This cohort study quantifies the relative importance of a polygenic risk score, fitness, activity, parental history of overweight, and body mass index in young adulthood regarding body mass index trends over 25 years.
Key Points
Question
What is the added value of polygenic risk in predicting body mass index (BMI) over time beyond young adulthood BMI, parental history of overweight, fitness, and activity?
Findings
Among 1608 white individuals and 909 black individuals in this cohort study of young adults in the United States, polygenic risk scores did not offer accurate prediction of BMI in midlife, whereas BMI in young adulthood (in their 20s) offered a more accurate prediction of long-term BMI trends.
Meaning
Comprehensive clinical risk profiles (incorporating BMI, its change over time, and behavioral factors), but not polygenic risk scores, offer substantial predictive ability for future BMI in the context of obesity prevention.
Abstract
Importance
Obesity is a major determinant of disease burden worldwide. Polygenic risk scores (PRSs) have been posited as key predictors of obesity. How a PRS can be translated to the clinical encounter (especially in the context of fitness, activity, and parental history of overweight) remains unclear.
Objective
To quantify the relative importance of a PRS, fitness, activity, parental history of overweight, and body mass index (BMI) (calculated as weight in kilograms divided by height in meters squared) in young adulthood on BMI trends over 25 years.
Design, Setting, and Participants
This population-based prospective cohort study at 4 US centers included white individuals and black individuals with assessments of polygenic risk of obesity, fitness, activity, and BMI in young adulthood (in their 20s) and up to 25 years of follow-up. Data collected between March 1985 and August 2011 were analyzed from April 25, 2019, to September 29, 2019.
Main Outcomes and Measures
Body mass index at the initial visit and 25 years later.
Results
This study evaluated an obesity PRS from a recently reported study of 1608 white individuals (848 women [52.7%]) and 909 black individuals (548 women [60.3%]) across the United States. At baseline (year 0), mean (SD) overall BMI was 24.2 (4.5), which increased to 29.6 (6.9) at year 25. Among white individuals, the PRS (combined with age, sex, self-reported parental history of overweight, and principal components of ancestry) explained 11.9% (at year 0) and 13.6% (at year 25) of variation in BMI. Although the addition of fitness increased the explanatory capability of the model (24.0% variance at baseline and up to 18.1% variance in BMI at year 25), baseline BMI in young adulthood was the strongest factor, explaining 52.3% of BMI in midlife in combination with age, sex, and self-reported parental history of overweight. Accordingly, models that included baseline BMI (especially BMI surveillance over time) were better in predicting BMI at year 25 compared with the PRS. In fully adjusted models, the effect sizes for fitness and the PRS on BMI were comparable in opposing directions. The added explanatory capacity of the PRS among black individuals was lower than among white individuals. Among white individuals, addition of baseline BMI and surveillance of BMI over time was associated with improved precision of predicted BMI at year 25 (mean error in predicted BMI 0 kg/m2 [95% CI, −11.4 to 11.4] to 0 kg/m2 [95% CI, −8.5 to 8.5] for baseline BMI and mean error 0 kg/m2 [95% CI, −5.3 to 5.3] for BMI surveillance).
Conclusions and Relevance
Cardiorespiratory fitness in young adulthood and a PRS are modestly associated with midlife BMI, although future BMI is associated with BMI in young adulthood. Fitness has a comparable association with future BMI as does the PRS. Caution should be exercised in the widespread use of polygenic risk for obesity prevention in adults, and close clinical surveillance and fitness may have prime roles in limiting the adverse consequences of elevated BMI on health.
Introduction
Body mass index (BMI) is a complex trait with inputs from environment (including diet, physical activity [PA], and socioecological factors) and genetics. Although some genomic studies1,2,3 of obesity suggest a heritability between 40% and 70%, most modern genome-wide association studies of obesity account for less than 4% of the variance in BMI, leading investigators to expand the search for susceptibility loci.4 The results of recent studies have suggested that a polygenic risk score (PRS) incorporating BMI associations ascribed to individual genetic variants across the human genome may be used to accurately predict the risk of obesity at a population level.1 These findings have prompted efforts to translate the PRS of obesity (and other complex human traits) to the clinical setting5 to increase diagnostic and therapeutic precision. In parallel, a widening body of literature has pointed toward sedentary lifestyle and decreased cardiorespiratory fitness as factors associated with obesity and obesity-related disease.6,7,8,9,10,11,12,13,14,15 Nevertheless, how these factors contribute (if at all) to BMI in midlife independent of BMI in young adulthood remains unclear. To understand how best to prevent (and treat) obesity, it is important to understand the contributions of each of these factors (environment and genetics) as they are viewed in the clinical setting to long-term development of increased BMI.
In this prospective cohort study, we sought to quantify the relative importance of an obesity PRS, cardiorespiratory fitness, PA, parental history of overweight, and BMI in young adulthood regarding BMI trends over 25 years. We used data from the Coronary Artery Risk Development in Young Adults (CARDIA) study to estimate the association of these variables with BMI and to evaluate model fit and prediction.
