This cohort study analyzes the neurodevelopmental assessment scores and possible implications for developmental growth among infants born after prenatal exposure to Zika virus in Colombia.
Key Points
Question
Do newborns with in utero Zika virus exposure, normal fetal imaging result, and average head circumference measurement at birth have normal neurodevelopmental outcomes?
Findings
In this cohort study of 70 Colombian infants with in utero Zika virus exposure but without congenital Zika syndrome at birth, multidomain neurodevelopmental assessment scores deviated from normal scores as the children became older.
Meaning
Findings from this study suggest long-term neurodevelopmental monitoring should be performed for all infants with Zika virus exposure to ascertain the neurodevelopmental implications of the virus that may manifest with older age.
Abstract
Importance
The number of children who were born to mothers with Zika virus (ZIKV) infection during pregnancy but who did not have apparent disability at birth is large, warranting the study of the risk for neurodevelopmental impairment in this population without congenital Zika syndrome (CZS).
Objective
To investigate whether infants without CZS but who were exposed to ZIKV in utero have normal neurodevelopmental outcomes until 18 months of age.
Design, Setting, and Participants
This cohort study prospectively enrolled a group of pregnant women with ZIKV in Atlántico Department, Colombia, and in Washington, DC. With this cohort, we performed a longitudinal study of infant neurodevelopment. Infants born between August 1, 2016, and November 30, 2017, were included if they were live born, had normal fetal brain findings on magnetic resonance imaging and ultrasonography, were normocephalic at birth, and had normal examination results without clinical evidence of CZS. Seventy-seven infants born in Colombia, but 0 infants born in the United States, met the inclusion criteria.
Exposures
Prenatal ZIKV exposure.
Main Outcomes and Measures
Infant development was assessed by the Warner Initial Developmental Evaluation of Adaptive and Functional Skills (WIDEA) and the Alberta Infant Motor Scale (AIMS) at 1 or 2 time points between 4 and 18 months of age. The WIDEA and AIMS scores were converted to z scores compared with normative samples. Longitudinal mixed-effects regression models based on bootstrap resampling methods estimated scores over time, accounting for gestational age at maternal ZIKV infection and infant age at assessment. Results were presented as slope coefficients with 2-tailed P values based on z statistics that tested whether the coefficient differed from 0 (no change).
Results
Of the 77 Colombian infants included in this cohort study, 70 (91%) had no CZS and underwent neurodevelopmental assessments. Forty infants (57%) were evaluated between 4 and 8 months of age at a median (interquartile range [IQR]) age of 5.9 (5.3-6.5) months, and 60 (86%) underwent assessment between 9 and 18 months of age at a median (IQR) age of 13.0 (11.2-16.4) months. The WIDEA total score (coefficients: age = –0.227 vs age2 = 0.006; P < .003) and self-care domain score (coefficients: age = –0.238 vs age2 = 0.01; P < .008) showed curvilinear associations with age. Other domain scores showed linear declines with increasing age based on coefficients for communication (–0.036; P = .001), social cognition (–0.10; P < .001), and mobility (–0.14; P < .001). The AIMS scores were similar to the normative sample over time (95% CI, –0.107 to 0.037; P = .34). Nineteen of 57 infants (33%) who underwent postnatal cranial ultrasonography had a nonspecific, mild finding. No difference was found in the decline of WIDEA z scores between infants with and those without cranial ultrasonography findings except for a complex interactive relationship involving the social cognition domain (P < .049). The AIMS z scores were lower in infants with nonspecific cranial ultrasonography findings (–0.49; P = .07).
Conclusions and Relevance
This study found that infants with in utero ZIKV exposure without CZS appeared at risk for abnormal neurodevelopmental outcomes in the first 18 months of life. Long-term neurodevelopmental surveillance of all newborns with ZIKV exposure is recommended.
Introduction
Infants who were exposed in utero to Zika virus (ZIKV) during the epidemic of 2015 to 2016 in Central and South America have aged into early childhood. Currently, there is a need to understand the spectrum of neurodevelopmental outcomes in children with congenital ZIKV exposure and to provide appropriate follow-up and neurodevelopmental interventions to at-risk children. Outcomes are poor in infants with severe neurologic abnormalities associated with congenital Zika syndrome (CZS), which includes microcephaly, global neurodevelopmental delay, epilepsy, visual impairment, and deafness.1,2 However, most newborns with prenatal ZIKV exposure do not have clinical manifestations of CZS.3 The risk and spectrum of neurodevelopmental impairment in infants without CZS after exposure to prenatal ZIKV are not yet known but are of major importance given the large number of children exposed to ZIKV without apparent neurologic abnormalities at birth.
According to multiple studies, not all fetuses exposed to ZIKV have overt neurologic outcomes and microcephaly evident before or at birth.2,4,5 Some ZIKV-exposed newborns with normocephaly have been reported to develop postnatal microcephaly.5,6 In these cases, substantial brain abnormalities consistent with CZS were found through neuroimaging,5,6 which likely would have been detected earlier had newborn imaging been performed. In children with CZS, visual and hearing impairments were seen in up to 17% of cases and may have occurred with or without microcephaly.7 Likewise, in other congenital infections, such as congenital cytomegalovirus, the fetal and neonatal brain may appear structurally normal, but neurologic and developmental defects can become evident during the first years of life.8 Because of the uncertainty regarding the long-term neurologic health of newborns exposed to ZIKV in utero, the Centers for Disease Control and Prevention recommended developmental follow-up of these newborns through early childhood.9 Thus, the international health care community remains on a steep learning curve given that many critical questions about ZIKV and its long-term consequences remain unanswered.
