Key Points
Question
Will the observed shortened life expectancy of adult survivors of childhood cancer lengthen over time given improvements in treatment and care?
Findings
Using a simulation model–based approach, this study estimates that children who received a diagnosis of and were treated for cancer in the 1990s will live longer into adulthood than those diagnosed in the 1970s. Despite improvements, these individuals remain at risk for a shortened lifespan owing to severe treatment-related late toxic effects.
Meaning
Evolving treatment approaches are projected to be associated with improved life expectancy after treatment for pediatric cancer, in particular among individuals who did not receive radiotherapy during childhood cancer treatment.
Abstract
Importance
Advances in childhood and adolescent cancer treatment have been associated with increased rates of cure during the past 3 decades; however, improvement in adult life expectancy for these individuals has not yet been reported.
Objectives
To project long-term survival and assess whether life expectancy will improve among adult survivors of childhood cancer who were treated in more recent decades.
Design, Setting, and Participants
A microsimulation model of competing mortality risks was developed using data from the Childhood Cancer Survivor Study on 5-year survivors of childhood cancer diagnosed between 1970 and 1999. The model included (1) late recurrence, (2) treatment-related late effects (health-related [subsequent cancers, cardiac events, pulmonary conditions, and other] and external causes), and (3) US background mortality rates.
Exposures
Treatment subgroups (no treatment or surgery only, chemotherapy alone, radiotherapy alone, and radiotherapy with chemotherapy) and individuals with acute lymphoblastic leukemia during childhood by era (1970-1979, 1980-1989, and 1990-1999).
Main Outcomes and Measures
Conditional life expectancy (defined as the number of years a 5-year survivor can expect to live), cumulative cause-specific mortality risk, and 10-year mortality risks conditional on attaining ages of 30, 40, 50, and 60 years.
Results
Among the hypothetical cohort of 5-year survivors of childhood cancer representative of the Childhood Cancer Survivor Study participants (44% female and 56% male; mean [SD] age at diagnosis, 7.3 [5.6] years), conditional life expectancy was 48.5 years (95% uncertainty interval [UI], 47.6-49.6 years) for 5-year survivors diagnosed in 1970-1979, 53.7 years (95% UI, 52.6-54.7 years) for those diagnosed in 1980-1989, and 57.1 years (95% UI, 55.9-58.1 years) for those diagnosed in 1990-1999. Compared with individuals without a history of cancer, these results represented a gap in life expectancy of 25% (95% UI, 24%-27%) (16.5 years [95% UI, 15.5-17.5 years]) for those diagnosed in 1970-1979, 19% (95% UI, 17%-20%) (12.3 years [95% UI, 11.3-13.4 years]) for those diagnosed in 1980-1989, and 14% (95% UI, 13%-16%) (9.2 years [95% UI, 8.3-10.4 years]) for those diagnosed in 1990-1999. During the 3 decades, the proportion of survivors treated with chemotherapy alone increased (from 18% in 1970-1979 to 54% in 1990-1999), and the life expectancy gap in this chemotherapy-alone group decreased from 11.0 years (95% UI, 9.0-13.1 years) to 6.0 years (95% UI, 4.5-7.6 years). In contrast, during the same time frame, only modest improvements in the gap in life expectancy were projected for survivors treated with radiotherapy (21.0 years [95% UI, 18.5-23.2 years] to 17.6 years [95% UI, 14.2-21.2 years]) or with radiotherapy and chemotherapy (17.9 years [95% UI, 16.7-19.2 years] to 14.8 years [95% UI, 13.1-16.7 years]). For the largest group of survivors by diagnosis—those with acute lymphoblastic leukemia—the gap in life expectancy decreased from 14.7 years (95% UI, 12.8-16.5 years) in 1970-1979 to 8.0 years (95% UI, 6.2-9.7 years).
Conclusions and Relevance
Evolving treatment approaches are projected to be associated with improved life expectancy after treatment for pediatric cancer, in particular among those who received chemotherapy alone for their childhood cancer diagnosis. Despite improvements, survivors remain at risk for shorter lifespans, especially when radiotherapy was included as part of their childhood cancer treatment.
This decision analytical model–based study uses data from the Childhood Cancer Survivor Study to project long-term survival and assess whether life expectancy will improve among adult survivors of childhood cancer who were treated in more recent decades.
Introduction
Advances in pediatric cancer therapy during the past few decades have been associated with remarkable increases in 5-year survival rates, with more than 80% of children and adolescents who receive a diagnosis today expected to live at least 5 years.1,2 By 2020, the population of childhood cancer survivors is projected to grow to more than 500 000 individuals.3,4 As adults, childhood cancer survivors face substantially elevated risks of serious long-term morbidity and premature death, with nearly 1 in 3 survivors reporting a severe or life-threatening condition 20 years after diagnosis.5 A study using a model-based approach estimated that late recurrence and excess mortality risks from secondary cancers, cardiac disease, and other late effects reduced projected life expectancy by as much as 28% among survivors who originally received a diagnosis of childhood cancer between 1970 and 1986.6 However, the association of childhood cancer with life expectancy after more modern therapy, often chosen based on concerns of late toxic effects, is not known.
