This case report compares in vivo 18F-flortaucipir at positron emission tomography with regions of tau pathology found at postmortem analysis in a former US football player with pathologically confirmed chronic traumatic encephalopathy.
Key Points
Question
Does in vivo binding of the tau positron emission tomography radioligand fluorine F 18–labeled (18F)–flortaucipir correspond to tau pathology in chronic traumatic encephalopathy?
Findings
In a patient with pathologically confirmed chronic traumatic encephalopathy, 18F-flortaucipir positron emission tomography performed 52 months prior to death showed a patchy, frontotemporal pattern corresponding qualitatively to regions with tau pathology found at autopsy. There was a modest correlation between regional 18F-flortaucipir standardized uptake value ratios and tau immunohistochemistry area fraction that did not reach statistical significance.
Meaning
Findings in this case suggest that 18F-flortaucipir may have limited utility as a biomarker of tau pathology in chronic traumatic encephalopathy.
Abstract
Importance
Biomarkers for chronic traumatic encephalopathy (CTE) are currently lacking. The radiotracer fluorine F 18–labeled (18F)–flortaucipir (FTP) detects tau pathology in Alzheimer disease, and positron emission tomography (PET) with FTP shows elevated binding in individuals at risk for CTE. No study, however, has assessed the correlation between in vivo FTP PET and postmortem tau in CTE.
Objective
To assess the regional association between in vivo FTP binding and postmortem tau pathology in a patient with pathologically confirmed CTE.
Design, Setting, and Participants
A white male former National Football League player with 17 years of US football exposure was clinically diagnosed with traumatic encephalopathy syndrome at a neurology tertiary referral center. 18F-Fludeoxyglucose, carbon 11–labeled Pittsburgh compound B, and FTP PET were performed 52 months prior to death, and magnetic resonance imaging, 50 months prior to death. Brain images were assessed qualitatively for abnormalities blinded to autopsy data. Autopsy was performed using a neurodegenerative research protocol. The FTP standardized uptake value ratios (inferior cerebellar gray reference region) and W-score (age-adjusted z-score) maps were compared with phosphorylated tau immunohistochemical analysis with monoclonal antibody CP13.
Main Outcomes and Measures
Qualitative and quantitative comparisons between antemortem FTP PET and tau pathology at autopsy.
Results
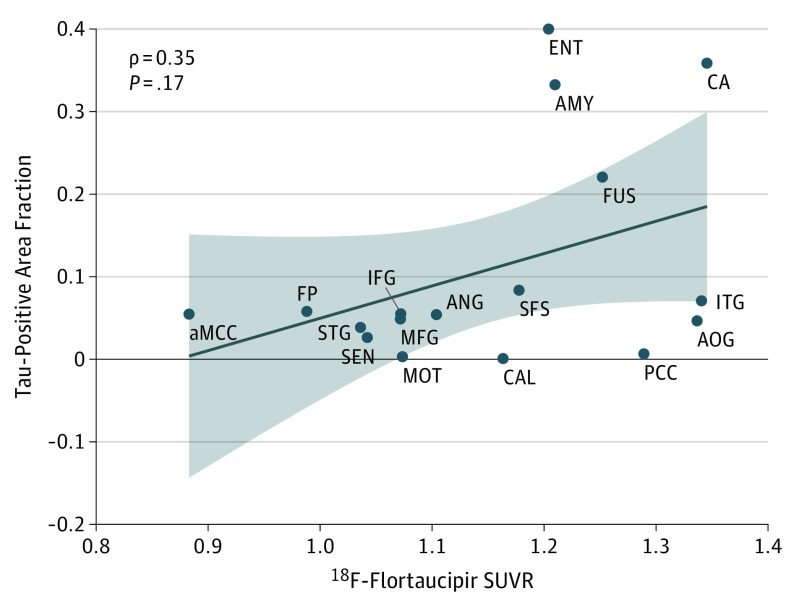
Flortaucipir uptake was distributed in a patchy, frontotemporal-predominant pattern that overlapped with regions showing neurodegeneration on magnetic resonance imaging and hypometabolism on 18F-fludeoxyglucose PET. Pathological assessment revealed stage 4 CTE; limbic argyrophilic grain disease; stage 2 limbic-predominant, age-related transactive response DNA-binding protein 43 encephalopathy; and Braak neurofibrillary tangle stage 3. 18F-Flortaucipir W-maps matched areas of high postmortem tau burden in left fusiform and inferior temporal gyri and juxtacortical frontal white matter. High FTP W-scores with low tau burden were found in the basal ganglia, thalamus, motor cortex, and calcarine cortex. No regions with low FTP W-scores corresponded to areas with high pathological tau burden. A modest correlation, which did not reach statistical significance (ρ = 0.35, P = .17), was found between FTP standardized uptake value ratio and tau area fraction at the regional level.