The infant cohort in the present study was large and well characterized to address the critical knowledge gap regarding the neurodevelopmental outcomes of infants without CZS born to mothers with positive ZIKV results during pregnancy. The objective of this study was to evaluate the early neurodevelopmental outcomes of infants with normocephaly who were exposed to ZIKV in utero and had normal fetal neuroimaging findings. Given that most of these infants also had postnatal brain imaging, we were able to compare the differences in postnatal neurologic outcomes between infants with normal cranial ultrasonography findings and those with nonspecific mild cranial ultrasonography findings.
Methods
Participants
In 2016 in Atlántico Department, Colombia, and in Washington, DC, we prospectively enrolled 82 pregnant women with symptomatic ZIKV infection and followed up on their pregnancies with serial fetal magnetic resonance imaging (MRI) and ultrasonography.10 With this cohort of pregnant women with sequential fetal and neonatal ultrasonography and MRI data, we conducted a longitudinal neurodevelopmental substudy of infants with fetal exposure to maternal symptomatic ZIKV infection but without apparent CZS.10 This cohort study received approval from the Children’s National Medical Center Institutional Review Board and the Institutional Review Committee and Independent Committee on Research Ethics. Women provided written informed consent for their infant’s participation in the study. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
The pregnant women included in the study met the Centers for Disease Control and Prevention clinical criteria for probable ZIKV infection and had laboratory evidence of ZIKV confirmed by 1 or more tests, including polymerase chain reaction, IgM, IgG, and plaque-reduction neutralization assay.10 Three of the 82 women (4%) had abnormal fetal neuroimaging findings, 1 (1%) had a stillbirth, and 1 (1%) had an infant with a postnatal infarction on brain MRI (but with normal fetal imaging results), leaving a cohort of 77 infants who had a clinically normal presentation at birth.10 Infants born between August 1, 2016, and November 30, 2017, were included in the study if they were live born, had normal fetal brain findings on MRI and ultrasonography, were normocephalic at birth, and had average examination results without clinical evidence of CZS. One infant with a postnatal cerebral infarction was excluded.10,11 Seventy-seven infants born in Colombia, but none of the US-born infants, met the inclusion criteria.
Biometry and Imaging
Birth weight, length, and head circumference measurements were collected for each infant.12 Most infants had previously undergone postnatal unsedated brain MRI and/or cranial ultrasonography scans, which were interpreted centrally at Children’s National Hospital, Washington, DC.10 Some infants did not undergo postnatal imaging because they did not return for neonatal appointments or because of their inability to naturally sleep for the MRI. Infant head circumference and weight were measured at each assessment. Head circumference values were converted to z scores based on standardized, sex-specific World Health Organization growth charts.12
Neurodevelopmental Assessments
Infant development was assessed with the Warner Initial Developmental Evaluation of Adaptive and Functional Skills (WIDEA) and the Alberta Infant Motor Scale (AIMS).13,14,15,16,17,18 One of us, a child neurologist (S.B.M.), traveled to Barranquilla and Sabanalarga in Atlántico Department, Colombia, to provide in-person WIDEA and AIMS training to the Colombian research staff. Infants had 1 to 2 assessments between 4 and 18 months of age. Owing to the distance between the towns of Barranquilla, the primary research site, and Sabanalarga, where a large number of the participants lived, a tight window for infant assessments was not feasible. Thus, either infants were brought to the research clinic (BIOMELAB in Barranquilla) by private car or public transportation or the research team traveled to Sabanalarga to provide assessments to multiple infants during a single day.
The WIDEA was selected because it has been validated as a measure of multidomain development, including adaptive and functional skills.14,15,17 It is a 50-item questionnaire comprising the domains of self-care, mobility, communication, and social cognition; can be used on children up to 30 months of age; and is available in the Spanish language. The WIDEA was administered orally by the research coordinator in the primary language of the parent (Spanish or English).
AIMS is an observational motor examination of the infant’s most mature movements in prone, supine, sitting, and standing positions and has been validated in Spanish-language populations.16,18 When children begin to walk independently, the AIMS score is maximized; thus, AIMS scores are not interpretable for infants older than 15 months. We recorded whether each infant was observed walking independently or not. The first 16 cases in Colombia were assessed in person by the neurologist (S.B.M.) and were used to train the Colombian research coordinators in the proper positioning of the infants for the examination.
Because AIMS is an observational examination, it provided the opportunity for remote, video-based scoring (C920 HD Pro WebCam; Logitech). The AIMS examinations were video recorded, and these videos were electronically transmitted using a secure file-sharing application (Sharebox; Bauhub) hosted at Children’s National Hospital for remote review and scoring by the neurologist. The interrater reliability of the AIMS assessments was ascertained by independent re-scoring by a second reader on our team (C.P. or M.E.M.). For 7 infants, the AIMS assessment could not be scored remotely because of our inability to observe all 4 body positions on video because of infant somnolence or irritability. For 3 infant AIMS examinations, the video files contained no recording or were damaged.