Methods
CARDIA Study Cohort
The CARDIA study is a prospective cohort study of 5115 white and black participants (self-identified) aged 18 to 30 years at baseline in 1985 to 1986 who were recruited from 4 field centers in the United States (Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California) to investigate the origins of cardiovascular disease. The study design has been previously described.16,17,18,19 Data collected between March 1985 and August 2011 were analyzed from April 25, 2019, to September 29, 2019. Data collected between the baseline examination (March 1985 to June 1986) and the year 25 follow-up examination (June 2010 to August 2011) were analyzed. This analysis included 1663 white participants from the CARDIA study in whom written informed consent had been provided for genetic analysis, DNA had been genotyped, and a PRS had been defined for a recent collaborative study1 with information on principal components of ancestry. In addition, we applied the PRS derived in white individuals to 955 black participants in the CARDIA study to test its predictive ability (with knowledge that the original PRS was not defined in black individuals). Excluded were individuals with missing (11 white individuals and 10 black individuals) or zero (20 white individuals and 18 black individuals) baseline treadmill exercise time, no baseline BMI (5 white individuals and 0 black individuals), or a history of bariatric surgery by year 25 (19 white individuals and 18 black individuals), leaving 2517 participants in our analytic cohort, including 1608 white individuals (848 women [52.7%]) and 909 black individuals (548 women [60.3%]). All individuals included in this study provided written informed consent, and the CARDIA study was approved by the institutional review boards at each participating institution (Northwestern University, Kaiser Permanente Northern California Division of Research, University of Minnesota, and The University of Alabama at Birmingham).
Exposure and Outcome Definition
Our exposures included BMI at baseline (measured by trained staff with the participant wearing light clothing and no shoes), self-reported parental history of overweight assessed at baseline (affirmative response to a question about the natural father or mother ever being very overweight), cardiorespiratory fitness at baseline (assessed using treadmill exercise time6), and a CARDIA study PA score at baseline (algorithm to assess usual moderate to vigorous PA using the CARDIA study20 exercise units). The PRS for obesity was constructed in the CARDIA study as described by Khera and colleagues.1 Samples were genotyped with the Affymetrix Genome-Wide Human SNP Array 6.0. Single-nucleotide polymorphisms (SNPs) passing quality control (minor allele frequency ≥2%, SNP call rate ≥95%, and Hardy-Weinberg equilibrium ≥10−4) were imputed to the 1000 Genomes Project phase 3 version 5 reference panel. Ten principal components of ancestry were used in regressions that included the PRS. Our primary outcome was BMI at the year 25 visit in the CARDIA study.
Statistical Modeling
Our primary objective was to evaluate the proportion of variability (variance) in BMI explained by the addition of fitness, activity, and/or the PRS to standard clinical risk variables of age, sex, and self-reported parental history of overweight. For mechanistic modeling, it is standard practice in the genetics literature not to adjust for potential mediators or baseline values of end points of interest. In contrast, for clinical prediction, adjustment for commonly available confounding variables, including baseline values of end points of interest, is necessary. Indeed, the CARDIA study participants engaged in routine clinical care would be expected to have serial assessment of BMI at multiple time points over the 25 years of follow-up, as in the present study. Given this dichotomy, we generated the following 3 sets of generalized linear models for BMI at each assessed time point as a function of clinical risk factors and fitness, activity, and/or the PRS: (1) not adjusted for baseline BMI, (2) adjusted for baseline BMI, or (3) adjusted for baseline and subsequent BMI at intermediate examinations. Each of these models was calculated over the subset of participants who had BMI data at the final BMI time point and all intermediate time points (Table).
Table. Baseline Characteristics of the Analytic Cohort by Race in the Coronary Artery Risk Development in Young Adults (CARDIA) Study.
| Characteristic | Mean (SD) | ||
|---|---|---|---|
| White Individuals (n = 1608) | Black Individuals (n = 909) | Overall (N = 2517) | |
| Age, y | |||
| Year 0 | 25.6 (3.3) | 24.4 (3.8) | 25.1 (3.6) |
| Year 25 | 50.8 (3.3) | 49.4 (3.9) | 50.3 (3.6) |
| Sex, No. (%) | |||
| Male | 760 (47.3) | 361 (39.7) | 1121 (44.5) |
| Female | 848 (52.7) | 548 (60.3) | 1396 (55.5) |
| BMI | |||
| Year 0 | 23.6 (3.8) | 25.3 (5.4) | 24.2 (4.5) |
| Year 2 | 24.2 (4.1) | 26.3 (5.8) | 24.9 (4.9) |
| Year 5 | 24.8 (4.4) | 27.3 (6.3) | 25.6 (5.3) |
| Year 7 | 25.3 (4.9) | 28.1 (6.5) | 26.3 (5.7) |
| Year 10 | 25.8 (5.2) | 29.0 (6.7) | 26.9 (5.9) |
| Year 15 | 27.0 (5.7) | 30.4 (7.1) | 28.2 (6.4) |
| Year 20 | 27.8 (5.8) | 31.4 (7.4) | 29.0 (6.6) |
| Year 25 | 28.2 (6.1) | 32.2 (7.7) | 29.6 (6.9) |
| Fitness and activitya | |||
| Exercise treadmill time, s | 645 (155) | 532 (168) | 604 (169) |
| Moderate to vigorous PA, exercise units | 460 (285) | 375 (304) | 429 (295) |
| Self-reported parental history of overweight, No. (%) | 747 (46.5) | 407 (44.8) | 1154 (45.8) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); PA, physical activity.
