This cohort study investigates the associations of low-carbohydrate and low-fat diets with total and cause-specific mortality among US adults.
Key Points
Question
What are the associations of types of low-carbohydrate and low-fat diets with mortality among US adults?
Findings
In this cohort study of 37 233 US adults 20 years or older, overall low-carbohydrate and low-fat diets were not associated with total mortality, but a healthy low-carbohydrate diet (lower amounts of low-quality carbohydrates and higher amounts of plant protein and unsaturated fat) and a healthy low-fat diet (lower amounts of saturated fat and higher amounts of high-quality carbohydrates and plant protein) were associated with lower total mortality.
Meaning
The associations of low-carbohydrate and low-fat diets with mortality may depend on the quality and food sources of macronutrients.
Abstract
Importance
It is crucial to incorporate quality and types of carbohydrate and fat when investigating the associations of low-fat and low-carbohydrate diets with mortality.
Objective
To investigate the associations of low-carbohydrate and low-fat diets with total and cause-specific mortality among US adults.
Design, Setting, and Participants
This prospective cohort study used data from the US National Health and Nutrition Examination Survey from 1999 to 2014 from 37 233 adults 20 years or older with 24-hour dietary recall data. Data were analyzed from July 5 to August 27, 2019.
Exposures
Overall, unhealthy, and healthy low-carbohydrate-diet and low-fat-diet scores based on the percentage of energy as total and subtypes of carbohydrate, fat, and protein.
Main Outcomes and Measures
All-cause mortality from baseline until December 31, 2015, linked to National Death Index mortality data.
Results
A total of 37 233 US adults (mean [SD] age, 49.7 [18.3] years; 19 598 [52.6%] female) were included in the present analysis. During 297 768 person-years of follow-up, 4866 total deaths occurred. Overall low-carbohydrate-diet and low-fat-diet scores were not associated with total mortality. The multivariable-adjusted hazard ratios for total mortality per 20-percentile increase in dietary scores were 1.07 (95% CI, 1.02-1.11; P = .01 for trend) for unhealthy low-carbohydrate-diet score, 0.91 (95% CI, 0.87-0.95; P < .001 for trend) for healthy low-carbohydrate-diet score, 1.06 (95% CI, 1.01-1.12; P = .04 for trend) for unhealthy low-fat-diet score, and 0.89 (95% CI, 0.85-0.93; P < .001 for trend) for healthy low-fat-diet score. The associations remained similar in the stratification and sensitivity analyses.
Conclusions and Relevance
In this study, overall low-carbohydrate-diet and low-fat-diet scores were not associated with total mortality. Unhealthy low-carbohydrate-diet and low-fat-diet scores were associated with higher total mortality, whereas healthy low-carbohydrate-diet and low-fat-diet scores were associated with lower total mortality. These findings suggest that the associations of low-carbohydrate and low-fat diets with mortality may depend on the quality and food sources of macronutrients.
Introduction
Diet plays an important role in the public health, and suboptimal diet is estimated as the first leading cause of death and the third leading cause of disability-adjusted life-years lost in the United States.1 Among most populations worldwide, carbohydrate is the primary source of energy, providing 50% or more of daily energy, with lesser amounts from fat and protein.2 Long-standing controversies have focused on the health consequences of dietary fat and carbohydrate. Some dietary guidelines have continued to recommend a low-fat diet (LFD) for prevention of chronic diseases,3 although inconsistent associations have been reported between total fat consumption and health outcomes.4,5,6,7 A low-carbohydrate diet (LCD) has become a popular strategy for weight loss and weight management in recent years,8 but the long-term associations of carbohydrate-restricted diets with health outcomes remain controversial.9,10
Beyond the quantity, evidence has indicated that quality and food sources of carbohydrate and fat play an important role in human diseases and health.2,8,11 Consumption of carbohydrates from refined grains and added sugars has been adversely associated with health outcomes, whereas consumption of carbohydrates from whole grains, nonstarchy vegetables, and whole fruits appears to be beneficial.2,12,13 Likewise, replacing saturated fat with unsaturated fat was associated with lower risk of heart disease and mortality.14,15 During the past 2 decades, the overall macronutrient composition among US adults generally remained stable, but types of carbohydrate and fat intake changed substantially.16 However, to our knowledge, no studies have investigated the associations of LFDs and LCDs with mortality by considering quality and types of carbohydrate and fat.
Using data from the US National Health and Nutrition Examination Survey (NHANES), we created overall, unhealthy, and healthy LCD and LFD scores based on total and types of macronutrients and investigated the associations of these dietary scores with total and cause-specific mortality. We hypothesized that unhealthy LCD and LFD scores were associated with higher mortality, whereas healthy LCD and LFD scores were associated with lower mortality.
Methods
Study Design and Population
This cohort study included data on adults 20 years or older who completed at least 1 dietary recall during the 8 cycles of NHANES from 1999 to 2014. NHANES is a nationally representative, cross-sectional study performed since 1999 by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention to assess information on health and nutritional status of the noninstitutionalized civilian population in the United States.17 Data were analyzed from July 5 to August 27, 2019. The NHANES study protocol was approved by the research ethics review board of the NCHS, and all participants provided written informed consent. The Institutional Review Board at the Harvard T.H. Chan School of Public Health determined that this analysis used public data sets that did not constitute human subjects research, so human subjects approval was not needed.
Information on NHANES study design, study protocol, and data collection methods has been reported previously.18 Sampling weights were used in NHANES to account for the complex study design (eg, oversampling of minorities), survey nonresponses, and poststratification.19 For this analysis, we excluded individuals with potentially unreliable dietary intake (n = 1697), defined as very low or high caloric intake (<800 or >4200 kcal/d in men and <600 or >3500 kcal/d in women) and individuals with no linked mortality data (n = 69).
Assessments of LCD and LFD Scores
In NHANES, diet was assessed using 24-hour dietary recalls (eAppendix in the Supplement). We used the percentage of energy from each macronutrient instead of absolute intake to reduce bias owing to underreporting of food consumption and to represent dietary composition.20,21 We divided the participants into 11 sex-specific strata each of percentage of energy from fat, protein, and carbohydrate (eTable 1 in the Supplement). For fat and protein, individuals in the highest stratum received 10 points and those in the lowest stratum received 0 points. For carbohydrate, the order of the strata was reversed. The points for the 3 macronutrients were then summed to create the overall LCD score, which ranged from 0 to 30. Therefore, the higher the score, the more closely the participant’s diet followed the pattern of an overall LCD. We also created 2 additional LCD scores: unhealthy LCD score was calculated according to the percentage of energy from high-quality carbohydrate, animal protein, and saturated fat; healthy LCD score was calculated according to the percentage of energy from low-quality carbohydrate, plant protein, and unsaturated fat (eTable 1 in the Supplement). Similar approaches were used to create overall, unhealthy, and healthy LFD scores (eTable 2 in the Supplement).
