This cohort study compares the risk of major adverse coronary events (MACE) associated with use of ticagrelor vs clopidogrel in patients with acute coronary syndrome treated with percutaneous coronary intervention.
Key Points
Question
What is the association between ticagrelor vs clopidogrel and major adverse coronary events, major bleeding, and dyspnea in patients with acute coronary syndrome treated with percutaneous coronary intervention?
Findings
In this cohort study of 11 185 patients, ticagrelor was not associated with a statistically significantly lower risk of major adverse coronary events compared with clopidogrel. However, it was associated with statistically significantly more major bleeding and dyspnea.
Meaning
Ticagrelor was not associated with a lower risk of major adverse coronary events in patients with acute coronary syndrome who underwent percutaneous coronary intervention.
Abstract
Importance
Guidelines currently recommend ticagrelor over clopidogrel for patients with acute coronary syndrome (ACS) based on randomized clinical trial data in which ticagrelor reduced major adverse coronary events (MACE) vs clopidogrel but increased bleeding and dyspnea.
Objective
To compare the risk of MACE with ticagrelor vs clopidogrel in patients with ACS treated with percutaneous coronary intervention (PCI), to compare major bleeding and dyspnea, and to evaluate the association between P2Y12 inhibitor adherence and MACE.
Design, Setting, and Participants
Population-based cohort study using data of patients discharged alive after PCI for ACS from the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease registry from April 1, 2012, to March 31, 2016, with follow-up to 1 year. Analysis began April 2018.
Exposures
Outpatient prescription for ticagrelor or clopidogrel within 31 days after PCI. Adherence was defined as a medication refill adherence value of 80% or higher.
Main Outcomes and Measures
Major adverse coronary events, a composite of all-cause death, hospitalization for ACS, unplanned coronary revascularization, or stent thrombosis within 365 days after index PCI. Secondary outcomes included hospitalization for major bleeding and emergency department visit for dyspnea.
Results
Of 11 185 individuals who underwent PCI, the median (interquartile range) age was 61 (54-71) years, and 2760 (24.7%) were women. Ticagrelor users (4076 [36.4%]) were generally younger and had fewer cardiac and noncardiac comorbidities than clopidogrel users. Ticagrelor was not associated with lower risk of MACE (adjusted hazard ratio [aHR], 0.97; 95% CI, 0.85-1.10); however, it was associated with an increased risk of major bleeding (aHR, 1.51; 95% CI, 1.29-1.78) and dyspnea (aHR, 1.98; 95% CI, 1.47-2.65). A total of 3328 ticagrelor users (81.6%) were adherent during the study vs 5256 of clopidogrel users (73.9%) (P < .001; χ2 = 86.4). In the full cohort, adherence was associated with a lower MACE risk (aHR, 0.79; 95% CI, 0.69-0.90 for adherence of ≥80% vs <80%). Differences in other secondary outcomes were not statistically significant. Sensitivity and subgroup analyses were consistent with primary analyses.
Conclusions and Relevance
In this population-based cohort study of patients with ACS who underwent PCI, outpatient use of ticagrelor was not associated with a statistically significant reduction in MACE vs clopidogrel; however, it was associated with more major bleeding and dyspnea.
Introduction
Guidelines recommend ticagrelor over clopidogrel as part of dual antiplatelet therapy with acetylsalicylic acid (or aspirin) in the treatment of individuals with acute coronary syndrome (ACS), regardless of plans for invasive management.1,2 This recommendation is primarily based on the Platelet Inhibition and Patient Outcomes (PLATO) trial,3 in which patients with ACS receiving ticagrelor had a 16% relative risk reduction in major adverse coronary events (MACE) and 22% relative risk reduction in all-cause death compared with those treated with clopidogrel. This improvement in efficacy was counterbalanced by a 19% relative risk increase in major bleeding not associated with coronary artery bypass grafting and an 84% relative risk increase in dyspnea, which was generally reported as mild and transient.3,4,5,6
Patients seen in routine practice may differ in several ways from those enrolled in clinical trials, including having higher risks of MACE and bleeding, more comorbidities, and lower likelihood of being prescribed and adhering to evidence-based therapies.7 One registry study reported that 32% of consecutively enrolled patients with myocardial infarction were ineligible for contemporary antiplatelet trials including PLATO.8 Observational studies can complement clinical trials by evaluating the associations of interventions in representative populations that reflect these factors and can identify barriers to replicating the results observed in randomized clinical trials.
The primary study objective was to assess the comparative association of ticagrelor and clopidogrel with reduced MACE using data from a population-based registry including all patients undergoing percutaneous coronary intervention (PCI) for ACS in a geographic region with universal access to health care. We hypothesized that ticagrelor would be associated with a lower risk of MACE than clopidogrel. Secondary objectives included the evaluation of the safety of ticagrelor compared with clopidogrel, as well as associations between adherence, persistence, and switching with MACE.
Methods
Data Sources
The Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease registry prospectively collects detailed clinical data on all patients undergoing coronary angiography in Alberta, Canada (population of approximately 4.3 million).9 There are 3 sites where coronary angiography is performed that serve the entire province. The Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease registry was used to define the study cohort, determine baseline clinical and procedural characteristics, and obtain data on death and coronary procedures. The Pharmaceutical Information Network contains data on all outpatient prescriptions filled at pharmacies in Alberta, including fill dates and quantities. The Pharmaceutical Information Network was used to collect data on exposure to P2Y12 inhibitors and other prescription medications, as well as data on P2Y12 inhibitor adherence, persistence, and switching. Data on baseline hemoglobin concentration, creatinine concentration, and estimated glomerular filtration rate were obtained from the Alberta Health Services Laboratory Services. The Discharge Abstract Database and National Ambulatory Care Reporting System, which contain data on emergency department visits and hospitalizations in Alberta (eg, admission and discharge dates and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10] diagnosis codes) were used to identify history of atrial fibrillation (ICD-10 code I48.x recorded within 2 years prior to index) and study outcomes. The University of Alberta Research Ethics Office approved this study with a waiver of informed consent because the data were deidentified when provided to the investigators.