Exercise treadmill time and moderate to vigorous PA were measured at study entry (year 0).
Because of multicollinearity between baseline and subsequent BMI, we initially fit a longitudinal generalized additive model for serial BMI as a function of age, sex, and baseline BMI using separate smoothing splines by sex and a random intercept by participant. Initial BMI was standardized to allow estimation of marginal mean splines for BMI as a function of age across various percentiles of initial BMI. Using this model, we generated growth curves for BMI as a function of age in the CARDIA study and used these to compute residuals at each subsequent examination, representing how much above or below the expected growth curve each individual was.
For our final generalized models for BMI at each time point, the total cumulative variance explained by all predictors (standard clinical variables, as well as fitness, activity, and/or the PRS [with ancestry expressed as 10 principal components]) was computed, as were β coefficients per SD in each predictor variable (eg, how much is BMI expected to increase per SD increase in the PRS). Each of these models was calculated over the subset of participants who had BMI data at the final BMI time point and all intermediate time points (to allow estimation of excess BMI using the growth curve method described previously).
Analyses were conducted separately by race given that the PRS was not derived in a racially heterogeneous population. As a sensitivity analysis, all key analyses were repeated omitting self-reported parental history of overweight. A type I error threshold of α < .05 was considered statistically significant, and all tests were 2 sided. All analyses were performed with R statistical software, version 3.6.1 (R Foundation for Statistical Computing) using the mgcv module for generalized additive models, emmeans for estimated marginal means, and ggplot2 for graphical plotting.
Results
Baseline Characteristics
The Table summarizes the distribution of age, sex, BMI, fitness and activity, and self-reported parental history of overweight for 1608 white (848 women [52.7%]) and 909 black (548 women [60.3%]) participants during follow-up in the CARDIA study. At study entry (year 0), the included white CARDIA study participants were young adults (mean [SD] age, 25.6 [3.3] years), equally distributed by sex, with a normal BMI (calculated as weight in kilograms divided by height in meters squared) (mean [SD], 23.6 [3.8]), with almost 1 in 2 reporting a subjective classification of overweight in either parent. There was a gradual increase in BMI during follow-up in the CARDIA study (eFigure 1 in the Supplement) such that by year 25 BMI had increased by approximately 5 in white individuals and 7 in black individuals (Figure 1). Individuals who were seen at sequential follow-up examinations in the CARDIA study generally had increasing BMI over time (eTable 1 in the Supplement). The PRS was normally distributed in individuals at study entry (eFigure 2 in the Supplement) but was differently calibrated in white individuals compared with black individuals (eFigure 3 in the Supplement). Although among white individuals the PRS differed statistically significantly between those individuals with self-reported parental history of overweight vs those without, actual differences were small and unlikely to be clinically meaningful, suggesting minimal collinearity between the PRS and parental history (mean [SD] PRS for BMI, 35.0 [0.1] vs 34.9 [0.1]; P < .001) (eFigure 4 in the Supplement). A comparison of individuals included in and excluded from the final analytic cohort (largely because of missing PRS) is summarized in eTable 2 in the Supplement.
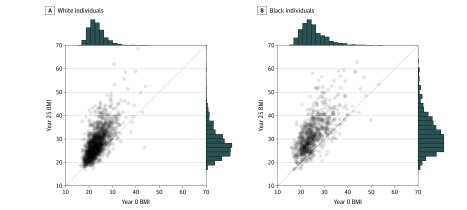
Figure 1. Distribution of Body Mass Index (BMI) Over 25 Years in the Coronary Artery Risk Development in Young Adults (CARDIA) Study.
A and B, The data represent individuals who had BMI data at study entry (year 0) and in follow-up (year 25). The marginal histograms show the distribution of BMI at each time point. Points above the dashed line represent individuals whose BMI increased from baseline to year 25. BMI is calculated as weight in kilograms divided by height in meters squared.
