Key Points
Question
Does preemptive treatment with acyclovir reduce the duration of mechanical ventilation in critically ill patients with herpes simplex virus oropharyngeal reactivation?
Findings
In this multicenter, randomized clinical trial of 238 adults, treatment with intravenous acyclovir vs placebo during 14 days did not significantly reduce ventilator-free days at day 60. Compared with placebo, intravenous acyclovir was not associated with higher incidence of adverse events.
Meaning
The findings of this study do not appear to support routine preemptive use of acyclovir in this setting.
This randomized clinical trial evaluates the effectiveness of preemptive treatment with intravenous acyclovir compared with placebo for reducing the duration of mechanical ventilation in patients with herpes simplex virus oropharyngeal reactivation.
Abstract
Importance
The role of herpes simplex virus (HSV) reactivation on morbidity and mortality in patients in the intensive care unit requiring mechanical ventilation remains unknown.
Objective
To determine whether preemptive treatment with intravenous acyclovir reduces the duration of mechanical ventilation in patients with HSV oropharyngeal reactivation.
Design, Setting, and Participants
A double-blind, placebo-controlled randomized clinical trial was conducted in 16 intensive care units in France. Participants included 239 adults (age, >18 years) who received mechanical ventilation for at least 96 hours and continued to receive mechanical ventilation for 48 hours or more, with HSV oropharyngeal reactivation. Patients were enrolled between February 2, 2014, and February 22, 2018.
Interventions
Participants were randomized to receive intravenous acyclovir, 5 mg/kg, 3 times daily for 14 days or a matching placebo.
Main Outcomes and Measures
The primary end point was ventilator-free days from randomization to day 60. Prespecified secondary outcomes included mortality at 60 days. Main analyses were conducted on an intention-to-treat basis.
Results
Of 239 patients enrolled and randomized, 1 patient withdrew consent, leaving 238 patients, with 119 patients in both the acyclovir and placebo (control) groups (median [IQR] age, 61 [50-70] years; 76 [32%] women) available for primary outcome measurement. On day 60, the median (IQR) numbers of ventilator-free days were 35 (0-53) for acyclovir recipients and 36 (0-50]) for controls (P = .17 for between-group comparison). Among secondary outcomes, 26 patients (22%) and 39 patients (33%) had died at day 60 (risk difference, 0.11, 95% CI, –0.004 to 0.22, P = .06). The adverse event frequency was similar for both groups (28% in the acyclovir group and 23% in the placebo group, P = .40), particularly acute renal failure post randomization affecting 3 acyclovir recipients (3%) and 2 controls (2%). Four patients (3%) in the acyclovir group vs none in the placebo group stopped the study drug for treatment-related adverse events.
Conclusions and Relevance
In patients receiving mechanical ventilation for 96 hours or more with HSV reactivation in the throat, use of acyclovir, 5 mg/kg, 3 times daily for 14 days, did not increase the number of ventilator-free days at day 60, compared with placebo. These findings do not appear to support routine preemptive use of acyclovir in this setting.
Trial Registration
ClinicalTrials.gov identifier: NCT02152358
Introduction
Among the Herpesviridae responsible for infections in humans, herpes simplex virus (HSV) is one of the most frequent, with 50% to 80% of healthy adults being infected, most during childhood, and carrying the virus. Critical illness and mechanical ventilation, with endotracheal tube–induced microtrauma, are factors strongly associated with reactivation HSV in nonimmunocompromised patients. Oropharyngeal HSV reactivation occurs in 20% to 50% of mechanically ventilated patients, with the virus being detectable in up to 64% of patients receiving prolonged mechanical ventilation. Some patients develop true HSV bronchopneumonitis.1,2 Moreover, HSV detection in the lower respiratory tract and HSV bronchopneumonitis seem to be associated with poorer outcomes in observational studies and a meta-analysis.1,2,3,4 However, whether HSV reactivation in intensive care unit (ICU) patients occurs in the most severely ill patients, thus considered a marker of severity, or is associated with morbidity and mortality and treatment with acyclovir could improve the prognosis remain to be determined. We designed the Preemptive Treatment for Herpesviridae trial to determine whether treating mechanically ventilated HSV-reactivation–positive patients with acyclovir would improve their outcomes.
Methods
Study Design
This randomized, multicenter, double-blind, placebo-controlled trial was conducted in 16 intensive care units (ICUs) in France from February 2, 2014, to February 22, 2018. An independent ethics committee (Comité de Protection des Personnes Sud Méditerranée 5) approved the study protocol, which is available with the statistical analysis plan in Supplement 1. Written informed consent was obtained from the patient or his or her legally authorized representative. For the latter, the patient’s follow-up informed consent was obtained when possible. Participants did not receive financial compensation. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for randomized clinical trials.5
During the study period, potentially eligible patients (ie, those mechanically ventilated for ≥96 hours) were screened twice weekly with quantitative polymerase chain reaction performed on whole blood for cytomegalovirus (CMV) and qualitative polymerase chain reaction performed on oropharyngeal swabs for HSV reactivations. Patients with HSV-positive oropharyngeal swabs were potentially eligible for this trial. Patients could not be included in another study. Patients and/or their relatives were informed of this screening until December 18, 2015, when screening became routine care and French law rendered informing them no longer necessary.
Study Participants
Patients aged 18 years or older who had been receiving mechanical ventilation for 96 hours or more, with a predicted mechanical ventilation duration of 48 hours or more and an HSV-positive oropharyngeal swab, were eligible for enrollment. Exclusion criteria were use of acyclovir, ganciclovir, or another antiviral with anti-HSV activity (eg, cidofovir or foscavir) at the time of randomization; known hypersensitivity to acyclovir; active HSV or CMV infection treated during the preceding month; pregnancy or lactation; pancytopenia; solid-organ or bone-marrow transplant; immunosuppressant therapy (including corticosteroids at doses ≥0.5 mg/kg per day of prednisone or its equivalent for >1 month); HIV infection; moribund condition, defined as a preinclusion Simplified Acute Physiology Score (SAPS) II of 75 or higher (possible score, 0-163; where higher scores indicate greater disease severity)6; decision made to withhold or withdraw life-sustaining therapies; and ICU readmission during the same hospital stay.