Statistical Analysis and Informatics Methods
Study data were entered into and managed with the Research Electronic Data Capture (REDCap; Vanderbilt University) database management system.19 The WIDEA and AIMS scores at each visit were converted to z scores based on normative samples.16,17 The change in WIDEA total, the 4 individual WIDEA domains, and the AIMS z scores in exposed infants were plotted over time. After descriptive analyses, longitudinal, multilevel, mixed-effects linear models based on bootstrap resampling methods were developed in Stata, version 15 (StataCorp LLC) to evaluate change in study outcomes over time, overall, and within subgroups.20 This approach adjusts SE estimates for the correlation between assessments in the same individual, and bootstrapping relaxes the need for transformation to meet the normality assumption. Before including age at assessments or gestational age at ZIKV infection as model covariables (adjusted main effects), we evaluated the need for and, as necessary, included interactive or higher-order effects to account for curvilinearity and effect modification involving these variables. Results were presented as slope coefficients with 2-tailed P values based on z statistics that tested whether the coefficient differed from 0 (no change). Two-way random-effects models were implemented in Stata, version 15, to estimate individual intraclass correlation coefficients to assess interrater reliability (agreement) in AIMS test scoring.21
A P = .05 was considered statistically significant, but we also included results that were borderline significant. Data analysis was performed from January 23, 2019, to August 22, 2019.
Results
Of the 77 Colombian infants included in this cohort study, 70 (91%) had no CZS and underwent neurodevelopmental assessments at 1 or 2 time points at age 4 to 8 months and/or 9 to 18 months (Table). Forty infants (57%) who were evaluated between 4 and 8 months of age had a median (interquartile range [IQR]) age of 5.9 (5.3-6.5) months. Sixty infants (86%) who underwent assessment between 9 and 18 months of age had a median (IQR) age of 13.0 (11.2-16.4) months. Head circumference z scores showed no decline over time (–0.013; P = .39), controlling for gestational age of ZIKV exposure.
Table. Clinical Characteristics of Infants Exposed to Zika Virus In Utero Without Congenital Zika Syndrome.
| Variable | No. (%) (n = 70) |
|---|---|
| Gestational wk of symptomatic maternal ZIKV infection, median (IQR) | 7 (4-10) |
| Birth, median (IQR) | |
| Gestational age, wk | 38.9 (37.9-39.1) |
| Weight, g | 3200 (2950-3420) |
| Head circumference, cm | 34 (33-36) |
| Male sex | 31 (44) |
| Postnatal neuroimaging with brain MRI and/or cranial ultrasonography | 57 (81) |
| Age, median (IQR), d | |
| Brain MRI (n = 50) | 18 (10.25-25) |
| Cranial ultrasonography (n = 53) | 16 (11-25) |
| Normal postnatal neuroimaging (n = 57) | 38 (67) |
| Finding on postnatal neuroimaging | 19 (33) |
| Lenticulostriate vasculopathy | 4 (21) |
| Subependymal or germinolytic cyst | 12 (63) |
| Choroid plexus cyst | 6 (32) |
| Isolated calcification | 1 (5) |
| Infant assessment at 2 time points | 30 (43) |
| Assessment between ages 4 and 8 mo | 40 (57) |
| Age at 4- to 8-mo assessment, median (IQR), mo | |
| WIDEA (n = 40) | 5.9 (5.3-6.5) |
| AIMS (n = 40) | 5.9 (5.3-6.5) |
| Assessment between ages 9 and 18 mo | 60 (86) |
| Age at 9- to 18-mo assessment, median (IQR), mo | |
| WIDEA (n = 60) | 13.0 (11.2-16.4) |
| AIMS (n = 53) | 13.9 (11.8-17.0) |
Abbreviations: AIMS, Alberta Infant Motor Scale; IQR, interquartile range; MRI, magnetic resonance imaging; WIDEA, Warner Initial Developmental Evaluation of Adaptive and Functional Skills; ZIKV, Zika virus.