Association of Polygenic Risk, Cardiorespiratory Fitness, and Clinical Risk in Young Adulthood With BMI Over 25 Years
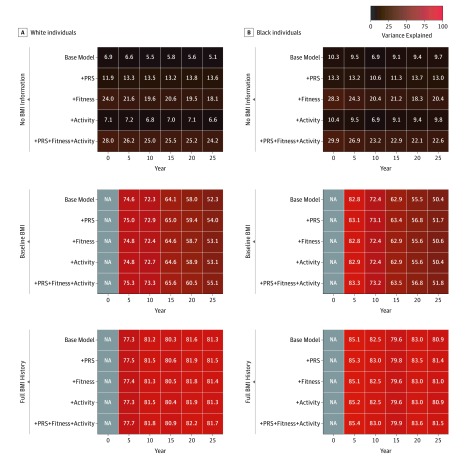
Age, sex, and self-reported parental history of overweight explained between 5.1% (year 25) and 6.9% (year 0) of population variation in BMI among white individuals and between 6.9% (year 10) and 10.3% (year 0) of population variation in BMI among black individuals (Figure 2). Among white individuals, the addition of the PRS and 10 principal components of ancestry increased the variance explained to between 11.9% (year 0) and 13.8% (year 20) (P < .001 for both) (eTable 3 in the Supplement), although activity more modestly increased the variance explained. In contrast, the addition of fitness increased the variance explained to between 18.1% (year 25) and 24.0% (year 0) (P < .001 for both). Baseline BMI in young adulthood was the strongest factor, explaining 74.6% of variation in BMI after 5 years (5.6-fold more than the PRS) and 52.3% of variation in BMI in midlife (3.8-fold more than the PRS) in combination with age, sex, and self-reported parental history of overweight. When all 3 factors were included together, fitness and the PRS had similar effect sizes per SD, although in opposing directions, with higher fitness associated with lower BMI and lower PRS associated with lower BMI. Similar findings were seen among black individuals, with the PRS resulting in a numerically smaller increase in variance explained from between 6.9% and 10.3% to between 10.6% and 14.7% (Figure 2B).
Figure 2. Variance in Body Mass Index (BMI) at Various Time Points Explained by Models With Increasing Information in the Coronary Artery Risk Development in Young Adults (CARDIA) Study.
A and B, The base model included age, sex, and self-reported parental history of overweight. The polygenic risk score (PRS), fitness, and/or activity were added to the base model. In the second block, baseline BMI was added to the base model. In the third block, preceding BMI growth curve history was also added to the base model. BMI is calculated as weight in kilograms divided by height in meters squared. NA indicates not applicable.
Once baseline BMI at year 0 was accounted for, the explained variance markedly increased to greater than 70% during young adulthood (in their 20s) and greater than 50% at midlife. However, added increases in the variance explained by fitness, activity, and the PRS beyond age, sex, and self-reported parental history of overweight were modest (<3%) (Figure 2). Serial surveillance of BMI for deviation from growth curve profiles derived from this population resulted in models durably accounting for approximately 80% of variation in BMI across young adulthood to midlife. Adjusted R2 to account for increasing numbers of parameters showed similar patterns (eFigure 5 in the Supplement).
No pairwise interactions were noted between the PRS and either fitness, activity, or baseline BMI on year 25 BMI. Sensitivity analysis examining the variance explained in models without self-reported parental history of overweight delivered similar incremental proportions of the variance explained for models with the PRS (eFigure 6 and eFigure 7 in the Supplement) and similar effect sizes (eTable 4 in the Supplement).
Prediction of Midlife BMI Using Polygenic Risk and Clinical Obesity Susceptibility
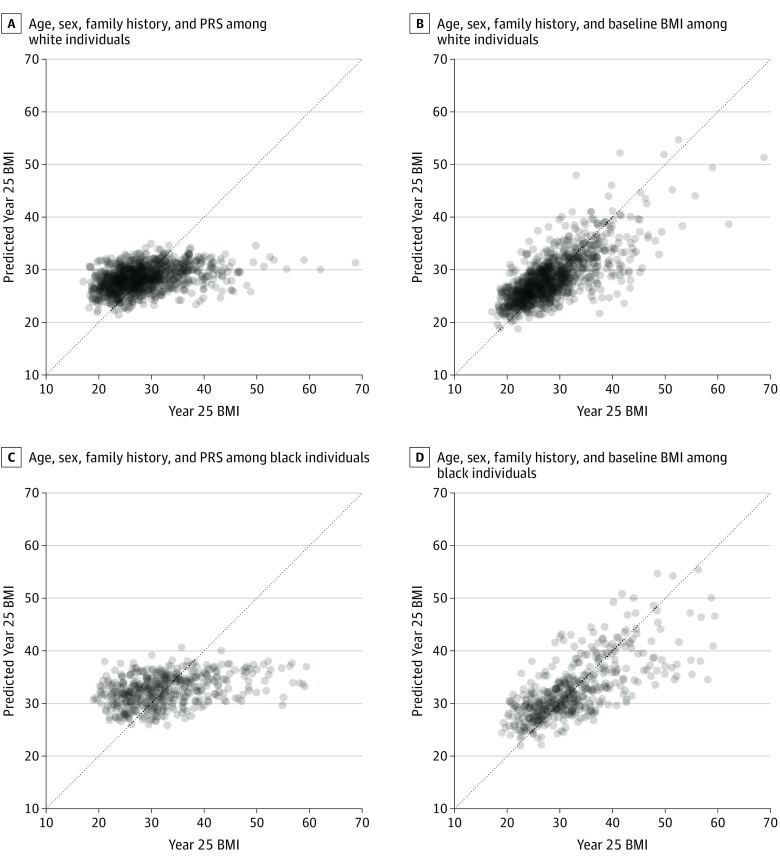
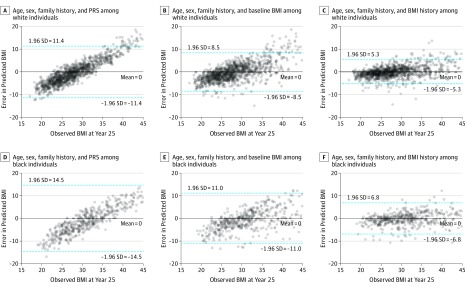
One of the major proposed clinical uses of an obesity PRS is as a clinical tool to predict how BMI changes over time in a clinical encounter. In this regard, we evaluated the predictive ability of the PRS alongside other relevant, easily obtained clinical parameters. Prediction of BMI at year 25 from age, sex, self-reported parental history of overweight, and the PRS was poor (Figure 3 and Figure 4), with systematic underestimation of BMI among participants with the highest BMIs and overestimation of BMI among participants with the lowest BMIs. Consequently, the limits of agreement were wide (mean error 0.0 kg/m2 [95% CI, −11.4 to 11.4] in white individuals, and mean error 0.0 kg/m2 [95% CI,−14.5 to 14.5] in black individuals), indicating poor predictive precision with the PRS. When BMI at the baseline study visit (year 0) was taken into account, prediction of BMI improved, with narrowed limits of agreement over 25 years (mean error 0.0 kg/m2 [95% CI, −8.5 to 8.5] in white individuals, and mean error 0.0 kg/m2 [−11.0 to 11.0] in black individuals). Notably, serial surveillance of BMI based on a growth curve approach further narrowed the prediction intervals (mean error 0.0 kg/m2 [95% CI, −5.3 to 5.3] in white individuals, and mean error 0.0 kg/m2 [−6.8 to 6.8] in black individuals). These results were consistent with models suggesting that the models with baseline BMI explained a much greater amount of variation in midlife BMI than the PRS. Similar findings were obtained if self-reported parental history of overweight was excluded from the models (eFigure 8 and eFigure 9 in the Supplement).