Ascertainment of Mortality
Mortality from all causes, heart disease, and cancer was identified through linkage to the National Death Index through December 31, 2015. The primary outcome was mortality from all causes, and the secondary outcome was mortality from heart diseases and cancer. Death from heart disease was defined as codes I00-09, I11, I13, and I20-51 using the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10), and death from cancer was defined as code C00-97. These codes were recorded when they were listed as the underlying cause of death. Follow-up time was defined as the interval from the 24-hour recall interview to the date of death for individuals who had died or to December 31, 2015, for participants who were censored.
Demographic and Lifestyle Factors and Comorbidity Conditions
Demographic and lifestyle factors, including age, sex, race/ethnicity, educational level, income, smoking, and physical activity, were collected during household interviews via standardized questionnaires. Alcohol intake, body weight, and height were obtained from participants who received physical examinations at a mobile examination center. Participants reported their race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, or other) according to categories provided by the NCHS. Mexican Americans and other Hispanic groups were combined to create the Hispanic group. Three educational levels were categorized (less than high school graduate, high school graduate or General Educational Development, or some college or above). Family income was classified as the ratio of family income to poverty and categorized into 3 levels (<1.30, 1.30-3.49, or ≥3.50). For missing data on educational level (n = 51) and income (n = 3037), data were imputed with median values. Smokers were defined as individuals who reported smoking at least 100 cigarettes during their lifetime, and participants who drank a minimum of 12 drinks in any given year were classified as drinkers. Physical activity was calculated by summarizing hours of self-reported moderate to vigorous activity during leisure time per week. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared.2 Comorbidity conditions (cancer, heart disease, stroke, diabetes, hypercholesterolemia, and hypertension) were defined if participants reported that they had ever been told by a health care professional that they had such conditions and/or to take prescribed medications because of these conditions.
Statistical Analysis
All analyses incorporated sample weights, stratification, and clustering of the complex sampling design to ensure nationally representative estimates. The multivariable Cox proportional hazards regression model was used to assess the hazard ratios (HRs) and 95% CIs of mortality associated with the LCD and LFD scores. The 6 LCD and LFD scores were categorized into quintiles. Person-years were calculated from the date of interview to the date of death or the end of follow-up, whichever occurred first. Model 1 was adjusted for age, sex, and race/ethnicity. In multivariate analyses, we further adjusted for educational level, family income, smoking, alcohol drinking, physical activity, total energy intake, BMI, family history of diabetes and heart disease, and histories of diabetes, heart disease, and cancer. The trends were estimated by treating the quintiles as a continuous variable. A 20-percentile increase in each score was used to estimate the HRs for mortality from heart disease, cancer, and other causes.
We further applied stratification analysis for associations between diet scores and total mortality according to several potential confounding factors at baseline. To evaluate the potential modification by subgroups, we used the survey-weighted Wald F statistic to test for an interaction between the diet score and subgroups variable. Given the large number of tests being performed, we adjusted the P value for multiple testing using the Bonferroni correction, and statistical significance was set at P < .001 (0.05/10 [subgroups] × 6 [dietary scores]) to account for type I error. We conducted several sensitivity analyses to test the robustness of our findings. First, we further adjusted the prevalence of hypertension and hypercholesterolemia at baseline, which could be the mediators of these associations. Second, we excluded the participants with a history of heart disease or cancer. Third, we excluded the participants who died during the first year of follow-up. All analyses were conducted using SAS statistical software, version 9.4 (SAS Institute Inc), and statistical significance was set at a 2-tailed P < .05.
Results
Participant Characteristics
A total of 37 233 US adults (mean [SD] age, 49.7 [18.3] years; 19 598 [52.6%] female) were included in the present analysis. During 297 768 person-years of follow-up, 4866 total deaths occurred, including 849 deaths from heart disease and 1068 deaths from cancer. Table 1 gives the characteristics of study participants at baseline according to quintiles of LCD and LFD scores. Participants with a higher overall LCD score were more likely to be older and non-Hispanic white; to have higher BMI, educational level, income level, and cholesterol intake; and to have lower total energy intake. With increasing overall LCD score, a greater proportion of participants had morbidity conditions (cancer, heart disease, diabetes, hypercholesterolemia, and hypertension). Similar results were observed for the healthy LCD score. In contrast, participants with a higher unhealthy LCD score tended to be younger and smokers and were unlikely to have morbidity conditions. Conversely, those with higher LFD scores tended to be minorities, to be nonsmokers, and to have lower BMI, intake of total energy, and cholesterol levels. With increasing overall and unhealthy LFD scores, a greater proportion of participants had lower educational and income levels. Significant correlations were found between LCD and LFD scores (r = −0.77 between unhealthy LCD and healthy LFD scores; r = −0.76 between healthy LCD and unhealthy LFD scores; r = 0.33 between the healthy LCD and LFD scores; and r = 0.33 between the healthy LCD and LFD scores) (eTable 3 in the Supplement).