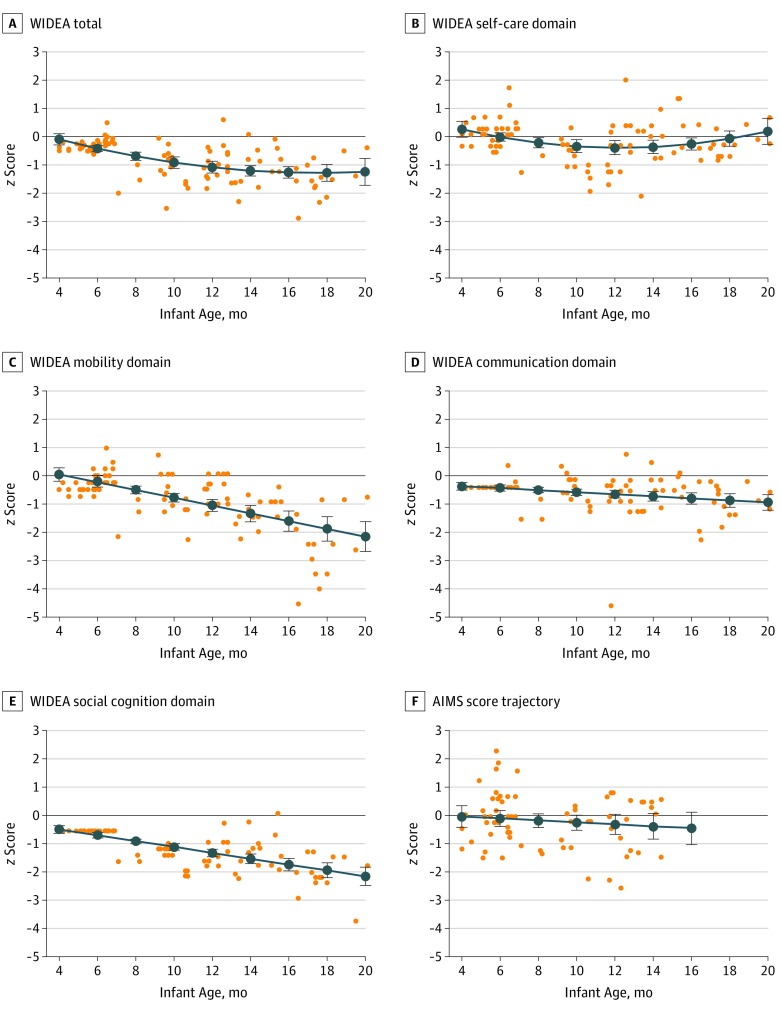
The median (IQR) WIDEA total score was 60.5 (58-62) at time point 1 and 102.5 (83.3-123.8) at time point 2 in the 70 infants. The WIDEA total z score showed a curvilinear pattern of decline over time (coefficients: age = –0.227 vs age2 = 0.006; P < .003) (Figure 1A). The WIDEA self-care domain had a similar curvilinear decline to 12 months of age, which thereafter increased to normative levels by 20 months of age (coefficients: age = –0.238 vs age2 = 0.01; P < .008) (Figure 1B). The WIDEA domain z scores of mobility with age coefficients (–0.14; P < .001), communication (–0.036; P = .001), and social cognition (–0.10; P < .001) decreased consistently and moderately over the assessment period (Figure 1C-E). The gestational age at maternal symptoms was not associated with the trajectory of change over time for WIDEA outcomes.
Figure 1. Infant Warner Initial Developmental Evaluation of Adaptive and Functional Skills (WIDEA) and Alberta Infant Motor Scale (AIMS) z Scores Over Time .
The WIDEA total, WIDEA domains, and AIMS z score trajectories were modeled over time in 70 infants with in utero Zika virus (ZIKV) exposure. z Scores at each assessment are shown as orange dots, and the modeled trajectory is shown as a blue line with 95% CI bars. The infants exposed to ZIKV in utero showed evidence of decline in WIDEA total z scores (A; coefficients: age = –0.227 vs age2 = 0.006; P < .003) and in the domains of self-care (B; coefficients: age = –0.238 vs age2 = 0.01; P < .008), mobility (C; –0.14; P < .001), communication (D; –0.036; P = .001), and social cognition (E; –0.10; P < .001). AIMS z scores (F) showed evidence consistent with modest decline, although not statistically significant, by infant age in months (excluding infants >15 months of age) (95% CI, –0.107 to 0.037; P = .34).
The median (IQR) total AIMS score was 25 (22-28) at time point 1 (40 [57%]) and 49 (45.5-57) at time point 2 (30 [43%]) among newborns with assessments at 15 months or younger. Although the AIMS score tended to decrease over time, it did not approach statistical significance (95% CI, −0.107 to 0.037; P = .34) (Figure 1F). Three infants (4%) underwent an AIMS assessment between 10 and 12.5 months of age that was more than 2 SDs below the norm (z scores: −2.57 for infant 1, −2.25 for infant 2, and −2.29 for infant 3) (Figure 1F). The WIDEA total z scores for these 3 infants were −1.36 for infant 1, −1.72 for infant 2, and −1.84 for infant 3. The WIDEA mobility z scores were −1.0 for infant 1, −1.18 for infant 2, and −1.36 for infant 3. Of the 3 infants with an AIMS z score below 2 SDs, only 1 had an ultrasonography finding. Twenty-three infants (33%) were 15 months or older at the time of an AIMS assessment. Twenty-one of these infants (91%) were walking independently in their videos, and given their age (>15 months), their AIMS scores were maximized. Interrater agreement under remote, video-based AIMS scoring reached the good range (individual intraclass correlation coefficient = 0.73; 95% CI, 0.42-0.87).