Figure 3. Predictive Accuracy of the Polygenic Risk Score (PRS) in the Coronary Artery Risk Development in Young Adults (CARDIA) Study Relative to the Clinical Risk Model That Includes Baseline and Serial Body Mass Index (BMI) Assessment.
Shown is the predicted vs observed year 25 BMI in white (A and B) and black (C and D) individuals in the PRS model and the baseline BMI model. The models are described in the Statistical Modeling subsection of the Methods section. Points above the dashed line represent individuals whose BMI increased from baseline to year 25. BMI is calculated as weight in kilograms divided by height in meters squared.
Figure 4. Error in Body Mass Index (BMI) Prediction With the Polygenic Risk Score (PRS) vs Clinical Models in the Coronary Artery Risk Development in Young Adults (CARDIA) Study.
Shown is the error in BMI prediction at year 25 vs the observed year 25 BMI in white (A-C) and black (D-F) individuals for the PRS model, baseline BMI model, and serial BMI model. BMI is calculated as weight in kilograms divided by height in meters squared.
Discussion
The principal findings of our study are 3-fold. First, although the PRS was statistically significantly associated with BMI in young adulthood and midlife, the association was modest (11%-14% of variance explained at best with age, sex, and self-reported parental history of overweight included), with insufficient precision for clinically relevant prediction of midlife BMI. Second, traditional clinical BMI assessment in young adulthood (age range, 18-30 years) offered more precision for prediction of an individual’s BMI 25 years later in midlife, with measurement of BMI serially offering the most precise predictions. Third, we found that fitness, activity, and self-reported parental history of overweight had a comparable association with BMI in midlife as the PRS (without evidence of interaction between fitness and the PRS), suggesting that higher levels of fitness and activity may limit obesity independent of polygenic risk. Ultimately, these findings emphasize that screening early in adulthood using a standard BMI measurement (and closer serial follow-up for those individuals at higher risk), as well as implementation of preventive strategies by increasing cardiorespiratory fitness, may be more clinically meaningful than reliance on genetic risk.
Given the importance of BMI in general health,21 efforts to predict the risk of obesity early in adulthood, when interventions may more easily have long-term ramifications, are of substantial clinical interest. Although useful in suggesting mechanisms of obesity in large populations, human genetics has to date displayed only a modest association with BMI (1%-9%).1,3,22 A landmark PRS (developed among 2.1 million genomic variants in >500 000 individuals) explained 9% to 10% of variation in BMI,1 with a statistically significant association between the PRS and BMI used to suggest that the PRS may predict long-term obesity development. Our results indicate that caution should be exercised in widespread use of polygenic risk in prediction of BMI. Using the most recent PRS in the CARDIA study, we found that knowing an individual’s BMI between ages 18 to 30 years provided substantially more information regarding BMI in midlife than did the PRS (almost 4-fold to 5-fold increased variance explained). In addition, extrapolation of future BMI on a genetic basis is likely to lead to biased estimates, systematically underestimating the BMI of the most obese members of the population. Furthermore, we found that the PRS had a similar effect size as cardiorespiratory fitness and moderate to vigorous PA (without evidence of effect modification), further highlighting that a PRS profile is not deterministic of obesity. Finally, we found that incorporation of serial assessment of BMI in clinic visits through young adulthood further augments the ability to discern BMI by midlife.