Randomization
A centralized, secure, web-based randomization system using minimization assigned patients at a 1:1 ratio, with stratification by study site, prerandomization mechanical ventilation duration (≤14 or >14 days), and number of organ failures according to the Sequential Organ-Failure Assessment7 (SOFA) score (<2 or ≥2). The SOFA score was calculated from 6 variables the day of randomization, taking into account the worst values of each parameter in the 24 hours preceding the randomization. Scores range from 0 to 24, with higher scores indicating more severe organ failure and higher mortality risk. Organ and system failure were deemed present when the corresponding SOFA score was greater than 2. All investigators, statisticians, and data analysts were blinded to arm assignments until the study and analysis were completed.
Study Interventions
Patients were randomized to receive intravenous acyclovir, 5 mg/kg, 3 times daily or a matching placebo preparation (controls) every 8 hours for a maximum duration of 14 days. That dose was chosen because it is recommended to treat immunocompromised patients’ cutaneous HSV infections and has been shown to effectively prevent HSV reactivation in a previous randomized clinical trial.8 For extubated patients discharged from the ICU before day 14 post randomization, the study agent was stopped at discharge. Acyclovir doses were adjusted to renal function according to the manufacturer’s recommendations.9 Placebo and acyclovir powders were conditioned in similar opaque bottles that were distributed post randomization and reconstituted in saline by the nurses before each administration.
By November 16, 2016, although 206 patients had been randomized, the dates of validity of the initially manufactured opaque bottles that contained either the placebo or acyclovir powder had expired. Because funding for replacing the powders was not available, it was decided to continue the trial only in the 2 centers with the highest inclusion rates—Hôpitaux Universitaires Pitié Salpêtrière-Charles Foix and Hôpitaux de Marseille—and to use a different placebo and acyclovir distribution procedure that would keep investigators blind without requiring additional budget. Accordingly, from November 16, 2016, to the end of the trial, when a patient was randomized in the trial, acyclovir was reconstituted in saline bags by a pharmacist or an independent research nurse (both not blinded to the randomization arm) outside of the ICU. The same process was used for the placebo, and bags were distributed to the nurse in charge of the patient. All bags were labeled using protocol labels of Preemptive Treatment for Herpesviridae study drug. As such, the nurses and physicians in charge of the patients in the ICU were not aware of the study drug during the entire study period.
Study Outcomes
The primary outcome was the number of ventilator-free days at day 60 (ie, days alive without mechanical ventilation). For patients who died before day 60, that number was zero, regardless of mechanical ventilation duration. For patients with multiple mechanical ventilation episodes during the 60-day follow-up period, days without mechanical ventilation were considered only after the last weaning process of mechanical ventilation. Secondary outcomes included day 60 mortality rate; mechanical ventilation duration; occurrence of HSV bronchopneumonitis2 or active CMV infection; secondary bacterial pneumonia, bacteremia, or fungemia; acute respiratory distress syndrome; and septic shock post randomization. The main safety end points were acute renal failure and neurotoxicity. The protocol specified that acyclovir could be stopped for stage 3 acute kidney injury as defined by the acute kidney injury network, if the treating physician judged it to be study agent related.10 Potential neurologic complications were accorded special attention. Physicians recorded other safety concerns in the electronic case report form.
The following post hoc analyses were conducted: per-protocol analysis, including patients who had received the study agent for 7 days or more; subgroup analyses according to the number of organ failures at randomization (≤2 or >2) and number of prerandomization days on mechanical ventilation (defined according to the median value of 10 days); subgroup analyses according to the time of randomization (ie, before and after November 16, 2016, corresponding to the time of dispensation of study drug changed); and sensitivity analysis comparing the cumulative incidence of each event (mechanical ventilation weaning and death) to take into account the competition between these 2 events (ie, the occurrence of one type of event [eg, death] will prevent the occurrence of the other [mechanical ventilation weaning]).11 Data on day 90 status and day 60 to 90 mechanical ventilation use were collected retrospectively to calculate day 90 mortality, day 90 ventilator-free days, and mechanical ventilation duration.
Statistical Analysis
According to a previous study evaluating HSV reactivation in patients with prolonged mechanical ventilation, the expected SD of ventilator-free days for controls was 20 days.2 We hypothesized that preemptive acyclovir administration could increase the number of ventilator-free days by 8 days. To have 80% power with a 5% α level, 112 patients had to be included per group after applying a correcting coefficient of 0.864 to adjust for asymptotic test efficiency.12 To account for potential losses to follow-up, that number was raised to 120 per group, meaning that 240 patients had to be included. Because 1 patient withdrew consent, the database was reset at 241 patients for stopping the inclusions. Two patients were inadvertently entered into the web randomization system but were not randomized because they fulfilled an exclusion criterion. When 241 patients were included in the web randomization system, the data manager stopped the inclusions, leaving 239 patients randomized and 2 not randomized.