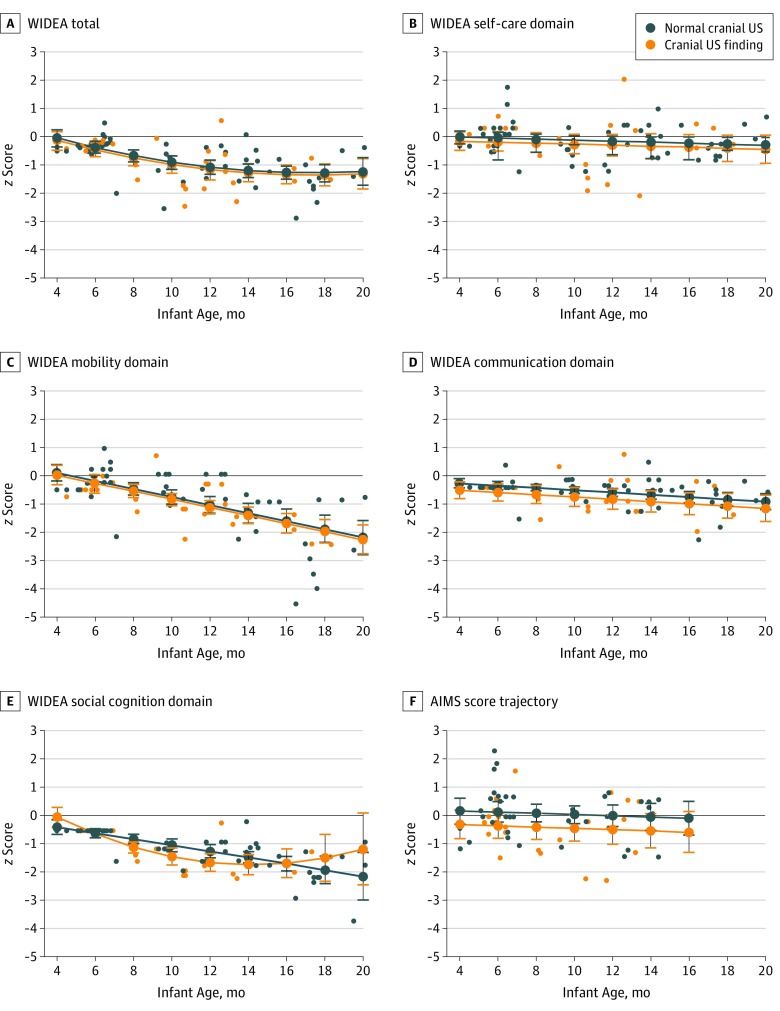
Nineteen of 57 infants (33%) who had previously undergone postnatal neuroimaging had mild, nonspecific findings (Table). Except for the WIDEA social cognition domain, no difference was found in the scores that decreased over time between the WIDEA total and WIDEA domain z scores for the 19 infants with nonspecific cranial ultrasonography findings compared with the 38 infants with normal cranial ultrasonography findings (−0.14; P = .22) (Figure 2A-E). For the WIDEA social cognition domain, a curvilinear decline was seen in infants with a nonspecific cranial ultrasonography finding in contrast to the linear decline seen in infants with normal cranial ultrasonography results (P = .049) (Figure 2E). The AIMS z scores were lower in infants with nonspecific cranial ultrasonography findings compared with infants with normal cranial ultrasonography findings (−0.49; P = .07) (Figure 2F).
Figure 2. Comparison of Warner Initial Developmental Evaluation of Adaptive and Functional Skills (WIDEA) and Alberta Infant Motor Scale (AIMS) z Scores for Infants With Postnatal Imaging.
The WIDEA total (A) and domains of self-care (B), mobility (C), communication (D), and social cognition (E) z score trajectories were modeled over time in the 57 infants of the cohort who had postnatal imaging. z Scores at each assessment are shown as dots, and the modeled trajectory is shown as a line with 95% CI bars. Infants with cranial ultrasonography (US) findings (n = 19) are shown in orange, and infants with normal cranial US (n = 38) are shown in blue. No difference was found in the WIDEA total or domain trajectories over time for the infants with mild, nonspecific cranial US findings (orange lines) compared with the infants with normal cranial US (blue line), except in the WIDEA social cognition domain, which showed a complex interactive association involving a curvilinear decline in infants with a cranial US finding compared with a linear decline in the infants with normal cranial US (P = .049). The AIMS z score trajectory (F) was lower among the 51 infants aged 15 months or younger with cranial US findings compared with infants with normal cranial US findings (–0.49; P = .07).
Discussion
Although many of the infants in this cohort study had normal neurodevelopmental scores through 18 months of age, scores in multiple areas of development for some infants decreased from normative mean scores over time. The infants included in the present cohort were well characterized, with laboratory-confirmed in utero exposure to ZIKV, normal fetal MRI and ultrasonography findings, and no evidence of CZS or microcephaly at birth. Thus, these infants were expected to have low risk for subsequent neurodevelopmental deficits, yet these deficits emerged in the first year of life and without a reduction in head circumference. We also found that nonspecific postnatal neuroimaging findings of lenticulostriate vasculopathy, germinolytic or subependymal cysts, and choroid plexus cysts, which were present in up to 37% of newborns exposed to ZIKV in utero,10 may be potential risk factors for worse early neurodevelopmental outcomes. To our knowledge, this study is the first to show that these nonspecific imaging findings may indicate subtle brain injury potentially associated with impaired neuromotor development.
The study population from the Caribbean coast of Colombia was unique compared with other cohorts of infants exposed to ZIKV in utero for several reasons.3,10,22,23,24 The infants in the present study received sequential neuroimaging during the fetal and postnatal periods.10 At birth, the infants were considered to have clinically normal presentation on the basis of fetal imaging, newborn examination, and postnatal neuroimaging results, and we achieved a high rate of follow-up (91%). Similarly, in other studies, most infants born to mothers with ZIKV infection during pregnancy did not have apparent impairments at birth.3,22,23 Nevertheless, it was important for parents to know whether their infant, who may have had a clinically normal presentation at birth, had increased risk for long-term neurologic or developmental impairment.
