Key Points
Question
What is the effectiveness of combining behavioral and drug therapy compared with each therapy alone for overactive bladder symptoms in men, and what is the best sequence for combining therapies?
Findings
In this 2-stage, multisite randomized clinical trial including 204 men with overactive bladder symptoms, reductions in voiding frequency were significantly greater in those receiving combined therapy compared with those receiving drug therapy alone but not compared with those receiving behavioral therapy alone and greater in those receiving behavioral therapy alone compared with those receiving drug therapy alone. After all groups received combined therapy, outcomes tended to be better for those initially receiving combined therapy, but there were no significant group differences.
Meaning
When using a stepped approach to combined therapy, it is reasonable to begin with behavioral therapy.
This randomized clinical trial evaluates whether combining behavioral and drug therapies improves outcomes compared with each therapy alone for overactive bladder symptoms in men and compares 3 sequences for implementing combined therapy.
Abstract
Importance
First-line behavioral and drug therapies for overactive bladder (OAB) symptoms in men are effective but not usually curative.
Objective
To determine whether combining behavioral and drug therapies improves outcomes compared with each therapy alone for OAB in men and to compare 3 sequences for implementing combined therapy.
Design, Setting, and Participants
In this 3-site, 2-stage, 3-arm randomized clinical trial, participants were randomized to 6 weeks of behavioral therapy alone, drug therapy alone, or combined therapy followed by step-up to 6 weeks of combined therapy for all groups. Participants were recruited from 3 outpatient clinics and included community-dwelling men 40 years or older with urinary urgency and 9 or more voids per 24 hours. Data were collected from July 2010 to July 2015 and analyzed from April 2016 to September 2019.
Interventions
Behavioral therapy consisted of pelvic floor muscle training with urge suppression strategies and delayed voiding. Drug therapy included an antimuscarinic (sustained-release tolterodine, 4 mg) plus an α-blocker (tamsulosin, 0.4 mg).
Main Outcomes and Measures
Seven-day bladder diaries completed before and after each 6-week treatment stage were used to calculate reduction in frequency of urination (primary outcome) and other symptoms (ie, urgency, urgency incontinence, and nocturia). Other secondary outcomes included validated patient global ratings of improvement and satisfaction, Overactive Bladder Questionnaire score, and International Prostate Symptom Score.
Results
Of the 204 included men, 133 (65.2%) were white, and the mean (SD) age was 64.1 (11.1) years. A total of 21 men discontinued treatment and 183 completed treatment. Mean (SD) voids per 24 hours decreased significantly in all 3 groups from baseline to 6-week follow-up (behavioral therapy: 11.7 [2.4] vs 8.8 [2.1]; change, 2.9 [2.4]; percentage change, 24.7%; P < .001; drug therapy: 11.8 [2.5] vs 10.3 [2.7]; change, 1.5 [2.3]; percentage change, 12.7%; P < .001; combined therapy: 11.8 [2.4] vs 8.2 [2.3]; change, 3.6 [2.1]; percentage change, 30.5%; P < .001). Intention-to-treat analyses indicated that posttreatment mean (SD) voiding frequencies were significantly lower in those receiving combined therapy compared with drug therapy alone (8.2 [2.3] vs 10.3 [2.7]; P < .001) but not significantly lower compared with those receiving behavioral therapy alone (8.2 [2.3] vs 8.8 [2.1]; P = .19) and were lower for behavioral therapy alone compared with drug therapy alone (8.8 [2.1] vs 10.3 [2.7]; P < .001). At 12-week follow-up, after all groups had received combined therapy, improvements in mean (SD) voids per 24 hours were also greatest for those receiving initial combined therapy compared with baseline (behavioral therapy: 11.7 [2.4] vs 8.0 [2.2]; change, 3.7 [2.3]; percentage change, 31.6%; P < .001; drug therapy: 11.8 [2.5] vs 8.6 [2.3]; change, 3.2 [2.5]; percentage change, 27.1%; P < .001; combined therapy: 11.8 [2.4] vs 8.0 [2.2]; change, 3.8 [2.1]; percentage change, 32.2%; P < .001), but there were no statistically significant group differences on primary or secondary measures.
Conclusions and Relevance
Combining behavioral and drug therapy yields greater improvements in OAB symptoms than drug therapy alone but not behavioral therapy alone. When using a stepped approach, it is reasonable to begin with behavioral therapy alone.
Trial Registration
ClinicalTrials.gov identifier: NCT01175382
Introduction
Overactive bladder (OAB) symptoms of urgency, frequent urination, urge incontinence, and/or nocturia1 are common, distressing symptoms that affect the lives of millions of men.2,3 Epidemiological studies indicate that these symptoms affect between 12% and 17% of community-dwelling adults and increase in prevalence with age in both men and women.2,3,4 Research on OAB symptoms and especially incontinence shows that they can have a major effect on quality of life5 and that frequency and/or urgency can be as bothersome as leakage.2
Overactive bladder symptoms are most often treated with drug or behavioral therapies. Common first-line drugs for men include α-adrenergic receptor antagonists (α-blockers) to reduce smooth muscle tone in the bladder outlet to decrease resistance as well as antimuscarinic agents to inhibit detrusor contractions.6,7 The efficacy and safety of combined 2-drug therapy in men with OAB has been demonstrated repeatedly.8,9,10,11,12,13,14,15,16
Behavioral therapies are a group of interventions recommended as first-line therapy for OAB by several guidelines and high-quality reviews.17,18,19,20 Studies demonstrate that behavioral interventions are free of adverse effects and effective for reducing OAB symptoms, particularly urge incontinence and voiding frequency, in both men and women.21,22 Behavioral training is one such intervention that combines pelvic floor muscle training and urge suppression strategies to improve bladder control by teaching patients to voluntarily control urgency and inhibit detrusor contractions.21,22,23,24
While behavioral and drug therapies reduce symptoms, most patients are not completely cured with either modality alone. Based on evidence that these treatments work by different mechanisms, it seems likely they could have additive effects.25 Evidence suggests that combining behavioral and drug therapy improves outcomes in women,26,27,28,29 yet the effectiveness of behavioral therapy in combination with drugs for men is less clear. Therefore, the objective of this study was to determine whether combining behavioral and drug therapy improves outcomes compared with behavioral or drug therapy alone in men with OAB. Further, the study compared 3 models for implementing combined treatment: (1) stepped therapy with behavioral therapy started first, (2) stepped therapy with drug therapy started first, and (3) combined therapy with both therapies initiated at the same time.
