11.5) | 371 (33.8) | 5267 (28.9) | 4403 (18.5) |
| Fifth | 23 099 (20.4) | 2325 (11.2) | 105 (9.6) | 5917 (32.5) | 3629 (15.2) |
| % With <high school education, quintilec | |||||
| First | 28 287 (25.0) | 2583 (12.4) | 407 (37.1) | 2689 (14.7) | 1692 (7.1) |
| Second | 25 368 (22.4) | 4561 (21.9) | 150 (13.7) | 3920 (21.5) | 2699 (11.3) |
| Third | 20 535 (18.2) | 3632 (17.4) | 174 (15.8) | 4905 (26.9) | 4653 (19.5) |
| Fourth | 21 305 (18.8) | 6365 (30.6) | 181 (16.5) | 2377 (13.0) | 5178 (21.7) |
| Fifth | 17 573 (15.5) | 3681 (17.7) | 186 (16.9) | 4340 (23.8) | 9622 (40.4) |
| % Living at <150% federal poverty level, quintiled | |||||
| First | 26 744 (23.7) | 2248 (10.8) | 123 (11.2) | 6010 (33.0) | 2844 (11.9) |
| Second | 24 177 (21.4) | 2791 (13.4) | 389 (35.4) | 3322 (18.2) | 2921 (12.2) |
| Third | 23 668 (20.9) | 3812 (18.3) | 144 (13.1) | 2987 (16.4) | 4589 (19.2) |
| Fourth | 18 486 (16.3) | 5403 (25.9) | 112 (10.2) | 4771 (26.2) | 8456 (35.5) |
| Fifth | 19 993 (17.7) | 6568 (31.5) | 330 (30.1) | 1141 (6.3) | 5034 (21.1) |
| % Living in language isolation, quintilee | |||||
| First | 28 485 (25.2) | 5422 (26.0) | 155 (14.1) | 494 (2.7) | 925 (3.9) |
| Second | 27 246 (24.1) | 5726 (27.5) | 450 (41.0) | 1651 (9.1) | 1644 (6.9) |
| Third | 22 820 (20.2) | 3961 (19.0) | 216 (19.7) | 3082 (16.9) | 4953 (20.8) |
| Fourth | 19 224 (17.0) | 2068 (9.9) | 176 (16.0) | 6097 (33.4) | 6899 (28.9) |
| Fifth | 15 293 (13.5) | 3645 (17.5) | 101 (9.2) | 6907 (37.9) | 9423 (39.5) |
| % Living in urban area, quintilef | |||||
| First | 28 757 (25.4) | 3609 (17.3) | 575 (52.4) | 644 (3.5) | 1899 (8.0) |
| Second | 26 229 (23.2) | 4103 (19.7) | 197 (17.9) | 2281 (12.5) | 4214 (17.7) |
| Third | 23 030 (20.4) | 272 (1.3) | 163 (14.8) | 2898 (15.9) | 6030 (25.3) |
| Fourth | 23 095 (20.4) | 6448 (31.0) | 125 (11.4) | 8624 (47.3) | 8450 (35.4) |
| Fifth | 11 957 (10.6) | 3910 (18.8) | 38 (3.5) | 3784 (20.8) | 3251 (13.6) |
Abbreviations: ACS, American Community Survey (2010-2014, 2012-2016) county-level variables; AJCC, American Joint Committee on Cancer; HR, hormone receptor.
Triple negative indicates ERBB2, estrogen, and progesterone receptor negative.
Median household income quintiles (range, $19 150-$123 970 in 2014 inflation-adjusted USD).
Percentage with less than high school education (range, 1.3%-53.3%).
Percentage of persons who are below 150% of the federal poverty level (range, 7.0%-69.3%).
Percentage of households in which no one aged 14 years or older speaks English only or speaks English “very well” (range, 0%-38.6%).
Percentage of persons living in census-defined urban areas (range, 0%-100%; https://www.census.gov/prod/cen2010/doc/sf1.pdf).
