This randomized clinical trial evaluates the safety and efficacy of rimabotulinumtoxinB injections in the management of sialorrhea in adults.
Key Points
Question
Are rimabotulinumtoxinB injections safe and effective for management of sialorrhea in adults?
Findings
In this randomized clinical trial of 187 adults with sialorrhea, rimabotulinumtoxinB injections (2500 U and 3500 U) appeared to statistically significantly reduce sialorrhea vs placebo (treatment effect, −0.30 for both doses vs placebo, P < .001). Therapeutic benefits were seen as early as 1 week after injection and persisted for 11 to 15 weeks.
Meaning
RimabotulinumtoxinB is a safe, effective, and well-tolerated treatment for sialorrhea in adults.
Abstract
Importance
RimabotulinumtoxinB (RIMA) may be preferable as an anti-sialorrhea treatment compared with current oral anticholinergic drugs in people with neurological disorders.
Objective
To assess the safety, efficacy, and tolerability of RIMA injections for the treatment of sialorrhea in adults.
Design, Setting, and Participants
This randomized, parallel, double-blind, placebo-controlled clinical trial of RIMA 2500 U and 3500 U was conducted from November 14, 2013, to January 23, 2017. A total of 249 adult patients with troublesome sialorrhea secondary to any disorder or cause were screened. Of them, 13 refused further participation in the study or were lost to follow-up and 49 did not fulfill the criteria for participation; 187 were ultimately enrolled. Patients had to have a minimum unstimulated salivary flow rate (USFR) of 0.2 g/min and a minimum Drooling Frequency and Severity Scale score of 4.
Exposures
Patients were randomized 1:1:1 to RIMA, 2500 U (n = 63); RIMA, 3500 U (n = 64); or placebo (n = 60).
Main Outcomes and Measures
Primary outcomes were the change in USFR from baseline to week 4 and the Clinical Global Impression of Change (CGI-C) at week 4. The CGI-C scores were recorded on a 7-point scale ranging from very much improved to very much worse. Adverse events were recorded throughout the trial period.
Results
Of 187 patients enrolled (147 men [78.6%]; mean [SD] age, 63.9 [13.3] years), 122 patients had Parkinson disease (65.2%), 13 (7.0%) were stroke survivors, 12 had amyotrophic lateral sclerosis (6.4%), 6 had medication-induced sialorrhea (3.2%), 4 had adult cerebral palsy (2.1%), and 30 had sialorrhea owing to other causes (16.0%). A total of 176 completed the study. Treatment with both doses of RIMA significantly reduced USFR at week 4 vs placebo (mean treatment difference, −0.30 g/min [95% CI, −0.39 to −0.21] for both doses vs placebo, P < .001). The CGI-C scores were statistically significantly improved at week 4 for both treatment groups vs placebo (−1.21 [95% CI, −1.56 to −0.87] for 2500 U, −1.14 [95% CI, −1.49 to −0.80] for 3500 U, both P < .001). Treatment benefits were seen as early as 1 week after injection and were maintained over the treatment cycle of approximately 13 weeks. The RIMA injections were well tolerated compared with placebo. The most common adverse events were self-limited mild to moderate dry mouth, dysphagia, and dental caries.
Conclusions and Relevance
Treatment with RIMA (2500 U and 3500 U) in adults was well tolerated and reduced sialorrhea, with the onset of the effect at 1 week after the injection. These data support the clinical use of RIMA in the management of sialorrhea in adults.
Trial Registration
ClinicalTrials.gov Identifier: NCT01994109
Introduction
Sialorrhea, defined as the excess spillage of saliva from the mouth, is a problematic symptom for many patients with neurological disorders. It affects up to 74% of people with Parkinson disease (PD)1 and is considered to be one of the most bothersome nonmotor symptoms of the disease.2 Up to half of the patients with amyotrophic lateral sclerosis (ALS)3 have sialorrhea; it is also commonly seen in stroke survivors.4 In these conditions, the sialorrhea is usually caused by inefficient elimination of oral saliva rather than increased saliva production. Physical consequences of sialorrhea include perioral chapping, excoriation of the skin around the mouth, oral hygiene problems, speech difficulties, and sleep interruption; in some cases, pooling of saliva at the back of the throat may lead to aspiration pneumonia.5,6 The embarrassment and social stigma associated with sialorrhea can be severe enough to result in social withdrawal.4
Medical treatment of sialorrhea is suboptimal and can be associated with systemic adverse effects. The mainstay of treatment has generally been oral antimuscarinic drugs. However, the use of these agents is often associated with dry mouth and can also cause systemic anticholinergic effects.4,7 Injection of botulinum toxin (BoNT) into the salivary glands inhibits saliva production by cleaving the SNAP receptor (SNARE) protein complex, inhibiting vesicular docking, and reducing the release of acetylcholine at the parasympathetic nerve terminals within the salivary gland.8 The potential use of BoNT therapy for sialorrhea was first reported by Pal and colleagues9 in 2000, who used BoNT type A (BoNT-A) treatment in 9 patients with PD. In 2004, Ondo and colleagues10 showed in a double-blind controlled trial that BoNT Type B (BoNT-B) injected into the parotid and submandibular glands effectively improved sialorrhea without causing dysphagia in patients with PD, and in 2009, Jackson and colleagues3 showed the utility of BoNT-B in patients with ALS. Since then, both BoNT-A and BoNT-B have been popular first-line treatment options,11 and the use of both serotypes incobotulinumtoxinA and rimabotulinumtoxinB (RIMA) was approved in July 2018 and August 2019,12,13 respectively, in the United States for the management of sialorrhea in adults. It has been suggested that BoNT-B may be particularly effective in the management of secretory disorders owing to greater sensitivity of cholinergic autonomic neurons to BoNT-B, especially postganglionic neurons containing M3 receptors (responsible for salivation).14,15,16 This predilection for salivary glands is also suggested clinically by the higher frequency of dry mouth reported as an adverse event (AE) when BoNT-B is injected into the cervical muscles to treat cervical dystonia.17
RimabotulinumtoxinB (MYOBLOC, Solstice Neuroscience) is the only commercially available BoNT-B injection. This phase 3, pivotal clinical trial was conducted to determine the efficacy and safety of RIMA (2500 U and 3500 U) compared with placebo in the treatment of sialorrhea. Both RIMA doses were hypothesized to achieve greater efficacy than placebo in improving sialorrhea at 4 weeks after injection.