Methods
Design
This trial was a 3-site, 2-stage, 3-arm, parallel-group randomized clinical trial conducted from July 2009 to July 2015. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. The trial protocol can be found in Supplement 1. Men with OAB symptoms were randomized to 6 weeks of behavioral training alone, drug therapy alone, or combined behavioral and drug therapy. After 6 weeks of treatment, participants receiving monotherapy were stepped up to combined behavioral and drug therapy for an additional 6 weeks (eFigure in Supplement 2). The study was approved by the University of Alabama at Birmingham Institutional Review Board for Human Use, and participants provided written informed consent.
Participants and Screening
Participants were community-dwelling men 40 years or older with urinary urgency and frequent urination with or without urge incontinence. After telephone screening, in-clinic evaluation consisted of a medical history, physical examination, mental status screening with the Mini-Cog,30 urinalysis, and, for participants with diabetes, testing for glycated hemoglobin level (unless there was documentation of test results of 9% or less of total hemoglobin within the past 3 months). Simple uroflowmetry was conducted (to measure average and peak urine flow), followed by postvoid residual determination by ultrasonography. Participants completed a 7-day bladder diary in which they recorded the time of every void and severity of urinary urgency associated with each. They also completed a 24-hour frequency/volume log in which they recorded the time and volume of each void. Inclusion required participants to have 9.0 or more voids per 24 hours (on average) on the 7-day baseline bladder diary. Exclusion criteria included indicators of outlet obstruction, positive dementia screening, and medical conditions that could have been contributing to urinary symptoms, such as diabetes, urinary tract infection, cancer, or neurological conditions. The complete list of exclusion criteria is included in eTable 1 in Supplement 2.
Randomization
To assure between-group comparability, participants were stratified on 2 dimensions: presence/absence of urge incontinence and frequency of urination based on baseline bladder diaries (9 to 12 voids per 24-hour day vs more than 12 voids per 24-hour day). Within each stratum, randomization was performed by a study biostatistician using a table of random numbers, a 1:1:1 ratio, sealed envelopes, and a variable block size to avoid large inequity in the total number of participants assigned to each group. Participants were then assigned to 6 weeks of behavioral therapy alone (to be followed by 6 weeks of combined therapy), drug therapy alone (to be followed by 6 weeks of combined therapy), or combined behavioral and drug therapy (to be sustained for 12 weeks).
Interventions
Stage 1: Behavioral Therapy Alone
Behavioral therapy was implemented in 3 clinic visits over a period of 6 weeks. It consisted of behavioral training, including skills and strategies for postponing urination, controlling urgency, and preventing urge incontinence. This included pelvic floor muscle training and daily bladder diaries to track increasing voiding intervals and enhance awareness of bladder habits.
Pelvic floor muscle training was conducted using verbal feedback based on anal palpation to teach participants how to contract and relax the pelvic floor muscles while keeping abdominal muscles relaxed. Participants were taught to contract their muscles during 2-second to 10-second periods separated by equal periods of relaxation, depending on initial ability. Recommendations for home practice included 45 exercises daily, usually 3 sessions of 15 exercises. Across sessions, duration was increased gradually to a maximum of 10 seconds.
In treatment visit 2 (at 2 weeks), a treatment adherence questionnaire was completed and reviewed to identify barriers to adherence and make recommendations for improving adherence. Participants were taught urge suppression strategies, ie, how to respond adaptively to urgency, using techniques such as relaxation and pelvic floor muscle contractions to diminish urgency, postpone urination, and prevent urine loss.22,24 Over the next several weeks, they were encouraged to incrementally delay voiding after suppressing the urge to void.22 In addition, nocturia was managed with fluid restriction (3 hours before bedtime and during the night) and with urge suppression strategies.31
Treatment visit 3 (at 4 weeks) was used to review progress, address adherence problems, reinforce and adjust participants’ home practice, and encourage persistence. During the 6 weeks of treatment, participants continued to keep a bladder diary so project staff could monitor progress and guide interventions.
Stage 1: Drug Therapy Alone
Participants in the drug therapy alone group received 2 drugs: an antimuscarinic (sustained-release tolterodine, 4 mg, once daily) and an α-blocker (tamsulosin, 0.4 mg, once daily before bed). In treatment visit 1, a baseline adverse effects profile was completed, and participants were educated about each drug and its possible adverse effects. They were provided with educational materials to assist with managing dry mouth and constipation, the most common adverse effects of antimuscarinics. They were given a 6-week supply of medication and an appointment for the posttreatment assessment visit. Bladder diaries were completed before randomization and following each stage of treatment.
At 3 weeks, the interventionist conducted a telephone evaluation to assess adverse effects and adherence. Minor adverse effects were treated symptomatically. If needed, the sustained-release tolterodine dose was reduced to 2 mg daily to improve tolerability.
Stage 1: Combined Behavioral and Drug Therapy
Participants receiving combined therapy were given behavioral and drug therapy as described above. Participants followed the same visit schedule as the behavioral therapy alone group.
Stage 2: Stepped Therapy
After the posttreatment assessment for stage 1 (6 weeks), participants in the behavioral therapy alone group had their behavioral instructions reinforced and then were stepped up to combined therapy. They were started on drug therapy and underwent follow-up as described above. Participants in the drug therapy alone group were also stepped up to combined therapy. They were instructed in pelvic floor muscle exercises followed by delayed voiding and behavioral strategies in the same visit sequence, as described above. They continued to take their medications at the doses established. Participants in the combined therapy group continued with combined therapy following the same visit schedule as the drug therapy alone group.
Measurement
Assessments were completed at baseline and after each 6-week treatment stage. The primary outcome was change in voiding frequency as documented in the bladder diaries.32 Changes in other symptoms, including urgency, incontinence, and nocturia, were secondary outcomes. For every void and incontinent episode, participants recorded the presence and severity of urgency using the Indevus Urgency Severity Scale,33 an event-specific scale embedded in the bladder diary.
Other outcome measures included (1) change from baseline in the Overactive Bladder Questionnaire score (to measure symptom bother and condition-specific health-related quality of life)34 and (2) change from baseline in the International Prostate Symptom Score (to measure frequency of lower urinary tract symptoms).35 Three validated single-question global ratings of improvement, the Patient Satisfaction Question, Estimated Percent Improvement, and Global Perception of Improvement, were used to assess patients’ perceptions of their treatment outcomes.36 All outcome measures were assessed and scored in a blinded fashion. Pill counts were used to assess adherence to drug therapy, and an adherence questionnaire was used to assess adherence to behavioral therapy.
Sample Size Calculations
Based on the results of an earlier trial,22 we assumed normally distributed outcomes, an average of 9 voids per day after 6 weeks of single therapy, and an SD of 2.5 for average number of voids per day. Under these conditions, sample sizes of 60 per group would provide 90% power to detect a difference of 1.5 voids per day with estimated group SDs of 2.5 for each and a significance level (α) of .025 using a 1-sided, 2-sample t test. The overall sample size was increased to 204 (68 per group) in anticipation of 10% loss to follow-up.