Table 1. Characteristics of Study Participants According to Quintiles of Low-Carbohydrate-Diet and Low-Fat-Diet Scoresa.
| Characteristic | Overall Low-Carbohydrate-Diet Score | Unhealthy Low-Carbohydrate-Diet Score | Healthy Low-Carbohydrate-Diet Score | Overall Low-Fat-Diet Score | Unhealthy Low-Fat-Diet Score | Healthy Low-Fat-Diet Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 5 | Quintile 1 | Quintile 5 | Quintile 1 | Quintile 5 | Quintile 1 | Quintile 5 | Quintile 1 | Quintile 5 | Quintile 1 | Quintile 5 | |
| Median score (IQR) | 4 (3-6) | 26 (24-28) | 7 (4-8) | 23 (22-25) | 5 (3-7) | 24 (23-26) | 7 (5-8) | 23 (22-25) | 8 (6-9) | 22 (21-24) | 6 (4-7) | 25 (23-27) |
| Participants, No. | 7239 | 7375 | 7674 | 7777 | 7685 | 7586 | 7845 | 7697 | 8410 | 8443 | 7993 | 7945 |
| Age, mean (SD), y | 47.8 (19.0) | 50.7 (17.1) | 57.0 (17.4) | 42.8 (16.3) | 40.8 (16.8) | 56.2 (16.2) | 49.4 (17.2) | 51.7 (18.6) | 55.3 (16.6) | 43.6 (17.9) | 39.8 (15.1) | 58.1 (16.7) |
| BMI, mean (SD) | 28.3 (6.47) | 29.7 (7.01) | 27.8 (5.81) | 29.6 (7.38) | 28.7 (6.90) | 29.1 (6.57) | 29.6 (7.29) | 28.1 (6.11) | 29.1 (6.78) | 28.5 (6.54) | 29.5 (7.61) | 27.9 (5.79) |
| Female | 3784 (52.3) | 3876 (52.6) | 4029 (52.5) | 4065 (52.3) | 4062 (52.9) | 3955 (52.1) | 4188 (53.4) | 4105 (53.3) | 4424 (52.6) | 4432 (52.5) | 4215 (52.7) | 4167 (52.4) |
| Race/ethnicity | ||||||||||||
| Non-Hispanic white | 3035 (41.9) | 4259 (57.7) | 2942 (38.3) | 4628 (59.5) | 3429 (44.6) | 4099 (54.0) | 5063 (64.5) | 2098 (27.3) | 5064 (60.2) | 3063 (36.3) | 4849 (60.7) | 2625 (33.0) |
| Non-Hispanic black | 1637 (22.6) | 1305 (17.7) | 1341 (17.5) | 1664 (21.4) | 1965 (25.6) | 1129 (14.9) | 1713 (21.8) | 1247 (16.2) | 1547 (18.4) | 1713 (20.3) | 2128 (26.6) | 1120 (14.1) |
| Hispanic | 2143 (29.6) | 1415 (19.2) | 2496 (32.5) | 1256 (16.2) | 1986 (25.8) | 1695 (22.3) | 912 (11.6) | 3177 (41.3) | 1355 (16.1) | 2936 (34.8) | 896 (11.2) | 2868 (36.1) |
| Other | 424 (5.9) | 396 (5.4) | 895 (11.7) | 229 (2.9) | 305 (4.0) | 663 (8.7) | 157 (2.0) | 1175 (15.3) | 444 (5.3) | 731 (8.7) | 120 (1.5) | 1332 (16.8) |
| Educational level | ||||||||||||
| Less than high school | 2207 (30.5) | 1783 (24.2) | 2321 (30.2) | 1868 (24.0) | 2253 (29.3) | 1783 (23.5) | 1635 (20.8) | 2973 (38.6) | 1754 (20.9) | 2938 (34.8) | 1843 (23.1) | 2642 (33.3) |
| High school graduate or GED | 1669 (23.1) | 1622 (22.0) | 1471 (19.2) | 2037 (26.2) | 2019 (26.3) | 1558 (20.5) | 2008 (25.6) | 1451 (18.9) | 1856 (22.1) | 1919 (22.7) | 2290 (28.7) | 1371 (17.3) |
| Some college or above | 3349 (46.3) | 3966 (53.8) | 3866 (50.4) | 3864 (49.7) | 3405 (44.3) | 4240 (55.9) | 4197 (53.5) | 3255 (42.3) | 4789 (56.9) | 3570 (42.3) | 3852 (48.2) | 3917 (49.3) |
| Family income to poverty ratio | ||||||||||||
| <1.3 | 2298 (31.7) | 1790 (24.3) | 1953 (25.4) | 2306 (29.7) | 2637 (34.3) | 1628 (21.5) | 1904 (24.3) | 2523 (32.8) | 1715 (20.4) | 3023 (35.8) | 2503 (31.3) | 2095 (26.4) |
| 1.3-3.49 | 2503 (34.6) | 2471 (33.5) | 2639 (34.4) | 2634 (33.9) | 2737 (35.6) | 2534 (33.4) | 2678 (34.1) | 2583 (33.6) | 2877 (34.2) | 2908 (34.4) | 2773 (34.7) | 2653 (33.4) |
| ≥3.5 | 1827 (25.2) | 2595 (35.2) | 2361 (30.8) | 2345 (30.2) | 1754 (22.8) | 2864 (37.8) | 2754 (35.1) | 1805 (23.5) | 3208 (38.1) | 1785 (21.1) | 2244 (28.1) | 2398 (30.2) |
| Dietary intake, mean (SD) | ||||||||||||
| Total energy, kcal/d | 2159 (408) | 1847 (354) | 2001 (394) | 1988 (394) | 2175 (419) | 1857 (367) | 2128 (389) | 1807 (356) | 2032 (388) | 1941 (410) | 2201 (403) | 1815 (367) |
| Total carbohydrate, % of total energy intake | 58.1 (2.38) | 46.3 (2.76) | 56.2 (3.49) | 48.0 (3.76) | 56.8 (3.00) | 47.9 (3.53) | 48.1 (3.19) | 56.4 (2.88) | 48.7 (4.00) | 56.0 (3.38) | 50.4 (4.44) | 54.6 (3.73) |
| High-quality carbohydrate | 8.64 (4.04) | 7.94 (3.16) | 12.40 (3.85) | 5.71 (1.78) | 6.41 (2.57) | 10.40 (4.07) | 6.87 (2.77) | 11.10 (4.43) | 9.43 (3.98) | 7.63 (3.18) | 4.96 (1.38) | 13.30 (3.63) |
| Low-quality carbohydrate | 49.5 (4.30) | 38.4 (3.65) | 43.8 (5.43) | 42.3 (4.48) | 50.4 (3.06) | 37.5 (3.32) | 41.2 (4.18) | 45.3 (5.22) | 39.3 (4.15) | 48.4 (4.13) | 45.5 (4.87) | 41.3 (4.91) |
| Total protein, % of total energy intake | 14.5 (1.15) | 17.6 (1.34) | 15.4 (1.48) | 16.9 (1.50) | 14.8 (1.38) | 17.2 (1.59) | 15.5 (1.28) | 16.8 (1.51) | 15.8 (1.53) | 16.3 (1.71) | 15.3 (1.59) | 16.9 (1.68) |
| Animal protein | 9.19 (1.00) | 11.80 (1.35) | 9.31 (1.07) | 11.60 (1.27) | 9.85 (1.25) | 10.90 (1.61) | 10.10 (1.25) | 10.80 (1.35) | 9.92 (1.44) | 10.90 (1.33) | 10.50 (1.48) | 10.30 (1.54) |
| Plant protein | 5.30 (0.76) | 5.81 (0.78) | 6.13 (0.93) | 5.29 (0.64) | 4.90 (0.48) | 6.33 (0.77) | 5.37 (0.67) | 6.06 (0.88) | 5.89 (0.87) | 5.41 (0.77) | 4.85 (0.44) | 6.55 (0.76) |
| Total fat, % of total energy intake | 27.4 (2.47) | 36.1 (2.82) | 28.4 (3.26) | 35.1 (3.29) | 28.4 (2.79) | 34.9 (3.37) | 36.4 (2.40) | 26.7 (2.11) | 35.5 (3.36) | 27.7 (2.73) | 34.2 (3.55) | 28.6 (3.25) |
| Saturated fat | 9.69 (1.25) | 12.90 (1.55) | 9.46 (1.15) | 13.10 (1.35) | 10.40 (1.49) | 11.90 (1.74) | 13.00 (1.44) | 9.34 (1.13) | 12.10 (1.79) | 10.20 (1.53) | 12.80 (1.49) | 9.50 (1.14) |
| Monounsaturated fat | 10.9 (1.19) | 14.5 (1.47) | 11.4 (1.62) | 14.0 (1.61) | 11.3 (1.25) | 14.1 (1.66) | 14.7 (1.34) | 10.6 (1.02) | 14.4 (1.64) | 11.0 (1.20) | 13.7 (1.66) | 11.4 (1.62) |