As proposed recently, we recalculated ventilator-free days in such a manner that death constituted a worse outcome than fewer days off the ventilator.13 Each patient was compared with every other patient in the study and assigned a score (tie: 0, win: +1, loss: −1) for each pairwise comparison based on who fared better. If one patient survived and the other did not, scores of +1 and −1 were assigned, respectively, for that pairwise comparison. If both patients in the pairwise comparison survived, the assigned score depended on which patient had more days free from mechanical ventilation: the patient with more days off the ventilator received a score of +1, and the patient with fewer days received a score of −1. If both patients survived and had the same number of days off the ventilator or if both patients died, they both were assigned a score of 0 for that pairwise comparison. For each patient, scores for all pairwise comparisons were summed, resulting in a cumulative score for each patient. These cumulative scores were ranked and compared between treatment groups via the Mann-Whitney technique. The probability of more favorable outcome is a ranked composite incorporating death and days free from mechanical ventilation among survivors. It represents the estimated probability that an individual randomly selected from 1 treatment group will have a higher score (more favorable outcome) than an individual randomly selected from the other group. It is mathematically equivalent to the area under the receiver operating characteristic curve for the Mann-Whitney test. A value of 50% indicates no difference between groups.13
Data are expressed as median (interquartile range [IQR]), mean (SD), or mean (95% CI), as appropriate. Between-group comparisons used a t test or the Mann-Whitney test for continuous variables according to the variable distribution (ie, normal or not). The normality assumption was tested for each quantitative variable using a Shapiro test if 1 of the numbers was less than 50 or an Anderson-Darling test. If normality was not rejected, we used the t test; otherwise, the Mann-Whitney test was used. For categorical variables, between-group comparisons used χ2 or Fischer exact tests. Censored outcomes (time to death and time to weaning-off mechanical ventilation) were described with the Kaplan-Meier method, with between-group log-rank test comparisons. Proportional hazards assumption was evaluated graphically using Shoenfeld residuals and the Harrell test. Main analyses were conducted on an intention-to-treat basis.
All analyses were computed with R software, version 3.5.1 (R Project for Statistical Computing), at a 2-sided, 5% α level of significance.
Results
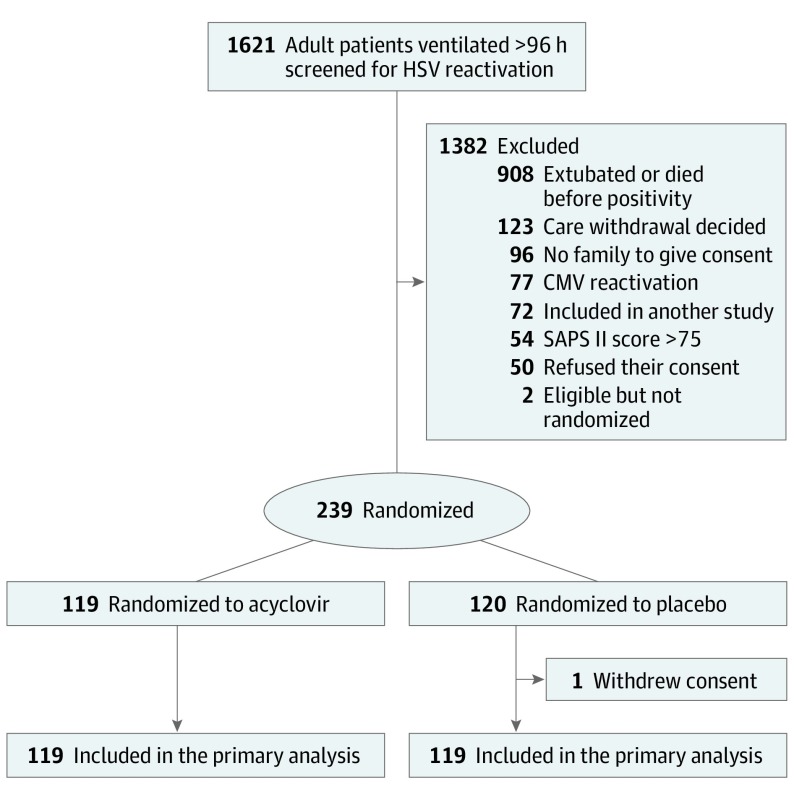
Among the 1621 patients screened for HSV and CMV, 239 were randomized (Figure 1). One placebo-group patient withdrew consent, leaving 238 assessable patients: 119 acyclovir and 119 placebo recipients. Participants’ baseline characteristics at ICU admission (Table 1) differed significantly only for body mass index, which was lower for controls (median [IQR]: acyclovir, 29.3 [26.1-34.3] vs control: 27.2 [23.5-32.1]; calculated as weight in kilograms divided by height in meters squared). Their characteristics at randomization were also comparable (Table 2). The median (IQR) prerandomization mechanical ventilation duration was 10 (8-14) days for both groups.
Figure 1. Study Flowchart.
The Simplified Acute Physiology Score (SAPS) II is assessed on a scale ranging from 0 to 163, with higher scores indicating greater disease severity. CMV indicates cytomegalovirus; HSV, herpes simplex virus.
Table 1. Patient Characteristics at ICU Admissiona.
| Characteristic | Study Group | |
|---|---|---|
| Acyclovir (n = 119) | Placebo (n = 119) | |
| Age, median (IQR), y | 61 (52-68) | 61 (48-71) |
| Men, No. (%) | 78 (66) | 84 (71) |
| Women, No. (%) | 41 (34) | 35 (29) |
| BMI, median (IQR) | 29.3 (26.1-34.3) | 27.2 (23.5-32.1) |
| McCabe and Jackson score >2, No. (%)b | 30 (25) | 26 (22) |
| Preexisting disease, No. (%) | ||
| NYHA III or IV heart failure | 5 (4) | 8 (7) |
| Cancer/hemopathy | 19 (16) | 16 (13) |
| Diabetes | 24 (20) | 31 (26) |
| COPD | 23 (19) | 14 (12) |
| Cirrhosis | 2 (2) | 4 (3) |
| Chronic renal failurec | 16 (13) | 13 (11) |
| Admission category, No. (%) | ||
| Medical | 99 (83) | 96 (81) |
| Emergency surgery | 12 (10) | 14 (12) |
| Planned surgery | 8 (7) | 9 (8) |
| Primary reason for mechanical ventilation, No. (%) | ||
| Septic shock | 16 (13) | 17 (14) |
| Cardiogenic shock | 12 (10) | 14 (12) |
| Shock, other | 1 (1) | 2 (2) |
| Acute respiratory failure | 31 (26) | 25 (21) |
| Postoperative acute respiratory failure | 6 (5) | 12 (10) |
| Exacerbation of chronic respiratory disease | 12 (10) | 9 (8) |
| Trauma | 5 (4) | 5 (4) |
| Coma | 6 (5) | 9 (8) |
| Cardiac arrest | 5 (4) | 5 (4) |
| Other | 25 (21) | 21 (18) |
| SAPS II, median (IQR)d | 45 (35-57) | 46 (37-57) |
| SOFA score, median (IQR)e | 10 (6-13) | 9 (7-12) |
| Organ or system failure, No. (%)f | ||
| Cardiovascular | 72/117 (62) | 77/117 (66) |
| Respiratory | 82/112 (73) | 80/113 (71) |
| Renal | 47 (40) | 43 (36) |
| Central nervous | 23/117 (20) | 27 (23) |
| Hepatic | 1 (1) | 4 (3) |
| Coagulation | 6 (5 | 8 (7) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; IQR, interquartile range; NYHA, New York Heart Association; SAPS, Simplified Acute Physiology Score; SOFA, Sequential Organ-Failure Assessment.