Methods
Study Conduct
This randomized, parallel, double-blind, placebo-controlled, 13-week clinical trial was conducted to evaluate the efficacy, safety, and tolerability of RIMA (2500 U and 3500 U) in the management of sialorrhea in adults (NCT01994109). The trial protocol is described in Supplement 1. The study was conducted from November 14, 2013, to January 23, 2017, at 33 sites in the United States, Ukraine, and Russia. Institutional review boards at the participating sites approved the protocol, and the trial was conducted in accordance with the Declaration of Helsinki18 and the International Conference on Harmonization Good Clinical Practice Guidelines.19 All patients provided written informed consent. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Study Population
Adults aged 18 to 85 years were eligible for this study if they reported troublesome sialorrhea for at least 3 months before screening. The sialorrhea could be secondary to any disorder or related to any cause, including but not limited to PD, ALS, stroke, adult cerebral palsy, traumatic brain injury, oral cancer, and adverse effects of other medications. To allow for open-label follow-up, patients had to be able to participate in the study for 1 year or more or 6 months for patients with ALS. Patients had to meet the inclusion criteria of a minimum unstimulated salivary flow rate (USFR)20 of 0.2 g/min and a minimum Drooling Frequency and Severity Scale (DFSS, range 2-9)21 score of 4.
Previous injection of BoNT into the salivary glands was a key exclusion criterion. Prior treatment with a BoNT-A injection at other anatomical sites was allowed provided that there was a treatment interval of 6 months or more and no AEs were reported. Patients diagnosed with moderate or severe dysphagia within the prior 6 months were excluded (Unified Parkinson Disease Rating Scale swallowing assessment rating of ≥3 for patients with PD); however, a percutaneous endoscopic gastrostomy tube for nutritional support in patients with ALS was not exclusionary. Other key exclusion criteria included any known sensitivity to BoNT or any of the RIMA solution components, prior surgery of the salivary glands, history of aspiration, aspiration pneumonia, a respiratory forced vital capacity of less than 20% predicted value, and any clinically significant disease that could affect participation in the study. Concomitant use of aminoglycoside antibiotics, curare-like agents, and warfarin was prohibited. Oral or transdermal pharmacological treatments for sialorrhea had to be discontinued for 30 days prior to baseline injection, although patients with ALS were permitted to use short-acting medications (including anticholinergics with duration <8 hours) to treat sialorrhea provided that they were receiving stable doses for 2 weeks or more prior to randomization.
Randomization and Blinding
Eligible patients were randomized at baseline using a computer-generated scheme in a ratio of 1:1:1 to RIMA, 2500 U; RIMA, 3500 U; or matched placebo using blocks stratified by site. Half of the patients in the placebo group received injection volumes equivalent to the 2500-U dose (total, 0.5 mL) and the other half received injection volumes equivalent to the 3500-U dose (total, 0.7 mL). To protect the double-blinding, injection syringes were prepared by personnel blinded to other study-related activities.
Study Design
After a screening visit, patients received study treatment on day 1 and were followed up by telephone within 24 hours after study injection. Thereafter, double-blind assessments were made at weeks 1, 2, 4, 8, and 11 to 15 weeks, which was the last observed visit (LOV). After completion of the study, patients were eligible to continue in a reinjection phase for up to 1 year.
Study Medications
Active medication was provided in a single-use, 1.0-mL glass vial containing 5000 U of RIMA per milliliter in human serum albumin, 0.05%, 0.01M sodium succinate, and 0.1M sodium chloride at a pH of 5.6. Matched placebo was supplied in identical vials containing identical excipients and matched pH.
Injections were guided by external anatomical landmarks first into the submandibular glands (right and then left) followed by the parotid glands using 4 separate syringes with needles (1.0-mL syringes with 0.5-inch, 30-G needles). Patients randomized to the RIMA 2500-U group received 250 U (0.05 mL) in each submandibular gland and 1000 U (0.2 mL) in each parotid gland. Patients randomized to the RIMA 3500-U group received 250 U (0.05 mL) in each submandibular gland and 1500 U (0.3 mL) in each parotid gland. Patients randomized to matched placebo received injection volumes equivalent to the 2500-U (total, 0.5 mL) and 3500-U (total, 0.7 mL) groups. At preselected trial sites that had prior experience using ultrasonography-guided salivary gland injection, half of the patients who had received injections had confirmation of needle placement using ultrasonography.