| Polyunsaturated fat | 6.79 (0.90) | 8.67 (1.24) | 7.51 (1.20) | 7.96 (1.27) | 6.70 (0.83) | 8.85 (1.13) | 8.73 (1.26) | 6.84 (0.85) | 8.95 (1.16) | 6.63 (0.77) | 7.75 (1.28) | 7.62 (1.16) |
| Cholesterol intake, mg/d | 220 (157) | 352 (216) | 198 (148) | 366 (224) | 259 (172) | 294 (200) | 366 (221) | 189 (142) | 318 (214) | 229 (163) | 369 (222) | 189 (140) |
| Nonsmoker | 4072 (56.3) | 3585 (48.6) | 4684 (61.0) | 3707 (47.7) | 4045 (52.6) | 3899 (51.4) | 3650 (46.5) | 4768 (61.9) | 4260 (50.7) | 4722 (55.9) | 3751 (46.9) | 4883 (61.5) |
| Nondrinker | 2341 (32.3) | 1767 (24.0) | 2758 (35.9) | 1849 (23.8) | 2244 (29.2) | 2050 (27.0) | 1878 (23.9) | 2873 (37.3) | 2228 (26.5) | 2671 (31.6) | 1876 (23.5) | 2902 (36.5) |
| Physically active | 1792 (24.8) | 1817 (24.6) | 1977 (25.8) | 1905 (24.5) | 1840 (23.9) | 1867 (24.6) | 1764 (22.5) | 1944 (25.3) | 2029 (24.1) | 2090 (24.8) | 1846 (23.1) | 2045 (25.7) |
| Family history of diabetes | 3046 (42.1) | 3263 (44.2) | 3134 (40.8) | 3411 (43.9) | 3403 (44.3) | 3363 (44.3) | 3489 (44.5) | 3097 (40.2) | 3699 (44.0) | 3534 (41.9) | 3541 (44.3) | 3258 (41.0) |
| Family history of heart disease | 869 (12.0) | 986 (13.4) | 762 (9.93) | 1117 (14.4) | 1011 (13.2) | 962 (12.7) | 1088 (13.9) | 781 (10.1) | 1076 (12.8) | 1005 (11.9) | 1174 (14.7) | 777 (9.8) |
| History of diabetes | 438 (6.0) | 1291 (17.5) | 926 (12.1) | 772 (9.9) | 349 (4.5) | 1600 (21.1) | 936 (11.9) | 965 (12.5) | 1309 (15.6) | 647 (7.7) | 470 (5.9) | 1338 (16.8) |
| History of heart disease | 761 (10.5) | 848 (11.5) | 1092 (14.2) | 626 (8.0) | 541 (7.0) | 1025 (13.5) | 776 (9.9) | 943 (12.3) | 1085 (12.9) | 739 (8.8) | 528 (6.6) | 1206 (15.2) |
| History of cancer | 631 (8.7) | 721 (9.8) | 916 (11.9) | 554 (7.1) | 487 (6.3) | 936 (12.3) | 807 (10.3) | 633 (8.2) | 1111 (13.2) | 538 (6.4) | 476 (6.0) | 898 (11.3) |
| Hypertension | 1763 (24.4) | 2374 (32.2) | 2451 (31.9) | 1857 (23.9) | 1477 (19.2) | 2753 (36.3) | 2336 (29.8) | 2117 (27.5) | 2924 (34.8) | 1813 (21.5) | 1619 (20.3) | 2706 (34.1) |
| Hypercholesterolemia | 1985 (27.4) | 2551 (34.6) | 2848 (37.1) | 1976 (25.4) | 1613 (21.0) | 3076 (40.5) | 2487 (31.7) | 2417 (31.4) | 3275 (38.9) | 1965 (23.3) | 1716 (21.5) | 3140 (39.5) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); GED, General Educational Development; IQR, interquartile range.
SI conversion factor: To convert cholesterol to millimoles per liter, multiply by 0.0259.
Data are presented as number (percentage) of study participants unless otherwise indicated.
LCD Scores and Mortality
The overall LCD score was not associated with total mortality (Table 2). The multivariable-adjusted HRs of total mortality from the lowest to highest quintiles of unhealthy LCD score were 1 (reference), 1.07 (95% CI, 0.94-1.21), 1.07 (95% CI, 0.93-1.23), 1.18 (95% CI, 1.03-1.35), and 1.16 (95% CI, 1.00-1.34) (P = .01 for trend). The multivariable-adjusted HRs of total mortality from the lowest to highest quintiles of healthy LCD score were 1 (reference), 0.83 (95% CI, 0.71-0.96), 0.86 (95% CI, 0.73-1.01), 0.73 (95% CI, 0.63-0.84), and 0.73 (95% CI, 0.63-0.85) (P < .001 for trend). A per 20-percentile increase in unhealthy LCD score was associated with a 7% higher risk of total mortality (HR, 1.07; 95% CI, 1.02-1.11), whereas a per 20-percentile increase in healthy LCD score was associated with 9% lower risk of total mortality (HR, 0.91; 95% CI, 0.87-0.95). The multivariable-adjusted HR per 20-percentile increase in unhealthy LCD score was 1.11 (95% CI, 1.00-1.23) for mortality from cancer, whereas the corresponding HR was 0.90 (95% CI, 0.83-0.98) per 20-percentile increase in healthy LCD score (eTable 4 in the Supplement).
Table 2. Associations Between Low-Carbohydrate-Diet Scores and Total Mortality.
| Characteristic | Quintiles of Low-Carbohydrate-Diet Scores | P for Trend | Per 20-Percentile Increase | ||||
|---|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |||
| Overall low-carbohydrate-diet scorea | |||||||
| Median score (IQR) | 4 (3-6) | 10 (9-11) | 15 (14-16) | 20 (19-21) | 26 (24-28) | NA | NA |
| Person-years of follow-up | 61 565 | 61 327 | 64 482 | 55 298 | 55 097 | NA | NA |
| Deaths, No. | 936 | 1047 | 1024 | 935 | 924 | NA | NA |
| Age- and sex-adjusted HR (95% CI) | 1 [Reference] | 1.00 (0.89-1.11) | 0.95 (0.83-1.08) | 1.00 (0.89-1.12) | 1.02 (0.90-1.16) | .76 | 1.01 (0.98-1.04) |
| Multivariable-adjusted HR (95% CI)b | 1 [Reference] | 0.95 (0.84-1.09) | 0.88 (0.76-1.02) | 0.90 (0.80-1.02) | 0.88 (0.76-1.02) | .06 | 0.97 (0.93-1.00) |
| Unhealthy low-carbohydrate-diet scorec | |||||||
| Median score (IQR) | 7 (4-8) | 12 (11-13) | 15 (14-16) | 18 (17-19) | 23 (22-25) | NA | NA |
| Person-years of follow-up | 58 890 | 61 281 | 51 158 | 63 288 | 63 152 | NA | NA |
| Deaths, No. | 1253 | 1181 | 845 | 918 | 669 | NA | NA |
| Age- and sex-adjusted HR (95% CI) | 1 [Reference] | 1.14 (1.01-1.27) | 1.18 (1.03-1.35) | 1.35 (1.16-1.56) | 1.36 (1.17-1.58) | <.001 | 1.13 (1.08-1.18) |
| Multivariable-adjusted HR (95% CI)b | 1 [Reference] | 1.07 (0.94-1.21) | 1.07 (0.93-1.23) | 1.18 (1.03-1.35) | 1.16 (1.00-1.34) | .01 | 1.07 (1.02-1.11) |
| Healthy low-carbohydrate-diet scored | |||||||
| Median score (IQR) | 5 (3-7) | 11 (10-12) | 16 (15-17) | 19 (18-20) | 24 (23-26) | NA | NA |
| Person-years of follow-up | 68 065 | 66 193 | 57 140 | 52 513 | 53 858 | NA | NA |
| Deaths, No. | 697 | 960 | 1096 | 1022 | 1091 | NA | NA |
| Age- and sex-adjusted HR (95% CI) | 1 [Reference] | 0.79 (0.68-0.92) | 0.83 (0.71-0.98) | 0.76 (0.65-0.89) | 0.74 (0.65-0.85) | <.001 | 0.92 (0.88-0.96) |
| Multivariable-adjusted HR (95% CI)b | 1 [Reference] | 0.83 (0.71-0.96) | 0.86 (0.73-1.01) | 0.73 (0.63-0.84) | 0.73 (0.63-0.85) | <.001 | 0.91 (0.87-0.95) |
Abbreviations: HR, hazard ratio; IQR, interquartile range; NA, not applicable.