There were no significant between-group differences in characteristics at ICU admission, except for body mass index (P = .002).
Denotes the severity of underlying medical conditions: 0 indicates no underlying disease; 1, nonfatal underlying disease; 2, ultimately fatal (survival >1 to <5 years) underlying disease; and 3, rapidly fatal (survival <1 year) underlying disease.
Creatinine clearance less than 60 mL/min, serum creatinine level greater than 1.7 mg/dL (to convert to micromoles per liter, multiply by 88.4), or chronic dialysis.
Possible score, 0 to 163; higher scores indicate greater disease severity.
Calculated from 6 variables the day of randomization, taking into account the worst values of each parameter in the 24 hours preceding the randomization. Scores range from 0 to 24, with higher scores indicating more severe organ failure and higher mortality risk. Patients with a SOFA score of 10 have a predicted mean chance of survival between 40% and 50%.
Organ or system failure was deemed present when the corresponding SOFA score was greater than 2. When data regarding organ/system failure were missing, number of assessable patients is reported.
Table 2. Patient Characteristics at Randomizationa.
| Characteristic | Study Group | |
|---|---|---|
| Acyclovir (n = 119) | Placebo (n = 119) | |
| MV duration before inclusion, median (IQR), d | 10 (8-14) | 10 (8-14) |
| Time between positive HSV test and randomization, median (IQR), d | 3 (1-4) | 2 (1-5) |
| Ongoing antimicrobial treatment, No. (%) | 88 (74) | 84 (71) |
| ECMO use, No. (%) | 40 (34) | 31 (26) |
| Renal replacement therapy, No. (%) | 36 (30) | 29 (24) |
| SOFA score, median (IQR)b | 7 (5-10) | 8 (5-11) |
| Organ or system failure, No. (%)c | ||
| Cardiovascular | 47 (40) | 53 (45) |
| Respiratory | 69 (58) | 76 (64) |
| Renal | 38 (32) | 32 (27) |
| Central nervous | 19 (16) | 26 (22) |
| Hepatic | 7 (6) | 7 (6) |
| Coagulation | 13 (11) | 9 (8) |
| Temperature, median (IQR), °C | 37.6 (36.6-38.2) | 37.8 (37.1-38.5) |
| White blood cell count, median (IQR), /μL | 12 900 (10 100-17 700) | 13 900 (10 400-20 300) |
| Neutrophil count, median (IQR), /μL | 9900 (7500-14 200) | 11 500 (8700-16 900) |
| Pao2/FiO2, median (IQR), mm Hg | 178 (87-246) | 174 (114-230) |
| Radiologic score, median (IQR) | 5 (2-8) | 6 (3-8) |
Abbreviations: ECMO, extracorporeal membrane oxygenation; HSV, herpes simplex virus; IQR, interquartile range; MV, mechanical ventilation; Pao2/FiO2 (fraction of inspired oxygen), partial oxygen pressure in arterial blood/fraction of inspired oxygen ratio; SOFA, Sequential Organ-Failure Assessment.
SI conversion: To convert neutrophils and white blood cells to ×109 per liter, multiply by 0.001.
There were no significant between-group differences in characteristics at randomization.
Calculated from 6 variables the day of randomization, taking into account the worst values of each parameter in the 24 hours preceding the randomization. Scores range from 0 to 24, with higher scores indicating more severe organ failure and higher mortality risk. Patients with a SOFA score of 7 to 8 have a predicted mean chance of survival between 15% and 20%.
Organ or system failure was deemed present when the corresponding SOFA score was greater than 2.
Study Drug
All patients received 1 or more doses of a study agent. Median (IQR) treatment durations were comparable (Table 3): 14 (11-14) days for acyclovir recipients and 14 (10-14) days for controls (P = .69). Ninety patients (43 acyclovir recipients and 47 controls) stopped the study agent earlier than scheduled. Reasons for discontinuation were 25 deaths (acyclovir, 9; control, 16), 41 ICU discharges (acyclovir, 21; control, 20), 4 acyclovir-related adverse events, HSV bronchopneumonitis in a control patient, active CMV infection or herpes zoster in 2 control patients, 9 physicians’ decisions (acyclovir, 4; control, 5), 4 errors (acyclovir, 3; control, 1), 3 unavailable study agents (acyclovir, 2; control, 1), and 1 unknown reason in a control patient.