The primary clinical implication of our results is the emphasis on the inclusion of BMI and potentially fitness and activity as vital signs for close clinical management and follow-up regardless of genetic risk.23,24 Despite widespread adoption of BMI and PA in modern risk assessment, measurement of either in the routine clinical encounter is not universal. In a study25 of visits to primary care physicians in Massachusetts, BMI was documented 60% of the time. In addition, only 1 in 3 adults may be counseled on PA recommendations.26 In view of these findings indicating that a high-dimensional PRS may not be clinically precise enough to inform risk, the rapid adoption and communication of these genetic risk assessments for complex traits (like obesity) by clinicians and industry partners to patients may be clinically counterproductive, drawing attention away from more precise factors that are easily assessed and mutable in the clinical setting. Indeed, evidence for strong, sustained consequences of genetic information on behavior change and long-term outcomes is lacking,27,28 and the findings of recent studies suggest that simply communicating a genetic risk may adversely alter an individual’s physiology itself.29 Finally, these clinical concerns run independently of emerging methodologic concerns with genetic risk construction, including a lack of data in non-European populations, the consequences of population stratification,30 and more generally the fundamental differences of an observed statistically significant association between the PRS and a trait vs its ability to predict that trait over the life course. Certainly, the application of the PRS at birth or early childhood as described in previous work1 may delineate individuals by weight. Nevertheless, by young adulthood, the genetic susceptibility is surmounted by BMI itself in understanding who may become obese in the future. Ultimately, interventions targeting the obesogenic environment via social and individual change in activity patterns, diet, stress, and other clinical contributors to obesity are more likely than genetic risk scores to alter BMI over time. Indeed, a substantial body of work exists in support of fitness and PA in obesity, type 2 diabetes, and cardiovascular disease.31,32
Limitations
This study has limitations. The results of this observational study should be interpreted in view of its design. Although we present our findings for both white and black participants in the CARDIA study, the original PRS was derived in a largely European population; therefore, further race-specific studies to understand the role of genetics in BMI is warranted. In addition, other genetic risk scores for BMI could have been used in this work,33 although most are limited by total variance in BMI explained to near 10% (without any major changes in these results expected). We did not include assessment of multiple domains of lifestyle over the life course and sociodemographic exposures (eg, educational level), but estimates of the association between the PRS and BMI in the CARDIA study are consistent with those from larger populations,1 and the phenotyping and longitudinal follow-up in the CARDIA study are unique to address our hypothesis. Although we had sufficient power to dissect relative associations of BMI, fitness, activity, and the PRS on long-term BMI, our results should be generalized in larger populations with harmonized measures of diet, lifestyle, and fitness or activity.
Conclusions
In a US-based cohort, we found that BMI in young adulthood provides much more precise prediction of midlife BMI compared with that afforded by modern polygenic risk estimates. We observed substantial prediction error in BMI with the use of the PRS, which was largely abrogated by consideration of BMI in young adulthood and its longitudinal course during young adulthood to midlife. Fitness, activity, and self-reported parental history of overweight had a similar magnitude of association with midlife BMI as the PRS without effect modification, suggesting that early fitness and activity may counteract the consequences of genetic risk on long-term BMI. Modern prevention efforts should focus on phenotypic characteristics (most prominently young adulthood BMI) that presage weight gain and obesity.