Statistical Analyses
The effectiveness of each intervention was calculated by comparing the frequency of voiding (primary) and other (secondary) symptoms during the final 7 days of the 6-week intervention period with the 7-day baseline period. The primary analysis was an effectiveness analysis based on the intention-to-treat approach, which included all randomized participants. For participants who missed visits, the outcomes were imputed using multiple imputation assuming missing at random. A complete cases analysis was also conducted for sensitivity by using only the participants who completed therapy.
To examine the effects of combined therapy (6 weeks), planned comparisons were conducted comparing combined therapy with each therapy alone. Analysis of covariance models were used to test the hypotheses after adjusting for baseline voiding frequency and age (based on prior evidence of their potential effects on outcomes). To examine the effects of combining behavioral and drug therapy from the beginning vs in a stepped fashion, outcomes at 12-week follow-up were compared. All tests were 2-sided, and the significance level was set as P < .025 for the primary outcome after Bonferroni correction and P < .05 for all secondary outcomes. Analyses were performed using SAS version 9.4 (SAS Institute).
Results
Overview of Participant Flow
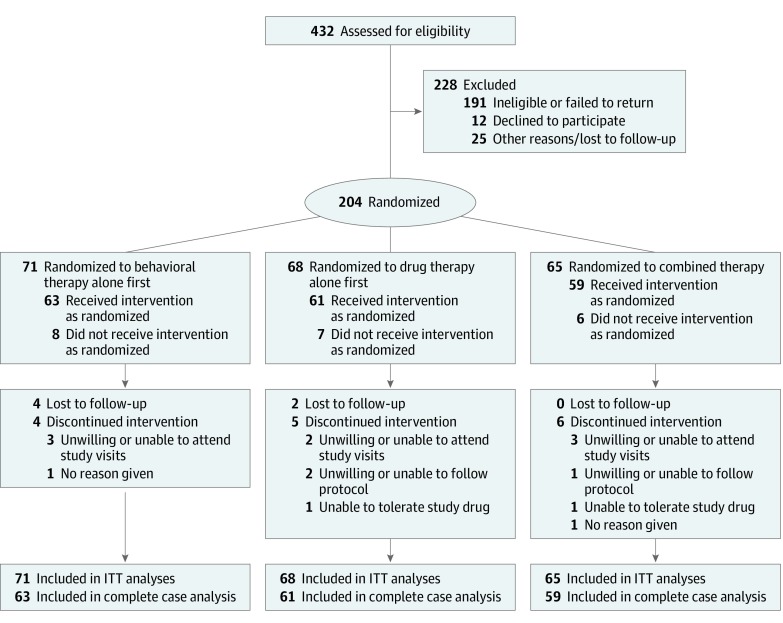
A total of 432 men were enrolled in the study. Of these, 228 were found to be ineligible, and 204 were randomized to behavioral therapy alone (n = 71), drug therapy alone (n = 68), or combined therapy (n = 65) (Figure 1). Twenty-one participants discontinued treatment, and 183 completed treatment and the stage 1 posttreatment assessment (63 in the behavioral therapy alone group, 61 in the drug therapy alone group, and 59 in the combined therapy group). Characteristics of the participants are presented in Table 1.
Figure 1. CONSORT Flow Diagram.
ITT indicates intention to treat.
Table 1. Baseline Characteristics of Participants.
| Variable | No. (%) | ||
|---|---|---|---|
| Behavioral Therapy (n = 71) | Drug Therapy (n = 68) | Combined Therapy (n = 65) | |
| Site | |||
| 1 | 27 (38) | 25 (37) | 26 (40) |
| 2 | 17 (24) | 19 (28) | 15 (23) |
| 3 | 27 (38) | 24 (35) | 24 (37) |
| Age, mean (SD), y | 63.6 (10.9) | 65.5 (11.0) | 63.2 (11.6) |
| Racea | |||
| Black | 14 (20) | 20 (29) | 20 (31) |
| Other | 9 (13) | 4 (6) | 4 (6) |
| White | 48 (68) | 44 (65) | 41 (63) |
| Marital status | |||
| Single | 13 (18) | 15 (22) | 16 (25) |
| Married | 40 (56) | 34 (50) | 41 (63) |
| Divorced | 16 (23) | 15 (22) | 7 (11) |
| Widowed | 2 (3) | 4 (6) | 1 (2) |
| Living status | |||
| Alone | 20 (28) | 28 (41) | 21 (32) |
| With someone | 51 (72) | 40 (59) | 44 (68) |
| Income, $ | |||
| ≤24 999 | 24 (34) | 18 (26) | 14 (22) |
| 25 000-49 999 | 21 (30) | 16 (24) | 22 (34) |
| 50 000-74 999 | 9 (13) | 10 (15) | 14 (22) |
| ≥75 000 | 17 (24) | 24 (35) | 15 (23) |
| Works for wages outside home | 28 (39) | 28 (41) | 30 (46) |
| Diabetes | 18 (25) | 7 (10) | 17 (26) |
| BMI, mean (SD)b | 30.9 (6.5) | 29.4 (5.6) | 30.1 (5.8) |
| PVR, mean (SD), mL | 42.2 (42.7) | 36.3 (41.0) | 36.1 (39.6) |
| Flow rate, mean (SD), mL/s | |||
| Peak | 20.4 (21.7) | 17.1 (9.5) | 19.3 (15.3) |
| Average | 7.3 (4.7) | 7.1 (3.8) | 9.4 (13.3) |
Abbreviations: BMI, body mass index; PVR, postvoid residual.
Participants self-identified race.
Calculated as weight in kilograms divided by height in meters squared.