Assessments
The primary efficacy end point was the USFR change. The USFR was determined in an unstimulated state (ie, at least 1 hour postprandial) just before injection of the study medication. Patients cleared their mouths of excess saliva and then tilted their head forward to allow the saliva to pool in the floor of their mouths. They then expectorated any saliva that accumulated in their mouths into a preweighed cup during a 5-minute collection period. The other primary end point was the Clinical Global Impressions of Change (CGI-C), which was assessed before saliva collection for USFR. The CGI-C was assessed at week 1 and then at every subsequent visit using a 7-item scale (from very much improved to very much worse).
Secondary end points included Clinical Global Impressions of Severity (CGI-S) assessed by a blinded rater on a 7-item scale (from normal to among the most extremely ill) at baseline before study drug administration and at each subsequent study visit; patient-rated global impressions of change (PGI-C) and severity (PGI-S); the Drooling Frequency and Severity Scale by investigator (DFSS-I) and patient (DFSS-P) based on the 4 weeks prior to assessment (range 2-9; frequency assessed as never, occasional, frequent, or constant; severity assessed as dry, mild, moderate, severe, or profuse); and the patient-rated Drooling Impact Score (DIS) (range 10-40, eTable 1 in Supplement 2).
Safety was assessed throughout the trial, and AE reports, vital signs, and laboratory test results were collected at each visit. Prespecified AEs of special interest were aspiration, aspiration pneumonia, choking, dysphagia, and symptoms suggesting distant spread of toxin effect. Patients also underwent a scheduled dental examination by an independent dentist at screening, week 4, and week 13.
Statistical Analysis
The safety population included all randomized patients who received a study injection. Efficacy was assessed in a modified intent-to-treat (mITT) population, which included all patients in the safety population who had 1 or more postinjection measurement recorded for both USFR and CGI-C up to week 4.
The primary efficacy end points, USFR change from baseline and CGI-C score at week 4, were compared by dose group using a 2-way analysis of covariance (ANCOVA) model that included dose group and site as factors and the corresponding baseline value as a covariate. For both ANCOVAs, the null hypothesis was not different among the 3 treatment groups, with the alternative of nonzero differences among them. The type I error rate for rejecting the null hypothesis was set at α = .05. Missing values at week 4 were imputed using the last observation carried forward. To avoid inflation of type I errors, pairwise comparisons between the active doses and placebo were performed with t tests using a hierarchical closed-test procedure by first comparing the 3500-U group vs placebo and then the 2500-U group vs placebo.
Secondary efficacy measures were analyzed using the same ANCOVA approach as described for the primary efficacy analysis. The proportion of treatment responders was compared between each active dose vs placebo using a Cochran-Mantel-Haenszel test. Onset of RIMA efficacy was defined as the point when the difference between the active dose vs placebo first became significant (ie, P < .05), and the duration of effectiveness was defined as the last assessment time with P < .05. In addition, subgroup analyses were performed to evaluate the effect of injection technique (external anatomical landmarks with confirmation on ultrasonography vs external anatomical landmarks alone), cause of sialorrhea (PD vs other), severity of illness, sex, and age group (<65 vs ≥65 years).
Assuming a dropout rate of 10%, a sample size of 59 patients per treatment group was expected to provide 80% power to detect a difference between RIMA and placebo of at least 0.55 (effect size) for both primary end points of USFR and CGI-C at the level of α = .05 (2-sided) based on a 2-sample t test. Anticipating a screen failure rate in the 25% to 35% range, we aimed at screening 240 patients.
Results
Patient Disposition
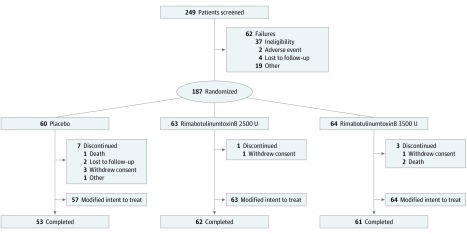
A total of 249 adult patients with troublesome sialorrhea secondary to any disorder or cause were screened. Of them, 13 refused further participation in the study or were lost to follow-up and 49 did not fulfill the criteria for participation. One hundred eighty-seven patients were enrolled and randomized 1:1:1 to receive RIMA, 2500 U (n = 63); RIMA, 3500 U (n = 64); or placebo (n = 60). The sample included 122 patients with PD (65.2%), 13 stroke survivors (7.0%), 12 with amyotrophic lateral sclerosis (6.4%), 6 with medication-induced sialorrhea (3.2%), 4 with adult cerebral palsy (2.1%), and 30 with sialorrhea owing to other causes (16.0%). Of the patients who were randomized, 176 (94.1%) completed the double-blind phase (Figure 1). The safety and mITT populations were identical for the RIMA treatment groups, whereas 3 patients with PD in the placebo group were excluded from the mITT population because they had no postbaseline USFR or CGI-C measures through week 4. Overall, 47 patients (25.5%) had confirmation on ultrasonography of their anatomically determined injection site.
Figure 1. CONSORT Diagram.