Cohort Definition
Included participants were older than 18 years, underwent PCI for ACS between April 1, 2012, and March 31, 2016, were discharged alive from index hospitalization, and filled a first prescription for clopidogrel or ticagrelor within 31 days after undergoing PCI. Both clopidogrel and ticagrelor were approved for ACS in Canada during the entire study, and ticagrelor was available on the Alberta acute care formulary from October 1, 2012. Prevalent P2Y12 inhibitor users, defined as individuals with a prescription for clopidogrel, prasugrel, or ticagrelor within 120 days prior to the index PCI, were excluded, as the present study was restricted to new users of P2Y12 inhibitor therapy.
Exposure
Exposure was defined by a prescription fill in the Pharmaceutical Information Network for clopidogrel or ticagrelor. Three additional measures of exposure were calculated: adherence, persistence, and switch, assuming an intended P2Y12 inhibitor duration of 12 months according to guideline recommendations.1,2 Adherence was estimated using the medication refill adherence (MRA),10,11 defined as: MRA (%) = (total days’ supply within an interval / total days in interval) × 100, where the days’ supply for clopidogrel equaled the number of tablets filled in that time interval, and the days’ supply for ticagrelor equaled half the total tablets filled (because it is taken twice daily). Medication refill adherence was censored at time of death, such that adherence for patients who died prior to the next fill could not exceed 100%. Patients with MRA of 80% or higher during the study were classified as adherent, as defined in previous ACS studies.3,12 Participants were classified as persistent during the 365 days after the index event if they had gaps between P2Y12 inhibitor prescription fills of less than the days’ supply plus a 15-day grace period.11 Switch was defined as 1 or more prescription fills for a P2Y12 inhibitor different from the first fill within 365 days after the index event. For patients who switched P2Y12 inhibitors during the study, all P2Y12 inhibitor fill information was considered for calculation of adherence and persistence (ie, patients were considered adherent if they filled the second P2Y12 inhibitor at appropriate intervals).
During the study, administration of acetylsalicylic acid, 81 mg, daily, for all patients without contraindication was the standard of care after ACS in Alberta and was incorporated in all standardized preprinted order sets for ACS management and post-ACS care. Acetylsalicylic acid, 81 mg, is not routinely recorded in the Pharmaceutical Information Network because it is available without a prescription in Alberta. For the purposes of this study, all patients were assumed to receive acetylsalicylic acid, 81 mg, daily, during follow-up.
Outcomes
The primary outcome was the first occurrence of MACE, defined as a composite of all-cause death, hospitalization with nonfatal ACS (ICD-10 codes I20.0, I21, or I22 as most responsible diagnosis), coronary revascularization excluding planned staged PCI procedures (which is a prospectively filled data field in the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease registry), or stent thrombosis within 365 days after the index hospitalization. Secondary outcomes included a composite of all-cause death, hospitalization with ACS, or ischemic stroke (ICD-10 codes I63.0 to I63.9 and I64); hospitalization for major bleeding (ICD-10 codes listed in eAppendix in the Supplement as first or second diagnosis code); and emergency department visit for dyspnea (ICD-10 code R06.0 as most responsible diagnosis). The ICD-10 codes for ACS and major bleeding have been validated.13,14,15
Statistical Analyses
Baseline characteristics were summarized for each group as proportions for categorical variables and as medians with interquartile ranges for continuous variables. Proportions and medians were compared using the χ2 and Mann-Whitney test, respectively. Hazard ratios (HR) and 95% confidence intervals for ticagrelor vs clopidogrel were obtained using Cox proportional hazard models, censored at time of P2Y12 inhibitor switch. Models for nonfatal outcomes were also censored at time of death. To account for baseline differences, fully adjusted Cox models were constructed for each outcome. Baseline characteristics, including all variables listed in Table 1, with a P value less than .20 in the univariable model, were included in a multivariable model; factors with a P value less than .05 in multivariable analysis remained in the final model. Additionally, the model was further adjusted for adherence using MRA as a continuous variable. To assess the association between adherence, persistence, and switching and outcomes, comparisons of MACE between ticagrelor and clopidogrel were stratified based on adherence of 80% or more vs less than 80%, persistence vs nonpersistence, and switching vs nonswitching. Furthermore, risk of MACE was compared between those with adherence of 80% or more and less than 80%, persistent vs nonpersistent, and switchers vs nonswitchers.
Table 1. Baseline Clinical and Angiographic Characteristics and P2Y12 Inhibitor Use During Study Follow-up.
| Characteristic | No. (%) | P Value | |
|---|---|---|---|
| Clopidogrel Group (n = 7109) | Ticagrelor Group (n = 4076) | ||
| Age, y | |||
| Median (IQR) | 62 (54-72) | 60 (53-69) | <.001 |
| ≥75 | 1434 (20.2) | 554 (13.6) | <.001 |
| Women | 1831 (25.8) | 929 (22.8) | <.001 |
| Fiscal yeara | |||
| 2013 | 2593 (93.6) | 177 (6.4) | <.001 |
| 2014 | 2105 (72.8) | 787 (27.2) | |
| 2015 | 1311 (48.3) | 1406 (51.7) | |