Effects of Individual and Combined Therapy (Stage 1: 6-Week Outcomes)
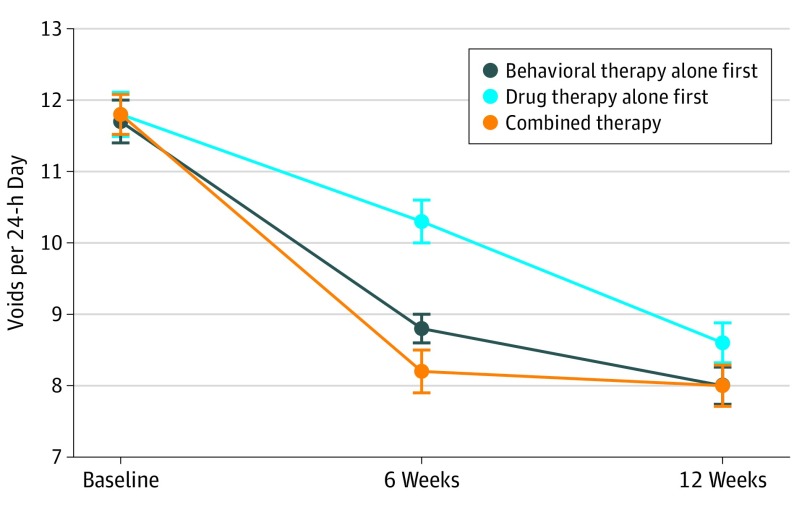
Mean (SD) voids per 24 hours decreased significantly in all 3 groups from baseline to 6-week follow-up (behavioral therapy: 11.7 [2.4] vs 8.8 [2.1]; change, 2.9 [2.4]; percentage change, 24.7%; P < .001; drug therapy: 11.8 [2.5] vs 10.3 [2.7]; change, 1.5 [2.3]; percentage change, 12.7%; P < .001; combined therapy: 11.8 [2.4] vs 8.2 [2.3]; change, 3.6 [2.1]; percentage change, 30.5%; P < .001). Intention-to-treat analyses indicated that posttreatment mean voiding frequencies were significantly lower in the combined therapy group compared with the drug therapy alone group (8.2 [2.3] vs 10.3 [2.7]; P < .001) but not significantly lower than the behavioral treatment alone group (8.2 [2.3] vs 8.8 [2.1]; P = .19) after adjustment for baseline voiding frequency and age (Table 2) (Figure 2). Further, mean voiding frequencies were lower for the behavioral therapy alone group compared with the drug therapy alone group (8.8 [2.1] vs 10.3 [2.7]; P < .001). Similar results were obtained from the complete cases analyses (eTables 2 and 3 in Supplement 2).
Table 2. Changes in Voiding Frequency and Other Urinary Symptoms .
| Bladder Diary Variable | Mean (SD) | P Value | ||
|---|---|---|---|---|
| Behavioral Therapy (n = 71) | Drug Therapy (n = 68) | Combined Therapy (n = 65) | ||
| After 6-wk Follow-up (Stage 1) | ||||
| Mean 24-h voiding frequency, voids/d | ||||
| Baseline | 11.7 (2.4) | 11.8 (2.5) | 11.8 (2.4) | >.99 |
| Posttreatment | 8.8 (2.1) | 10.3 (2.7) | 8.2 (2.3) | <.001a |
| Change (baseline to 6-wk follow-up) | 2.9 (2.4) | 1.5 (2.3) | 3.6 (2.1) | <.001a |
| Change, % | 24.7 | 12.7 | 30.5 | NA |
| Within-group P valueb | <.001 | <.001 | <.001 | NA |
| Mean nocturia frequency, episodes/nightc | ||||
| Baseline | 2.1 (1.2) | 2.2 (1.1) | 2.1 (1.3) | .85 |
| Posttreatment | 1.3 (0.8) | 1.8 (1.2) | 1.3 (1.0) | <.001a |
| Change (baseline to 6-wk follow-up) | 0.7 (1.0) | 0.4 (1.0) | 0.9 (1.1) | <.001a |
| Within-group P valueb | <.001 | .004 | <.001 | NA |
| Mean urgency scored | ||||
| Baseline | 1.6 (0.6) | 1.6 (0.5) | 1.5 (0.6) | .40 |
| Posttreatment | 1.6 (0.6) | 1.5 (0.5) | 1.3 (0.7) | .004a |
| Change (baseline to 6-wk follow-up) | −0.1 (0.5) | 0.1 (0.5) | 0.2 (0.6) | .004a |
| Within-group P valueb | .19 | .10 | .03 | NA |
| Maximum urgency scored | ||||
| Baseline | 2.3 (0.7) | 2.4 (0.6) | 2.4 (0.6) | .70 |
| Posttreatment | 2.2 (0.7) | 2.2 (0.6) | 1.8 (0.8) | <.001a |
| Change (baseline to 6-wk follow-up) | 0.1 (0.6) | 0.2 (0.6) | 0.5 (0.7) | <.001a |
| Within-group P valueb | .14 | .002 | <.001 | NA |
| Incontinence, episodes/wk | ||||
| Baseline | 6.8 (11.3) | 5.5 (10.4) | 6.6 (10.1) | .78 |
| Posttreatment | 3.1 (6.0) | 2.6 (6.6) | 1.5 (4.3) | .15a |
| Change (baseline to 6-wk follow-up) | 3.6 (8.0) | 2.9 (10.6) | 5.2 (8.9) | .15a |
| Change, % | 52.9 | 52.7 | 78.8 | NA |
| Within-group P valueb | <.001 | .03 | <.001 | NA |
| Mean Overactive Bladder Questionnaire score | ||||
| Baseline | 65.5 (34.0) | 61.1 (32.4) | 62.6 (34.8) | .74 |
| Posttreatment | 43.0 (28.2) | 39.5 (30.0) | 23.8 (22.1) | <.001a |
| Change (baseline to 6-wk follow-up) | 22.5 (22.0) | 21.6 (27.6) | 38.8 (31.3) | <.001a |
| Within-group P valueb | <.001 | <.001 | <.001 | NA |
| Mean International Prostate Symptom Scoree | ||||
| Baseline | 15.9 (5.5) | 16.3 (5.9) | 17.6 (6.4) | .21 |
| Posttreatment | 11.4 (5.3) | 11.5 (5.8) | 9.2 (4.8) | <.001a |
| Change (baseline to 6-wk follow-up) | 4.6 (4.1) | 4.7 (5.9) | 8.5 (4.9) | <.001a |
| Within-group P valueb | <.001 | <.001 | <.001 | NA |
| After 12-wk Follow-up (Stage 2) | ||||
| Mean 24-h voiding frequency, voids/d | ||||
| Baseline | 11.7 (2.4) | 11.8 (2.5) | 11.8 (2.4) | >.99 |
| 12-wk treatment | 8.0 (2.2) | 8.6 (2.3) | 8.0 (2.2) | .33a |
| Change (baseline to 12-wk follow-up) | 3.7 (2.3) | 3.2 (2.5) | 3.8 (2.1) | .33a |
| Change, % | 31.6 | 27.1 | 32.2 | NA |
| Within-group P valueb | <.001 | <.001 | <.001 | NA |
| Mean nocturia frequency, episodes/nightc | ||||
| Baseline | 2.1 (1.2) | 2.2 (1.1) | 2.1 (1.3) | .85 |
| 12-wk treatment | 1.2 (0.9) | 1.4 (1.1) | 1.2 (1.0) | .34a |
| Change (baseline to 12-wk follow-up) | 0.9 (1.0) | 0.8 (1.0) | 1.0 (1.0) | .34a |
| Within-group P valueb | <.001 | <.001 | <.001 | NA |
| Mean urgency scored | ||||
| Baseline | 1.6 (0.6) | 1.6 (0.5) | 1.5 (0.6) | .40 |
| 12-wk treatment | 1.5 (0.6) | 1.5 (0.6) | 1.2 (0.7) | .06a |
| Change (baseline to 12-wk follow-up) | 0.1 (0.6) | 0.2 (0.6) | 0.3 (0.6) | .06a |
| Within-group P valueb | .34 | .06 | .002 | NA |
| Maximum urgency scored | ||||
| Baseline | 2.3 (0.7) | 2.4 (0.6) | 2.4 (0.6) | .70 |
| 12-wk treatment | 2.0 (0.8) | 2.0 (0.8) | 1.8 (0.8) | .07a |
| Change (baseline to 12-wk follow-up) | 0.3 (0.7) | 0.4 (0.7) | 0.6 (0.8) | .07a |
| Within-group P valueb | <.001 | <.001 | <.001 | NA |
| Incontinence, episodes/wk | ||||
| Baseline | 6.8 (11.3) | 5.5 (10.4) | 6.6 (10.1) | .78 |
| 12-wk treatment | 1.2 (2.5) | 1.1 (3.0) | 1.2 (3.1) | >.99a |
| Change (baseline to 12-wk follow-up) | 5.5 (10.0) | 4.4 (10.1) | 5.4 (9.1) | >.99a |
| Change, % | 80.9 | 80.0 | 81.8 | NA |
| Within-group P valueb | <.001 | <.001 | <.001 | NA |
| Mean Overactive Bladder Questionnaire score | ||||
| Baseline | 65.5 (34.0) | 61.1 (32.4) | 62.6 (34.8) | .74 |