eFigure 1. Longitudinal Models for BMI Over Time in CARDIA
eFigure 2. Polygenic Risk Score for BMI
eFigure 3. Calibration of Polygenic Risk Score vs Body Mass Index (BMI) at Study Entry
eFigure 4. Histogram of Polygenic Risk Score for Body Mass Index by Self-reported Parental Overweight/Obesity Status
eFigure 5. Adjusted R2 for Models of BMI at Various Time Points With Increasing Information
eFigure 6. Variance in BMI at Various Time Points Explained by Models With Increasing Information
eFigure 7. Adjusted R2 for Models of BMI at Various Time Points With Increasing Information
eFigure 8. Predictive Accuracy of PRS in CARDIA, Relative to Clinical Risk Model Including Baseline and Serial BMI Assessment
eFigure 9. Error in BMI Prediction With PRS vs Clinical Models
eTable 1. Characteristics of Analytic Cohort Over Time
eTable 2. Characteristics of Subjects Included and Excluded From Analytic Cohort by Race
eTable 3. Linear Regression Models for BMI at Specific CARDIA Follow-up Times
eTable 4. Linear Regression Models for BMI at Specific CARDIA Follow-up Times
References
- 1.Khera AV, Chaffin M, Wade KH, et al. . Polygenic prediction of weight and obesity trajectories from birth to adulthood. Cell. 2019;177(3):587-596.e9. doi: 10.1016/j.cell.2019.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belsky DW, Moffitt TE, Sugden K, et al. . Development and evaluation of a genetic risk score for obesity. Biodemography Soc Biol. 2013;59(1):85-100. doi: 10.1080/19485565.2013.774628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Locke AE, Kahali B, Berndt SI, et al. ; LifeLines Cohort Study; ADIPOGen Consortium; AGEN-BMI Working Group; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GLGC; ICBP; MAGIC Investigators; MuTHER Consortium; MIGen Consortium; PAGE Consortium; ReproGen Consortium; GENIE Consortium; International Endogene Consortium . Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197-206. doi: 10.1038/nature14177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herrera BM, Lindgren CM. The genetics of obesity. Curr Diab Rep. 2010;10(6):498-505. doi: 10.1007/s11892-010-0153-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunter DJ, Drazen JM. Has the genome granted our wish yet? N Engl J Med. 2019;380(25):2391-2393. doi: 10.1056/NEJMp1904511 [DOI] [PubMed] [Google Scholar]
- 6.Shah RV, Murthy VL, Colangelo LA, et al. . Association of fitness in young adulthood with survival and cardiovascular risk: the Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA Intern Med. 2016;176(1):87-95. doi: 10.1001/jamainternmed.2015.6309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bild DE, Sholinsky P, Smith DE, Lewis CE, Hardin JM, Burke GL. Correlates and predictors of weight loss in young adults: the CARDIA study. Int J Obes Relat Metab Disord. 1996;20(1):47-55. [PubMed] [Google Scholar]
- 8.Sidney S, Sternfeld B, Haskell WL, Quesenberry CP Jr, Crow RS, Thomas RJ. Seven-year change in graded exercise treadmill test performance in young adults in the CARDIA study: cardiovascular risk factors in young adults. Med Sci Sports Exerc. 1998;30(3):427-433. doi: 10.1097/00005768-199803000-00014 [DOI] [PubMed] [Google Scholar]
- 9.Fung MD, Canning KL, Mirdamadi P, Ardern CI, Kuk JL. Lifestyle and weight predictors of a healthy overweight profile over a 20-year follow-up. Obesity (Silver Spring). 2015;23(6):1320-1325. doi: 10.1002/oby.21087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carnethon MR, Gidding SS, Nehgme R, Sidney S, Jacobs DR Jr, Liu K. Cardiorespiratory fitness in young adulthood and the development of cardiovascular disease risk factors. JAMA. 2003;290(23):3092-3100. doi: 10.1001/jama.290.23.3092 [DOI] [PubMed] [Google Scholar]
- 11.Carnethon MR, Sternfeld B, Schreiner PJ, et al. . Association of 20-year changes in cardiorespiratory fitness with incident type 2 diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) fitness study. Diabetes Care. 2009;32(7):1284-1288. doi: 10.2337/dc08-1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarzynski MA, Schuna JM Jr, Carnethon MR, et al. . Association of fitness with incident dyslipidemias over 25 years in the Coronary Artery Risk Development in Young Adults study. Am J Prev Med. 2015;49(5):745-752. doi: 10.1016/j.amepre.2015.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chow LS, Odegaard AO, Bosch TA, et al. . Twenty year fitness trends in young adults and incidence of prediabetes and diabetes: the CARDIA study. Diabetologia. 2016;59(8):1659-1665. doi: 10.1007/s00125-016-3969-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandey A, Allen NB, Ayers C, et al. . Fitness in young adulthood and long-term cardiac structure and function: the CARDIA study. JACC Heart Fail. 2017;5(5):347-355. doi: 10.1016/j.jchf.2016.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennedy AB, Lavie CJ, Blair SN. Fitness or fatness: which is more important? JAMA. 2018;319(3):231-232. doi: 10.1001/jama.2017.21649 [DOI] [PubMed] [Google Scholar]
- 16.Wagenknecht LE, Perkins LL, Cutter GR, et al. . Cigarette smoking behavior is strongly related to educational status: the CARDIA study. Prev Med. 1990;19(2):158-169. doi: 10.1016/0091-7435(90)90017-E [DOI] [PubMed] [Google Scholar]