Three patients in the placebo group were included in the safety population but excluded from the modified intent-to-treat analyses because they had no postbaseline unstimulated salivary flow rate or Clinical Global Impression of Change measures up to or at week 4.
Baseline Characteristics
Baseline characteristics for the 184 patients in the mITT population were generally comparable between groups (Table 1). There was an overall predominance of men (144 [78.6%]), and the mean (SD) age of all patients was 63.9 (13.3) years. A smaller proportion of patients had a diagnosis of PD in the RIMA 3500-U group (38 [59.4%]) vs the other 2 treatment groups (RIMA, 2500 U, 43 [68.3%]; placebo, 38 [66.7%]). Patients had a mean USFR of 0.64 to 0.66 g/min and DFSS score ranging from 6.46 to 6.60, indicating moderate to severe drooling.
Table 1. Demographic and Baseline Disease Characteristics in the Modified Intent-to-Treat Population.
| Parameter | Treatment Groups | ||
|---|---|---|---|
| Placebo (n = 57) | RIMA 2500 U (n = 63) | RIMA 3500 U (n = 64) | |
| Male, No. (%) | 41 (71.9) | 48 (76.2) | 55 (85.9) |
| Female, No. (%) | 16 (28.1) | 15 (23.8) | 9 (14.1) |
| Age, mean (SD), y | 64.1 (13.1) | 62.6 (13.0) | 64.6 (14.0) |
| Race/ethnicity, No. (%) | |||
| White | 55 (96.5) | 60 (95.2) | 60 (93.8) |
| Asian | 1 (1.8) | 1 (1.6) | 0 |
| Black/African American | 1 (1.8) | 2 (3.2) | 1 (1.6) |
| Other | 0 | 3 (4.7) | 0 |
| Diagnosis, No. (%) | |||
| Parkinson disease | 38 (66.7) | 43 (68.3) | 38 (59.4) |
| Stroke | 4 (7.0) | 4 (6.3) | 5 (7.8) |
| ALS | 4 (7.0) | 4 (6.3) | 4 (6.3) |
| Medication induced | 2 (3.5) | 2 (3.2) | 2 (3.1) |
| Adult cerebral palsy | 1 (1.8) | 2 (3.2) | 1 (1.6) |
| Othera | 8 (14.0) | 8 (12.7) | 14 (21.9) |
| Previous treatment for sialorrhea, No. (%) | 6 (10.5) | 8 (12.7) | 12 (18.8) |
| Years since sialorrhea diagnosis, mean (SD) | 2.8 (3.6) | 3.7 (7.4) | 3.1 (3.6) |
| Unstimulated salivary flow rate, mean (SD), g/min | 0.65 (0.55) | 0.66 (0.52) | 0.64 (0.40) |
| Drooling Frequency and Severity Scale, mean (SD) | 6.60 (1.19) | 6.46 (1.39) | 6.58 (1.33) |
Abbreviations: ALS, amyotrophic lateral sclerosis; RIMA, rimabotulinumtoxinB.
Other causes occurred in more than 2 patients in any group.
Primary End Points
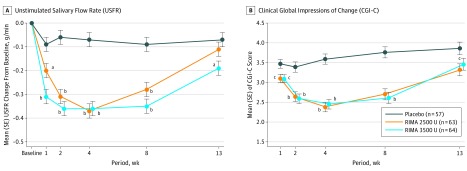
Both doses of RIMA (2500 U and 3500 U) statistically significantly reduced USFR vs placebo at 4 weeks after injection. The treatment difference of −0.30 g/min (95% CI, −0.39 to −0.21; both P < .001) was identical in both RIMA groups vs placebo (Figure 2A). The mean CGI-C improved at week 4 after injection in both RIMA groups vs placebo; the treatment difference was −1.21 (95% CI, −1.56 to −0.87; P < .001) for the 2500-U group vs placebo and −1.14 (95% CI, −1.49 to −0.80; P < .001) for the 3500-U group vs placebo (Figure 2B).
Figure 2. Least Squares Mean Changes From Baseline.
Analysis of covariance model including dosage group and site as factors and the corresponding baseline value as a covariate. RIMA indicates rimabotulinumtoxinB. Error bars indicate the SE.
aP < .01 vs placebo.
bP < .001 vs placebo.
cP < .05 vs placebo
Secondary End Points
The reductions in USFR were observed with both RIMA doses compared with placebo beginning at week 1 and continuing through week 8. The significant treatment effect of the RIMA 3500-U dose, but not the 2500-U dose, continued through to the LOV (week 11 to 15). Compared with placebo, the mean adjusted CGI-C scores also improved with RIMA beginning at week 1 (treatment difference, −0.38 vs placebo for the 2500-U dose, P = .01; −0.37 vs placebo for the 3500-U dose, P = .01) and continuing through week 8 (treatment differences of −1.06 and −1.15 vs placebo, P < .001 for both the 2500-U and 3500-U doses, respectively). However, although the significant treatment effect of the 2500-U dose on CGI-C continued through to the study end (treatment difference of −0.54 vs placebo, P = .0146), the effects of RIMA, 3500 U, were not significant at the LOV. Investigator ratings of severity (CGI-S and DFFS-I) also showed considerable improvements with both RIMA doses compared with placebo (eTable 2 in Supplement 2).