The WIDEA was a valuable tool for assessing development across multiple domains in this cohort, and its modeled trajectory showed an evolving decline from test norms. Although the differences in the communication and self-care domains were not dramatic, differences in the social cognition and mobility domains grew significantly larger over time (Figure 1A-E). The substantial decrease in mobility domain z scores compared with the AIMS z scores may be owing to the WIDEA evaluating more complex mobility functions beyond independent ambulation, which is the highest achieved motor skill assessed in AIMS (Figure 1F). This factor may also explain the relatively low variability in mobility z scores among infants aged between 4 and 8 months and the high variability among infants at older ages of assessment, when mobility tasks, such as walking independently and going up stairs, were assessed. Low motor scores at this age may reflect typical variability in timing for myelination of the motor tracts.25 In these children, mobility would be expected to normalize if evaluated at an older age. Using a general movement assessment tool at age 12 weeks, a study found that early motor function deficits in Brazilian infants exposed to ZIKV in utero were associated with 2-year cognitive, language, and motor outcomes.24 Also of concern in the present study cohort was the decline in the WIDEA social cognition domain over time, which may indicate impaired neurocognitive development. Because infant and child neurodevelopment occurs over many years, with increasingly complex neurologic and cognitive functions required as children age and enter school, longer-term neurodevelopmental evaluations are necessary.
Remote scoring of video-recorded AIMS examinations is an innovative approach to neurodevelopment evaluation that may potentially be highly valuable for international populations who do not have direct access to pediatric specialists. To our knowledge, the AIMS test has not been recorded on video for remote scoring in a research setting. With this method, a child neurologist with expertise in infant neurodevelopment was able to view and score each infant’s motor maturity. Scoring video-recorded AIMS remotely has unique advantages, including less environmental distractions for the scorer, the ability to observe and review infant movements repeatedly to ensure accuracy, and the opportunity to quantify the reliability by comparing assessments from independent reviewers. Additional advantages of using AIMS are that it does not require standardized manipulatives and requires less training to properly administer the items compared with other tests, such as the Bayley Scales of Infant and Toddler Development. AIMS was reliable in this study despite the training occurring at different sites and countries and the research team, with the exception of a Colombian team member, having no previous experience in neurodevelopmental assessments. The identification of neurodevelopmental assessment tools that can be used in low-resource settings is important for research and patient care.26 In the future, these tools could be expanded to include a telemedicine approach to enable multinational experts to inform the care for high-risk infants.
Lenticulostriate vasculopathy can be seen on cranial ultrasonography in association with infectious and noninfectious etiologies, including congenital cytomegalovirus infection.26,27 The appearance of lenticulostriate vasculopathy may indicate mild injury to the basal ganglia and thalami and may be associated with neurodevelopmental delays in children. However, separating lenticulostriate vasculopathy from the neurodevelopmental outcomes of the underlying condition (ie, congenital infection or hypoxemia) is often difficult.27,28 In this study, finding lenticulostriate vasculopathy on ultrasonography raised the concern that ZIKV may have direct or inflammatory implications for the lenticulostriate vessels of the immature brain. Zika virus appears to be vasotropic and has been associated with fetal and infant stroke.11,29,30 Similarly, subependymal cysts can be seen on cranial ultrasonography in newborns in association with congenital infection and other conditions, such as resolving grade I intraventricular hemorrhage, but in isolation these cysts have not been reported to correlate with lower neurodevelopmental outcomes.31,32 In this study, even in the absence of other brain findings by ultrasonography and MRI, the relatively high number of these nonspecific changes on cranial ultrasonography in infants exposed to ZIKV in utero as well as the trend toward lower AIMS scores in infants with nonspecific imaging findings may indicate mild ZIKV damage to the developing brain. This finding may be important from a public health standpoint and needs to be evaluated in a larger cohort. With a limited sample size, only the WIDEA social cognition domain provided evidence of a difference in trajectory, indicating a deficit from age 6 to 16 months between those with cranial ultrasonography findings and those without ultrasonography findings. Longer-term neurodevelopmental follow-up and evaluation in larger cohorts are needed for a better understanding of the implications of these findings.
This study supports the recommendations by the Centers for Disease Control and Prevention to perform long-term follow-up of all infants exposed to ZIKV in utero, not just those with manifestations of CZS at birth.33 Neurodevelopmental delays may be mild and subclinical and can influence multiple areas of development. Without standardized assessment, developmental abnormalities may not be detected, and opportunities to optimize early developmental intervention may be missed. In the United States, not all infants with in utero exposure to ZIKV receive the recommended evaluations.34 Thus, the high rate of follow-up visits in the Colombian cohort enables a more complete picture of the spectrum of outcomes for infants who were exposed to ZIKV in utero but did not have CZS.