- 17.Dyer AR, Cutter GR, Liu KQ, et al. . Alcohol intake and blood pressure in young adults: the CARDIA study. J Clin Epidemiol. 1990;43(1):1-13. doi: 10.1016/0895-4356(90)90050-Y [DOI] [PubMed] [Google Scholar]
- 18.Bild DE, Jacobs DR Jr, Sidney S, Haskell WL, Anderssen N, Oberman A. Physical activity in young black and white women: the CARDIA study. Ann Epidemiol. 1993;3(6):636-644. doi: 10.1016/1047-2797(93)90087-K [DOI] [PubMed] [Google Scholar]
- 19.Sidney S, Jacobs DR Jr, Haskell WL, et al. . Comparison of two methods of assessing physical activity in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Epidemiol. 1991;133(12):1231-1245. doi: 10.1093/oxfordjournals.aje.a115835 [DOI] [PubMed] [Google Scholar]
- 20.Smith AW, Cronin KA, Bowles H, et al. . Reproducibility of physical activity recall over fifteen years: longitudinal evidence from the CARDIA study. BMC Public Health. 2013;13:180. doi: 10.1186/1471-2458-13-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Twig G, Tirosh A, Leiba A, et al. . BMI at age 17 years and diabetes mortality in midlife: a nationwide cohort of 2.3 million adolescents. Diabetes Care. 2016;39(11):1996-2003. doi: 10.2337/dc16-1203 [DOI] [PubMed] [Google Scholar]
- 22.Speliotes EK, Willer CJ, Berndt SI, et al. ; MAGIC; Procardis Consortium . Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42(11):937-948. doi: 10.1038/ng.686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khera AV, Emdin CA, Kathiresan S. Genetic risk, lifestyle, and coronary artery disease. N Engl J Med. 2017;376(12):1194-1195. [DOI] [PubMed] [Google Scholar]
- 24.Kilpeläinen TO, Qi L, Brage S, et al. . Physical activity attenuates the influence of FTO variants on obesity risk: a meta-analysis of 218,166 adults and 19,268 children. PLoS Med. 2011;8(11):e1001116. doi: 10.1371/journal.pmed.1001116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rose SA, Turchin A, Grant RW, Meigs JB. Documentation of body mass index and control of associated risk factors in a large primary care network. BMC Health Serv Res. 2009;9:236. doi: 10.1186/1472-6963-9-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berra K, Rippe J, Manson JE. Making physical activity counseling a priority in clinical practice: the time for action is now. JAMA. 2015;314(24):2617-2618. doi: 10.1001/jama.2015.16244 [DOI] [PubMed] [Google Scholar]
- 27.Hollands GJ, French DP, Griffin SJ, et al. . The impact of communicating genetic risks of disease on risk-reducing health behaviour: systematic review with meta-analysis. BMJ. 2016;352:i1102. doi: 10.1136/bmj.i1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vassy JL, He W, Florez JC, Meigs JB, Grant RW. Six-year diabetes incidence after genetic risk testing and counseling: a randomized clinical trial. Diabetes Care. 2018;41(3):e25-e26. doi: 10.2337/dc17-1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turnwald BP, Goyer JP, Boles DZ, Silder A, Delp SL, Crum AJ. Learning one’s genetic risk changes physiology independent of actual genetic risk. Nat Hum Behav. 2019;3(1):48-56. doi: 10.1038/s41562-018-0483-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sohail M, Maier RM, Ganna A, et al. . Polygenic adaptation on height is overestimated due to uncorrected stratification in genome-wide association studies. Elife. 2019;8:8. doi: 10.7554/eLife.39702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fletcher GF, Landolfo C, Niebauer J, Ozemek C, Arena R, Lavie CJ. Promoting physical activity and exercise: JACC Health Promotion Series. J Am Coll Cardiol. 2018;72(14):1622-1639. doi: 10.1016/j.jacc.2018.08.2141 [DOI] [PubMed] [Google Scholar]
- 32.Lavie CJ, Laddu D, Arena R, Ortega FB, Alpert MA, Kushner RF. Healthy weight and obesity prevention: JACC Health Promotion Series. J Am Coll Cardiol. 2018;72(13):1506-1531. doi: 10.1016/j.jacc.2018.08.1037 [DOI] [PubMed] [Google Scholar]
- 33.Yengo L, Sidorenko J, Kemper KE, et al. ; GIANT Consortium . Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum Mol Genet. 2018;27(20):3641-3649. doi: 10.1093/hmg/ddy271 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Longitudinal Models for BMI Over Time in CARDIA
eFigure 2. Polygenic Risk Score for BMI
eFigure 3. Calibration of Polygenic Risk Score vs Body Mass Index (BMI) at Study Entry
eFigure 4. Histogram of Polygenic Risk Score for Body Mass Index by Self-reported Parental Overweight/Obesity Status
eFigure 5. Adjusted R2 for Models of BMI at Various Time Points With Increasing Information
eFigure 6. Variance in BMI at Various Time Points Explained by Models With Increasing Information
eFigure 7. Adjusted R2 for Models of BMI at Various Time Points With Increasing Information
eFigure 8. Predictive Accuracy of PRS in CARDIA, Relative to Clinical Risk Model Including Baseline and Serial BMI Assessment
eFigure 9. Error in BMI Prediction With PRS vs Clinical Models
eTable 1. Characteristics of Analytic Cohort Over Time
eTable 2. Characteristics of Subjects Included and Excluded From Analytic Cohort by Race
eTable 3. Linear Regression Models for BMI at Specific CARDIA Follow-up Times
eTable 4. Linear Regression Models for BMI at Specific CARDIA Follow-up Times