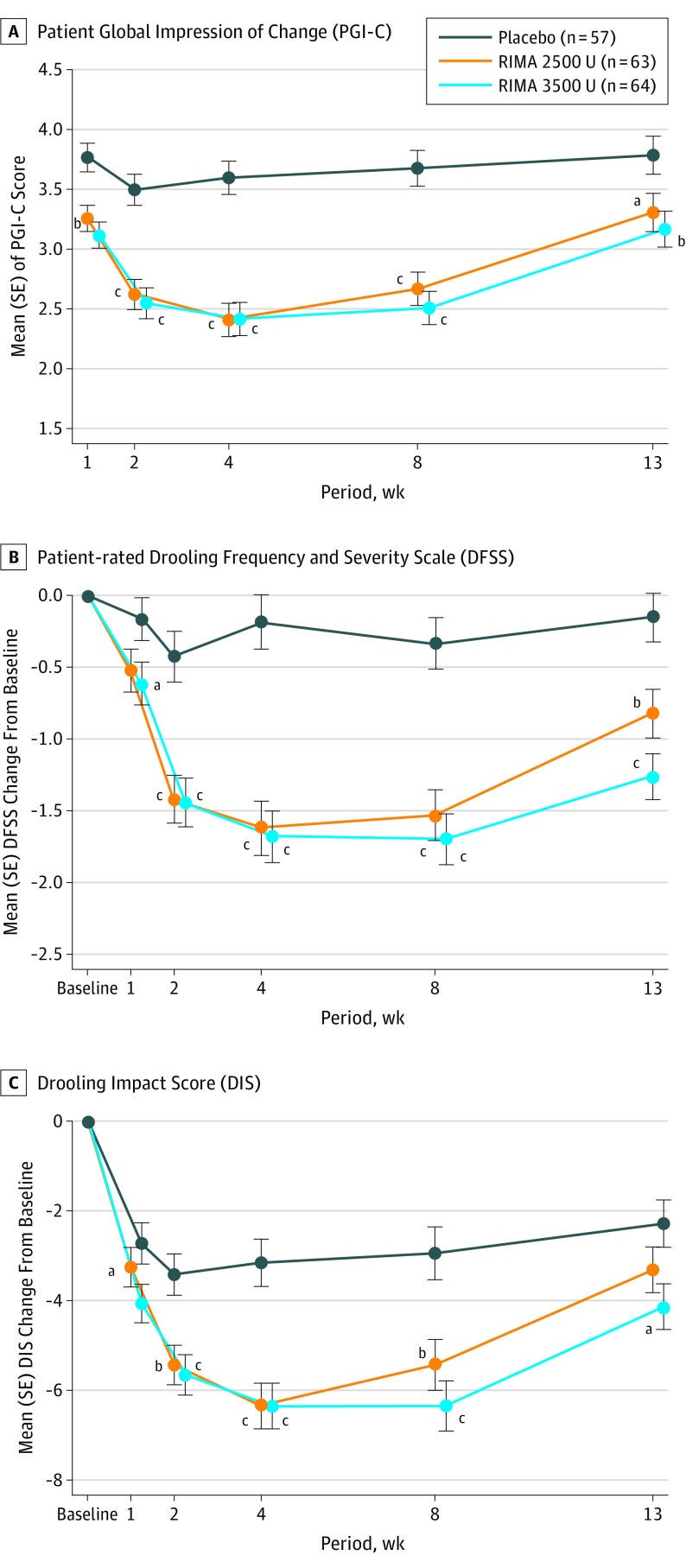
Patient-reported outcome measures mirrored the primary efficacy findings. Patient ratings of overall disease severity (PGI-C and DFSS-P) and treatment effect (DIS) demonstrated early improvements with both RIMA doses compared with placebo (Figure 3). A greater proportion of participants rated themselves on PGI-C as improved or very much improved with RIMA, 2500 U, compared with placebo beginning at week 2 and continuing through week 8. For the 3500-U dose, significance on PGI-C vs placebo was noted as early as week 1 and was maintained through to LOV.
Figure 3. Secondary End Points: Changes From Baseline in Patient-Reported Outcomes.
Analysis of covariance model including dose group and site as factors and the corresponding baseline value as a covariate. RIMA indicates rimabotulinumtoxinB. Error bars indicate the SE.
aP < .05 vs placebo.
bP < .01 vs placebo.
cP < .001 vs placebo.
Safety and Tolerability
Most patients in each RIMA group experienced 1 or more AEs, which were usually mild or moderate in intensity. The most common AEs with RIMA treatment were dry mouth, dysphagia, and dental caries (Table 2). Reported AEs were generally considered treatment related (2500-U RIMA, 34 of 63 [54.0%]; 3500 U-RIMA, 36 of 64 [56.3%]; and placebo, 12 of 60 [20.0%]). One event of moderate dry mouth but no events of dysphagia led to study discontinuation. One patient in the 2500-U group had severe dry mouth (resolved), and 1 patient in the placebo group had severe dysphagia (ongoing at end of study).