| 12-wk treatment | 22.8 (21.9) | 26.4 (24.5) | 18.9 (18.6) | .11a |
| Change (baseline to 12-wk follow-up) | 42.7 (29.9) | 34.7 (29.6) | 43.7 (33.6) | .11a |
| Within-group P valueb | <.001 | <.001 | <.001 | NA |
| Mean International Prostate Symptom Scoree | ||||
| Baseline | 15.9 (5.5) | 16.3 (5.9) | 17.6 (6.4) | .21 |
| 12-wk treatment | 8.2 (5.3) | 8.8 (4.7) | 8.0 (4.8) | .32a |
| Change (baseline to 12-wk follow-up) | 7.7 (5.3) | 7.5 (5.9) | 9.6 (5.6) | .32a |
| Within-group P valueb | <.001 | <.001 | <.001 | NA |
Abbreviation: NA, not applicable.
F test adjusting for baseline values and age.
Within-group P value compared baseline vs posttreatment, ie, treatment effects, with Wilcoxon signed rank sum test.
Nocturia was defined as the number of voids between the time the participant went to bed with the intention of sleeping and just prior to morning awakening (as recorded in the diary).
Scores ranged from 0 to 3, with 0 indicating none (no urgency); 1, mild (awareness of urgency but is easily tolerated); 2, moderate (enough urgency discomfort that it interferes with or shortens usual activity); and 3, severe (extreme urgency discomfort that abruptly stops all activities or tasks).
Scores ranged from 0 to 35, with higher scores indicating more frequent lower urinary tract symptoms.
Figure 2. Changes in 24-Hour Voiding Frequencies With Individual and Combined Therapy.
Mean 24-hour voiding frequencies at baseline; after 6 weeks of behavioral therapy alone, drug therapy alone, or initial combined therapy; and after an additional 6 weeks of step-up combined therapy for all arms. Error bars indicate SEs.
Mean frequency of nocturia decreased significantly in all 3 groups (Table 2). Analysis of covariance indicated significant between-group differences in favor of combined therapy, with drug therapy alone showing the smallest changes (mean [SD]: behavioral therapy alone, 1.3 [0.8]; drug therapy alone, 1.8 [1.2]; combined therapy, 1.3 [1.0]; P < .001). Mean urgency scores decreased significantly in the combined therapy group but not in the behavioral therapy alone or drug therapy alone groups. Using maximum daily urgency scores, decreases were significant in both the combined therapy group and the drug therapy alone group but not in the behavioral therapy alone group.
Scores on the Overactive Bladder Questionnaire and the International Prostate Symptom Score decreased significantly in all 3 groups (Table 2). Analysis of covariance yielded significant group differences, with combined therapy being superior (mean [SD] Overactive Bladder Questionnaire score: behavioral therapy alone, 43.0 [28.2]; drug therapy alone, 39.5 [30.0]; combined therapy, 23.8 [22.1]; P < .001; mean [SD] International Prostate Symptom Score: behavioral therapy alone, 11.4 [5.3]; drug therapy alone, 11.5 [5.8]; combined therapy, 9.2 [4.8]; P < .001).
On patient global ratings, more participants receiving combined therapy rated themselves as better or much better, and more were completely satisfied (Table 3). Adverse effects were lowest in the behavioral therapy group. No adverse effects or no bothersome adverse effects were reported by 86% (55 of 64) in the behavioral therapy alone group compared with 32% (21 of 65) in the drug therapy alone group and 34% (21 of 61) in the combined therapy group (P < .001).
Table 3. Patient Global Ratings.
| Rating Scale | No. (%) | P Valuea | ||
|---|---|---|---|---|
| Behavioral Therapy | Drug Therapy | Combined Therapy | ||
| After 6-wk Follow-up (Stage 1) | ||||
| Total, No. | 64 | 65 | 61 | NA |
| Global perception of improvement | ||||
| Much better | 13 (20) | 9 (14) | 24 (39) | <.001 |
| Better | 39 (61) | 33 (51) | 31 (51) | |
| About the same | 12 (19) | 22 (34) | 6 (10) | |
| Worse | 0 | 1 (2) | 0 | |
| Satisfaction with progress | ||||
| Completely | 18 (28) | 13 (20) | 30 (49) | <.001 |
| Somewhat satisfied | 42 (66) | 41 (63) | 30 (49) | |
| Somewhat dissatisfied | 3 (5) | 8 (12) | 1 (2) | |
| Very dissatisfied | 1 (2) | 3 (5) | 0 | |
| How bothersome were adverse effects? | ||||
| No adverse effects | 33 (52) | 12 (18) | 8 (13) | <.001 |
| Not at all bothersome | 22 (34) | 9 (14) | 13 (21) | |
| A little | 6 (9) | 20 (31) | 29 (48) | |
| Somewhat | 3 (5) | 18 (28) | 10 (16) | |
| Extremely | 0 | 6 (9) | 1 (2) | |
| Wish to receive another form of treatment | 44 (69) | 38 (58) | 27 (44) | .02 |
| After 12-wk Follow-up (Stage 2) | ||||
| Total, No. | 62 | 61 | 59 | NA |
| Global perception of improvement | ||||
| Much better | 28 (45) | 26 (43) | 30 (51) | .32 |
| Better | 27 (43) | 26 (43) | 26 (44) | |
| About the same | 5 (8) | 9 (15) | 3 (5) | |
| Worse | 2 (3) | 0 | 0 | |
| Satisfaction with progress | ||||
| Completely | 32 (52) | 32 (52) | 28 (47) | .82 |
| Somewhat satisfied | 26 (42) | 28 (46) | 29 (49) | |
| Somewhat dissatisfied | 3 (5) | 0 | 1 (2) | |
| Very dissatisfied | 1 (2) | 1 (2) | 1 (2) | |
| How bothersome were adverse effects? | ||||
| No adverse effects | 13 (21) | 9 (15) | 12 (20) | .55 |
| Not at all bothersome | 17 (27) | 12 (20) | 10 (17) | |
| A little | 16 (26) | 27 (44) | 16 (27) | |
| Somewhat | 14 (23) | 12 (20) | 19 (32) | |
| Extremely | 2 (3) | 1 (2) | 2 (3) | |
| Wish to receive another form of treatment | 23 (37) | 20 (33) | 16 (27) | .50 |
Abbreviation: NA, not applicable.