In multivariable models associating race/ethnicity with risk of locally advanced breast cancer (Table 2), racial/ethnic minority women—including NHB (odds ratio [OR], 1.46; 95% CI, 1.40-1.53), American Indian or Alaskan Native (OR, 1.31 [95% CI, 1.07-1.61]), and Hispanic (OR, 1.35 [95% CI, 1.30-1.42]) women—were more likely to be diagnosed at stage III vs stages I or II after adjustment for age, SEER registry, year of diagnosis, and breast cancer subtype. These associations were attenuated toward the null when we fully adjusted for census tract–level SES factors and health insurance status (NHB: OR, 1.29 [95% CI, 1.23-1.35]; American Indian or Alaskan Native: OR, 1.11 [95% CI, 0.91-1.35]; Hispanic: OR, 1.17 [95% CI, 1.12-1.22]). In multivariable analyses, after adjusting for demographic and clinical characteristics and county-level SES factors (Table 3), racial/ethnic minority women all had between a 2-fold and 4-fold higher odds of being uninsured or having Medicaid at the time of breast cancer diagnosis compared with NHW women (NHB: OR, 2.11 [95% CI, 2.02-2.21]; American Indian or Alaskan Native: OR, 3.46 [95% CI, 2.96-4.06]; Asian or Pacific Islander: OR, 2.32 [95% CI, 2.21-2.44]; Hispanic: OR, 4.21 [95% CI, 4.05-4.38]).
Table 2. Results From Multivariable Logistic Models Associating Race/Ethnicity With Risk of Locally Advanced Breast Cancer.
| AJCC Stage III vs Stages I or II | Crude Model | Adjusted Model | ||||||
|---|---|---|---|---|---|---|---|---|
| Multivariablea | Multivariable and SESb | Multivariable, SES, and Mediatorc | ||||||
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| White | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Black | 1.57 (1.52-1.65) | <.001 | 1.46 (1.40-1.53) | <.001 | 1.36 (1.30-1.42) | <.001 | 1.29 (1.23-1.35) | <.001 |
| American Indian or Alaskan Native | 1.33 (1.13-1.57) | .001 | 1.31 (1.07-1.61) | .01 | 1.24 (1.02-1.52) | .03 | 1.11 (0.91-1.35) | .32 |
| Asian or Pacific Islander | 1.03 (0.99-1.09) | .17 | 0.99 (0.94-1.04) | .67 | 1.01 (0.96-1.07) | .68 | 0.96 (0.90-1.01) | .11 |
| Hispanic | 1.47 (1.40-1.52) | <.001 | 1.35 (1.30-1.42) | <.001 | 1.33 (1.27-1.38) | <.001 | 1.17 (1.12-1.22) | <.001 |
Abbreviations: AJCC, American Joint Committee on Cancer; NA, not applicable; OR, odds ratio; SEER, Surveillance, Epidemiology, and End Results Program; SES, socioeconomic status.
Multivariable model adjusted for age, SEER registry, year of diagnosis, and breast cancer subtype.
Multivariable model above additionally adjusted for marital status and county-level SES variables (median income, educational level, poverty level, language isolation, and urban residence).
Multivariable model above additionally adjusted for the mediator, health insurance status.
Table 3. Results From Multivariable Logistic Models Associating Race/Ethnicity With Health Insurance Status (Uninsured or Medicaid Coverage vs Insured).
| AJCC Stage III vs Stages I or II | Crude Model | Adjusted Model | ||||
|---|---|---|---|---|---|---|
| Multivariablea | Multivariable and SESb | |||||
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| White | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Black | 2.85 (2.75-2.96) | <.001 | 2.85 (2.74-2.96) | <.001 | 2.11 (2.02-2.21) | <.001 |
| American Indian or Alaskan Native | 6.11 (5.42-6.91) | <.001 | 4.37 (3.78-5.05) | <.001 | 3.46 (2.96-4.06) | <.001 |
| Asian or Pacific Islander | 1.72 (1.65-1.80) | <.001 | 1.90 (1.82-1.99) | <.001 | 2.32 (2.21-2.44) | <.001 |
| Hispanic | 4.22 (4.08-4.36) | <.001 | 4.15 (4.00-4.30) | <.001 | 4.21 (4.05-4.38) | <.001 |
Abbreviations: AJCC, American Joint Committee on Cancer; NA, not applicable; OR, odds ratio; SEER, Surveillance, Epidemiology, and End Results Program; SES, socioeconomic status.
Multivariable model adjusted for age, SEER registry, year of diagnosis, and breast cancer subtype.
Multivariable model additionally adjusted for marital status and county-level SES variables (median income, educational level, poverty level, language isolation, and urban residence).
Based on the regression coefficients estimated in our multivariable logistic regression models, eTable 2 in the Supplement reports the calculated natural direct effects, indirect effects, and TE for NHB, American Indian or Alaskan Native, and Hispanic women on the risk of locally advanced stage III breast cancer. The TE was similar to that found in the regression model that treated adequate health insurance status as a mediator (Table 2). Approximately half the observed association with higher stage was explained by being uninsured or receiving Medicaid in NHB (45%), American Indian or Alaskan Native (46%), and Hispanic (47%) women.