Table 2. Summary of Treatment-Emergent Adverse Events.
| Adverse Event | Treatment Group | ||
|---|---|---|---|
| Placebo (n = 60) | RIMA 2500 U (n = 63) | RIMA 3500 U (n = 64) | |
| Patients, No. (%) | |||
| With ≥1 adverse event | 26 (43.3) | 46 (73.0) | 44 (68.8) |
| With a serious adverse event | 4 (6.7) | 1 (1.6) | 4 (6.3) |
| With adverse event leading to discontinuation | 2 (3.3) | 0 | 4 (6.3) |
| Death, No. (%) | 1 (1.7) | 0 | 2 (3.1) |
| Adverse events affecting >5% in any group, No. (%) | |||
| Dry mouth | 5 (8.3) | 24 (38.1) | 29 (45.3) |
| Dysphagia | 1 (1.7) | 7 (11.1) | 3 (4.7) |
| Dental caries | 2 (3.3) | 5 (7.9) | 3 (4.7) |
| Fall | 4 (6.7) | 4 (6.3) | 3 (4.7) |
| Dizziness | 0 | 4 (6.3) | 0 |
| Cough | 4 (6.7) | 0 | 0 |
Abbreviation: RIMA, rimabotulinumtoxinB.
The number of serious AEs was similar between the 3 groups. Serious AEs were reported for 1 patient treated with RIMA, 2500 U (urinary tract infection); 4 patients treated with RIMA, 3500 U (syncope, aspiration pneumonia, metastatic brain cancer resulting in death, and pneumonia and cardio-respiratory arrest resulting in death); and 4 patients treated with placebo (urosepsis, nodular melanoma, small-bowel obstruction, and cardiac arrest resulting in death). Only the single serious events of moderate aspiration pneumonia (which resolved) and severe pneumonia were considered to be possibly related to treatment (with RIMA, 3500 U). The serious event of severe pneumonia was judged to have resolved; however, the patient who had a history of diabetes mellitus, hypertension, and transient ischemic attack died 14 days later owing to cardiorespiratory arrest. Choking was always considered as possibly or probably related to treatment and was reported at similar rates with RIMA, 2500 U, and placebo (both n = 2) and was not reported in the RIMA 3500-U group. No clinically relevant changes were noted in laboratory measures or vital signs; there was no statistically significant change in respiratory forced vital capacity.
Subgroup analyses of the primary end points (USFR and CGI-C) showed that the efficacy and safety of RIMA was not affected by the injection technique (ie, confirmation of needle placement on ultrasonography), cause of sialorrhea (PD vs non-PD), severity of illness, sex, or age (eFigure 1 and eFigure 2 in Supplement 2).
Discussion
This phase 3 trial showed that both RIMA treatment groups significantly reduced saliva production and reported drooling compared with placebo. This reduction was deemed to be clinically relevant per clinician- and patient-reported validated outcomes. Therapeutic effects of RIMA for troublesome sialorrhea were observed as early as week 1 after injection, and the treatment benefits were maintained over the duration of the treatment cycle. The observed effect size was higher than anticipated at around 0.9 for USFR and as high as 1.2 for CGI-C. Injections of RIMA were generally well tolerated, with a satisfactory safety profile.
In current clinical practice, patients are often treated empirically with anticholinergic therapies, including sublingual atropine drops, oral glycopyrrolate, oral benztropine, and oral or transdermal scopolamine.22 However, evidence supporting the use of these medications is limited,23 and their associated risks, including constipation, bradycardia, cognitive impairment, drowsiness, and urinary retention, can be especially problematic in patients with neurological diseases.22,23 The lack of these AEs in this study suggests that RIMA may be preferable as an antisialorrhea treatment compared with current oral anticholinergic drugs in people with neurological disorders.
Evidence-based reviews have concluded that there is level B evidence for the safety and efficacy of abobotulinumtoxinA and onabotulinumtoxinA in managing sialorrhea.23 Recently, the US Food and Drug Administration approved the use of incobotulinumtoxinA for treating sialorrhea in adults based on the results of a phase 3 study, which also demonstrated efficacy, safety, and tolerability.24 The efficacy of RIMA has already been demonstrated in a number of small, randomized, placebo-controlled studies (most focusing on PD),3,10,25,26,27 but confirmation of the findings in a large prospective study of adequate power was lacking. Like the incobotulinumtoxinA study,24 the present study mostly comprised patients with PD, but in contrast to that pivotal study, we also included patients with ALS, medication (neuroleptic)-induced sialorrhea, and other causes, such as adult cerebral palsy and oral cancer. Although the sample sizes for each individual cause were too small to analyze separately, no significant differences were seen in the efficacy or safety of RIMA in patients with PD vs those with other causes. One head-to-head crossover study comparing RIMA with abobotulinumtoxinA found equivalent efficacy in controlling sialorrhea but a quicker onset of action after RIMA injections.28 In that study of 29 patients with PD or ALS, the mean latency to RIMA benefit was 3.2 days, which was an earlier time than was measured in the present study.28 The earliest USFR time reported in the recent study with incobotulinumtoxinA was at 4 weeks, although the effects on patient impression as early as 1 week (nonsignificant vs placebo) were also reported.24
The RIMA injection was generally well tolerated in this study. The AEs experienced by patients suggest little distant systemic spread or local diffusion after intraglandular injection of RIMA. The most common AE was dry mouth. Most instances of dry mouth were mild to moderate in severity and only led to 1 patient discontinuing the study treatment. The safety and tolerability profile was generally similar between patients with different causes of sialorrhea, including ALS, for which disease-related dysphagia is associated with a potentially higher risk for BoNT chemodenervation. Dental caries was reported at a higher incidence than in previous studies, which may reflect the close supervision and dedicated scrutiny of patients by independent dentists at the participating sites. Studies of children who are neurologically impaired have shown a direct association of BoNT-A treatment for sialorrhea with dental caries.29 However, it should also be noted that the frequency of untreated caries and periodontal disease is reported to be higher in patients with PD (65% of enrolled patients) than in age-matched healthy control individuals.30
Strengths and Limitations
Strengths of this study are its size, inclusive population in terms of cause of sialorrhea, and the use of blinded raters. Efficacy was consistently demonstrated across a range of objective and subjective outcome measures that were rated by both investigators and patients. Limitations include the fixed-dose design and predominance of white participants. Herein we report the primary results for a single treatment cycle; questions regarding continued efficacy and safety, including potential immunogenicity, require evaluation over several cycles. The relative importance of ultrasonography guidance may also change as the size of glands potentially decreases owing to atrophy with repeated injections over time.31 Results for the open-label extension of this study and for another open-label study will be reported separately. The present study was not intended to show a significant difference between the 2 RIMA doses tested, and we did not see a clear differentiation of safety or efficacy at the 2 doses studied. One small study previously demonstrated a dose-related efficacy between 1500 U and 3500 U25; flexibility in doses is warranted given that the 3500 U dose lasted longer and was well tolerated. Other important clinical questions such as best dose distribution between the submandibular and parotid glands, head-to-head comparisons with BoNT-A formulations, and the association of treatment with quality of life also merit further investigation.