Clinical trialists in pediatric oncology, who recognized the risk for life-threatening late effects, have focused modern clinical trials to study the outcome of treatments that reduce or eliminate exposure to radiotherapy and/or lower cumulative dose exposures to anthracyclines and other chemotherapies. Lower treatment exposures have been associated with an observed decrease in late mortality among survivors in the 15 years immediately after their cancer diagnosis,7 yet their association with length of life is unknown. We sought to leverage newly available late mortality data on childhood cancer survivors who received a diagnosis between 1970 and 1999 to update previous estimates of projected life expectancy and examine whether survivors of childhood and adolescent cancer diagnosed in recent treatment eras can expect to live longer as a result of changes in pediatric cancer treatment.
Methods
Model Overview
We developed a microsimulation model to estimate projections of long-term survival for childhood and adolescent cancer survivors diagnosed between 1970 and 1999 using data from the Childhood Cancer Survivor Study (CCSS). The CCSS is a multi-institutional retrospective cohort study with longitudinal follow-up of North American 5-year survivors of common cancers (leukemia, central nervous system tumors, Hodgkin lymphoma, non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, soft-tissue sarcoma, and bone tumors) diagnosed prior to 21 years of age and between 1970 and 1999.8 In the microsimulation model, a cohort of 5-year survivors representative of the CCSS enters the model (eFigure 1 in the Supplement). Each year, individuals face competing risks of dying from background mortality (age-specific mortality rates in the general population), their original cancer (late recurrence), and late effects (including health-related [subsequent cancers, cardiac events, pulmonary conditions, and other causes] and external causes [accidents, suicides, and poisonings]). Individuals are followed up throughout their lifetime (or until 100 years of age). The study was determined not to be human participants research as defined in federal regulations by the Boston Children’s Hospital Institutional Review Board.
Using the model, we projected long-term survival outcomes for a cohort of survivors over three 10-year treatment eras (1970-1979, 1980-1989, and 1990-1999). Subgroup analyses included 4 mutually exclusive treatment subgroups (no treatment or surgery only, chemotherapy alone, radiotherapy alone, and chemotherapy combined with radiotherapy [chemoradiotherapy]), as well as a subset of survivors with a diagnosis of acute lymphoblastic lymphoma (ALL), the only diagnosis with a sufficient number of participants (n = 5794) to model as an independent group. In addition, we simulated a hypothetical parallel cohort of individuals representative of the general population matched 1 to 1 with the observed cohort of cancer survivors (based on age at diagnosis, sex, and year of diagnosis). These individuals were therefore only at risk of dying from background mortality rates and served as the general population comparator in all analyses.
Model outcomes included conditional life expectancy (defined as the number of additional years a 5-year survivor can expect to live); cumulative cause-specific mortality risk at the ages of 30, 40, 50, 60, and 70 years; and 10-year mortality risk conditional on reaching the ages of 30, 40, 50, and 60 years. We calculated the difference in simulated life expectancy between the cohort of survivors and the parallel cohort of matched general population individuals to estimate the difference, or gap, in life expectancy between the 2 groups. Relative measures were calculated as a percentage of the life expectancy of the matched general population individuals. Excess cause-specific mortality risks among survivors (defined as the risk in addition to baseline risks in the general population) were estimated using a similar approach.
To more fully account for uncertainty in late mortality risks, we conducted 1000 simulations in which we bootstrapped the cohort members and sampled from parameter sets identified via model calibration that produced model outcomes consistent with observed CCSS data. For all modeled outcomes, we report the mean and 95% uncertainty interval (UI; calculated as the 2.5 and 97.5 percentile among 1000 simulations). The model was programmed in Java, version 1.8.0 (Oracle Corp); statistical analyses were performed in Stata, version 14 (StataCorp LLC) and R, version 3.3.1 (R Foundation for Statistical Computing). See eMethods in the Supplement for additional details on model development.
Data Sources
We used data from 22 150 CCSS participants as the basis of the model (eTable 1 in the Supplement). For generalizability to the larger population of US childhood cancer survivors for the diagnoses included in the CCSS, we used inverse probability weighting (based on sex, institution where the survivor was treated, primary cancer diagnosis, age at diagnosis, and year of diagnosis among all survivors eligible to participate in the CCSS, as well as sampling weights to account for undersampling of survivors of ALL diagnosed between 1987 and 1999) in the calculation of all outcomes to account for potential nonparticipation bias.9 Treatment subgroup was based on CCSS medical record abstraction that recorded cancer treatments received within 5 years of initial diagnosis. For survivors with missing treatment information, the treatment subgroup was based on multiple-imputation methods.7,10
Because excess treatment-related mortality risks are determined largely by treatment exposures associated with original cancer treatment, we focused on the following broad treatment subgroups by era: no treatment or surgery only, chemotherapy alone, radiotherapy alone, and chemoradiotherapy. Owing to limited sample size, we evaluated only 1 diagnostic subgroup—patients with ALL—independently by era. For each treatment subgroup and era, as previously described,7 we estimated annual mortality rates due to late recurrence or progression of original cancer; absolute excess mortality rates for external causes, including accidents, suicides, and poisonings; and absolute excess mortality rates due to health-related causes, including subsequent (primary) cancers, cardiac causes, pulmonary causes, and all other causes (see eMethods in the Supplement for International Classification of Diseases, Ninth Revision and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes). Background mortality rates were based on age-, sex-, and calendar year–specific mortality rates for the US population.11
Model Assumptions
Although the CCSS provides estimates of late mortality risks during the earlier decades after diagnosis, data for longer follow-up times are not available. Furthermore, follow-up time varied by treatment era, with survivors who received a diagnosis during the 1990-1999 era having shorter follow-up time than those who received a diagnosis during the 1970-1979 era. Consistent with trends observed in the CCSS7 and Surveillance, Epidemiology, and End Results Program (SEER),12 we made the following assumptions: (1) imputed (ie, censored) excess mortality rates for health-related causes were equal to or lower than rates observed for earlier treatment eras, (2) excess mortality rates due to external causes did not vary by subgroup or treatment decade (owing to the small number of observed related deaths), (3) mortality rates due to late recurrence decreased with number of years since diagnosis (within treatment era), and (4) all late mortality rates remained constant at rates observed during the interval of 35 or more years since diagnosis (because data beyond this time point were not yet available).