| 2016 | 1100 (39.2) | 1706 (60.8) | |
| ACS type | |||
| STEMI | 3170 (44.6) | 1783 (43.7) | .007 |
| NSTEMI | 2829 (39.8) | 1683 (41.3) | |
| Unstable angina | 1081 (15.2) | 576 (14.1) | |
| Unknown | 29 (0.4) | 34 (0.8) | |
| Cardiovascular history and risk factors | |||
| Prior MI | 858 (12.1) | 287 (7.0) | <.001 |
| Prior coronary artery bypass grafting | 294 (4.1) | 172 (4.2) | .83 |
| Prior PCI | 951 (13.4) | 478 (11.7) | .01 |
| Cerebrovascular disease | 326 (4.6) | 120 (2.9) | <.001 |
| Peripheral vascular disease | 696 (9.8) | 135 (3.3) | <.001 |
| Heart failure | 434 (6.1) | 126 (3.1) | <.001 |
| Atrial fibrillation | 662 (9.3) | 147 (3.6) | <.001 |
| Diabetes mellitus | 1807 (25.4) | 968 (23.7) | .05 |
| Hyperlipidemia | 4163 (58.6) | 2091 (51.3) | <.001 |
| Hypertension | 4857 (68.3) | 2532 (62.1) | <.001 |
| Smoking status | |||
| Current | 2301 (32.4) | 1060 (26.0) | <.001 |
| Former | 1580 (22.2) | 715 (17.5) | |
| Never/not recorded | 3228 (45.4) | 2301 (56.5) | |
| Other comorbidities | |||
| Chronic pulmonary disease | 714 (10.0) | 147 (3.6) | <.001 |
| Renal disease | 295 (4.1) | 108 (2.6) | <.001 |
| Dialysis dependence | 62 (0.9) | 14 (0.3) | .001 |
| Liver disease | 51 (0.7) | 15 (0.4) | .02 |
| Malignancy | 239 (3.4) | 95 (2.3) | .002 |
| Coronary anatomy | |||
| 1 Vessel | 2395 (33.7) | 1325 (32.5) | .54 |
| 2 Vessels | 1849 (26.0) | 1107 (27.2) | |
| 2 Vessels including proximal LAD | 551 (7.8) | 300 (7.4) | |
| 3 Vessels | 1159 (16.3) | 641 (15.7) | |
| 3 Vessels including proximal LAD | 908 (12.8) | 555 (13.6) | |
| Left main | 228 (3.2) | 136 (3.3) | |
| Minimal CAD | 19 (0.3) | 12 (0.3) | |
| Ejection fraction, % | |||
| >50 | 2638 (37.1) | 1298 (31.8) | <.001 |
| 35-50 | 1220 (17.2) | 482 (11.8) | |
| 20-34 | 198 (2.8) | 67 (1.6) | |
| <20 | 19 (0.3) | 4 (0.1) | |
| Missing | 3034 (42.7) | 2225 (54.6) | |
| Meets PLATO trial eligibility criteria | 5639 (79.3) | 3223 (79.1) | .75 |
| TIMI Risk Score for Secondary Prevention | |||
| Median (IQR) | 2 (1-3) | 1 (1-2) | <.001 |
| ≥3 | 2014 (28.3) | 770 (18.9) | <.001 |
| Stent placement | 6747 (94.9) | 3827 (93.9) | .02 |
| Drug-eluting stent | 3989 (56.1) | 3127 (76.7) | <.001 |
| Laboratory values, median (IQR) | |||
| Hemoglobin, g/dL | 13.7 (12.5-14.8) | 14.1 (13.0-15.1) | <.001 |
| Serum creatinine, mg/dL | 0.95 (0.81-1.11) | 0.95 (0.81-1.09) | .41 |
| eGFR, mL/min | 62 (61-85) | 81 (65-93) | <.001 |
| Other medications ≤31 d after PCI | |||
| Oral anticoagulant | 1060 (14.9) | 134 (3.3) | <.001 |
| Proton pump inhibitor | 2630 (37.0) | 1270 (31.2) | <.001 |
| Study P2Y12 inhibitor utilization | |||
| MRA, median (IQR), % | 98 (78-101) | 99 (88-102) | <.001 |
| At month 0 to 6 | 100 (89-104) | 101 (96-107) | <.001 |
| At month 7 to 12 | 95 (67-100) | 95 (81-100) | <.001 |
| MRA ≥80% | 5256 (73.9) | 3328 (81.6) | <.001 |
| At months 0 to 6 | 5732 (80.6) | 3602 (88.4) | <.001 |
| At months 7 to 12 | 4892 (68.8) | 3048 (74.8) | <.001 |
| Persistence | 4186 (58.9) | 2579 (63.3) | <.001 |
| Switch | 162 (2.3) | 571 (14.0) | <.001 |
Abbreviations: ACS, acute coronary syndrome; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate; IQR, interquartile range; LAD, left anterior descending artery; MI, myocardial infarction; MRA, medication refill adherence; NSTEMI, non–ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; PLATO, Platelet Inhibition and Patient Outcomes; STEMI, ST-segment elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction.
SI conversion factors: To convert hemoglobin to g/L, multiply by 10.0; serum creatinine to µmol/L, multiply by 88.4.
Percentages represent proportion of cohort initially prescribed that P2Y12 inhibitor during the indicated fiscal year.
To ensure robustness of the primary analysis, several sensitivity analyses were performed. First, ticagrelor and clopidogrel were compared in a propensity score–matched cohort, in which logistic regression was used to estimate the probability of ticagrelor use based on baseline variables listed in Table 1, and patients treated with ticagrelor were matched with those who received clopidogrel using 1:1 nearest-neighbor matching. Outcomes were compared using χ2 tests and HRs from proportional hazards models stratified by matched pairs to account for correlation between each matched pair. Second, the primary analysis was repeated with adjustment for a cardiac-specific comorbidity index.16 Subgroup analyses for MACE, major bleeding, and dyspnea were performed for the following: age (<65, 65-74, or ≥75 years), diabetes, estimated glomerular filtration rate (≥60 vs <60 mL/min), by fiscal year, with exclusion of patients receiving oral anticoagulants or with atrial fibrillation at baseline, exclusion of patients who switched P2Y12 inhibitors, and high-risk criteria using 2 definitions: PLATO inclusion criteria (defined as either ST-segment elevation myocardial infarction, excluding those treated with rescue PCI following failed fibrinolysis, or non–ST-segment elevation myocardial infarction plus any of the following: age ≥60 years, multivessel coronary artery disease on coronary angiography, prior myocardial infarction, coronary artery bypass grafting, cerebrovascular disease, peripheral vascular disease, diabetes or renal disease, or estimated glomerular filtration rate <60 mL/min), and the TIMI Risk Score for Secondary Prevention.17 The threshold for statistical significance was set at 2-sided P values less than .05. All analyses were performed using SAS, version 9.4 (SAS Institute) and R, version 3.4.3 (R Project for Statistical Computing). Analysis began April 2018.