Low total carbohydrates and high total protein and fat.
Adjusted for age (20-34, 35-49, 50-64, and ≥65 years), sex (male or female), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and other), educational level (less than high school, high school graduate or General Educational Development, and some college or above), ratio of family income to poverty (<1.30, 1.30-3.49, or ≥3.5), family history of diabetes mellitus (yes or no), family history of heart disease (yes or no), history of diabetes (yes or no), history of heart disease (yes or no), history of cancer (yes or no), physical activity (0, 0.1-0.9, 1.0-3.4, 3.5-5.9, or ≥6 hours per week), alcohol consumption (0, 0.1-4.9, 5-14.9, 15-29.9, or ≥30 g/d), smoking status (never smoker, former smoker, or current smoker [1-14, 15-24, or ≥25 cigarettes per day]), cholesterol (quintiles), and body mass index (calculated as weight in kilograms divided by height in meters squared) (<21, 21-24.9, 25-29.9, 30-35, and >35).
Low high-quality carbohydrate and high animal protein and saturated fat.
Low low-quality carbohydrate and high plant protein and unsaturated fat.
Low-Fat-Diet Scores and Mortality
The overall LFD score was not associated with total mortality (Table 3). The multivariable-adjusted HRs of total mortality from the lowest to highest quintiles of unhealthy LFD score were 1 (reference), 0.95 (95% CI, 0.86-1.05), 1.05 (95% CI, 0.92-1.20), 1.08 (95% CI, 0.94-1.24), and 1.12 (95% CI, 0.97-1.30) (P = .04 for trend). The multivariable-adjusted HRs of total mortality from the lowest to highest quintiles of healthy LFD score were 1 (reference), 1.03 (95% CI, 0.85-1.24), 0.85 (95% CI, 0.72-1.01), 0.84 (95% CI, 0.72-0.98), and 0.73 (95% CI, 0.61-0.87) (P < .001 for trend). A per 20-percentile increase in unhealthy LFD score was associated with 6% higher risk of total mortality (HR, 1.06; 95% CI, 1.01-1.12), whereas a per 20-percentile increase in healthy LFD score was associated with 11% lower risk of total mortality (HR, 0.89; 95% CI, 0.85-0.93). A per 20-percentile increase in healthy LFD score was associated with a 15% (95% CI, 7%-22%) lower risk of mortality from cancer (eTable 4 in the Supplement).
Table 3. Associations Between Low-Fat-Diet Scores and Total Mortality.
| Characteristic | Quintiles of Low-Fat-Diet Scores | P for Trend | Per 20-Percentile Increase | ||||
|---|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |||
| Overall low-fat-diet scorea | |||||||
| Median score (IQR) | 7 (5-8) | 11 (10-12) | 14 (13-15) | 19 (17-20) | 23 (22-25) | NA | NA |
| Person-years of follow-up | 63 246 | 48 159 | 63 771 | 63 927 | 58 666 | NA | NA |
| Deaths, No. | 899 | 759 | 1079 | 1042 | 1087 | NA | NA |
| Age- and sex-adjusted HR (95% CI) | 1 [Reference] | 1.04 (0.94-1.15) | 1.00 (0.90-1.11) | 1.00 (0.89-1.11) | 0.96 (0.85-1.09) | .49 | 0.98 (0.94-1.03) |
| Multivariable-adjusted HR (95% CI)b | 1 [Reference] | 1.04 (0.94-1.15) | 1.01 (0.90-1.15) | 0.99 (0.88-1.12) | 0.94 (0.81-1.08) | .34 | 0.97 (0.93-1.02) |
| Unhealthy low-fat-diet scorec | |||||||
| Median score (IQR) | 8 (6-9) | 12 (11-13) | 15 (14-16) | 18 (17-19) | 22 (21-24) | NA | NA |
| Person-years of follow-up | 63 297 | 52 125 | 56 175 | 56 650 | 69 521 | NA | NA |
| Deaths, No. | 1198 | 949 | 947 | 884 | 888 | NA | NA |
| Age- and sex-adjusted HR (95% CI) | 1 [Reference] | 0.93 (0.83-1.05) | 1.05 (0.92-1.20) | 1.10 (0.96-1.25) | 1.22 (1.07-1.40) | .002 | 1.10 (1.05-1.16) |
| Multivariable-adjusted HR (95% CI)b | 1 [Reference] | 0.95 (0.86-1.05) | 1.05 (0.92-1.20) | 1.08 (0.94-1.24) | 1.12 (0.97-1.30) | .04 | 1.06 (1.01-1.12) |
| Healthy low-fat-diet scored | |||||||
| Median score (IQR) | 6 (4-7) | 11 (10-11) | 14 (13-15) | 19 (18-20) | 25 (23-27) | NA | NA |
| Person-years of follow-up | 69 214 | 56 351 | 56 905 | 58 807 | 56 492 | NA | NA |
| Deaths, No. | 545 | 796 | 968 | 1274 | 1283 | NA | NA |
| Age- and sex-adjusted HR (95% CI) | 1 [Reference] | 1.01 (0.85-1.20) | 0.83 (0.70-0.98) | 0.80 (0.69-0.91) | 0.69 (0.59-0.82) | <.001 | 0.88 (0.84-0.91) |
| Multivariable-adjusted HR (95% CI)b | 1 [Reference] | 1.03 (0.85-1.24) | 0.85 (0.72-1.01) | 0.84 (0.72-0.98) | 0.73 (0.61-0.87) | <.001 | 0.89 (0.85-0.93) |
Abbreviations: HR, hazard ratio; IQR, interquartile range; NA, not applicable.
Low total fat, high total carbohydrate and total fat.
Adjusted for age (20-34, 35-49, 50-64, and ≥65 years), sex (male or female), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and other), educational level (less than high school, high school graduate or General Educational Development, and some college or above), ratio of family income to poverty (<1.30, 1.30-3.49, or ≥3.5), family history of diabetes mellitus (yes or no), family history of heart disease (yes or no), history of diabetes (yes or no), history of heart disease (yes or no), history of cancer (yes or no), physical activity (0, 0.1-0.9, 1.0-3.4, 3.5-5.9, or ≥6 hours per week), alcohol consumption (0, 0.1-4.9, 5-14.9, 15-29.9, or ≥30 g/d), smoking status (never smoker, former smoker, or current smoker [1-14, 15-24, or ≥25 cigarettes per day]), cholesterol (quintiles), and body mass index (calculated as weight in kilograms divided by height in meters squared) (<21, 21-24.9, 25-29.9, 30-35, and >35).