Limitations
This cohort study was limited by the small sample size and the lack of a normative Colombian population for the WIDEA and AIMS assessments, although both tools have been used or validated in other Hispanic populations.17,18 Some inherent differences likely existed between the populations from which the WIDEA and AIMS were normed and the population of Colombia. Although the Colombian community in which most of the participating infants lived was a semirural, low-income neighborhood, the family network and support appeared strong. The high rate of compliance with neurodevelopmental follow-up visits was a testament to the parents’ concern for their children. We believed the differences that developed over time in this cohort were unlikely to be solely associated with the differences in neurodevelopmental trajectories between the normative population and children in Colombia. The population in Atlántico Department is low to middle income, with the city of Barranquilla being densely populated and Sabanalarga being rural. Dengue virus and ZIKV both circulate in this region. Some infants lacked complete AIMS data owing to their fussiness during examinations or incomplete video assessments, which was a limitation to video-based remote scoring.
Conclusions
This study showed that the outcomes for a cohort of newborns with in utero exposure to ZIKV appeared better than the early expectations of widespread neurodevelopmental consequences among infants and young children who were exposed to this virus. However, with older age, these children began to show decreased neurodevelopmental scores, suggesting possible developmental implications. These findings warrant longer-term surveillance of all infants who have been exposed to ZIKV in utero to enable the assessment of the full neurodevelopmental trajectory and childhood outcomes.
References
- 1.Alves LV, Paredes CE, Silva GC, Mello JG, Alves JG. Neurodevelopment of 24 children born in Brazil with congenital Zika syndrome in 2015: a case series study. BMJ Open. 2018;8(7):e021304. doi: 10.1136/bmjopen-2017-021304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melo AS, Aguiar RS, Amorim MM, et al. Congenital Zika virus infection: beyond neonatal microcephaly. JAMA Neurol. 2016;73(12):1407-1416. doi: 10.1001/jamaneurol.2016.3720 [DOI] [PubMed] [Google Scholar]
- 3.Hoen B, Schaub B, Funk AL, et al. Pregnancy outcomes after ZIKV infection in French territories in the Americas. N Engl J Med. 2018;378(11):985-994. doi: 10.1056/NEJMoa1709481 [DOI] [PubMed] [Google Scholar]
- 4.França GV, Schuler-Faccini L, Oliveira WK, et al. Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet. 2016;388(10047):891-897. doi: 10.1016/S0140-6736(16)30902-3 [DOI] [PubMed] [Google Scholar]
- 5.van der Linden V, Pessoa A, Dobyns W, et al. Description of 13 infants born during October 2015-January 2016 with congenital Zika virus infection without microcephaly at birth - Brazil. MMWR Morb Mortal Wkly Rep. 2016;65(47):1343-1348. doi: 10.15585/mmwr.mm6547e2 [DOI] [PubMed] [Google Scholar]
- 6.Aragao MFVV, Holanda AC, Brainer-Lima AM, et al. Nonmicrocephalic infants with congenital Zika syndrome suspected only after neuroimaging evaluation compared with those with microcephaly at birth and postnatally: how large is the Zika virus “iceberg”? AJNR Am J Neuroradiol. 2017;38(7):1427-1434. doi: 10.3174/ajnr.A5216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lage M-LC, Carvalho AL, Ventura PA, et al. Clinical, neuroimaging, and neurophysiological findings in children with microcephaly related to congenital Zika virus infection. Int J Environ Res Public Health. 2019;16(3):E309. doi: 10.3390/ijerph16030309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amir J, Atias J, Linder N, Pardo J. Follow-up of infants with congenital cytomegalovirus and normal fetal imaging. Arch Dis Child Fetal Neonatal Ed. 2016;101(5):F428-F432. doi: 10.1136/archdischild-2015-308357 [DOI] [PubMed] [Google Scholar]
- 9.Russell K, Oliver SE, Lewis L, et al. Update: interim guidance for the evaluation and management of infants with possible congenital Zika virus infection - United States, August 2016. MMWR Morb Mortal Wkly Rep. 2016;65(33):870-878. doi: 10.15585/mmwr.mm6533e2 [DOI] [PubMed] [Google Scholar]
- 10.Mulkey SB, Bulas DI, Vezina G, et al. Sequential neuroimaging of the fetus and newborn with in utero Zika virus exposure. JAMA Pediatr. 2019;173(1):52-59. doi: 10.1001/jamapediatrics.2018.4138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulkey SB, Vezina G, Bulas DI, et al. Neuroimaging findings in normocephalic newborns with intrauterine Zika virus exposure. Pediatr Neurol. 2018;78:75-78. doi: 10.1016/j.pediatrneurol.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. WHO growth standards are recommended for use in the U.S. for infants and children 0 to 2 years of age. https://www.cdc.gov/growthcharts/who_charts.htm. Updated September 9, 2010. Accessed April 24, 2019.