In sensitivity analyses that excluded patients with Medicaid coverage, differences in demographic and clinical characteristics were similar to those in our main analyses. Compared with insured women, the women lacking insurance were younger, had higher proportions of NHB and Hispanic women, and presented with later-stage disease. The proportion mediated calculated for NHB, American Indian or Alaskan Native, and Hispanic women were lower compared with our main analysis, between 39% and 41%.
Discussion
Having a more advanced stage at breast cancer diagnosis is a major factor in the disparity between NHB and NHW women in breast cancer mortality and morbidity. Women from racial/ethnic minority populations in the United States present with a more advanced stage of breast cancer. Our study quantifies the extent to which insurance mediates this difference. To our knowledge, this is one of the first studies to apply this novel mediation analysis to a large, nationally representative database to demonstrate that nearly half the disparity between NHB and NHW women in breast cancer stage at diagnosis could be explained by lack of insurance or having Medicaid coverage. Insurance is a modifiable risk factor and having adequate health insurance for all could reduce the persistent racial outcome disparities in breast cancer.
Without insurance coverage, the lack of prevention, screening, and access to care, as well as delays in diagnosis lead to a later stage of disease at diagnosis and thus worse survival.22 However, the consequences of having a later-stage cancer extend beyond 5-year survival statistics, with an extensive body of literature documenting the tangible detriment of delay to diagnosis and treatment in breast cancer.23,24,25 Patients with a diagnosis of later-stage cancer require more intensive treatment and are at higher risk for treatment-associated morbidity and poorer overall quality of life. This finding is especially true for women who receive chemotherapy.26 Studies demonstrate a correlation between history of cancer and future risk for unemployment.27,28 Risk factors for unemployment include having received chemotherapy and undergoing a mastectomy, both of which are more common with later-stage breast cancer (compared with early-stage breast cancer).29 Lack of insurance across the cancer care continuum can negatively influence a patient’s ability to be optimally treated, survive cancer, and live a productive life in survivorship.
Many studies verify the influence of insurance on breast cancer outcomes, but few have been able to quantify this effect. Sineshaw et al30 conducted a similar study among patients in the National Cancer Database with colon cancer that sought to investigate the association of insurance and treatment with disparities. Akin to our results, they demonstrate that insurance coverage was associated with half the survival disparity seen between black and white patients. Insurance coverage is critical at diagnosis, and the ability to maintain insurance across the entire continuum of care is equally essential. Losing insurance coverage compromises completion of care, including adjuvant treatment recommendations, lasting years beyond the initial breast cancer diagnosis. Gorey31 makes the case that the inequity seen in breast cancer outcomes in the United States compared with Canada is owing to Canada’s universal health coverage. A recent study by Jemal et al32 demonstrates the association that the Patient Protection and Affordable Care Act has had with cancer stage at diagnosis. They do not focus on race/ethnicity, but report on the trend toward early-stage diagnosis in low-income patients given adequate insurance. Beyond racial/ethnic minority populations that experience worse outcomes in breast cancer because a lack of insurance, NHW individuals living in rural areas are another group that may also benefit from expansion of insurance coverage. These studies and others demonstrate that insurance coverage has a direct association with breast cancer outcomes.22,33,34
Late stage at diagnosis and lack of health insurance have negative repercussions for patients with cancer and their families. Studies have examined the association of premature cancer-related mortality with lost productivity. Bradley and colleagues35 modeled the cost from premature cancer mortality on annual productivity and estimated the 2020 figures at $147.6 billion. When they factored in lost productivity owing to loss of caregivers, that figure exceeded $308 billion. Therefore, inadequate health insurance coverage also mediates the growing survivorship gap experienced by racial/ethnic minorities with cancer. Thus, the negative effects of late-stage cancer are likely to have lasting repercussions for patients with cancer and their families.
Finally, health care spending is significantly associated with late-stage diagnosis. Mittmann et al36 demonstrated that costs of breast cancer care are increased by stage. Blumen and colleagues37 used insurance claims to determine the cost of treating breast cancer by stage. Stage III breast cancer was 58% more costly to treat than stage I or II breast cancer ($129 387 vs $82 121). Overall, earlier stage at diagnosis of breast cancer is not only beneficial for individual patients and families but also on society as a whole to decrease costs and equity among all populations.