Results
Conditional Life Expectancy
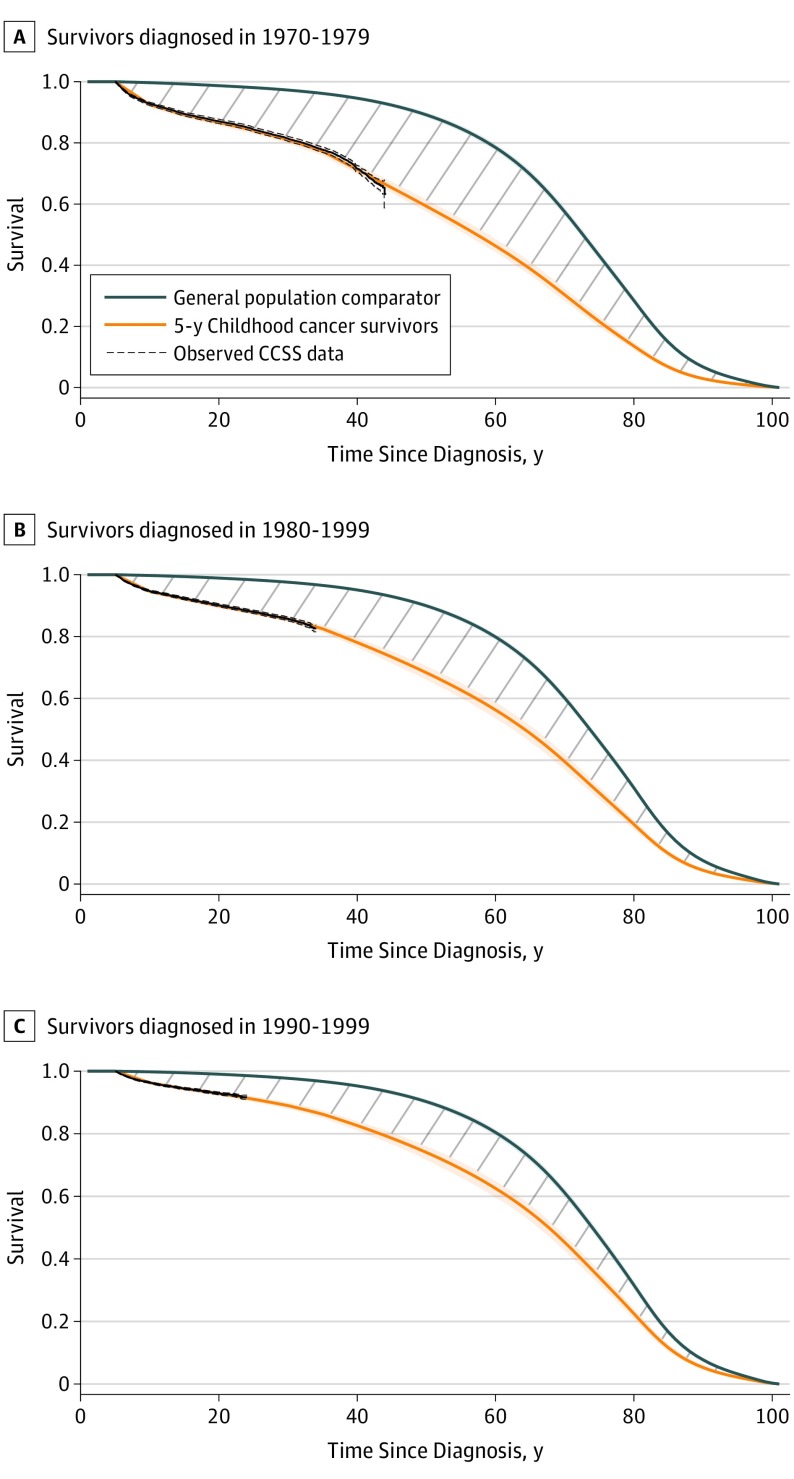
Figure 1 shows projected overall survival for the overall cohort of 5-year childhood and adolescent cancer survivors (44% female and 56% male; mean [SD] age at diagnosis, 7.3 [5.6] years) and the general population comparator by 10-year treatment era. Conditional life expectancy, or the number of years of survival starting at 5 years from diagnosis, improved during the 3 decades. Conditional life expectancy was 48.5 years (95% UI, 47.6-49.6 years) for survivors who received a diagnosis during the 1970-1979 era, 53.7 years (95% UI, 52.6-54.7 years) for survivors who received a diagnosis during the 1980-1989 era, and 57.1 years (95% UI, 55.9-58.1 years) for survivors who received a diagnosis during the 1990-1999 era (Table). Represented by the area between the survival curves, the gap in life expectancy of the survivors compared with the general population comparator was 16.5 years (95% UI, 15.5-17.5 years) or 25% (95% UI, 24%-27%) for those who received diagnosis during the 1970-1979 era, 12.3 years (95% UI, 11.3-13.4 years) or 19% (95% UI, 17%-20%) for those who received a diagnosis during the 1980-1989 era, and 9.2 years (95% UI, 8.3-10.4 years) or 14% (95% UI, 13%-16%) for those who received a diagnosis during the 1990-1999 era (eFigure 2A in the Supplement).