Results
Cohort Characteristics
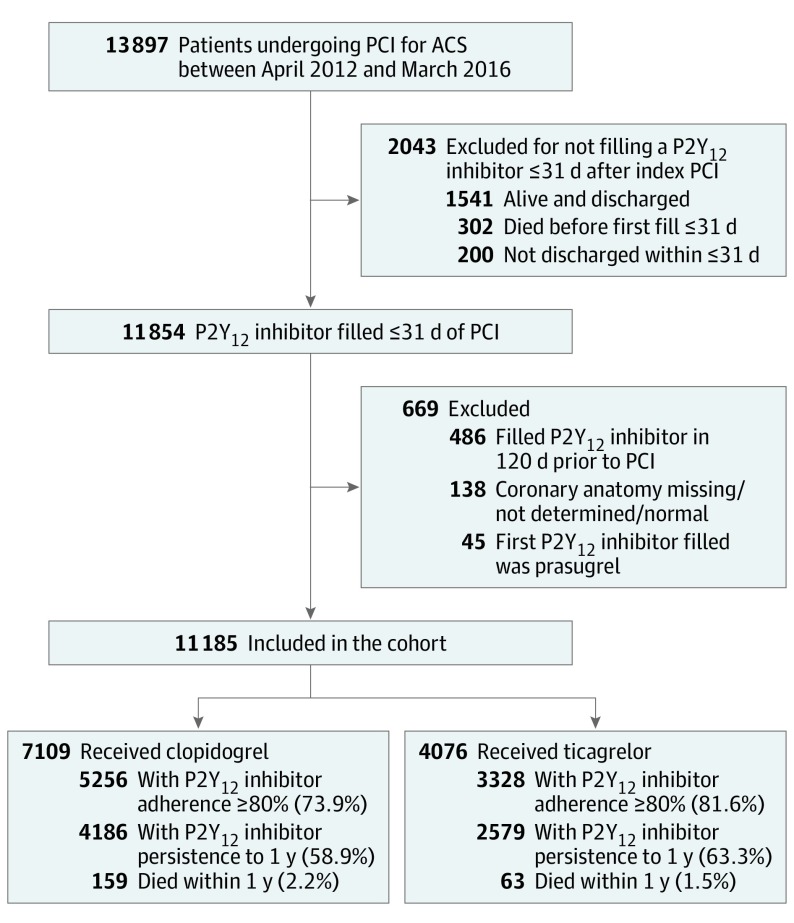
From April 1, 2012, to March 31, 2016, a total of 13 897 patients underwent PCI for ACS in Alberta, Canada. Of these, 11 185 patients (80.5%) filled at least 1 prescription for a P2Y12 inhibitor within 31 days of PCI and met all study eligibility criteria (Figure). Overall, clopidogrel was the most frequently prescribed P2Y12 inhibitor during the study (7109 of 11 185 [63.6%]); however, ticagrelor use steadily increased and was used in 3112 of 5523 patients (56.3%) by the second half of the study (Table 1 and eFigure in the Supplement).
Figure. Cohort Derivation.
Derivation of the study cohort from APPROACH between April 1, 2012, and March 31, 2016, is shown. Included patients from Alberta, Canada, who were 18 years or older who underwent percutaneous coronary intervention (PCI) for an acute coronary syndrome (ACS), were not receiving a P2Y12 inhibitor in the 120 days preceding the ACS, and filled a prescription for clopidogrel or ticagrelor within 31 days after their PCI.
The median (interquartile range) age was 61 (54-71) years, 2760 (24.7%) were women, and 4953 (44.3%) presented with ST-segment elevation myocardial infarction. Patients filled their first outpatient prescription for a P2Y12 inhibitor a median (interquartile range) of 3 (1-4) days after index PCI and 1 (0-3) day after hospital discharge. Table 1 lists baseline characteristics by P2Y12 inhibitor group. Patients receiving ticagrelor were statistically significantly younger, less likely to be women, and had a lower prevalence of prior myocardial infarction, other cardiovascular disease, cardiac risk factors, and comorbidities compared with clopidogrel users. Ticagrelor users were also more likely to receive a drug-eluting stent than clopidogrel users and less likely to receive a prescription for an oral anticoagulant within 31 days after PCI.
Major Adverse Coronary Events
In unadjusted analyses, outpatient use of ticagrelor was associated with a lower risk of MACE than clopidogrel (HR, 0.84; 95% CI, 0.74-0.95) (Table 2). Unadjusted death rates were also lower with ticagrelor but not hospitalization for ACS, coronary revascularization or the composite of death, ACS, or ischemic stroke. After multivariable adjustment including baseline characteristics listed in Table 1 and adherence, differences in MACE (adjusted HR [aHR], 0.97; 95% CI, 0.85-1.10) and other cardiovascular outcomes were no longer statistically significant between ticagrelor and clopidogrel (Table 2).
Table 2. Association of Clopidogrel vs Ticagrelor With Outcomes Within 1 Year After Percutaneous Coronary Intervention for Acute Coronary Syndrome.
| Outcome | No. (%) | P Value | HR (95% CI) | |||
|---|---|---|---|---|---|---|
| Clopidogrel Group (n = 7109) | Ticagrelor Group (n = 4076) | Unadjusted | Adjusted for Age and Sex | Fully Adjusteda | ||
| MACE | 828 (11.6) | 419 (10.3) | .03 | 0.84 (0.74-0.95)b | 0.88 (0.78-0.995)b | 0.97 (0.85-1.10) |
| All-cause death | 159 (2.2) | 63 (1.5) | .01 | 0.73 (0.54-0.98)b | 0.91 (0.67-1.23) | 1.19 (0.87-1.62) |
| ACS | 505 (7.1) | 259 (6.4) | .13 | 0.85 (0.73-1.00) | 0.88 (0.75-1.03) | 0.93 (0.79-1.09) |
| Coronary revascularization | 338 (4.8) | 178 (4.4) | .35 | 0.83 (0.69-1.01) | 0.85 (0.70-1.04) | 0.90 (0.73-1.10) |
| PCI | 249 (3.5) | 133 (3.3) | .50 | 0.87 (0.70-1.09) | 0.88 (0.71-1.11) | 0.87 (0.69-1.09) |
| Coronary artery bypass grafting | 94 (1.3) | 46 (1.1) | .38 | 0.72 (0.49-1.06) | 0.75 (0.51-1.11) | 1.01 (0.68-1.52) |
| Stent thrombosis | 25 (0.4) | 19 (0.5) | .35 | 1.30 (0.71-2.38) | 1.29 (0.70-2.36) | 1.66 (0.88-3.12) |
| Composite of death, ACS, or ischemic stroke | 676 (9.5) | 328 (8.0) | .009 | 0.82 (0.72-0.94)b | 0.87 (0.76-1.00) | 0.93 (0.80-1.09) |
| Ischemic stroke | 40 (0.6) | 20 (0.5) | .62 | 0.91 (0.52-1.57) | 1.02 (0.59-1.78) | 1.35 (0.75-2.40) |
| Major bleeding | 449 (6.3) | 277 (6.8) | .32 | 1.09 (0.93-1.27) | 1.23 (1.05-1.43)b | 1.51 (1.29-1.78)b |
| Intracranial | 11 (0.2) | 5 (0.1) | .67 | 0.68 (0.22-2.15) | 0.71 (0.22-2.24) | 0.88 (0.26-2.90) |
| Gastrointestinal | 135 (1.9) | 99 (2.4) | .06 | 1.33 (1.02-1.74)b | 1.55 (1.19-2.04)b | 2.02 (1.52-2.68)b |
| Pulmonary | 175 (2.5) | 111 (2.7) | .40 | 1.12 (0.88-1.43) | 1.24 (0.97-1.58) | 1.49 (1.15-1.93)b |
| Urologic | 74 (1.0) | 38 (0.9) | .58 | 0.86 (0.58-1.30) | 1.01 (0.67-1.52) | 1.16 (0.76-1.78) |
| Other | 85 (1.2) | 42 (1.0) | .43 | 0.89 (0.61-1.30) | 0.99 (0.68-1.44) | 1.18 (0.79-1.75) |
| Dyspnea | 116 (1.6) | 119 (2.9) | <.001 | 1.80 (1.39-2.34)b | 2.01 (1.54-2.61)b | 1.98 (1.47-2.65)b |
Abbreviations: ACS, acute coronary syndrome; HR, hazard ratio; MACE, major adverse coronary event; PCI, percutaneous coronary intervention.