Cochran-Mantel-Haenszel procedure used to account to the ordinal nature of response.
Comparison of Combined Therapy Models (Stage 2: 12-Week Outcomes)
At 12-week follow-up, after all groups had received combined therapy, improvements in mean (SD) voids per 24 hours were also greatest for those receiving initial combined therapy compared with baseline (behavioral therapy: 11.7 [2.4] vs 8.0 [2.2]; change, 3.7 [2.3]; percentage change, 31.6%; P < .001; drug therapy: 11.8 [2.5] vs 8.6 [2.3]; change, 3.2 [2.5]; percentage change, 27.1%; P < .001; combined therapy: 11.8 [2.4] vs 8.0 [2.2]; change, 3.8 [2.1]; percentage change, 32.2%; P < .001) (Table 2). There were no longer differences between the groups on the primary outcome.
Following a similar pattern, at 12-week follow-up, improvements were greatest in the combined therapy group but without between-group differences on the other bladder diary and questionnaire measures. Patient perception of improvement as better or much better was similar across groups (89% [55 of 62] in the group receiving behavioral therapy alone first; 85% [52 of 61] in the group receiving drug therapy alone first; 95% [56 of 59] in the group receiving combined therapy; P = .32) (Table 3). Similarly, the percentage of patients completely satisfied after 12 weeks was similar across groups (52% [32 of 62] in the group receiving behavioral therapy alone first; 52% [32 of 61] in the group receiving drug therapy alone first; 47% [28 of 59] in the group receiving combined therapy throughout; P = .82). Adverse effects no longer differed by group; 21% (13 of 62) in the group receiving behavioral therapy alone first reported no adverse effects compared with 15% (9 of 61) in the group receiving drug therapy alone first and 20% (12 of 59) in the group receiving combined therapy throughout (P = .55).
Discussion
This 2-stage, 3-arm, multisite randomized clinical trial explored the effects of combining behavioral and drug therapy compared with each therapy alone for men with OAB symptoms. The results show that combining behavioral and drug therapy yielded significantly greater short-term reductions in voiding frequency compared with drug therapy alone but not compared with behavioral therapy alone. Combined therapy also yielded the best outcomes for nocturia and urinary urgency as well as other secondary outcomes, including patient-perceived improvement and satisfaction, but with more adverse effects than behavioral therapy alone.
These findings help to address the paucity of data on combining behavioral and drug therapy for OAB in men.37 They are consistent with prior studies of combining behavioral therapy with drug therapy in predominantly female samples.26,27,28,29 Similar to our trial, 2 studies of adding delayed voiding (one component of behavioral therapy in the current trial) to antimuscarinic therapy for men and women with OAB27,29 also demonstrated significant decreases in voiding frequency with combined therapy compared with drug therapy alone. One other study in men with OAB who had insufficient improvement with an α-blocker alone22 demonstrated that adding behavioral therapy was at least as effective as adding an antimuscarinic medication and was well tolerated. To our knowledge, the current study is the fourth randomized clinical trial to show the efficacy of behavioral therapy for reducing nocturia31,38,39 and the third demonstrating that behavioral therapy offers statistically superior nocturia reductions compared with drug therapy alone.21,22
In the present study, the 3 approaches to combined therapy all resulted in similar 12-week outcomes. When using a stepped approach, it is reasonable to begin with behavioral therapy alone, not just because of the better adverse effect profile but also because behavioral therapy yields better 6-week outcomes than drug therapy and, thus, results are achieved more quickly than starting with drug therapy alone. A stepped approach starting with behavioral therapy alone allows patients to evaluate its effect before discussing with their clinicians the advantages and disadvantages of adding drugs with accompanying adverse effects for a small degree of additional improvement. Adding to the adverse effects and cost considerations, a 2015 study40 demonstrated that only 29% of men prescribed α-blockers for lower urinary tract symptoms are adherent after 1 year, and rates may be even lower among men using more than 1 drug. In contrast, a 2011 study of behavioral therapy for postprostatectomy incontinence23 showed 91% adherence at 1 year. Thus, offering behavioral therapy first may be important to patients in making decisions about drug therapy.
Limitations
A limitation of this trial was that participants and interventionists could not be blinded. However, all participants knew they were receiving an active therapy with proven efficacy and could be expected to have a reasonable expectation of benefit. Further, there were salient features of the protocol that were common across the 3 treatment arms and supported the internal validity of the study. Participants in all groups interacted with the interventionist, completed diaries, and received individualized adjustment of their treatment program. The behavioral treatment did involve more in-person visits and bladder diaries, making it more intensive in these aspects. However, these features are intrinsic to behavioral treatment, which depends on active patient engagement and self-monitoring to be minimally effective and are not essential to usual drug therapy. Therefore, we designed this trial to represent how both therapies are usually implemented in a real-world clinical setting.
Another limitation is that each stage of therapy was 6 weeks in duration, while similar trials test 12 weeks of therapy. The rationale for choosing 6 weeks was based on previous work showing a flattening of symptom improvement curves after 4 to 6 weeks with drug therapy11 and behavioral therapy21 and enabled participants to step up to combined therapy in an additional 6 weeks of the study. Because the results of the different sequences for combining therapy were similar at 12 weeks, our study highlights the opportunity to integrate patient preferences (for or against behavioral or drug therapy) in shared decision-making.