Figure 1. Projected Survival Curves for Childhood Cancer Survivors and General Population Comparators.
A, Survivors who received a childhood cancer diagnosis during the 1970-1979 treatment era. Life expectancy gap, 16.5 years (95% uncertainty interval [UI], 15.5-17.5 years). B, Survivors who received a childhood cancer diagnosis during the 1980-1989 treatment era. Life expectancy gap, 12.3 years (95% UI, 11.3-13.4 years). C, Survivors who received a childhood cancer diagnosis during the 1990-1999 treatment era. Life expectancy gap, 9.2 years (95% UI, 8.3-10.4 years). Shaded regions indicate the 95% UIs. The areas under the curves represent the projected life expectancy for the general population comparator and 5-year survivors. The area between the curves, as indicated by the diagonal gray lines, represents the gap in life expectancy between the 5-year cancer survivors and the general population comparator. Observed data from the Childhood Cancer Survivor Study (CCSS) are shown by the dashed lines.
Table. Conditional Life Expectancy Among Survivors and General Population Comparator.
| Subgroup | Diagnosis Age, Mean, y | Conditional Life Expectancy (95% Uncertainty Interval)a | |||
|---|---|---|---|---|---|
| General Population Comparator, y | 5-y Survivors of Childhood and Adolescent Cancer, y | Gap in Life Expectancyb | |||
| Years | % | ||||
| Overall cohort | |||||
| 1970-1979 | 8.1 | 65.1 (64.6-65.5) | 48.5 (47.6-49.6) | 16.5 (15.5-17.5) | 25.4 (23.8-26.8) |
| 1980-1989 | 7.2 | 66.0 (65.7-66.3) | 53.7 (52.6-54.7) | 12.3 (11.3-13.4) | 18.6 (17.1-20.3) |
| 1990-1999 | 6.9 | 66.3 (65.9-66.7) | 57.1 (55.9-58.1) | 9.2 (8.3-10.4) | 13.9 (12.5-15.6) |
| No treatment or surgery only | |||||
| 1970-1979 | 7.3 | 65.9 (64.2-67.7) | 58.9 (55.5-62.2) | 7.0 (4.2-10.1) | 10.6 (6.4-15.3) |
| 1980-1989 | 6.0 | 67.3 (66.0-68.6) | 61.0 (58.6-63.3) | 6.3 (4.2-8.4) | 9.3 (0.06-0.12) |
| 1990-1999 | 6.4 | 67.1 (66.1-68.0) | 61.2 (59.3-63.2) | 5.8 (4.0-7.5) | 8.7 (6.3-12.4) |
| Chemotherapy alone | |||||
| 1970-1979 | 7.8 | 65.3 (64.4-66.3) | 54.3 (52.1-56.5) | 11.0 (9.0-13.1) | 16.8 (13.8-20.0) |
| 1980-1989 | 6.2 | 67.0 (66.4-67.7) | 59.2 (57.2-60.9) | 7.9 (6.2-9.8) | 11.7 (9.3-14.5) |
| 1990-1999 | 6.1 | 67.1 (66.4-67.7) | 61.1 (59.5-62.7) | 6.0 (4.5-7.6) | 8.9 (6.6-11.3) |
| Radiotherapy alone | |||||
| 1970-1979 | 10.9 | 62.3 (61.3-63.4) | 41.3 (39.2-43.8) | 21.0 (18.5-23.2) | 33.7 (29.7-37.1) |
| 1980-1989 | 9.9 | 63.4 (62.4-64.5) | 44.5 (41.7-47.1) | 18.9 (16.4-21.7) | 29.8 (25.9-34.2) |
| 1990-1999 | 9.6 | 63.8 (62.2-65.5) | 46.2 (42.8-49.4) | 17.6 (14.2-21.2) | 27.5 (22.5 -33.0) |
| Chemoradiotherapy | |||||
| 1970-1979 | 7.5 | 65.6 (65.0-66.1) | 47.6 (46.4-48.9) | 17.9 (16.7-19.2) | 27.3 (25.5-29.2) |
| 1980-1989 | 7.6 | 65.5 (65.0-66.0) | 50.1 (48.4-51.6) | 15.4 (14.0-17.1) | 23.5 (21.4-26.1) |
| 1990-1999 | 8.2 | 65.0 (64.4-65.7) | 50.3 (48.4-52.0) | 14.8 (13.1-16.7) | 22.7 (20.1-25.6) |
| Acute lymphoblastic leukemia only | |||||
| 1970-1979 | 5.6 | 67.6 (66.8-68.3) | 52.9 (51.0-54.8) | 14.7 (12.8-16.5) | 21.8 (18.9-24.5) |
| 1980-1989 | 5.7 | 67.8 (67.1-68.4) | 56.8 (54.9-59.2) | 10.9 (8.5-12.9) | 16.1 (12.5-18.9) |
| 1990-1999 | 7.1 | 67.7 (66.8-68.6) | 59.7 (57.9-61.6) | 8.0 (6.2-9.7) | 11.8 (9.1-14.2) |
Conditional on surviving cancer for 5 years and the matched time points for the general population comparator.