Table 3. Outcomes.
| Parameter | Study Group | P Value | |
|---|---|---|---|
| Acyclovir (n = 119) | Placebo (n = 119) | ||
| Primary outcome | |||
| Ventilator-free days at day 60, median (IQR) | 35 (0-53) | 36 (0-50) | .17 |
| Secondary outcomes post randomization | |||
| Day 60 mortality, No. (%) | 26 (22) | 39 (33) | .06 |
| Duration of MV, median (IQR) | 17 (7-30) | 13 (7-23) | .41 |
| Probability of more favorable outcome, %a | 50.78 | 40.48 | .16 |
| ICU length of stay, median (IQR) | 20 (12-41) | 17 (11-31) | .10 |
| HSV bronchopneumonitis, No. (%) | 1 (1) | 4 (3) | .37 |
| Cytomegalovirus infection, No. (%) | 1 (1) | 5 (4) | .21 |
| Hospital-acquired bacterial pneumonia, No. (%) | 58 (49) | 53 (45) | .52 |
| Secondary bacteremia or fungemia, No. (%) | 29 (24) | 27 (23) | .75 |
| ARDS postrandomization, No. (%)b | 14 (12) | 7 (6) | |
| Mild | 2 | 0 | .11 |
| Moderate | 7 | 2 | |
| Severe | 5 | 4 | |
| Septic shock post randomization, No. (%) | 22 (18) | 27 (23) | .42 |
| No. of days with study drug, median (IQR) | 14 (11-14) | 14 (10-14) | .69 |
Abbreviations: ARDS, acute respiratory distress syndrome; HSV, herpes simplex virus; ICU, intensive care unit; IQR, interquartile range; MV, mechanical ventilation; Pao2/FiO2 (fraction of inspired oxygen) partial oxygen pressure in arterial blood/fraction of inspired oxygen ratio; PEEP positive end-expiratory pressure.
Probability of more favorable outcome is a ranked composite incorporating death and days free from mechanical ventilation among survivors. It represents the estimated probability that an individual randomly selected from 1 treatment group will have a higher score (more favorable outcome) than an individual randomly selected from the other group. It is mathematically equivalent to the area under the receiver operating characteristic curve for the Mann-Whitney test. A value of 50% indicates no difference between groups.13
The Berlin definition of ARDS is as follows: mild: Pao2/FiO2 greater than 200 but 300 or less, with positive end expiratory pressure (PEEP) or continuous positive airway pressure (CPAP) 5 cm H2O or more; moderate: Pao2/FiO2 greater than 100 but 200 or less, with PEEP or CPAP 5 cm H2O or more; severe: Pao2/FiO2 100 or less with PEEP 5 cm H2O or more.14
Primary and Secondary End Points
On day 60, the median (IQR) numbers of ventilator-free days were 35 (0-53) for acyclovir recipients and 36 (0-50) for controls (P = .17 for between-group comparison).
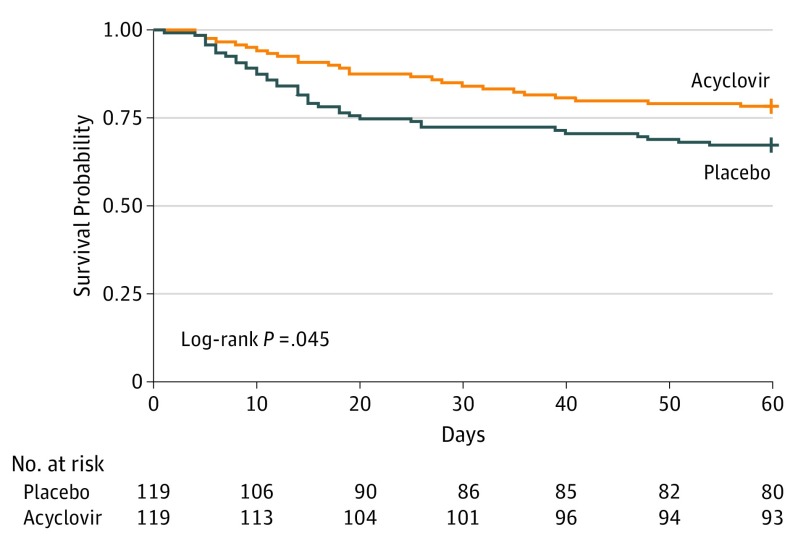
On day 60, 26 acyclovir recipients (22%) and 39 controls (33%) had died (risk difference, 0.11; 95% CI, –0.004 to 0.22; P = .06). The hazard ratio for death within 60 days post randomization for the acyclovir group vs controls was 0.61 (95% CI, 0.37-0.99; P = .047) (Figure 2). No significant violation from the proportional hazards assumption was observed (P = .25). On day 60, 33 acyclovir recipients (28%) and 48 control patients (40%) had died or were still receiving mechanical ventilation (P = .08). Median (IQR) mechanical ventilation duration for day-60 survivors was 17 (6-32) days for acyclovir recipients and 14 (825) days for controls (P = .89). Other secondary end points (Table 3) did not differ between groups. Time to weaning from mechanical ventilation was comparable for the 2 groups (eFigure 1 in Supplement 2).
Figure 2. Survival Analysis Through 60 Days.
Survival estimates in the intention-to-treat population.
Patients’ clinical courses, as assessed by temperature, white blood cell count, radiologic score,15 and modified Clinical Pulmonary Infection Score16 from randomization to day 14, and SOFA score and Pao2/fraction of inspired oxygen ratio kinetics from randomization to day 28 were also comparable for the 2 study groups (eFigures 2-7 in Supplement 2). Microorganisms responsible for bacteremia/fungemia post randomization were also similar between groups (eTable 1 in Supplement 2).
Post Hoc Analyses
The ranked composite end point, incorporating death and days free from mechanical ventilation through day 28, was not significantly different between treatment groups (Table 3). The probability of more favorable outcome with acyclovir compared with placebo was 51%. Subgroup analyses, reported in eTable 2, eTable 3, and eFigure 8 in Supplement 2, showed a significant interaction between day 60 mortality and number of organ failures at randomization but not mechanical ventilation duration before randomization, no significant interaction between ventilator-free days and the number of organ failures at randomization or mechanical ventilation duration before randomization, and no significant interaction between time of randomization and ventilator-free days or day 60 mortality. Sensitivity analysis comparing the cumulative incidence of mechanical ventilation weaning and death displayed similar results (eFigure 9 in Supplement 2).
On day 90, 33 acyclovir recipients (28%) and 41 controls (34%) had died (P = .26 for between-group comparison) (eFigure 10 in Supplement 2). The median (IQR) numbers of day 90 ventilator-free days were comparable for acyclovir recipients (32 [0-53]) and controls (36 [0-50]) (P = .43).