- 13.Wu YW, Mathur AM, Chang T, et al. High-dose erythropoietin and hypothermia for hypoxic-ischemic encephalopathy: a phase II trial. Pediatrics. 2016;137(6):e20160191. doi: 10.1542/peds.2016-0191 [DOI] [PubMed] [Google Scholar]
- 14.Msall ME, Tremont MR, Ottenbacher KJ. Functional assessment of preschool children: optimizing development and family supports in early intervention. Infants Young Child. 2001;14:46-66. doi: 10.1097/00001163-200114010-00008 [DOI] [Google Scholar]
- 15.Msall ME. Measuring functional skills in preschool children at risk for neurodevelopmental disabilities. Ment Retard Dev Disabil Res Rev. 2005;11(3):263-273. doi: 10.1002/mrdd.20073 [DOI] [PubMed] [Google Scholar]
- 16.Piper MC, Darrah J. Motor Assessment of the Developing Infant. Philadelphia, PA: Saunders; 1994. [Google Scholar]
- 17.Park JJ. Development of a functional assessment tool in children birth to 36 months: validation study of Warner Initial Developmental Evaluation of Adaptive and Functional Skills (Warner IDEA-FS) in typical and atypical children [master’s thesis]. Chicago, IL: University of Chicago; 2010. [Google Scholar]
- 18.Morales-Monforte E, Bagur-Calafat C, Suc-Lerin N, Fornaguera-Martí M, Cazorla-Sánchez E, Girabent-Farrés M. The Spanish version of the Alberta Infant Motor Scale: validity and reliability analysis. Dev Neurorehabil. 2017;20(2):76-82. doi: 10.3109/17518423.2015.1066461 [DOI] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mooney CZ, Duval RD. Bootstrapping: A Nonparametric Approach to Statistical Inference. Newbury Park, CA: Sage; 1993. doi: 10.4135/9781412983532 [DOI] [Google Scholar]
- 21.McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficient. Psychol Methods. 1996;1(1):30-46. doi: 10.1037/1082-989X.1.1.30 [DOI] [Google Scholar]
- 22.Adhikari EH, Nelson DB, Johnson KA, et al. Infant outcomes among women with Zika virus infection during pregnancy: results of a large prenatal Zika screening program. Am J Obstet Gynecol. 2017;216(3):292.e1-292.e8, e298. doi: 10.1016/j.ajog.2017.01.018 [DOI] [PubMed] [Google Scholar]
- 23.Shapiro-Mendoza CK, Rice ME, Galang RR, et al. ; Zika Pregnancy and Infant Registries Working Group . Pregnancy outcomes after maternal Zika virus infection during pregnancy - U.S. territories, January 1, 2016-April 25, 2017. MMWR Morb Mortal Wkly Rep. 2017;66(23):615-621. doi: 10.15585/mmwr.mm6623e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Einspieler C, Utsch F, Brasil P, et al. ; GM Zika Working Group . Association of infants exposed to prenatal Zika virus infection with their clinical, neurologic, and developmental status evaluated via the General Movement Assessment Tool. JAMA Netw Open. 2019;2(1):e187235. doi: 10.1001/jamanetworkopen.2018.7235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lebel C, Deoni S. The development of brain white matter microstructure. Neuroimage. 2018;182:207-218. doi: 10.1016/j.neuroimage.2017.12.097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connery AK, Berrios-Siervo G, Arroyave P, et al. Responding to the Zika epidemic: preparation of a neurodevelopmental testing protocol to evaluate young children in rural Guatemala. Am J Trop Med Hyg. 2019;100(2):438-444. doi: 10.4269/ajtmh.18-0713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cantey JB, Sisman J. The etiology of lenticulostriate vasculopathy and the role of congenital infections. Early Hum Dev. 2015;91(7):427-430. doi: 10.1016/j.earlhumdev.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 28.Shin HJ, Kim MJ, Lee HS, Namgung R, Park KI, Lee MJ. Imaging patterns of sonographic lenticulostriate vasculopathy and correlation with clinical and neurodevelopmental outcome. J Clin Ultrasound. 2015;43(6):367-374. doi: 10.1002/jcu.22196 [DOI] [PubMed] [Google Scholar]
- 29.Mulkey SB, DeBiasi RL, du Plessis AJ. Cerebral infarction due to Zika virus. J Neurol Sci. 2018;387:109-110. doi: 10.1016/j.jns.2018.01.032 [DOI] [PubMed] [Google Scholar]
- 30.Landais A, Césaire A, Fernandez M, et al. ZIKA vasculitis: a new cause of stroke in children? J Neurol Sci. 2017;383:211-213. doi: 10.1016/j.jns.2017.10.045 [DOI] [PubMed] [Google Scholar]
- 31.Larcos G, Gruenewald SM, Lui K. Neonatal subependymal cysts detected by sonography: prevalence, sonographic findings, and clinical significance. AJR Am J Roentgenol. 1994;162(4):953-956. doi: 10.2214/ajr.162.4.8141023 [DOI] [PubMed] [Google Scholar]
- 32.Yamashita Y, Outani Y, Kawano Y, Horikawa M, Matsuishi T, Hashimoto T. Clinical analyses and short-term prognoses of neonates with subependymal cysts. Pediatr Neurol. 1990;6(6):375-378. doi: 10.1016/0887-8994(90)90003-J [DOI] [PubMed] [Google Scholar]
- 33.Adebanjo T, Godfred-Cato S, Viens L, et al. ; Contributors . Update: interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection—United States, October 2017. MMWR Morb Mortal Wkly Rep. 2017;66(41):1089-1099. doi: 10.15585/mmwr.mm6641a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rice ME, Galang RR, Roth NM, et al. Vital signs: Zika-associated birth defects and neurodevelopmental abnormalities possibly associated with congenital Zika virus infection—U.S. territories and freely associated states, 2018. MMWR Morb Mortal Wkly Rep. 2018;67(31):858-867. doi: 10.15585/mmwr.mm6731e1 [DOI] [PMC free article] [PubMed] [Google Scholar]