Compared with the general population comparator.
To understand what factors may be associated with the narrowing of the gap in life expectancy among survivors treated in more recent decades, we examined the composition of diagnoses and treatments by treatment era. The distribution of most cancer diagnoses in the survivor cohorts remained largely stable across eras (eTable 1 and eFigure 3A in the Supplement) except for a slight increase in the proportion of survivors of ALL. In contrast, the composition of the cohort in terms of treatment subgroups changed over time. The proportion treated with chemotherapy alone increased during the 3 decades from 18% during the 1970-1979 era to 37% during the 1980-1989 era to 54% during the 1990-1999 era (eFigure 3B in the Supplement); the proportion treated with any radiotherapy (alone or with chemotherapy) decreased from 77% during the 1970-1979 era to 56% during the 1980-1989 era to 36% during the 1990-1999 era.
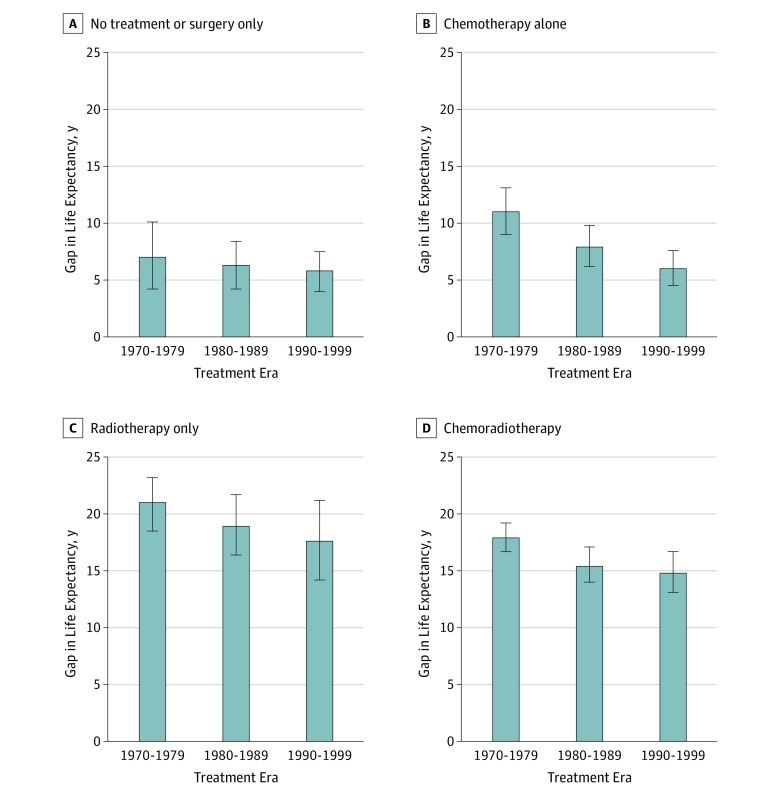
Changes in the gap in life expectancy varied by treatment subgroup (Figure 2). Between the 1970-1979 era and the 1990-1999 era, the gap in life expectancy among the chemotherapy-alone subgroup decreased by nearly half from 11.0 years (95% UI, 9.0-13.1 years) to 6.0 years (95% UI, 4.5-7.6 years). Only modest improvements in the gap in life expectancy between the 1970-1979 era and the 1990-1999 era were projected for survivors treated with radiotherapy alone (21.0 years [95% UI, 18.5-23.2 years] to 17.6 years [95% UI, 14.2-21.2 years]) or chemoradiotherapy (17.9 years [95% UI, 16.7-19.2 years] to 14.8 years [95% UI, 13.1-16.7 years]). The gap in life expectancy remained stable between the 1970-1979 era and the 1990-1999 era for the no treatment or surgery-only subgroup (7.0 years [95% UI, 4.2-10.1 years] to 5.8 years [95% UI, 4.0-7.5 years]).
Figure 2. Projected Gap in Life Expectancy Among Childhood Cancer Survivors.
A, Projected gaps in life expectancy for survivors who received no treatment or surgery only vs the age-, sex-, and decade-matched general population comparator. B, Projected gaps in life expectancy for survivors who received chemotherapy alone vs the general population comparator. C, Projected gaps in life expectancy for survivors who received radiotherapy alone vs the general population comparator. D, Projected gaps in life expectancy for survivors who received chemoradiotherapy vs the general population comparator. Error bars indicate 95% uncertainty intervals.
For the ALL subgroup, the gap in life expectancy between the 1970-1979 era and the 1990-1999 era decreased from 14.7 years (95% UI, 12.8-16.5 years) to 8.0 years (95% UI, 6.2-9.7 years) (eFigure 2B in the Supplement). During this period, treatment for most survivors of ALL shifted from chemoradiotherapy (83%) to chemotherapy alone (78%).