Adjusted for age, sex, and medication refill adherence as a continuous variable over the entire year and statistically significant variables from stepwise variable selection: MACE (prior PCI, diabetes, hyperlipidemia, coronary anatomy, drug-eluting stent, hemoglobin, creatinine, proton pump inhibitor use); major bleeding (malignancy, hemoglobin, creatinine, oral anticoagulant use); dyspnea (fiscal year, prior myocardial infarction, heart failure, diabetes, chronic pulmonary disease, renal disease); and ischemic stroke (diabetes, hypertension, oral anticoagulant use).
Statistically significant results.
Safety
In unadjusted analyses, ticagrelor was not associated with a statistically significantly greater risk of major bleeding compared with clopidogrel (HR, 1.09; 95% CI, 0.93-1.27). However, ticagrelor was associated with a higher risk of major bleeding compared with clopidogrel in the fully adjusted model (aHR, 1.51; 95% CI, 1.29-1.78) (Table 2). This difference was mainly driven by an increase in gastrointestinal hemorrhage (aHR, 2.02; 95% CI, 1.52-2.68) and pulmonary hemorrhage (aHR, 1.49; 95% CI, 1.15-1.93). Furthermore, ticagrelor was associated with a statistically significantly greater risk of an emergency department visit for dyspnea, which persisted in the fully adjusted model (aHR, 1.98; 95% CI, 1.47-2.65) (Table 2).
Association of Adherence, Persistence, and Switching With Major Adverse Coronary Events
Ticagrelor users were more likely to be adherent to P2Y12 inhibitor therapy during the entire study than clopidogrel users (3328 [81.6%] vs 5256 [73.9%]; P < .001; χ2 = 86.4). Median MRA declined in both groups between months 0 to 6 and months 7 to 12, and 2579 ticagrelor users (63.3%) vs 4186 clopidogrel users (58.9%) persisted on a P2Y12 inhibitor for 1 year (P < .001; χ2 = 20.9) (Table 1). Major adverse coronary events were not statistically significantly different between ticagrelor and clopidogrel users regardless of adherence, persistence, and switching (Table 3). In the full cohort, MACE was lower in patients with P2Y12 inhibitor adherence of 80% or higher compared with adherence under 80% (aHR, 0.79; 95% CI, 0.69-0.90).
Table 3. Major Adverse Coronary Events Within 1 Year After Percutaneous Coronary Intervention for Acute Coronary Syndrome Based on Adherence, Persistence, and Switching of Study P2Y12 Inhibitor.
| Characteristic | MACE, No./Total No. (%) | HR (95% CI)a | ||
|---|---|---|---|---|
| Full Cohort (N = 11 185) | Clopidogrel Group (n = 7109) | Ticagrelor Group (n = 4076) | Ticagrelor vs Clopidogrel | |
| Adherence | ||||
| MRA ≥80% | 881/8584 (10.3) | 550/5256 (10.5) | 311/3328 (9.9) | 1.00 (0.86-1.16) |
| MRA <80% | 366/2601 (14.1) | 278/1853 (15.0) | 88/748 (11.8) | 0.88 (0.68-1.14) |
| MRA ≥80% vs <80%, HR (95% CI)a | 0.79 (0.69-0.90)b | 0.77 (0.66-0.89)b | 0.86 (0.67-1.11) | NA |
| Persistence | ||||
| Yes | 697/6765 (10.3) | 433/4186 (10.3) | 264/2579 (10.2) | 1.02 (0.87-1.20) |
| No | 550/4420 (12.4) | 395/2923 (13.5) | 155/1497 (10.4) | 0.89 (0.73-1.09) |
| Persistent vs nonpersistent, HR (95% CI)a | 0.90 (0.80-1.01) | 0.85 (0.74-0.98)b | 1.01 (0.81-1.24) | NA |
| Switchc | ||||
| Yes | NA | 6/92 (6.5) | 47/521 (9.0) | 1.49 (0.62-3.61) |
| No | NA | 752/6947 (10.8) | 322/3505 (9.2) | 0.98 (0.86-1.12) |
| Switch vs no switch, HR (95% CI)a | NA | 0.56 (0.25-1.25) | 0.88 (0.65-1.20) | NA |
Abbreviations: HR, hazard ratio; MACE, major adverse coronary event; MRA, medication refill adherence; NA, not applicable.
Adjusted for age, sex, prior percutaneous coronary intervention, diabetes, hyperlipidemia, coronary anatomy, drug-eluting stent, hemoglobin, creatinine, and proton pump inhibitor use.
Statistically significant results.
Excludes switches occurring after MACE event.