Conclusions
This study provides class I evidence that treatment with RIMA (2500 U and 3500 U) safely reduced saliva production as early as 1 week after injection. These data support the clinical use of RIMA for management of troublesome sialorrhea in adult patients.
Trial Protocol
eTable 1. Drooling Impact Scale
eTable 2. Secondary Efficacy Measures
eFigure 1. Comparison of Subgroups for Unstimulated Salivary Flow Rate (USFR) at Week 4
eFigure 2. Comparison of Subgroups for Clinical Global Impression of Change (CGI-C) at Week 4
Data Sharing Statement
References
- 1.Kalf JG, de Swart BJ, Borm GF, Bloem BR, Munneke M. Prevalence and definition of drooling in Parkinson’s disease: a systematic review. J Neurol. 2009;256(9):1391-1396. doi: 10.1007/s00415-009-5098-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Politis M, Wu K, Molloy S, G Bain P, Chaudhuri KR, Piccini P. Parkinson’s disease symptoms: the patient’s perspective. Mov Disord. 2010;25(11):1646-1651. doi: 10.1002/mds.23135 [DOI] [PubMed] [Google Scholar]
- 3.Jackson CE, Gronseth G, Rosenfeld J, et al. ; Muscle Study Group . Randomized double-blind study of botulinum toxin type B for sialorrhea in ALS patients. Muscle Nerve. 2009;39(2):137-143. doi: 10.1002/mus.21213 [DOI] [PubMed] [Google Scholar]
- 4.Hockstein NG, Samadi DS, Gendron K, Handler SD. Sialorrhea: a management challenge. Am Fam Physician. 2004;69(11):2628-2634. [PubMed] [Google Scholar]
- 5.Postma AG, Heesters M, van Laar T. Radiotherapy to the salivary glands as treatment of sialorrhea in patients with parkinsonism. Mov Disord. 2007;22(16):2430-2435. doi: 10.1002/mds.21752 [DOI] [PubMed] [Google Scholar]
- 6.Leibner J, Ramjit A, Sedig L, et al. The impact of and the factors associated with drooling in Parkinson’s disease. Parkinsonism Relat Disord. 2010;16(7):475-477. doi: 10.1016/j.parkreldis.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 7.Potulska A, Friedman A. Controlling sialorrhoea: a review of available treatment options. Expert Opin Pharmacother. 2005;6(9):1551-1554. doi: 10.1517/14656566.6.9.1551 [DOI] [PubMed] [Google Scholar]
- 8.Bhidayasiri R, Truong DD. Expanding use of botulinum toxin. J Neurol Sci. 2005;235(1-2):1-9. doi: 10.1016/j.jns.2005.04.017 [DOI] [PubMed] [Google Scholar]
- 9.Pal PK, Calne DB, Calne S, Tsui JK. Botulinum toxin A as treatment for drooling saliva in PD. Neurology. 2000;54(1):244-247. doi: 10.1212/WNL.54.1.244 [DOI] [PubMed] [Google Scholar]
- 10.Ondo WG, Hunter C, Moore W. A double-blind placebo-controlled trial of botulinum toxin B for sialorrhea in Parkinson’s disease. Neurology. 2004;62(1):37-40. doi: 10.1212/01.WNL.0000101713.81253.4C [DOI] [PubMed] [Google Scholar]
- 11.Vashishta R, Nguyen SA, White DR, Gillespie MB. Botulinum toxin for the treatment of sialorrhea: a meta-analysis. Otolaryngol Head Neck Surg. 2013;148(2):191-196. doi: 10.1177/0194599812465059 [DOI] [PubMed] [Google Scholar]
- 12.Drugs@FDA. Highlights of prescribing information: xeomin. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125360s074lbl.pdf. Accessed December 2, 2019.