Additionally, the results of this study provide new evidence for behavioral therapy in men to inform changes in practice guidelines. Although treatment guidelines for OAB recommend behavioral therapy as first-line treatment,16,17 this has been based on research on women. Further, men’s OAB symptoms overlap considerably with benign prostatic hyperplasia, and benign prostatic hyperplasia treatment guidelines recommend drug therapy as first-line treatment.6 Men with symptoms attributed to benign prostatic hyperplasia but with primarily urgency, frequency, and nocturia may well be treated more satisfactorily with behavioral therapy alone, avoiding adverse effects of drug therapy.
Conclusions
This study yields important information related to optimizing treatment of OAB symptoms in men. Although some clinicians advocate combined treatment, most do not integrate behavioral components, such as pelvic floor muscle training or delayed voiding, into standard therapy. Behavioral therapy can be implemented by nurses, nurse practitioners, and physical therapists and has potential for widespread application in a variety of outpatient settings.
Trial protocol
eTable 1. Exclusion criteria.
eTable 2. Changes in voiding frequency and other urinary symptoms of participants who completed the study: baseline to 6 weeks of therapy.
eTable 3. Changes in voiding frequency and other urinary symptoms of participants who completed the study: baseline to 12 weeks of therapy.
eFigure. Overview of study design.
Data sharing statement
References
- 1.Abrams P, Cardozo L, Fall M, et al. ; Standardisation Sub-Committee of the International Continence Society . The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61(1):37-49. doi: 10.1016/S0090-4295(02)02243-4 [DOI] [PubMed] [Google Scholar]
- 2.Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108(7):1132-1138. doi: 10.1111/j.1464-410X.2010.09993.x [DOI] [PubMed] [Google Scholar]
- 3.Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327-336. [DOI] [PubMed] [Google Scholar]
- 4.Markland AD, Goode PS, Redden DT, Borrud LG, Burgio KL. Prevalence of urinary incontinence in men: results from the national health and nutrition examination survey. J Urol. 2010;184(3):1022-1027. doi: 10.1016/j.juro.2010.05.025 [DOI] [PubMed] [Google Scholar]
- 5.Jackson S. The patient with an overactive bladder—symptoms and quality-of-life issues. Urology. 1997;50(6A)(suppl):18-22. doi: 10.1016/S0090-4295(97)00580-3 [DOI] [PubMed] [Google Scholar]
- 6.McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185(5):1793-1803. doi: 10.1016/j.juro.2011.01.074 [DOI] [PubMed] [Google Scholar]
- 7.Madhuvrata P, Cody JD, Ellis G, Herbison GP, Hay-Smith EJ. Which anticholinergic drug for overactive bladder symptoms in adults. Cochrane Database Syst Rev. 2012;(1):CD005429. doi: 10.1002/14651858.CD005429.pub2 [DOI] [PubMed] [Google Scholar]
- 8.Chung DE, Te AE, Staskin DR, Kaplan SA. Efficacy and safety of tolterodine extended release and dutasteride in male overactive bladder patients with prostates >30 grams. Urology. 2010;75(5):1144-1148. doi: 10.1016/j.urology.2009.12.010 [DOI] [PubMed] [Google Scholar]
- 9.Chung SD, Chang HC, Chiu B, Liao CH, Kuo HC. The efficacy of additive tolterodine extended release for 1-year in older men with storage symptoms and clinical benign proastatic hyperplasia. Neurourol Urodyn. 2011;30(4):568-571. doi: 10.1002/nau.20923 [DOI] [PubMed] [Google Scholar]
- 10.Chapple C, Herschorn S, Abrams P, Sun F, Brodsky M, Guan Z. Tolterodine treatment improves storage symptoms suggestive of overactive bladder in men treated with alpha-blockers. Eur Urol. 2009;56(3):534-541. doi: 10.1016/j.eururo.2008.11.026 [DOI] [PubMed] [Google Scholar]
- 11.Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA. 2006;296(19):2319-2328. doi: 10.1001/jama.296.19.2319 [DOI] [PubMed] [Google Scholar]
- 12.Lee SH, Chung BH, Kim SJ, Kim JH, Kim JC, Lee JY. Initial combined treatment with anticholinergics and α-blockers for men with lower urinary tract symptoms related to BPH and overactive bladder: a prospective, randomized, multi-center, double-blind, placebo-controlled study. Prostate Cancer Prostatic Dis. 2011;14(4):320-325. doi: 10.1038/pcan.2011.22 [DOI] [PubMed] [Google Scholar]
- 13.Drake MJ, Chapple C, Sokol R, et al. ; NEPTUNE Study Group . Long-term safety and efficacy of single-tablet combinations of solifenacin and tamsulosin oral controlled absorption system in men with storage and voiding lower urinary tract symptoms: results from the NEPTUNE study and NEPTUNE II open-label extension. Eur Urol. 2015;67(2):262-270. doi: 10.1016/j.eururo.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi O, Kakizaki H, Homma Y, et al. ; ASSIST Study Group . Solifenacin as add-on therapy for overactive bladder symptoms in men treated for lower urinary tract symptoms—ASSIST, randomized controlled study. Urology. 2011;78(1):126-133. doi: 10.1016/j.urology.2011.02.055 [DOI] [PubMed] [Google Scholar]
- 15.Kaplan SA, Roehrborn CG, Abrams P, Chapple CR, Bavendam T, Guan Z. Antimuscarinics for treatment of storage lower urinary tract symptoms in men: a systematic review. Int J Clin Pract. 2011;65(4):487-507. doi: 10.1111/j.1742-1241.2010.02611.x [DOI] [PubMed] [Google Scholar]
- 16.Oelke M, Bachmann A, Descazeaud A, et al. ; European Association of Urology . EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2013;64(1):118-140. doi: 10.1016/j.eururo.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 17.Gormley EA, Lightner DJ, Faraday M, Vasavada SP; American Urological Association; Society of Urodynamics, Female Pelvic Medicine . Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment. J Urol. 2015;193(5):1572-1580. doi: 10.1016/j.juro.2015.01.087 [DOI] [PubMed] [Google Scholar]
- 18.Dumoulin C, Adewuyi T, Booth J, et al. Adult conservative management In: Abrams P, Cardozo L, Wagg A, Wein A, eds. Incontinence. 6th ed Bristol, UK: International Continence Society; 2017:1443-1628. [Google Scholar]
- 19.Hartmann KE, McPheeters ML, Biller DH, et al. Treatment of overactive bladder in women. Evid Rep Technol Assess (Full Rep). 2009;(187):1-120, v. [PMC free article] [PubMed] [Google Scholar]