Switching P2Y12 inhibitors occurred in 571 ticagrelor users (14.0%) vs 162 clopidogrel users (2.3%) (Table 1) and was not associated with an increased risk of MACE (aHR, 0.56; 95% CI, 0.25-1.25 with initial clopidogrel use and aHR, 0.88; 95% CI, 0.65-1.20 with initial ticagrelor use) (Table 3). Within 30 days after an emergency department visit for dyspnea, 28 of 112 ticagrelor users (25.0%) switched P2Y12 inhibitors compared with 1 of 115 clopidogrel users (0.9%) (P < .001; χ2 = 29.6). Similarly, more ticagrelor users than clopidogrel users switched their P2Y12 inhibitor within 30 days after a major bleed (18 of 255 [7.1%] vs 2 of 439 [0.5%]; P < .001; χ2 = 25.1). Moreover, among ticagrelor users, switching occurred more frequently in those with an emergency department visit for dyspnea (54 of 119 [45.4%]) vs those without dyspnea (517 of 3957 [13.1%]) (P < .001; χ2 = 100.1) and among those with hospitalization for major bleed (53 of 277 [19.1%]) vs those without major bleed (518 of 3799 [13.6%]) (P = .01; χ2 = 6.5).
Sensitivity and Subgroup Analyses
Propensity score matching created a well-balanced cohort (n = 7422) with standardized differences for all baseline characteristics less than 0.1 (eTable 1 in the Supplement). Findings from the propensity score–matched analysis (Table 4) were consistent with the primary, multivariable-adjusted analyses, not demonstrating a statistically significant difference between ticagrelor and clopidogrel for MACE (HR, 1.00; 95% CI, 0.86-1.17) but higher risks of major bleeding (HR, 1.52; 95% CI, 1.24-1.87) and dyspnea (HR, 2.42; 95% CI, 1.70-3.45) with ticagrelor. Similarly, differences between ticagrelor and clopidogrel were not observed for MACE or any of its components when adjusted for a cardiac-specific comorbidity index (eTable 2 in the Supplement).
Table 4. Association of Clopidogrel vs Ticagrelor With Outcomes Within 1 Year After Percutaneous Coronary Intervention for Acute Coronary Syndrome in Propensity Score–Matched Cohort.
| Outcome | No. (%) | P Value | HR (95% CI) | |
|---|---|---|---|---|
| Clopidogrel Group (n = 3711) | Ticagrelor Group (n = 3711) | |||
| MACE | 368 (9.9) | 380 (10.2) | .64 | 1.00 (0.86-1.17) |
| All-cause death | 54 (1.5) | 61 (1.6) | .51 | 1.10 (0.75-1.61) |
| ACS | 228 (6.1) | 235 (6.3) | .74 | 1.02 (0.84-1.24) |
| Coronary revascularization | 168 (4.5) | 157 (4.2) | .53 | 0.86 (0.67-1.09) |
| PCI | 121 (3.3) | 114 (3.1) | .64 | 0.90 (0.68-1.19) |
| CABG | 50 (1.3) | 44 (1.2) | .53 | 0.74 (0.47-1.15) |
| Stent thrombosis | 7 (0.2) | 18 (0.5) | .03 | 2.57 (1.07-6.16)a |
| Composite of all-cause death, ACS, or stroke | 290 (7.8) | 299 (8.1) | .70 | 1.02 (0.86-1.21) |
| Ischemic stroke | 18 (0.5) | 17 (0.5) | .87 | 0.94 (0.48-1.86) |
| Major bleed | 182 (4.9) | 261 (7.0) | <.001 | 1.52 (1.24-1.87)a |
| Intracranial | 3 (0.1) | 3 (0.1) | >.99 | 1.00 (0.14-7.10) |
| Gastrointestinal | 53 (1.4) | 95 (2.6) | <.001 | 2.10 (1.44-3.06)a |
| Pulmonary | 81 (2.2) | 105 (2.8) | .08 | 1.32 (0.97-1.80) |
| Urologic | 29 (0.8) | 37 (1.0) | .32 | 1.32 (0.79-2.22) |
| Other | 32 (0.9) | 38 (1.0) | .47 | 1.29 (0.78-2.11) |
| Dyspnea | 46 (1.2) | 116 (3.1) | <.001 | 2.42 (1.70-3.45)a |
Abbreviations: ACS, acute coronary syndrome; CABG, coronary artery bypass grafting; HR, hazard ratio; MACE, major adverse coronary event; PCI, percutaneous coronary intervention.
Statistically significant results.
Subgroup analyses for MACE, major bleeding, and dyspnea based on age, diabetes, estimated glomerular filtration rate, high-risk criteria based on PLATO or TIMI Risk Score for Secondary Prevention criteria, fiscal year, and exclusion of patients with atrial fibrillation, receiving oral anticoagulants, or switching P2Y12 inhibitors were all consistent with results for the overall cohort (eTable 3 in the Supplement). eTable 4 in the Supplement provides the full details of the multivariate models for MACE, major bleeding, and dyspnea. eTable 5 in the Supplement illustrates the change in the hazard ratio with stepwise addition of variables to the multivariate model for MACE.
Discussion
In this large, contemporary, population-based cohort study of patients who underwent PCI for ACS, outpatient use of ticagrelor was not associated with a lower risk of MACE compared with clopidogrel; however, it was associated with a higher risk of major bleeding and dyspnea. Conversely, adherence to any P2Y12 inhibitor therapy was associated with 21% lower relative risk of MACE compared with P2Y12 inhibitor nonadherence.