Cumulative Cause-Specific Mortality Risks
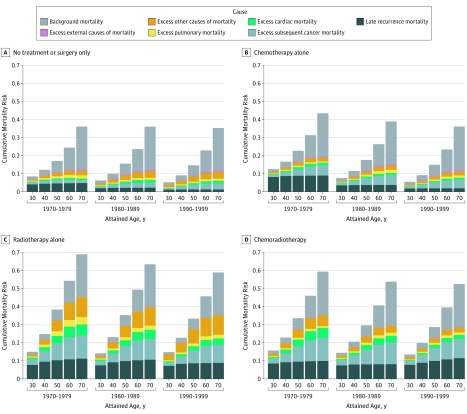
As the cohort aged, cumulative late recurrence and excess late mortality risks for secondary cancers, cardiac events, pulmonary, and other causes (at the ages of 30, 40, 50, 60, and 70 years) decreased by treatment era (eFigure 4A and eTable 2 in the Supplement). Simultaneously, a slight increase in the cumulative excess mortality risk for external causes was projected owing to competing mortality risks (ie, lower rates of other nonrecurrence late mortality risks associated with survivors living longer and being at risk for dying from accidents, suicides, and poisonings). Results for treatment subgroups are shown in Figure 3, and results for survivors of ALL are shown in in eFigure 4B in the Supplement.
Figure 3. Projected Cumulative Mortality Risks Among Childhood Cancer Survivors.
A, Cumulative cause-specific mortality risks between the ages of 30 and 70 years for patients receiving no treatment or surgery only. B, Cumulative cause-specific mortality risks between the ages of 30 and 70 years for patients receiving chemotherapy alone. C, Cumulative cause-specific mortality risks between the ages of 30 and 70 years for patients receiving radiotherapy alone. D, Cumulative cause-specific mortality risks between the ages of 30 and 70 years for patients receiving chemoradiotherapy.
Conditional 10-Year Mortality Risks by Attained Age
Compared with the general population, the relative risk of dying within 10 years for survivors at each age decreased by treatment era (eFigure 5 in the Supplement); relative risks were lowest among the surgery-alone and chemotherapy-only subgroups. For instance, for a patient treated in 1970 with either no treatment or surgery alone who reached the age of 30 years, the relative risk of dying in the next decade was 3.4 (95% UI, 1.4-9.8) compared with the general population risk, and for a patient treated in 1990, the relative risk was 2.9 (95% UI, 1.8-4.9). In contrast, the relative risk of dying in the next decade for a patient treated in 1970 with chemoradiotherapy was 5.7 (95% UI, 4.3-7.5), and for a patient treated in 1990, the relative risk was 5.1 (95% UI, 3.5-7.3). Background mortality risk surpassed childhood cancer-related mortality risks at the age of 40 years for survivors who received no treatment or were treated with surgery only and at the age of 50 years for those treated with chemotherapy alone (eFigure 6 in the Supplement). Background mortality risk did not surpass childhood cancer mortality until the age of 60 years for those treated with chemoradiotherapy (all eras) or radiotherapy alone (beginning in the 1990-1999 treatment era only). Background mortality risk surpassed cancer-related mortality risks at the age of 60 years for survivors of ALL diagnosed in the 1970s, whereas for those treated in the 1980s and 1990s, background mortality risk surpassed cancer-related mortality risks at 50 years of age.
Discussion
Using a model-based approach, we found that changes in treatment for pediatric cancer during the past 3 decades are projected to be associated with improved life expectancy among 5-year survivors of childhood and adolescent cancer. Compared with individuals without a history of cancer, the projected life expectancy of survivors diagnosed in the 1970s is approximately 25% lower than in the general population owing to late recurrence and health-related late mortality risks. However, this projected gap in life expectancy is expected to narrow to 14% among more recently diagnosed survivors of childhood cancer. These model-based observations are likely to be associated with reductions in the use of radiotherapy and changes in chemotherapy regimens associated with late mortality and were generally consistent with observed changes in therapeutic exposures (eTables 3, 4, 5, and 6 in the Supplement). Although our findings suggest that long-term survival is expected to improve, adult survivors of childhood cancer remain at risk for a shortened lifespan.
Our findings of improved life expectancy based on the decade of diagnosis and treatment are consistent with known changes in pediatric cancer treatment during the past decades, including the omission of radiotherapy for ALL, acute myeloid leukemia, and non-Hodgkin lymphoma and the shift away from the use of radiotherapy for some solid tumors.13,14 Although previous studies have reported decreasing late mortality risk ratios7,15 or have described or estimated late effects risks,5,16,17 our study is the first, to our knowledge, to project changes in life expectancy among childhood cancer survivors. By quantifying the effect of late mortality risks using metrics comparable to the general population (or other disease populations), these estimates provide important and useful benchmarks for research and can aid in clinical care. Care of childhood cancer survivors who are now adults requires informed conversations about long-term expectations, including reassurance of young adult survivors about mortality risks that can guide preventive medical care recommendations and discussions around family planning and other life choices. Our findings emphasize the importance for survivors to adhere to follow-up care and risk reduction behaviors because subsequent primary cancers and cardiac events were the leading causes of early mortality in all subgroups. Guidelines for care based on specific exposures during childhood are provided by the Children’s Oncology Group (http://www.survivorshipguidelines.org/) and include recommendations for secondary breast cancer screening, colon cancer screening, and heart disease assessments for at-risk survivors.