Limitations
This study has limitations associated with our use of data collected from the SEER registries. First, women aged 40 to 64 years sampled for our specific analysis may not be reflective of the general population of women in the United States. The SEER registries include 18 population-based regions, and the data may not be generalizable to other regions not covered by SEER. Information on insurance status collected from cancer registries has been used to conduct epidemiologic studies and inform policy, but others note limitations on the accuracy of insurance status in registry data and the possibility of uninsured patients with cancer enrolling in Medicaid shortly after diagnosis.38,39 Given that we grouped uninsured women and those enrolled in Medicaid together compared with insured women, we believe the outcome of such misclassification in our study to be minimal. In our sensitivity analysis restricted to insured and uninsured women only, the proportion mediated was slightly lower. This finding may indicate that, for women entirely lacking insurance coverage, a considerable amount of the disparity is explained by insurance status but that other important, perhaps unmeasured, factors result in diagnosis of breast cancer at later stages. As with any observational study, unmeasured confounding is possible and our analysis is limited in its measurement of all exposure-outcome and mediator-outcome confounders that may influence our findings.40 Although we are able to control for many sociodemographic factors at the county level as proxies for SES, we were unable to measure individual-level SES factors and other important behavioral factors, such as obesity or parity. Finally, we are not able to describe specific patient-level or provider-level data that could identify and confirm reasons for upstaging of the diagnosis of breast cancer.
Conclusions
In the setting of ongoing and persistent racial/ethnic disparities in breast cancer outcomes, we found that insurance coverage mediates nearly half of the increased risk for later-stage breast cancer diagnosis seen among racial/ethnic minorities. We acknowledge that our findings do not suggest that insurance alone will eliminate racial/ethnic disparities in breast cancer, as studies have demonstrated that equal insurance coverage and access to care will not fix the problem.41 However, the ability to quantify the association that insurance has with breast cancer stage is relevant to potential policy changes regarding insurance and a prioritization of solutions for the increased burden of cancer mortality and morbidity disproportionately placed on racial/ethnic minority populations. Adequate insurance coverage for all patients with cancer is an important consideration and one major systemic change that can be pursued to ameliorate consistent disparities. However, reasons for disparities in breast cancer are multifactorial, with opportunities for research across many disciplines. Although we present insurance as a potential large factor, we acknowledge that there are other factors to account for the remaining percentage. Specifically, there are emerging data to suggest a biological component that could also play a large role. The scope of this problem is large, with many necessary but insufficient solutions. As such, we recommend a comprehensive, multifaceted approach to solve breast cancer disparities. Future studies should continue to examine the direct and indirect costs of inadequate health insurance to patients of all racial/ethnic backgrounds, their families, and society as a whole.
eTable 1. Descriptive Characteristics of Women Aged 40-64 Years Diagnosed with Incident, Invasive Stage I-III Breast Cancer in the Surveillance, Epidemiology, and End Results Program by Health Insurance Status, 2010-2016
eTable 2. Causal Mediation Analyses of Indirect and Direct Effects of Race/Ethnicity on Risk of Locally Advanced Breast Cancer and Proportion Mediated by Health Insurance Status
References
- 1.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67(6):439-448. doi: 10.3322/caac.21412 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30. doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 3.Richardson JL, Langholz B, Bernstein L, Burciaga C, Danley K, Ross RK. Stage and delay in breast cancer diagnosis by race, socioeconomic status, age and year. Br J Cancer. 1992;65(6):922-926. doi: 10.1038/bjc.1992.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halpern MT, Bian J, Ward EM, Schrag NM, Chen AY. Insurance status and stage of cancer at diagnosis among women with breast cancer. Cancer. 2007;110(2):403-411. doi: 10.1002/cncr.22786 [DOI] [PubMed] [Google Scholar]