Low unsaturated fat and high low-quality carbohydrate and animal protein.
Low saturated fat and high high-quality carbohydrate and plant protein.
Subgroup and Sensitivity Analyses
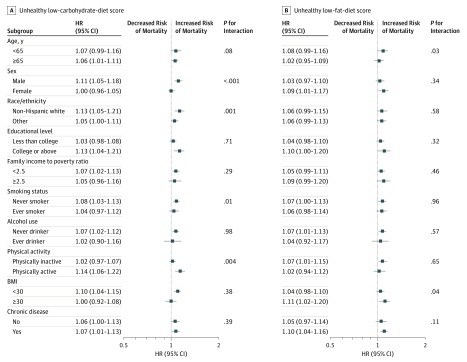
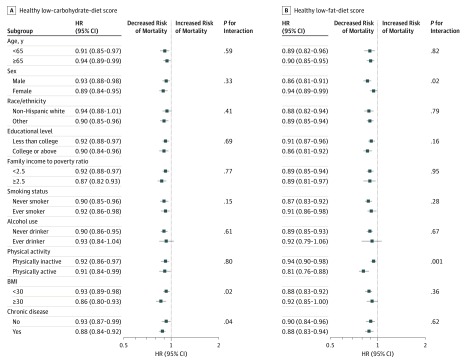
In stratified analysis, the associations remained persistent in most subgroups (Figure 1 and Figure 2 and eFigure 1 in the Supplement). After multiple testing corrections, a statistically significant interaction on total mortality was detected between unhealthy LCD score and sex (P < .001 for interaction); the HRs per 20-percentile increase were 1.11 (95% CI, 1.05-1.18) among male participants vs 1.00 (95% CI, 0.96-1.05) among female participants (eFigure in the Supplement). In sensitivity analyses, the associations remained similar when we further adjusted for the prevalence of hypertension and hypercholesterolemia at baseline (eTable 5 in the Supplement) and excluded deaths during 1-year follow-up baseline (eTable 6 in the Supplement). When we excluded participants with heart disease or cancer at baseline, statistically significant associations per 20-percentile increase remained in healthy LCD and LFD scores (eTable 7 in the Supplement).
Figure 1. Hazard Ratios (HRs) of Total Mortality per 20-Percentile Increase in Unhealthy Low-Carbohydrate-Diet and Low-Fat-Diet Scores by Subgroups.
Results were adjusted for age (20-34, 35-49, 50-64, and ≥65 years), sex (male or female), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and other), educational level (less than high school, high school graduate or General Educational Development, and some college or above), ratio of family income to poverty (<1.30, 1.30-3.49, or ≥3.5), family history of diabetes mellitus (yes or no), family history of heart disease (yes or no), history of diabetes (yes or no), history of heart disease (yes or no), history of cancer (yes or no), physical activity (0, 0.1-0.9, 1.0-3.4, 3.5-5.9, or ≥6 hours per week), alcohol consumption (0, 0.1-4.9, 5-14.9, 15-29.9, or ≥30 g/d), smoking status (never smoker, former smoker, current smoker [1-14, 15-24, or ≥25 cigarettes per day]), dietary cholesterol intake (quintiles), and body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) (<21, 21-24.9, 25-29.9, 30-35, and >35) except the corresponding subgroup variates.
Figure 2. Hazard Ratios (HRs) of Total Mortality per 20-Percentile Increase in Healthy Low-Carbohydrate-Diet and Low-Fat-Diet Scores by Subgroups.
Results were adjusted for age (20-34, 35-49, 50-64, and ≥65 years), sex (male or female), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and other), educational level (less than high school, high school graduate or General Educational Development, and some college or above), ratio of family income to poverty (<1.30, 1.30-3.49, or ≥3.5), family history of diabetes mellitus (yes or no), family history of heart disease (yes or no), history of diabetes (yes or no), history of heart disease (yes or no), history of cancer (yes or no), physical activity (0, 0.1-0.9, 1.0-3.4, 3.5-5.9, or ≥6 hours per week), alcohol consumption (0, 0.1-4.9, 5-14.9, 15-29.9, or ≥30 g/d), smoking status (never smoker, former smoker, or current smoker [1-14, 15-24, or ≥25 cigarettes per day]), dietary cholesterol intake (quintiles), and body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) (<21, 21-24.9, 25-29.9, 30-35, and >35) except the corresponding subgroup variate.
Discussion
In a nationally representative sample of US adults, overall LCD and LFD scores were not associated with risk of total mortality. However, unhealthy LCD and LFD scores were associated with higher total mortality, whereas healthy LCD and LFD scores were associated with lower total mortality.
Despite variance in macronutrient composition, LCDs and LFDs have shown similar associations with weight loss and metabolic biomarkers, with similar intensity of energy restriction and adherence to the intervention.17,22 However, the associations between 2 types of diets and long-term health outcomes were inconsistent. Our results were in accordance with previous observational studies20,21 that reported no association between overall LCD and health outcomes. On the basis of repeated dietary measurements and long-term follow-up from the Nurses’ Health Study, no consistent associations were observed between overall LCD and coronary heart disease and mortality.20,21 In a recent meta-analysis of cohort studies, Seidelmann et al18 reported a U-shaped association between carbohydrate intake and mortality and suggested that sources of food modified the association. In contrast to these findings, Mazidi et al23 reported a positive association between the overall LCD and mortality among US adults using NHANES data from 1999 to 2010. However, the LCD score in that study was based on the absolute intake of macronutrients directly from 24-hour dietary recalls, without controlling for potential bias owing to underreporting of food consumption. For dietary fat, emerging evidence has documented that the type of dietary fat is associated with human health independent of total fat intake.24 The significant association between total fat and health outcomes in cohort studies could be potentially attributable to the quantity of saturated fat or unsaturated fat.15,25 In the Women’s Health Initiative Dietary Modification Trial, the overall low-fat intervention (20% energy as fat) did not significantly reduce the risk of cardiovascular disease.5
Consistent with our results on unhealthy and healthy LFDs, higher intake of saturated fat was associated with higher mortality, but unsaturated fats were associated with lower mortality among 126 233 men and women followed up to 32 years.25 In a recent meta-analysis, LCD that favored animal-derived protein and fat sources was associated with higher mortality, whereas LCD based on plant-derived protein and fat intake was associated with lower mortality.18 However, a moderate LCD typically still contains more than 40% energy as carbohydrate, and an LFD typically contains up to 30% energy as fat and even a very LFD up to 20%.8 Our study extended the previous evidence and suggests that the health benefits of an LCD or LFD may depend not only on the types of protein and fat or carbohydrate but also on the quality of carbohydrate or fat remaining in the diet. The association between unhealthy LCD and total mortality appeared to be stronger among male participants compared with female participants. According to previous findings,16 males tended to consume a higher percentage of energy from low-quality carbohydrates, animal protein, and saturated fat, which might partly explain the stronger association. However, because of the remaining variations of individual characteristics across the unhealthy LCD score categories, residual confounding may contribute to the observed interaction.