Our findings highlight the need for new therapeutic approaches for cancer diagnoses for which radiotherapy remains an integral component of local control of the disease and for the vigilant care of survivors who received radiotherapy as children. Although the percentage of survivors in the CCSS treated with radiotherapy alone has decreased to less than 5%, the projected risk of treatment-related late mortality remains high for this subgroup. Improvements in life expectancy for survivors treated with chemoradiotherapy, a group constituting nearly one-third of survivors, have also been modest. New precision approaches—possibly using genetic markers to guide treatment, such as risk markers for susceptibility to radiogenic cancers or heart failure—may serve to minimize late effect risks in the future.18 In addition, our findings suggest that, as health-related late mortality risks decrease, more survivors are at risk for dying from external causes, including accidents, suicides, and poisonings. Although these findings are suggestive, they highlight the need for additional data on these risks and a greater understanding of survivors’ mental health needs.19,20
Limitations
Our study has several limitations. First, our findings are based on data from the CCSS, which does not include less common childhood cancer diagnoses, such as retinoblastoma, but represent the best available data on late mortality risks among US childhood cancer survivors. In addition, for the diagnoses included, CCSS participants are similar in terms of age at diagnosis, sex, race/ethnicity, and major cancer type to those reported in SEER.3 Second, because CCSS participants who received a more recent (1980-1999) diagnosis had shorter follow-up time, simplifying assumptions were necessary to project late mortality risks. We assumed that absolute excess late mortality rates remained constant 35 years after diagnosis21,22 and were equal to or lower than rates observed in the prior treatment decade as data suggesting otherwise were not yet available,7,12 potentially underestimating mortality risks and thereby overestimating the projected improvement in survivor life expectancy. For example, with a longer follow-up time, it may become apparent that observed decreases in cause-specific deaths among survivors who received a diagnosis in the 1990s are only delayed. In addition, as background mortality rates increase with age, we could have potentially overestimated total mortality rates by assuming constant absolute excess mortality. Third, the composition of diagnoses within each treatment subgroup changed over time, which may limit direct comparability across eras. We were unable to assess data on the comparability of treatment patterns between the CCSS and SEER because SEER lacks complete and detailed treatment information. Fourth, given the sample size needed for stable late mortality estimates, we were unable to stratify the treatment subgroups into specific diagnoses (except for ALL) or detailed treatment exposure levels (eg, cumulative anthracycline dose, chest radiotherapy dose, or evolving radiotherapy dose and field). Fifth, treatment data for relapsed cancer or secondary cancers occurring 5 years after diagnosis were not captured in the treatment exposure data used to define treatment subgroups. Although not likely a large number, some survivors in the chemotherapy-alone subgroup may have received radiotherapy as a part of relapse therapy occurring more than 5 years after initial diagnosis. Sixth, our estimates are based on survivors treated prior to 2000 and may not reflect even newer therapeutic approaches, such as immunotherapy and increasingly response-adapted or risk-adapted regimens. As longer follow-up data become available, our model can be refined and updated. Seventh, our estimates do not consider a number of factors that might be associated with survivor well-being (aside from mortality); next steps in our work include incorporating quality-of-life aspects to more completely portray long-term health for children and adolescents with a diagnosis of cancer.
Conclusions
Our model-based findings suggest that advances in treatment for cancer during the past decades can be expected to improve life expectancy for survivors of childhood and adolescent cancer, in particular for those treated with chemotherapy alone. However, life expectancy among adult survivors of childhood cancer remains compromised even decades after initial diagnosis. Our findings highlight the need for continued monitoring of survivors’ health to manage late mortality risks and underscore the need for new therapeutic approaches to minimize early mortality risks, especially for cancer diagnoses for which radiotherapy remains a key component of therapy.
eMethods. Additional Methodological Details on Model Development
eFigure 1. Model Diagram
eFigure 2. Projected Gap in Life Expectancy Among Survivors Diagnosed in 1970s, 1980s, and 1990s: Overall Cohort and Acute Lymphoblastic Leukemia (ALL) Subgroup
eFigure 3. Cohort Composition by Treatment Era
eFigure 4. Projected Cumulative Mortality Risks Among Survivors Diagnosed in 1970s, 1980s, and 1990s: Overall Cohort and Acute Lymphoblastic Leukemia (ALL) Subgroup
eFigure 5. Projected Relative 10-Year Mortality Risk Among Survivors Diagnosed in 1970-79, 1980-89, and 1990-99 by Attained Age (Compared to General Population)
eFigure 6. Cause-Specific Attributable Proportion of Projected 10-Year Mortality Risk Conditional Upon Surviving to Age 30, 40, 50, and 60 Among Survivors Diagnosed in 1970-79, 1980-89, and 1990-99
eTable 1. Patient Characteristics and Treatment Subgroup: Overall Cohort of Survivors of Childhood and Adolescent Cancer
eTable 2. Model Results: Cause-Specific Cumulative Risks by Age 30, 40, 50, 60, and 70
eTable 3. Patient Characteristics and Therapeutic Exposures: Chemotherapy Alone Subgroup
eTable 4. Patient Characteristics and Therapeutic Exposures: Radiotherapy Alone Subgroup
eTable 5. Patient Characteristics and Therapeutic Exposures: Chemoradiotherapy Subgroup
eTable 6. Patient Characteristics and Therapeutic Exposures: Acute Lymphoblastic Leukemia Subgroup
References
- 1.Hewitt M, Greenfield S, Stovall E, eds; National Research Council . From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academies Press; 2005. [Google Scholar]
- 2.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):83-103. doi: 10.3322/caac.21219 [DOI] [PubMed] [Google Scholar]
- 3.Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24(4):653-663. doi: 10.1158/1055-9965.EPI-14-1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14(1):61-70. doi: 10.1038/nrc3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibson TM, Mostoufi-Moab S, Stratton KL, et al. Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970-99: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2018;19(12):1590-1601. doi: 10.1016/S1470-2045(18)30537-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeh JM, Nekhlyudov L, Goldie SJ, Mertens AC, Diller L. A model-based estimate of cumulative excess mortality in survivors of childhood cancer. Ann Intern Med. 2010;152(7):409-417, W131-W138. doi: 10.7326/0003-4819-152-7-201004060-00005 [DOI] [PMC free article] [PubMed]
- 7.Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;374(9):833-842. doi: 10.1056/NEJMoa1510795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute–supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308-2318. doi: 10.1200/JCO.2009.22.3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robins J. Marginal structural models versus structural nested models as tools for causal inference. In: Halloran M, Berry D, eds. Statistical Models in Epidemiology: The Environment and Clinical Trials. Vol IMA. Vol 116. New York: Springer-Verlag; 1999:95-134. doi: 10.1007/978-1-4612-1284-3_2 [DOI] [Google Scholar]
- 10.Little RJA, Rubin DB. Statistical Analysis With Missing Data. 2nd ed. New York: John Wiley; 2002. doi: 10.1002/9781119013563 [DOI] [Google Scholar]
- 11.Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics . Compressed mortality file: years 1968-1978 with ICD-8 codes, 1979-1998 with ICD-9 codes and 1999-2016 with ICD-10 codes. https://wonder.cdc.gov/wonder/help/cmf.html. Updated November 19, 2019. Accessed September 18, 2016.