- 5.Mols F, Vingerhoets AJJM, Coebergh JW, van de Poll-Franse LV. Quality of life among long-term breast cancer survivors: a systematic review. Eur J Cancer. 2005;41(17):2613-2619. doi: 10.1016/j.ejca.2005.05.017 [DOI] [PubMed] [Google Scholar]
- 6.Brawley OW, Berger MZ. Cancer and disparities in health: perspectives on health statistics and research questions. Cancer. 2008;113(7)(suppl):1744-1754. doi: 10.1002/cncr.23800 [DOI] [PubMed] [Google Scholar]
- 7.Hunt BR, Whitman S, Hurlbert MS. Increasing black:white disparities in breast cancer mortality in the 50 largest cities in the United States. Cancer Epidemiol. 2014;38(2):118-123. doi: 10.1016/j.canep.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 8.Hamood R, Hamood H, Merhasin I, Keinan-Boker L. Chronic pain and other symptoms among breast cancer survivors: prevalence, predictors, and effects on quality of life. Breast Cancer Res Treat. 2018;167(1):157-169. doi: 10.1007/s10549-017-4485-0 [DOI] [PubMed] [Google Scholar]
- 9.Yedjou CG, Tchounwou PB, Payton M, et al. Assessing the racial and ethnic disparities in breast cancer mortality in the United States. Int J Environ Res Public Health. 2017;14(5):E486. doi: 10.3390/ijerph14050486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daly B, Olopade OI. A perfect storm: how tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015;65(3):221-238. doi: 10.3322/caac.21271 [DOI] [PubMed] [Google Scholar]
- 11.Reeder-Hayes KE, Anderson BO. Breast cancer disparities at home and abroad: a review of the challenges and opportunities for system-level change. Clin Cancer Res. 2017;23(11):2655-2664. doi: 10.1158/1078-0432.CCR-16-2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayanian JZ, Kohler BA, Abe T, Epstein AM. The relation between health insurance coverage and clinical outcomes among women with breast cancer. N Engl J Med. 1993;329(5):326-331. doi: 10.1056/NEJM199307293290507 [DOI] [PubMed] [Google Scholar]
- 13.Amini A, Jones BL, Yeh N, et al. Disparities in disease presentation in the four screenable cancers according to health insurance status. Public Health. 2016;138:50-56. doi: 10.1016/j.puhe.2016.03.014 [DOI] [PubMed] [Google Scholar]
- 14.Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Trends in cancer survival by health insurance status in California from 1997 to 2014. JAMA Oncol. 2018;4(3):317-323. doi: 10.1001/jamaoncol.2017.3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kagawa-Singer M, Pourat N. Asian American and Pacific Islander breast and cervical carcinoma screening rates and healthy people 2000 objectives. Cancer. 2000;89(3):696-705. doi: [DOI] [PubMed] [Google Scholar]
- 16.US Department of Commerce 2014 ACS 1-year and 2010-2014 ACS 5-year data releases. https://www2.census.gov/programs-surveys/acs/summary_file/2014/documentation/tech_docs/2014_SummaryFile_Tech_Doc.pdf. Accessed December 3, 2019.
- 17.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173-1182. doi: 10.1037/0022-3514.51.6.1173 [DOI] [PubMed] [Google Scholar]
- 18.VanderWeele TJ. Mediation and mechanism. Eur J Epidemiol. 2009;24(5):217-224. doi: 10.1007/s10654-009-9331-1 [DOI] [PubMed] [Google Scholar]
- 19.VanderWeele TJ. Mediation analysis: a practitioner’s guide. Annu Rev Public Health. 2016;37:17-32. doi: 10.1146/annurev-publhealth-032315-021402 [DOI] [PubMed] [Google Scholar]
- 20.Emsley R, Liu H. PARAMED: Stata module to perform causal mediation analysis using parametric regression models In: Baum CF, ed. Statistical Software Components. Boston, MA: Boston College Department of Economics; 2013. [Google Scholar]
- 21.Stata, release 14. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 22.Coburn N, Fulton J, Pearlman DN, Law C, DiPaolo B, Cady B. Treatment variation by insurance status for breast cancer patients. Breast J. 2008;14(2):128-134. doi: 10.1111/j.1524-4741.2007.00542.x [DOI] [PubMed] [Google Scholar]
- 23.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94(5):334-357. doi: 10.1093/jnci/94.5.334 [DOI] [PubMed] [Google Scholar]
- 24.Hershman DL, Wang X, McBride R, Jacobson JS, Grann VR, Neugut AI. Delay of adjuvant chemotherapy initiation following breast cancer surgery among elderly women. Breast Cancer Res Treat. 2006;99(3):313-321. doi: 10.1007/s10549-006-9206-z [DOI] [PubMed] [Google Scholar]