Our findings differ from prior studies on this topic, and this may be due to differences in methodology, patient populations, and advances in interventional cardiology. The randomized clinical trial that established the use of ticagrelor in ACS, PLATO,3 was a multinational trial including all ACS subtypes regardless of planned invasive management, in whom PCI was performed predominantly using bare-metal stents and first-generation drug-eluting stents. Furthermore, outcome ascertainment in the PLATO trial included in-hospital outcomes. The large observational SWEDEHEART registry study,18 which was also discordant with our findings, was not restricted to patients with ACS who underwent PCI, did not specify the proportion of patients receiving second-generation drug-eluting stents, and defined exposure based on intended choice and duration of P2Y12 inhibitor therapy on discharge without confirmation of use and adherence using prescription fills or patient interview. Like our study, the SWEDEHEART study18 did not include in-hospital events and only included patients who survived to discharge. Conversely, our observational study was restricted to patients with ACS who all underwent PCI, survived to discharge, were new users of P2Y12 inhibitor therapy, and primarily received second-generation drug-eluting stents, which have an improved safety profile, including a lower risk of stent thrombosis than bare-metal and older-generation drug-eluting stents.19 The Dutch CHANGE DAPT cohort study, which enrolled patients with ACS who underwent PCI exclusively with second-generation drug-eluting stents, also found no statistically significant difference in MACE between ticagrelor and clopidogrel.20 In aggregate, these study findings suggest that the increased potency of ticagrelor may not translate to improved efficacy in the era of second-generation drug-eluting stents, particularly after patients are hospital discharged. A 2019 randomized clinical trial of patients with ACS undergoing PCI found ticagrelor to be inferior to the once-daily potent P2Y12 inhibitor prasugrel.21
Although the greater antiplatelet potency of ticagrelor did not translate to reduction in MACE in this study, it was associated with an increased risk of major bleeding. Given that patients who underwent coronary artery bypass grafting during the index hospitalization were excluded from this study, these findings emulate the increased risk of non–coronary artery bypass grafting–related major bleeding seen in PLATO.3 Moreover, our cohort included patients at higher risk of bleeding than those generally included in clinical trials, as indicated by a rate of major bleeding in the ticagrelor group that was higher than that reported in PLATO (6.8% vs 4.5%) despite the stricter bleeding definition used in our study and exclusion of events (including periprocedural bleeding) occurring during the index hospitalization.3 Similarly, patients in the ticagrelor group of this cohort had a 2-fold higher risk of emergency department visits for dyspnea vs those in the clopidogrel group. Although ticagrelor-related dyspnea is generally mild and transient, it persists and impairs quality of life in a subset of patients, leading to increased health care utilization, nonadherence, and premature discontinuation.3,4,5,6,22
This study found that adherence to a P2Y12 inhibitor was more strongly associated with risk of MACE than choice of the P2Y12 inhibitor itself. These findings extend prior observations that premature discontinuation of P2Y12 inhibitors is associated with a greater risk of death, rehospitalization, and stent thrombosis.23,24 Several factors associated with lower adherence to P2Y12 inhibitors,24 including greater comorbidity burden and use of oral anticoagulation, were more prevalent in clopidogrel users within the present study. This may have accounted in part for the lower adherence and persistence in clopidogrel users compared with ticagrelor users. These results should encourage clinicians to routinely ask patients whether they are taking their medications as prescribed and identify and resolve barriers to adherence, including cost, adverse events (including dyspnea with ticagrelor), and burden from number or frequency of medications administered. The steady decline in adherence during the course of the present study, consistent with prior studies,25 warrants ongoing assessment of medication adherence starting at hospital discharge and continuing at every follow-up visit.
Limitations
This study has limitations inherent to its observational design. First, residual unmeasured confounding may persist despite measurement and adjustment for a variety of known clinical, angiographic, and laboratory variables. Second, we excluded outcomes occurring during the index hospitalization, as information on in-hospital P2Y12 inhibitor use was not available within our databases. Third, the definitions for exposure, adherence, and persistence assume that patients took their P2Y12 inhibitors as filled and may overestimate true adherence. However, these definitions have been validated, are consistent with those used in multiple prior studies, and provide the closest surrogate to medication use available using administrative data.10,11 Fourth, the adherence definition assumed that all patients were intended to receive a P2Y12 inhibitor for at least 12 months, which was routine standard of care during the study. Fifth, we used all-cause death for the composite primary outcome because cause of death was not reliably ascertained within available databases. Therefore, these results are not directly comparable with the primary outcome of PLATO. However, we included the secondary outcome of all-cause death, ACS, or stroke, which was also evaluated in PLATO. Sixth, we used a novel outcome definition for dyspnea using a nonspecific ICD-10 symptom code that requires further validation. However, post hoc analyses further evaluating this outcome revealed higher switch rates in the ticagrelor group among those with dyspnea and more switches from ticagrelor than clopidogrel within 30 days after an emergency department visit for dyspnea, supporting an association between ticagrelor use and these events.
Conclusions
In a large, representative population-based cohort of patients who underwent PCI for ACS primarily using second-generation drug-eluting stents, ticagrelor was not associated with a lower risk of MACE compared with clopidogrel; however, it was associated with a higher risk of major bleeding and emergency department visits for dyspnea.