- 12.Armstrong GT, Pan Z, Ness KK, Srivastava D, Robison LL. Temporal trends in cause-specific late mortality among 5-year survivors of childhood cancer. J Clin Oncol. 2010;28(7):1224-1231. doi: 10.1200/JCO.2009.24.4608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hudson MM, Neglia JP, Woods WG, et al. Lessons from the past: opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr Blood Cancer. 2012;58(3):334-343. doi: 10.1002/pbc.23385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green DM, Kun LE, Matthay KK, et al. Relevance of historical therapeutic approaches to the contemporary treatment of pediatric solid tumors. Pediatr Blood Cancer. 2013;60(7):1083-1094. doi: 10.1002/pbc.24487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fidler MM, Reulen RC, Winter DL, et al. ; British Childhood Cancer Survivor Study Steering Group . Long term cause specific mortality among 34 489 five year survivors of childhood cancer in Great Britain: population based cohort study. BMJ. 2016;354:i4351. doi: 10.1136/bmj.i4351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Essig S, Li Q, Chen Y, et al. Risk of late effects of treatment in children newly diagnosed with standard-risk acute lymphoblastic leukaemia: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2014;15(8):841-851. doi: 10.1016/S1470-2045(14)70265-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet. 2017;390(10112):2569-2582. doi: 10.1016/S0140-6736(17)31610-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chow EJ, Antal Z, Constine LS, et al. New agents, emerging late effects, and the development of precision survivorship. J Clin Oncol. 2018;36(21):2231-2240. doi: 10.1200/JCO.2017.76.4647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunnes MW, Lie RT, Bjørge T, et al. Suicide and violent deaths in survivors of cancer in childhood, adolescence and young adulthood—a national cohort study. Int J Cancer. 2017;140(3):575-580. doi: 10.1002/ijc.30474 [DOI] [PubMed] [Google Scholar]
- 20.Brinkman TM, Zhang N, Recklitis CJ, et al. Suicide ideation and associated mortality in adult survivors of childhood cancer. Cancer. 2014;120(2):271-277. doi: 10.1002/cncr.28385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mertens AC. Cause of mortality in 5-year survivors of childhood cancer. Pediatr Blood Cancer. 2007;48(7):723-726. doi: 10.1002/pbc.21114 [DOI] [PubMed] [Google Scholar]
- 22.Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2328-2338. doi: 10.1200/JCO.2008.21.1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Additional Methodological Details on Model Development
eFigure 1. Model Diagram
eFigure 2. Projected Gap in Life Expectancy Among Survivors Diagnosed in 1970s, 1980s, and 1990s: Overall Cohort and Acute Lymphoblastic Leukemia (ALL) Subgroup
eFigure 3. Cohort Composition by Treatment Era
eFigure 4. Projected Cumulative Mortality Risks Among Survivors Diagnosed in 1970s, 1980s, and 1990s: Overall Cohort and Acute Lymphoblastic Leukemia (ALL) Subgroup
eFigure 5. Projected Relative 10-Year Mortality Risk Among Survivors Diagnosed in 1970-79, 1980-89, and 1990-99 by Attained Age (Compared to General Population)
eFigure 6. Cause-Specific Attributable Proportion of Projected 10-Year Mortality Risk Conditional Upon Surviving to Age 30, 40, 50, and 60 Among Survivors Diagnosed in 1970-79, 1980-89, and 1990-99
eTable 1. Patient Characteristics and Treatment Subgroup: Overall Cohort of Survivors of Childhood and Adolescent Cancer
eTable 2. Model Results: Cause-Specific Cumulative Risks by Age 30, 40, 50, 60, and 70
eTable 3. Patient Characteristics and Therapeutic Exposures: Chemotherapy Alone Subgroup
eTable 4. Patient Characteristics and Therapeutic Exposures: Radiotherapy Alone Subgroup
eTable 5. Patient Characteristics and Therapeutic Exposures: Chemoradiotherapy Subgroup
eTable 6. Patient Characteristics and Therapeutic Exposures: Acute Lymphoblastic Leukemia Subgroup