Several possible mechanisms could be involved in the associations of types of LCD and LFD with mortality. Fat provides more than twice as much energy as carbohydrates and protein by weight. A high-saturated-fat diet is highly palatable and may have a weak effect on satiation, potentially leading to overconsumption and obesity.8 Low-quality carbohydrates, such as refined grains and added sugars, provide limited nutritional value, and their high glycemic load could be associated with high postprandial glucose and insulin, inflammation, insulin resistance, and dyslipidemia.26,27,28 In a recent clinical trial, replacing refined carbohydrates with saturated fat improved the overall lipid profile among healthy adults.29 In addition to macronutrients, the observed associations in our study may be in part attributable to food sources and components in these foods. The association between red and processed meat intake and mortality has been well established and may partly explain the higher risk of death among participants with higher unhealthy LCD and LFD.30,31,32 The food sources of high-quality carbohydrates, plant protein, and unsaturated fat include whole grains, nonstarchy vegetables, whole fruits, and nuts. These foods and their bioactive components (such as fiber, vitamins and minerals, and phytochemicals), rather than the macronutrients, may be involved in the associations between healthy LCD and LFD scores and mortality.33,34,35,36
Strengths and Limitations
Strengths of our study include use of a nationally representative sample of US adults, longitudinal study design, and collection of data using validated measures. However, this study has several limitations. First, all dietary scores in this study were not designed to mimic any particular versions of diets. Therefore, the results could not be directly translated to the assessment of health benefit or risk associated with the popular versions of the diets. Second, self-reported dietary intake is subject to measurement error. We applied the National Cancer Institute method to reduce measurement error and improve estimates of usual intake.37 Third, changes in methods for assessing dietary intake and dietary database during the study period may affect estimated macronutrient intake level. Still, the same protocols were followed to derive each macronutrient from different foods across all cycles. Fourth, dietary information was based on a single assessment at baseline, and participants may change their diets during the follow-up. Thus, misclassification of dietary intake was possible. Fifth, because of the observational nature of the study design, we could not determine any causality.
Conclusions
In a nationally representative sample of US adults, overall LCD and LFD scores were not associated with total mortality. Unhealthy LCD and LFD scores were associated with higher total mortality, whereas healthy LCD and LFD scores were associated with lower total mortality. These findings suggest that the associations of LCDs and LFDs with mortality may depend on the quality and food sources of macronutrients.
eAppendix. Assessment of Dietary Intake
eTable 1. Criteria for Determining the Low-Carbohydrate-Diet Scores
eTable 2. Criteria for Determining the Low-Fat-Diet Scores
eTable 3. Correlation Matrix Between Six Low-Carbohydrate and Low-Fat Diets Scores
eTable 4. Hazard Ratios (HRs) of Mortality from Heart Disease, Cancer, and Other Causes Per 20-Percentile Increase in Low-Carbohydrate and Low-Fat Diet Scores
eTable 5. Hazard Ratios (HRs) of Mortality from All-Cause, Heart Disease, Cancer, and Other Causes Per 20-Percentile Increase in Low-Carbohydrate and Low-Fat Diet Scores Further Adjusted for the Prevalence of Hypertension and Hypercholesterolemia at Baseline
eTable 6. Hazard Ratios (HRs) of Mortality from All-Cause, Heart Disease, Cancer, and Other Causes Per 20-Percentile Increase in Low-Carbohydrate and Low-Fat Diet Scores by Excluding Deaths During First-Year Follow-up
eTable 7. Hazard Ratios (HRs) of Mortality from All-Cause, Heart Disease, Cancer, and Other Causes Per 20-Percentile Increase in Low-Carbohydrate and Low-Fat Diet Scores by Excluding Patients With Heart Disease and Cancer at Baseline
Reference
- 1.Mokdad AH, Ballestros K, Echko M, et al. ; US Burden of Disease Collaborators . The state of US health, 1990-2016: burden of diseases, injuries, and risk factors among US states. JAMA. 2018;319(14):1444-1472. doi: 10.1001/jama.2018.0158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ludwig DS, Hu FB, Tappy L, Brand-Miller J. Dietary carbohydrates: role of quality and quantity in chronic disease. BMJ. 2018;361:k2340. doi: 10.1136/bmj.k2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Healthy diet. 2018. https://www.who.int/nutrition/publications/nutrientrequirements/healthydiet_factsheet/en/. Accessed June 2, 2019.
- 4.Dehghan M, Mente A, Zhang X, et al. ; Prospective Urban Rural Epidemiology (PURE) Study Investigators . Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet. 2017;390(10107):2050-2062. doi: 10.1016/S0140-6736(17)32252-3 [DOI] [PubMed] [Google Scholar]
- 5.Howard BV, Van Horn L, Hsia J, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative randomized controlled dietary modification trial. JAMA. 2006;295(6):655-666. doi: 10.1001/jama.295.6.655 [DOI] [PubMed] [Google Scholar]
- 6.de Souza RJ, Mente A, Maroleanu A, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ. 2015;351:h3978. doi: 10.1136/bmj.h3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chowdhury R, Warnakula S, Kunutsor S, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. 2014;160(6):398-406. doi: 10.7326/M13-1788 [DOI] [PubMed] [Google Scholar]
- 8.Ludwig DS, Willett WC, Volek JS, Neuhouser ML. Dietary fat: from foe to friend? Science. 2018;362(6416):764-770. doi: 10.1126/science.aau2096 [DOI] [PubMed] [Google Scholar]
- 9.Mann J, McLean R, Skeaff M, Morenga LT. Low carbohydrate diets: going against the grain. Lancet. 2014;384(9953):1479-1480. doi: 10.1016/S0140-6736(14)61413-6 [DOI] [PubMed] [Google Scholar]
- 10.Rubin R. High-fiber diet might protect against range of conditions. JAMA. 2019;321(17):1653-1655. doi: 10.1001/jama.2019.2539 [DOI] [PubMed] [Google Scholar]
- 11.Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019;393(10170):434-445. doi: 10.1016/S0140-6736(18)31809-9 [DOI] [PubMed] [Google Scholar]
- 12.AlEssa HB, Cohen R, Malik VS, et al. Carbohydrate quality and quantity and risk of coronary heart disease among US women and men. Am J Clin Nutr. 2018;107(2):257-267. doi: 10.1093/ajcn/nqx060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muraki I, Wu H, Imamura F, et al. Rice consumption and risk of cardiovascular disease: results from a pooled analysis of 3 U.S. cohorts. Am J Clin Nutr. 2015;101(1):164-172. doi: 10.3945/ajcn.114.087551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacks FM, Lichtenstein AH, Wu JHY, et al. ; American Heart Association . Dietary fats and cardiovascular disease: a Presidential Advisory from the American Heart Association. Circulation. 2017;136(3):e1-e23. doi: 10.1161/CIR.0000000000000510 [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Hruby A, Bernstein AM, et al. Saturated fats compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease: a prospective cohort study. J Am Coll Cardiol. 2015;66(14):1538-1548. doi: 10.1016/j.jacc.2015.07.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shan Z, Rehm CD, Rogers G, et al. Trends in dietary carbohydrate, protein, and fat intake and diet quality among US adults, 1999-2016. JAMA. 2019;322(12):1178-1187. doi: 10.1001/jama.2019.13771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston BC, Kanters S, Bandayrel K, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA. 2014;312(9):923-933. doi: 10.1001/jama.2014.10397 [DOI] [PubMed] [Google Scholar]
- 18.Seidelmann SB, Claggett B, Cheng S, et al. Dietary carbohydrate intake and mortality: a prospective cohort study and meta-analysis. Lancet Public Health. 2018;3(9):e419-e428. doi: 10.1016/S2468-2667(18)30135-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US Centers for Disease Control and Prevention Key concepts about the NHANES sample weights. https://www.cdc.gov/nchs/tutorials/nhanes/SurveyDesign/SampleDesign/Info1.htm. Accessed June 2, 2019.