eAppendix. Major bleeding ICD-10 codes
eFigure. Study drug by fiscal year
eTable 1. Baseline characteristics by antiplatelet group in propensity score-matched cohort
eTable 2. Major adverse coronary events within 1 year after percutaneous coronary intervention for acute coronary syndrome adjusted for the Cardiac-Specific Comorbidity Index
eTable 3. Subgroup analyses
eTable 4. Multivariate model for effectiveness and safety outcomes within 1 year after percutaneous coronary intervention for acute coronary syndrome
eTable 5. Sensitivity analysis: Stepwise addition of covariates to multivariate analysis of ticagrelor versus clopidogrel on major adverse coronary events within 1 year after percutaneous coronary intervention for acute coronary syndrome
References
- 1.Mehta SR, Bainey KR, Cantor WJ, et al. ; members of the Secondary Panel . 2018 Canadian Cardiovascular Society/Canadian Association of Interventional Cardiology focused update of the guidelines for the use of antiplatelet therapy. Can J Cardiol. 2018;34(3):214-233. doi: 10.1016/j.cjca.2017.12.012 [DOI] [PubMed] [Google Scholar]
- 2.Levine GN, Bates ER, Bittl JA, et al. . 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. 2016;134(10):e123-e155. doi: 10.1161/CIR.0000000000000404 [DOI] [PubMed] [Google Scholar]
- 3.Wallentin L, Becker RC, Budaj A, et al. ; PLATO Investigators . Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045-1057. doi: 10.1056/NEJMoa0904327 [DOI] [PubMed] [Google Scholar]
- 4.Storey RF, Becker RC, Harrington RA, et al. . Characterization of dyspnoea in PLATO study patients treated with ticagrelor or clopidogrel and its association with clinical outcomes. Eur Heart J. 2011;32(23):2945-2953. doi: 10.1093/eurheartj/ehr231 [DOI] [PubMed] [Google Scholar]
- 5.Storey RF, Bliden KP, Patil SB, et al. ; ONSET/OFFSET Investigators . Incidence of dyspnea and assessment of cardiac and pulmonary function in patients with stable coronary artery disease receiving ticagrelor, clopidogrel, or placebo in the ONSET/OFFSET study. J Am Coll Cardiol. 2010;56(3):185-193. doi: 10.1016/j.jacc.2010.01.062 [DOI] [PubMed] [Google Scholar]
- 6.Bonaca MP, Bhatt DL, Cohen M, et al. ; PEGASUS-TIMI 54 Steering Committee and Investigators . Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372(19):1791-1800. doi: 10.1056/NEJMoa1500857 [DOI] [PubMed] [Google Scholar]
- 7.Kennedy-Martin T, Curtis S, Faries D, Robinson S, Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials. 2015;16(1):495. doi: 10.1186/s13063-015-1023-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Udell JA, Wang TY, Li S, et al. . Clinical trial participation after myocardial infarction in a national cardiovascular data registry. JAMA. 2014;312(8):841-843. doi: 10.1001/jama.2014.6217 [DOI] [PubMed] [Google Scholar]
- 9.Ghali WA, Knudtson ML. Overview of the Alberta provincial project for outcome assessment in coronary heart disease: on behalf of the APPROACH investigators. Can J Cardiol. 2000;16(10):1225-1230. [PubMed] [Google Scholar]
- 10.Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40(7-8):1280-1288. doi: 10.1345/aph.1H018 [DOI] [PubMed] [Google Scholar]
- 11.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15(8):565-574. doi: 10.1002/pds.1230 [DOI] [PubMed] [Google Scholar]
- 12.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297(2):177-186. doi: 10.1001/jama.297.2.177 [DOI] [PubMed] [Google Scholar]
- 13.McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of myocardial infarction diagnoses in administrative databases: a systematic review. PLoS One. 2014;9(3):e92286. doi: 10.1371/journal.pone.0092286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomes T, Mamdani MM, Holbrook AM, Paterson JM, Hellings C, Juurlink DN. Rates of hemorrhage during warfarin therapy for atrial fibrillation. CMAJ. 2013;185(2):E121-E127. doi: 10.1503/cmaj.121218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnason T, Wells PS, van Walraven C, Forster AJ. Accuracy of coding for possible warfarin complications in hospital discharge abstracts. Thromb Res. 2006;118(2):253-262. doi: 10.1016/j.thromres.2005.06.015 [DOI] [PubMed] [Google Scholar]
- 16.Azzalini L, Chabot-Blanchet M, Southern DA, et al. . A disease-specific comorbidity index for predicting mortality in patients admitted to hospital with a cardiac condition. CMAJ. 2019;191(11):E299-E307. doi: 10.1503/cmaj.181186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bohula EA, Bonaca MP, Braunwald E, et al. . Atherothrombotic risk stratification and the efficacy and safety of vorapaxar in patients with stable ischemic heart disease and previous myocardial infarction. Circulation. 2016;134(4):304-313. doi: 10.1161/CIRCULATIONAHA.115.019861 [DOI] [PubMed] [Google Scholar]
- 18.Sahlén A, Varenhorst C, Lagerqvist B, et al. . Outcomes in patients treated with ticagrelor or clopidogrel after acute myocardial infarction: experiences from SWEDEHEART registry. Eur Heart J. 2016;37(44):3335-3342. doi: 10.1093/eurheartj/ehw284 [DOI] [PubMed] [Google Scholar]
- 19.Palmerini T, Biondi-Zoccai G, Della Riva D, et al. . Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012;379(9824):1393-1402. doi: 10.1016/S0140-6736(12)60324-9 [DOI] [PubMed] [Google Scholar]
- 20.Zocca P, van der Heijden LC, Kok MM, et al. . Clopidogrel or ticagrelor in acute coronary syndrome patients treated with newer-generation drug-eluting stents: CHANGE DAPT. EuroIntervention. 2017;13(10):1168-1176. doi: 10.4244/EIJ-D-17-00634 [DOI] [PubMed] [Google Scholar]
- 21.Schüpke S, Neumann F-J, Menichelli M, et al. ; ISAR-REACT 5 Trial Investigators . Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med. 2019;381(16):1524-1534. doi: 10.1056/NEJMoa1908973 [DOI] [PubMed] [Google Scholar]
- 22.Gaubert M, Laine M, Richard T, et al. . Effect of ticagrelor-related dyspnea on compliance with therapy in acute coronary syndrome patients. Int J Cardiol. 2014;173(1):120-121. doi: 10.1016/j.ijcard.2014.02.028 [DOI] [PubMed] [Google Scholar]
- 23.Spertus JA, Kettelkamp R, Vance C, et al. . Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER registry. Circulation. 2006;113(24):2803-2809. doi: 10.1161/CIRCULATIONAHA.106.618066 [DOI] [PubMed] [Google Scholar]
- 24.Airoldi F, Colombo A, Morici N, et al. . Incidence and predictors of drug-eluting stent thrombosis during and after discontinuation of thienopyridine treatment. Circulation. 2007;116(7):745-754. doi: 10.1161/CIRCULATIONAHA.106.686048 [DOI] [PubMed] [Google Scholar]
- 25.Czarny MJ, Nathan AS, Yeh RW, Mauri L. Adherence to dual antiplatelet therapy after coronary stenting: a systematic review. Clin Cardiol. 2014;37(8):505-513. doi: 10.1002/clc.22289 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Major bleeding ICD-10 codes
eFigure. Study drug by fiscal year
eTable 1. Baseline characteristics by antiplatelet group in propensity score-matched cohort
eTable 2. Major adverse coronary events within 1 year after percutaneous coronary intervention for acute coronary syndrome adjusted for the Cardiac-Specific Comorbidity Index
eTable 3. Subgroup analyses
eTable 4. Multivariate model for effectiveness and safety outcomes within 1 year after percutaneous coronary intervention for acute coronary syndrome
eTable 5. Sensitivity analysis: Stepwise addition of covariates to multivariate analysis of ticagrelor versus clopidogrel on major adverse coronary events within 1 year after percutaneous coronary intervention for acute coronary syndrome