- 13.Drugs@FDA. FDA approved drug products: BLA 103846. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=103846. Accessed December 2, 2019.
- 14.Arezzo JC. NeuroBloc/Myobloc: unique features and findings. Toxicon. 2009;54(5):690-696. doi: 10.1016/j.toxicon.2009.03.009 [DOI] [PubMed] [Google Scholar]
- 15.Bentivoglio AR, Del Grande A, Petracca M, Ialongo T, Ricciardi L. Clinical differences between botulinum neurotoxin type A and B. Toxicon. 2015;107(pt A):77-84. doi: 10.1016/j.toxicon.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 16.Dashtipour K, Bhidayasiri R, Chen JJ, Jabbari B, Lew M, Torres-Russotto D. RimabotulinumtoxinB in sialorrhea: systematic review of clinical trials. J Clin Mov Disord. 2017;4:9. doi: 10.1186/s40734-017-0055-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Comella CL, Jankovic J, Shannon KM, et al. ; Dystonia Study Group . Comparison of botulinum toxin serotypes A and B for the treatment of cervical dystonia. Neurology. 2005;65(9):1423-1429. doi: 10.1212/01.wnl.0000183055.81056.5c [DOI] [PubMed] [Google Scholar]
- 18.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 19.Dixon JR. The International Conference on Harmonization Good Clinical Practice guideline. Qual Assur. 1998;6(2):65-74. doi: 10.1080/105294199277860 [DOI] [PubMed] [Google Scholar]
- 20.Proulx M, de Courval FP, Wiseman MA, Panisset M. Salivary production in Parkinson’s disease. Mov Disord. 2005;20(2):204-207. doi: 10.1002/mds.20189 [DOI] [PubMed] [Google Scholar]
- 21.Thomas-Stonell N, Greenberg J. Three treatment approaches and clinical factors in the reduction of drooling. Dysphagia. 1988;3(2):73-78. doi: 10.1007/BF02412423 [DOI] [PubMed] [Google Scholar]
- 22.McGeachan AJ, Mcdermott CJ. Management of oral secretions in neurological disease. Pract Neurol. 2017;17(2):96-103. doi: 10.1136/practneurol-2016-001515 [DOI] [PubMed] [Google Scholar]
- 23.Lakraj AA, Moghimi N, Jabbari B. Sialorrhea: anatomy, pathophysiology and treatment with emphasis on the role of botulinum toxins. Toxins (Basel). 2013;5(5):1010-1031. doi: 10.3390/toxins5051010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jost WH, Friedman A, Michel O, et al. SIAXI: placebo-controlled, randomized, double-blind study of incobotulinumtoxinA for sialorrhea. Neurology. 2019;92(17):e1982-e1991. doi: 10.1212/WNL.0000000000007368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chinnapongse R, Gullo K, Nemeth P, Zhang Y, Griggs L. Safety and efficacy of botulinum toxin type B for treatment of sialorrhea in Parkinson’s disease: a prospective double-blind trial. Mov Disord. 2012;27(2):219-226. doi: 10.1002/mds.23929 [DOI] [PubMed] [Google Scholar]
- 26.Lagalla G, Millevolte M, Capecci M, Provinciali L, Ceravolo MG. Long-lasting benefits of botulinum toxin type B in Parkinson’s disease-related drooling. J Neurol. 2009;256(4):563-567. doi: 10.1007/s00415-009-0085-1 [DOI] [PubMed] [Google Scholar]
- 27.Steinlechner S, Klein C, Moser A, Lencer R, Hagenah J. Botulinum toxin B as an effective and safe treatment for neuroleptic-induced sialorrhea. Psychopharmacology (Berl). 2010;207(4):593-597. doi: 10.1007/s00213-009-1689-y [DOI] [PubMed] [Google Scholar]
- 28.Guidubaldi A, Fasano A, Ialongo T, et al. Botulinum toxin A versus B in sialorrhea: a prospective, randomized, double-blind, crossover pilot study in patients with amyotrophic lateral sclerosis or Parkinson’s disease. Mov Disord. 2011;26(2):313-319. doi: 10.1002/mds.23473 [DOI] [PubMed] [Google Scholar]
- 29.Ferraz Dos Santos B, Dabbagh B, Daniel SJ, Schwartz S. Association of onabotulinum toxin A treatment with salivary pH and dental caries of neurologically impaired children with sialorrhea. Int J Paediatr Dent. 2016;26(1):45-51. doi: 10.1111/ipd.12156 [DOI] [PubMed] [Google Scholar]
- 30.Cicciù M, Risitano G, Lo Giudice G, Bramanti E. Periodontal health and caries prevalence evaluation in patients affected by Parkinson’s disease. Parkinsons Dis. 2012;2012:541908. doi: 10.1155/2012/541908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Younis RE, Abou Elkhier M, Mourad M, Elnahas W. Ultrastructural changes in the parotid gland of rats after intraglandular injection of botulinum toxin A. Ann Oral & Maxillofac Surg. 2013;1(4):38. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Drooling Impact Scale
eTable 2. Secondary Efficacy Measures
eFigure 1. Comparison of Subgroups for Unstimulated Salivary Flow Rate (USFR) at Week 4
eFigure 2. Comparison of Subgroups for Clinical Global Impression of Change (CGI-C) at Week 4
Data Sharing Statement