- 20.Fung TT, van Dam RM, Hankinson SE, Stampfer M, Willett WC, Hu FB. Low-carbohydrate diets and all-cause and cause-specific mortality: two cohort studies. Ann Intern Med. 2010;153(5):289-298. doi: 10.7326/0003-4819-153-5-201009070-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halton TL, Willett WC, Liu S, et al. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med. 2006;355(19):1991-2002. doi: 10.1056/NEJMoa055317 [DOI] [PubMed] [Google Scholar]
- 22.Tobias DK, Chen M, Manson JE, Ludwig DS, Willett W, Hu FB. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3(12):968-979. doi: 10.1016/S2213-8587(15)00367-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazidi M, Katsiki N, Mikhailidis DP, Sattar N, Banach M. Lower carbohydrate diets and all-cause and cause-specific mortality: a population-based cohort study and pooling of prospective studies. Eur Heart J. 2019;40(34):2870-2879. doi: 10.1093/eurheartj/ehz174 [DOI] [PubMed] [Google Scholar]
- 24.Forouhi NG, Krauss RM, Taubes G, Willett W. Dietary fat and cardiometabolic health: evidence, controversies, and consensus for guidance. BMJ. 2018;361:k2139. doi: 10.1136/bmj.k2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang DD, Li Y, Chiuve SE, et al. Association of specific dietary fats with total and cause-specific mortality. JAMA Intern Med. 2016;176(8):1134-1145. doi: 10.1001/jamainternmed.2016.2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welsh JA, Sharma A, Abramson JL, Vaccarino V, Gillespie C, Vos MB. Caloric sweetener consumption and dyslipidemia among US adults. JAMA. 2010;303(15):1490-1497. doi: 10.1001/jama.2010.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musa-Veloso K, Poon T, Harkness LS, O’Shea M, Chu Y. The effects of whole-grain compared with refined wheat, rice, and rye on the postprandial blood glucose response: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2018;108(4):759-774. doi: 10.1093/ajcn/nqy112 [DOI] [PubMed] [Google Scholar]
- 28.Vanegas SM, Meydani M, Barnett JB, et al. Substituting whole grains for refined grains in a 6-wk randomized trial has a modest effect on gut microbiota and immune and inflammatory markers of healthy adults. Am J Clin Nutr. 2017;105(3):635-650. doi: 10.3945/ajcn.116.146928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shih CW, Hauser ME, Aronica L, Rigdon J, Gardner CD. Changes in blood lipid concentrations associated with changes in intake of dietary saturated fat in the context of a healthy low-carbohydrate weight-loss diet: a secondary analysis of the Diet Intervention Examining The Factors Interacting with Treatment Success (DIETFITS) trial. Am J Clin Nutr. 2019;109(2):433-441. doi: 10.1093/ajcn/nqy305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng Y, Li Y, Satija A, et al. Association of changes in red meat consumption with total and cause specific mortality among US women and men: two prospective cohort studies. BMJ. 2019;365:l2110. doi: 10.1136/bmj.l2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guasch-Ferré M, Satija A, Blondin SA, et al. Meta-analysis of randomized controlled trials of red meat consumption in comparison with various comparison diets on cardiovascular risk factors. Circulation. 2019;139(15):1828-1845. doi: 10.1161/CIRCULATIONAHA.118.035225 [DOI] [PubMed] [Google Scholar]
- 32.Song M, Fung TT, Hu FB, et al. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern Med. 2016;176(10):1453-1463. doi: 10.1001/jamainternmed.2016.4182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zong G, Gao A, Hu FB, Sun Q. Whole grain intake and mortality from all causes, cardiovascular disease, and cancer: a meta-analysis of prospective cohort studies. Circulation. 2016;133(24):2370-2380. doi: 10.1161/CIRCULATIONAHA.115.021101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller V, Mente A, Dehghan M, et al. ; Prospective Urban Rural Epidemiology (PURE) Study Investigators . Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): a prospective cohort study. Lancet. 2017;390(10107):2037-2049. doi: 10.1016/S0140-6736(17)32253-5 [DOI] [PubMed] [Google Scholar]
- 35.Bao Y, Han J, Hu FB, et al. Association of nut consumption with total and cause-specific mortality. N Engl J Med. 2013;369(21):2001-2011. doi: 10.1056/NEJMoa1307352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Budhathoki S, Sawada N, Iwasaki M, et al. ; Japan Public Health Center–based Prospective Study Group . Association of animal and plant protein intake with all-cause and cause-specific mortality in a Japanese cohort. JAMA Intern Med. 2019;179(11):1509-1518. doi: 10.1001/jamainternmed.2019.2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tooze JA, Midthune D, Dodd KW, et al. A new statistical method for estimating the usual intake of episodically consumed foods with application to their distribution. J Am Diet Assoc. 2006;106(10):1575-1587. doi: 10.1016/j.jada.2006.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Assessment of Dietary Intake
eTable 1. Criteria for Determining the Low-Carbohydrate-Diet Scores
eTable 2. Criteria for Determining the Low-Fat-Diet Scores
eTable 3. Correlation Matrix Between Six Low-Carbohydrate and Low-Fat Diets Scores
eTable 4. Hazard Ratios (HRs) of Mortality from Heart Disease, Cancer, and Other Causes Per 20-Percentile Increase in Low-Carbohydrate and Low-Fat Diet Scores
eTable 5. Hazard Ratios (HRs) of Mortality from All-Cause, Heart Disease, Cancer, and Other Causes Per 20-Percentile Increase in Low-Carbohydrate and Low-Fat Diet Scores Further Adjusted for the Prevalence of Hypertension and Hypercholesterolemia at Baseline
eTable 6. Hazard Ratios (HRs) of Mortality from All-Cause, Heart Disease, Cancer, and Other Causes Per 20-Percentile Increase in Low-Carbohydrate and Low-Fat Diet Scores by Excluding Deaths During First-Year Follow-up
eTable 7. Hazard Ratios (HRs) of Mortality from All-Cause, Heart Disease, Cancer, and Other Causes Per 20-Percentile Increase in Low-Carbohydrate and Low-Fat Diet Scores by Excluding Patients With Heart Disease and Cancer at Baseline