The per-protocol analysis that included patients treated for 7 days or more retained 227 patients: 111 acyclovir recipients and 116 controls. On day 60, the median numbers of ventilator-free days were 31 (0-50) for acyclovir recipients and 34 (0-50) for controls (P = .42). Among those 237 patients, 26 acyclovir recipients (23%) and 39 controls (34%) had died by day 60 (P = .09).
Safety
The adverse event rates were comparable for the 2 groups (eTable 4 in Supplement 2), notably with 3 acyclovir recipients (3%) and 2 controls (2%) experiencing acute renal failure after randomization. Moreover, creatinine levels from randomization to day 14 for the 2 groups did not differ significantly (eFigure 11 in Supplement 2) and the percentages of patients requiring renal replacement therapy from randomization to end of treatment were comparable (eTable 5 in Supplement 2). No neurologic adverse event was reported by the investigators during the study, and Glasgow Coma Scale score changes from day 1 to day 14 were comparable for the groups (eFigure 12 in Supplement 2). Total bilirubin levels and platelet counts from day 1 to day 28 did not differ between groups (eFigure 13 and eFigure 14 in Supplement 2, respectively). Four patients (3%) in the acyclovir group vs none in the placebo group stopped the study drug for treatment-related adverse events.
Discussion
Our study results show that preemptive acyclovir administration, compared with placebo for mechanically ventilated patients with oropharyngeal HSV reactivation, was not associated with shorter mechanical ventilation durations, as assessed by the number of ventilator-free days. Moreover, other secondary outcomes did not differ between acyclovir recipients and controls. Nonsignificant lower day 60 mortality was observed for acyclovir recipients compared with controls. Acyclovir was well tolerated at the dose administered (5 mg/kg 3 times daily), without renal or neurologic adverse events, although our study was underpowered to assess such major complications.
Reactivation of HSV is frequent in ICU patients and associated with increased morbidity2 and mortality.4 However, the exact effect of HSV reactivation in ICU patients remains controversial: it could be a bystander (ie, a marker of disease severity) effect or a pathogen with independent morbidity and mortality.17,18 Reactivation of HSV is the first step before HSV-associated bronchopneumonitis, which can worsen lung inflammation or injury and pave the way for nosocomial bacterial pneumonia, thereby prolonging mechanical ventilation.2,19
Research on HSV reactivation and its prognosis has been published4; however, to our knowledge, Tuxen et al8 conducted the only randomized, double-blind, placebo-controlled trial on acyclovir efficacy against HSV reactivation. They randomized 45 patients with acute respiratory distress syndrome to receive prophylactic acyclovir or placebo and found that acyclovir was associated with significantly less HSV reactivation (6% vs 71%, P < .001), but not with shorter mechanical ventilation durations or lower mortality. Because some patients, even those who are HSV seropositive, will never experience virus reactivation during their ICU stays, this prophylactic strategy of treating before reactivation may unnecessarily expose patients to acyclovir. Because of renal and neurologic toxic effects,20,21 the applicability of prophylactic acyclovir may be questionable. Luyt et al2 compared 42 ICU patients who developed HSV bronchopneumonitis—19 patients given acyclovir, 10 mg/kg, 3 times per day for 5 to 14 days and 23 patients without antiviral treatment. The investigators found that the clinical courses of all patients were similar, including hospital mortality rates (acyclovir, 37%; control, 57%). However, the criteria for treating patients with acyclovir were not defined by the authors. Although treating only patients with documented HSV bronchopneumonitis has the advantage of limiting acyclovir exposure to patients with true disease, it raises several issues, including a complicated definition of disease.2 In addition, Traen et al19 retrospectively analyzed 212 patients with HSV-1–positive respiratory tract or bronchoalveolar lavage-fluid cultures and compared the outcomes of acyclovir recipients (n = 106) vs those not given the drug (n = 106). Acyclovir was associated with lower in-ICU and in-hospital mortality rates, even after adjustment in multivariable Cox or propensity-score analyses. However, that analysis was retrospective and, despite adjustment, potential confounders might not have been taken into account (notably, the decision to administer acyclovir based on unknown criteria).
To our knowledge, no published study has evaluated the effect of a preemptive strategy for ICU patients with HSV reactivation. Such a strategy has several advantages. First, acyclovir is given only to patients with HSV reactivation, therefore, not exposing those without reactivation to this potentially toxic drug. Second, patients are treated before lung disease occurs, and some authors have suggested that HSV might induce lung injury, paving the way for bacterial infection.2,17 This kind of strategy is applied successfully for CMV in solid-organ recipients; preemptive treatment of CMV reactivation seems to be, at least for some transplanted organs, noninferior to a prophylactic approach.22 However, use of preemptive treatment implies implementing regular screening of patients susceptible to HSV reactivation.
We were unable to demonstrate any beneficial effect of acyclovir in ICU patients. This result could have been due to a lack of power of our study. As indicated above, some investigators have postulated that acyclovir could improve the prognosis of ICU patients having reactivated HSV by limiting the bronchioalveolar damage secondary to viral infection, and/or by preventing CMV replication, even the anti-CMV effect of acyclovir is usually observed with higher doses than the ones used in our study.1,2,4,17 Although the acyclovir group had a nonsignificant lower day 60 mortality, the number of ventilator-free days was comparable to that of placebo-treated patients, implying that survivors in the acyclovir group had a longer mechanical ventilation duration (ie, fewer ventilator-free days) than the placebo recipient survivors. This longer duration of mechanical ventilation in acyclovir survivors might be due to the higher number of patients with extracorporeal membrane oxygenation support and the higher percentage of patients developing acute respiratory distress syndrome after randomization. Whether acyclovir could improve the survival of mechanically ventilated patients with reactivated HSV at the cost of a prolonged mechanical ventilation duration is unknown and remains to be explored. Whether targeting a more narrowly defined population (ie, patients with <2 organ failures) might have changed our results also remains to be determined.