- 25.Gagliato D de M, Gonzalez-Angulo AM, Lei X, et al. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol. 2014;32(8):735-744. doi: 10.1200/JCO.2013.49.7693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganz PA, Desmond KA, Leedham B, Rowland JH, Meyerowitz BE, Belin TR. Quality of life in long-term, disease-free survivors of breast cancer: a follow-up study. J Natl Cancer Inst. 2002;94(1):39-49. doi: 10.1093/jnci/94.1.39 [DOI] [PubMed] [Google Scholar]
- 27.Carlsen K, Ewertz M, Dalton SO, Badsberg JH, Osler M. Unemployment among breast cancer survivors. Scand J Public Health. 2014;42(3):319-328. doi: 10.1177/1403494813520354 [DOI] [PubMed] [Google Scholar]
- 28.Tevaarwerk AJ, Lee JW, Sesto ME, et al. Employment outcomes among survivors of common cancers: the Symptom Outcomes and Practice Patterns (SOAPP) study. J Cancer Surviv. 2013;7(2):191-202. doi: 10.1007/s11764-012-0258-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jagsi R, Hawley ST, Abrahamse P, et al. Impact of adjuvant chemotherapy on long-term employment of survivors of early-stage breast cancer. Cancer. 2014;120(12):1854-1862. doi: 10.1002/cncr.28607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sineshaw HM, Ng K, Flanders WD, Brawley OW, Jemal A. Factors that contribute to differences in survival of black vs white patients with colorectal cancer. Gastroenterology. 2018;154(4):906-915.e7. doi: 10.1053/j.gastro.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorey KM. Breast cancer survival in Canada and the USA: meta-analytic evidence of a Canadian advantage in low-income areas. Int J Epidemiol. 2009;38(6):1543-1551. doi: 10.1093/ije/dyp193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jemal A, Lin CC, Davidoff AJ, Han X. Changes in insurance coverage and stage at diagnosis among nonelderly patients with cancer after the Affordable Care Act. J Clin Oncol. 2017;35(35):3906-3915. doi: 10.1200/JCO.2017.73.7817 [DOI] [PubMed] [Google Scholar]
- 33.Roetzheim RG, Gonzalez EC, Ferrante JM, Pal N, Van Durme DJ, Krischer JP. Effects of health insurance and race on breast carcinoma treatments and outcomes. Cancer. 2000;89(11):2202-2213. doi: [DOI] [PubMed] [Google Scholar]
- 34.Kuzmiak CM, Haberle S, Padungchaichote W, Zeng D, Cole E, Pisano ED. Insurance status and the severity of breast cancer at the time of diagnosis. Acad Radiol. 2008;15(10):1255-1258. doi: 10.1016/j.acra.2008.04.011 [DOI] [PubMed] [Google Scholar]
- 35.Bradley CJ, Yabroff KR, Dahman B, Feuer EJ, Mariotto A, Brown ML. Productivity costs of cancer mortality in the United States: 2000-2020. J Natl Cancer Inst. 2008;100(24):1763-1770. doi: 10.1093/jnci/djn384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mittmann N, Porter JM, Rangrej J, et al. Health system costs for stage-specific breast cancer: a population-based approach. Curr Oncol. 2014;21(6):281-293. doi: 10.3747/co.21.2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blumen H, Fitch K, Polkus V. Comparison of treatment costs for breast cancer, by tumor stage and type of service. Am Health Drug Benefits. 2016;9(1):23-32. [PMC free article] [PubMed] [Google Scholar]
- 38.Sabik LM, Bradley CJ. Understanding the limitations of cancer registry insurance data—implications for policy. JAMA Oncol. 2018;4(10):1432-1433. doi: 10.1001/jamaoncol.2018.2436 [DOI] [PubMed] [Google Scholar]
- 39.Bradley CJ, Gardiner J, Given CW, Roberts C. Cancer, Medicaid enrollment, and survival disparities. Cancer. 2005;103(8):1712-1718. doi: 10.1002/cncr.20954 [DOI] [PubMed] [Google Scholar]
- 40.Richiardi L, Bellocco R, Zugna D. Mediation analysis in epidemiology: methods, interpretation and bias. Int J Epidemiol. 2013;42(5):1511-1519. doi: 10.1093/ije/dyt127 [DOI] [PubMed] [Google Scholar]
- 41.Yood MU, Johnson CC, Blount A, et al. Race and differences in breast cancer survival in a managed care population. J Natl Cancer Inst. 1999;91(17):1487-1491. doi: 10.1093/jnci/91.17.1487 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Descriptive Characteristics of Women Aged 40-64 Years Diagnosed with Incident, Invasive Stage I-III Breast Cancer in the Surveillance, Epidemiology, and End Results Program by Health Insurance Status, 2010-2016
eTable 2. Causal Mediation Analyses of Indirect and Direct Effects of Race/Ethnicity on Risk of Locally Advanced Breast Cancer and Proportion Mediated by Health Insurance Status