- 20.Shamliyan TA, Kane RL, Wyman J, Wilt TJ. Systematic review: randomized, controlled trials of nonsurgical treatments for urinary incontinence in women. Ann Intern Med. 2008;148(6):459-473. doi: 10.7326/0003-4819-148-6-200803180-00211 [DOI] [PubMed] [Google Scholar]
- 21.Burgio KL, Locher JL, Goode PS, et al. Behavioral vs drug treatment for urge urinary incontinence in older women: a randomized controlled trial. JAMA. 1998;280(23):1995-2000. doi: 10.1001/jama.280.23.1995 [DOI] [PubMed] [Google Scholar]
- 22.Burgio KL, Goode PS, Johnson TM, et al. Behavioral versus drug treatment for overactive bladder in men: the Male Overactive Bladder Treatment in Veterans (MOTIVE) trial. J Am Geriatr Soc. 2011;59(12):2209-2216. doi: 10.1111/j.1532-5415.2011.03724.x [DOI] [PubMed] [Google Scholar]
- 23.Goode PS, Burgio KL, Johnson TM II, et al. Behavioral therapy with or without biofeedback and pelvic floor electrical stimulation for persistent postprostatectomy incontinence: a randomized controlled trial. JAMA. 2011;305(2):151-159. doi: 10.1001/jama.2010.1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgio KL, Goode PS, Locher JL, et al. Behavioral training with and without biofeedback in the treatment of urge incontinence in older women: a randomized controlled trial. JAMA. 2002;288(18):2293-2299. doi: 10.1001/jama.288.18.2293 [DOI] [PubMed] [Google Scholar]
- 25.Goode PS, Burgio KL, Locher JL, Umlauf MG, Lloyd LK, Roth DL. Urodynamic changes associated with behavioral and drug treatment of urge incontinence in older women. J Am Geriatr Soc. 2002;50(5):808-816. doi: 10.1046/j.1532-5415.2002.50204.x [DOI] [PubMed] [Google Scholar]
- 26.Burgio KL, Locher JL, Goode PS. Combined behavioral and drug therapy for urge incontinence in older women. J Am Geriatr Soc. 2000;48(4):370-374. doi: 10.1111/j.1532-5415.2000.tb04692.x [DOI] [PubMed] [Google Scholar]
- 27.Mattiasson A, Blaakaer J, Høye K, Wein AJ; Tolterodine Scandinavian Study Group . Simplified bladder training augments the effectiveness of tolterodine in patients with an overactive bladder. BJU Int. 2003;91(1):54-60. doi: 10.1046/j.1464-410X.2003.03076.x [DOI] [PubMed] [Google Scholar]
- 28.Burgio KL, Kraus SR, Menefee S, et al. ; Urinary Incontinence Treatment Network . Behavioral therapy to enable women with urge incontinence to discontinue drug treatment: a randomized trial. Ann Intern Med. 2008;149(3):161-169. doi: 10.7326/0003-4819-149-3-200808050-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattiasson A, Masala A, Morton R, Bolodeoku J; SOLAR Study Group . Efficacy of simplified bladder training in patients with overactive bladder receiving a solifenacin flexible-dose regimen: results from a randomized study. BJU Int. 2010;105(8):1126-1135. doi: 10.1111/j.1464-410X.2009.08910.x [DOI] [PubMed] [Google Scholar]
- 30.Borson S, Scanlan JM, Chen P, Ganguli M. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51(10):1451-1454. doi: 10.1046/j.1532-5415.2003.51465.x [DOI] [PubMed] [Google Scholar]
- 31.Johnson TM II, Vaughan CP, Goode PS, et al. Pilot results from a randomized trial in men comparing alpha-adrenergic antagonist versus behavior and exercise for nocturia and sleep [published online October 28, 2016]. Clin Ther. doi: 10.1016/j.clinthera.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 32.Wyman JF, Choi SC, Harkins SW, Wilson MS, Fantl JA. The urinary diary in evaluation of incontinent women: a test-retest analysis. Obstet Gynecol. 1988;71(6, pt 1):812-817. [PubMed] [Google Scholar]
- 33.Nixon A, Colman S, Sabounjian L, et al. A validated patient reported measure of urinary urgency severity in overactive bladder for use in clinical trials. J Urol. 2005;174(2):604-607. doi: 10.1097/01.ju.0000165461.38088.7b [DOI] [PubMed] [Google Scholar]
- 34.Coyne K, Revicki D, Hunt T, et al. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: the OAB-q. Qual Life Res. 2002;11(6):563-574. doi: 10.1023/A:1016370925601 [DOI] [PubMed] [Google Scholar]
- 35.Badia X, Rodríguez F, Carballido J, et al. ; ESECI-98 Group . Influence of sociodemographic and health status variables on the American Urological Association symptom scores in patients with lower urinary tract symptoms. Urology. 2001;57(1):71-77. doi: 10.1016/S0090-4295(00)00894-3 [DOI] [PubMed] [Google Scholar]
- 36.Burgio KL, Goode PS, Richter HE, Locher JL, Roth DL. Global ratings of patient satisfaction and perceptions of improvement with treatment for urinary incontinence: validation of three global patient ratings. Neurourol Urodyn. 2006;25(5):411-417. doi: 10.1002/nau.20243 [DOI] [PubMed] [Google Scholar]
- 37.Norton JM, Bavendam TG, Elwood W, et al. Research needs to understand self-management of lower urinary tract symptoms: summary of NIDDK workshop. J Urol. 2018;199(6):1408-1410. doi: 10.1016/j.juro.2017.11.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson TM II, Burgio KL, Redden DT, Wright KC, Goode PS. Effects of behavioral and drug therapy on nocturia in older incontinent women. J Am Geriatr Soc. 2005;53(5):846-850. doi: 10.1111/j.1532-5415.2005.53260.x [DOI] [PubMed] [Google Scholar]
- 39.Johnson TM II, Markland AD, Goode PS, et al. Efficacy of adding behavioural treatment or antimuscarinic drug therapy to α-blocker therapy in men with nocturia. BJU Int. 2013;112(1):100-108. doi: 10.1111/j.1464-410X.2012.11736.x [DOI] [PubMed] [Google Scholar]
- 40.Cindolo L, Pirozzi L, Sountoulides P, et al. Patient’s adherence on pharmacological therapy for benign prostatic hyperplasia (BPH)-associated lower urinary tract symptoms (LUTS) is different: is combination therapy better than monotherapy? BMC Urol. 2015;15:96. doi: 10.1186/s12894-015-0090-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eTable 1. Exclusion criteria.
eTable 2. Changes in voiding frequency and other urinary symptoms of participants who completed the study: baseline to 6 weeks of therapy.
eTable 3. Changes in voiding frequency and other urinary symptoms of participants who completed the study: baseline to 12 weeks of therapy.
eFigure. Overview of study design.
Data sharing statement