Limitations
One major limitation of our study was the lack of serial monitoring of HSV shedding in respiratory tract secretions. It is known that HSV reactivation begins in the throat within the first 10 days of mechanical ventilation,1 followed by a descending contamination of the bronchial tree. Using quantitative real-time polymerase chain reaction testing for the quantitative detection of HSV DNA, De Vos et al23 described the time-course of HSV shedding in lower respiratory tract secretions and found that HSV emerged in tracheal and bronchial aspirates after a median of 7 (IQR, 5-11 days) days of intubation, followed by an exponential increase to reach very high HSV peaks (106-1010 copies/mL) in 78% of the HSV DNA-positive patients.24 Therefore, although we cannot exclude that patients with HSV oropharyngeal reactivation could evolve toward a rapid, spontaneous resolution of HSV reactivation without acyclovir, this resolution appears to be uncommon in ICU patients requiring prolonged mechanical ventilation. Conversely, we cannot be sure whether acyclovir, 5 mg/kg, 3 times daily was able to stop virus reactivation and prevent lower respiratory tract colonization and/or infection in our study, although this dose has been shown to be effective to prevent HSV reactivation in a previous randomized clinical trial.8
Our study has other limitations. First, whether the study population and standard of care in the ICUs that participated in the Preemptive Treatment for Herpesviridae trial were similar to those observed elsewhere could be debatable. However, patients’ clinical characteristics in our study were comparable to those reported in a large, nonselected database of patients who were mechanically ventilated.25 Second, although our treatment groups did not differ significantly, acyclovir recipients received slightly more frequent, but not statistically significant, extracorporeal membrane oxygenation support and renal replacement therapy at randomization, and more developed acute respiratory tract distress syndrome post randomization, it remains unknown whether those findings could have affected the results. Third, using ventilator-free days as the primary outcome may be debated.24 Fourth, we cannot exclude the possibility that some patients may have had HSV viremia or HSV organ disease without previous oropharyngeal reactivation and that we could have missed these patients. In addition, our primary hypothesis (8-day increase of ventilator-free days) was perhaps too optimistic, and our sample size may have limited the study’s ability to detect a true effect.
Conclusions
It appears that for ICU patients with HSV reactivation in the throat while receiving mechanical ventilation for 96 hours or more, acyclovir, 5 mg/kg, 3 times daily compared with placebo was unable to increase the day 60 number of ventilator-free days.
Study Protocol
eTable 1. Microorganisms Responsible for Bacteremia/Fungemia Post-Randomization According to Study Group
eTable 2. Ventilator-Free Days and Mortality at Day 60 According to Subgroup Analysis
eTable 3. Ventilator-Free Days and Day-60 Mortality According to Time of Randomization
eTable 4. Adverse Events
eTable 5. Percentages of Patients Receiving Renal Replacement Therapy According to Study Group From Randomization to Day 21
eFigure 1. Time to Weaning Off Mechanical Ventilation According to Study Group
eFigure 2. Temperature Kinetics from Randomization to Day 14 According to Study Group
eFigure 3. White Blood-Cell–Count Kinetics from Randomization to Day 14 According to Study Group
eFigure 4. Kinetics of Radiologic Score1 from Randomization to Day 14 According to Study Group
eFigure 5. Evolution of Modified Clinical Pulmonary Infection Score (mCPIS) From Randomization to Day 14 According to Study Group
eFigure 6. Kinetics of the Sequential Organ-Failure Assessment (SOFA) Score From Randomization to Day 28 According to Study Group
eFigure 7. Evolution of PaO2/FiO2 Ratio from Randomization to Day 28 According to Study Group
eFigure 8. Subgroup Analyses of Day 60 Mortality
eFigure 9. Sensitivity Analysis Comparing the Cumulative Incidence of Each Event; Weaning of Mechanical Ventilation and Death
eFigure 10. Kaplan-Meier 90-Day Survival Estimates for the Intention-To-Treat Population
eFigure 11. Creatinine-Level Kinetics from Randomization to Day 28 According to Study Group
eFigure 12. Glasgow Coma Score Kinetics from Randomization to Day 14 According to Study Group
eFigure 13. Bilirubin-Level Kinetics from Randomization to Day 28 According to Study Group
eFigure 14. Platelet-Count Kinetics from Randomization to Day 28 According to Study Group
eReferences.
Data Sharing Statement
References
- 1.Bruynseels P, Jorens PG, Demey HE, et al. Herpes simplex virus in the respiratory tract of critical care patients: a prospective study. Lancet. 2003;362(9395):1536-1541. doi: 10.1016/S0140-6736(03)14740-X [DOI] [PubMed] [Google Scholar]
- 2.Luyt C-E, Combes A, Deback C, et al. Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. Am J Respir Crit Care Med. 2007;175(9):935-942. doi: 10.1164/rccm.200609-1322OC [DOI] [PubMed] [Google Scholar]
- 3.Linssen CFM, Jacobs JA, Stelma FF, et al. Herpes simplex virus load in bronchoalveolar lavage fluid is related to poor outcome in critically ill patients. Intensive Care Med. 2008;34(12):2202-2209. doi: 10.1007/s00134-008-1231-4 [DOI] [PubMed] [Google Scholar]
- 4.Coisel Y, Bousbia S, Forel J-M, et al. Cytomegalovirus and herpes simplex virus effect on the prognosis of mechanically ventilated patients suspected to have ventilator-associated pneumonia. PLoS One. 2012;7(12):e51340. doi: 10.1371/journal.pone.0051340 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Begg C, Cho M, Eastwood S, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276(8):637-639. doi: 10.1001/jama.1996.03540080059030 [DOI] [PubMed] [Google Scholar]
- 6.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957-2963. doi: 10.1001/jama.1993.03510240069035 [DOI] [PubMed] [Google Scholar]
- 7.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure; on behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707-710. doi: 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 8.Tuxen DV, Wilson JW, Cade JF. Prevention of lower respiratory herpes simplex virus infection with acyclovir in patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1987;136(2):402-405. doi: 10.1164/ajrccm/136.2.402 [DOI] [PubMed] [Google Scholar]
- 9.Résumé Des Caractéristiques Du Produit http://agence-prd.ansm.sante.fr/php/ecodex/rcp/R0229767.htm. Accessed.
- 10.Mehta RL, Kellum JA, Shah SV, et al. ; Acute Kidney Injury Network . Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141-1154. doi: 10.1214/aos/1176350951 [DOI] [Google Scholar]
- 12.Hollander M, Wolfe D. Nonparametric Statistical Methods. 2nd ed New York: John Wiley and Sons; 1999. [Google Scholar]
- 13.Beitler JR, Sarge T, Banner-Goodspeed VM, et al. ; EPVent-2 Study Group . Effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure-guided strategy vs an empirical high PEEP-FiO2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2019;321(9):846-857. doi: 10.1001/jama.2019.0555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranieri VM, Rubenfeld GD, Thompson BT, et al. ; ARDS Definition Task Force . Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533. doi: 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 15.Weinberg PF, Matthay MA, Webster RO, Roskos KV, Goldstein IM, Murray JF. Biologically active products of complement and acute lung injury in patients with the sepsis syndrome. Am Rev Respir Dis. 1984;130(5):791-796. doi: 10.1164/arrd.1984.130.5.791 [DOI] [PubMed] [Google Scholar]
- 16.Luyt C-E, Chastre J, Fagon J-Y. Value of the clinical pulmonary infection score for the identification and management of ventilator-associated pneumonia. Intensive Care Med. 2004;30(5):844-852. doi: 10.1007/s00134-003-2125-0 [DOI] [PubMed] [Google Scholar]
- 17.Luyt C-E, Bréchot N, Chastre J. What role do viruses play in nosocomial pneumonia? Curr Opin Infect Dis. 2014;27(2):194-199. doi: 10.1097/QCO.0000000000000049 [DOI] [PubMed] [Google Scholar]
- 18.Forel J-M, Martin-Loeches I, Luyt C-E. Treating HSV and CMV reactivations in critically ill patients who are not immunocompromised: pro. Intensive Care Med. 2014;40(12):1945-1949. doi: 10.1007/s00134-014-3445-y [DOI] [PubMed] [Google Scholar]
- 19.Traen S, Bochanen N, Ieven M, et al. Is acyclovir effective among critically ill patients with herpes simplex in the respiratory tract? J Clin Virol. 2014;60(3):215-221. doi: 10.1016/j.jcv.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 20.Ryan L, Heed A, Foster J, Valappil M, Schmid ML, Duncan CJA. Acute kidney injury (AKI) associated with intravenous aciclovir in adults: Incidence and risk factors in clinical practice. Int J Infect Dis. 2018;74:97-99. doi: 10.1016/j.ijid.2018.07.002 [DOI] [PubMed] [Google Scholar]
- 21.Ernst ME, Franey RJ. Acyclovir- and ganciclovir-induced neurotoxicity. Ann Pharmacother. 1998;32(1):111-113. doi: 10.1345/aph.17135 [DOI] [PubMed] [Google Scholar]
- 22.Kotton CN, Kumar D, Caliendo AM, et al. ; The Transplantation Society International CMV Consensus Group . The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation. 2018;102(6):900-931. doi: 10.1097/TP.0000000000002191 [DOI] [PubMed] [Google Scholar]
- 23.De Vos N, Van Hoovels L, Vankeerberghen A, et al. Monitoring of herpes simplex virus in the lower respiratory tract of critically ill patients using real-time PCR: a prospective study. Clin Microbiol Infect. 2009;15(4):358-363. doi: 10.1111/j.1469-0691.2009.02704.x [DOI] [PubMed] [Google Scholar]
- 24.Bodet-Contentin L, Frasca D, Tavernier E, Feuillet F, Foucher Y, Giraudeau B. Ventilator-free day outcomes can be misleading. Crit Care Med. 2018;46(3):425-429. doi: 10.1097/CCM.0000000000002890 [DOI] [PubMed] [Google Scholar]
- 25.Bellani G, Laffey JG, Pham T, et al. ; LUNG SAFE Investigators; ESICM Trials Group . Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788-800. doi: 10.1001/jama.2016.0291 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol
eTable 1. Microorganisms Responsible for Bacteremia/Fungemia Post-Randomization According to Study Group
eTable 2. Ventilator-Free Days and Mortality at Day 60 According to Subgroup Analysis
eTable 3. Ventilator-Free Days and Day-60 Mortality According to Time of Randomization
eTable 4. Adverse Events
eTable 5. Percentages of Patients Receiving Renal Replacement Therapy According to Study Group From Randomization to Day 21
eFigure 1. Time to Weaning Off Mechanical Ventilation According to Study Group
eFigure 2. Temperature Kinetics from Randomization to Day 14 According to Study Group
eFigure 3. White Blood-Cell–Count Kinetics from Randomization to Day 14 According to Study Group
eFigure 4. Kinetics of Radiologic Score1 from Randomization to Day 14 According to Study Group
eFigure 5. Evolution of Modified Clinical Pulmonary Infection Score (mCPIS) From Randomization to Day 14 According to Study Group
eFigure 6. Kinetics of the Sequential Organ-Failure Assessment (SOFA) Score From Randomization to Day 28 According to Study Group
eFigure 7. Evolution of PaO2/FiO2 Ratio from Randomization to Day 28 According to Study Group
eFigure 8. Subgroup Analyses of Day 60 Mortality
eFigure 9. Sensitivity Analysis Comparing the Cumulative Incidence of Each Event; Weaning of Mechanical Ventilation and Death
eFigure 10. Kaplan-Meier 90-Day Survival Estimates for the Intention-To-Treat Population
eFigure 11. Creatinine-Level Kinetics from Randomization to Day 28 According to Study Group
eFigure 12. Glasgow Coma Score Kinetics from Randomization to Day 14 According to Study Group
eFigure 13. Bilirubin-Level Kinetics from Randomization to Day 28 According to Study Group
eFigure 14. Platelet-Count Kinetics from Randomization to Day 28 According to Study Group
eReferences.
Data Sharing Statement