Conclusions and Relevance
In this patient, FTP PET findings during life showed a modest correspondence with postmortem pathology in CTE. These findings suggest that FTP may have limited utility as a tau biomarker in CTE.
Introduction
Chronic traumatic encephalopathy (CTE) is a neurodegenerative disease associated with a history of repetitive head traumas.1 Accurately diagnosing CTE during life is challenging given clinical overlap with other neurodegenerative syndromes. Antemortem biomarkers are needed to facilitate clinical diagnosis during life and accelerate research on disease mechanisms and drug development. Two recent studies in former professional US ball players meeting clinical criteria for traumatic encephalopathy syndrome suggested that fluorine F 18–labeled (18F)–flortaucipir (FTP) positron emission tomography (PET) may be a potential in vivo CTE biomarker.2,3 We describe the association between antemortem FTP PET and postmortem tau pathology in a retired US football player diagnosed with traumatic encephalopathy syndrome and found to have CTE at autopsy.
Methods
The institutional review boards at the University of California San Francisco, University of California Berkeley, and Lawrence Berkeley National Laboratory approved the study. Written informed consent for participation in research and autopsy was obtained from the patient. The analyses were performed from May 5, 2011, to December 7, 2018.
Clinical and Neuropsychological Testing
Neurological assessment and neuropsychological testing were performed at the University of California San Francisco Alzheimer Disease Research Center, San Francisco, as previously described.4
Neuroimaging
Magnetic resonance imaging (MRI) was performed at the University of California, San Francisco, and PET imaging with 18F-fludeoxyglucose (FDG) (glucose metabolism), carbon 11–labeled Pittsburgh compound B (PiB) (β-amyloid), and 18F-FTP (tau) were performed at Lawrence Berkeley National Laboratory, Berkeley, California (eMethods in the Supplement).
Neuropathology
Neuropathological examination was performed at the University of California, San Francisco Neurodegenerative Disease Brain Bank as previously described5 (eMethods in the Supplement).
FTP-Pathology Correspondence
We first visually compared FTP W-score maps with neuropathological tau burden. Quantitative analysis was performed by comparing FTP standardized uptake value ratios (SUVRs) in pathologically sampled regions5 with postmortem tau area fraction, defined as the proportion of the region staining positively for phosphorylated tau (CP13) immunohistochemistry (eMethods in the Supplement).
Results
Clinical History
The patient was a white male former National Football League defensive end. His football career started in high school and spanned 17 years. After retiring from football, he worked as a professional stuntman. He reported having experienced innumerable head impacts. At age 57 years, he developed depression, social withdrawal, episodic rage, anxiety, and a reduced ability to multitask. At age 63 years, he developed postural tremor, stooped posture, and a shuffling gait. Neurological examinations (at ages 65 and 68 years) revealed mild parkinsonism. Neuropsychological testing demonstrated mild impairments in attention and executive functioning (eTable in the Supplement). Clinical presentation met criteria for traumatic encephalopathy syndrome with mixed behavioral, cognitive, mood, and motor features.6 At age 72 years, the patient developed recurrent seizures and was hospitalized in an intensive care unit, where he died (eFigure in the Supplement).
MRI and PET Imaging
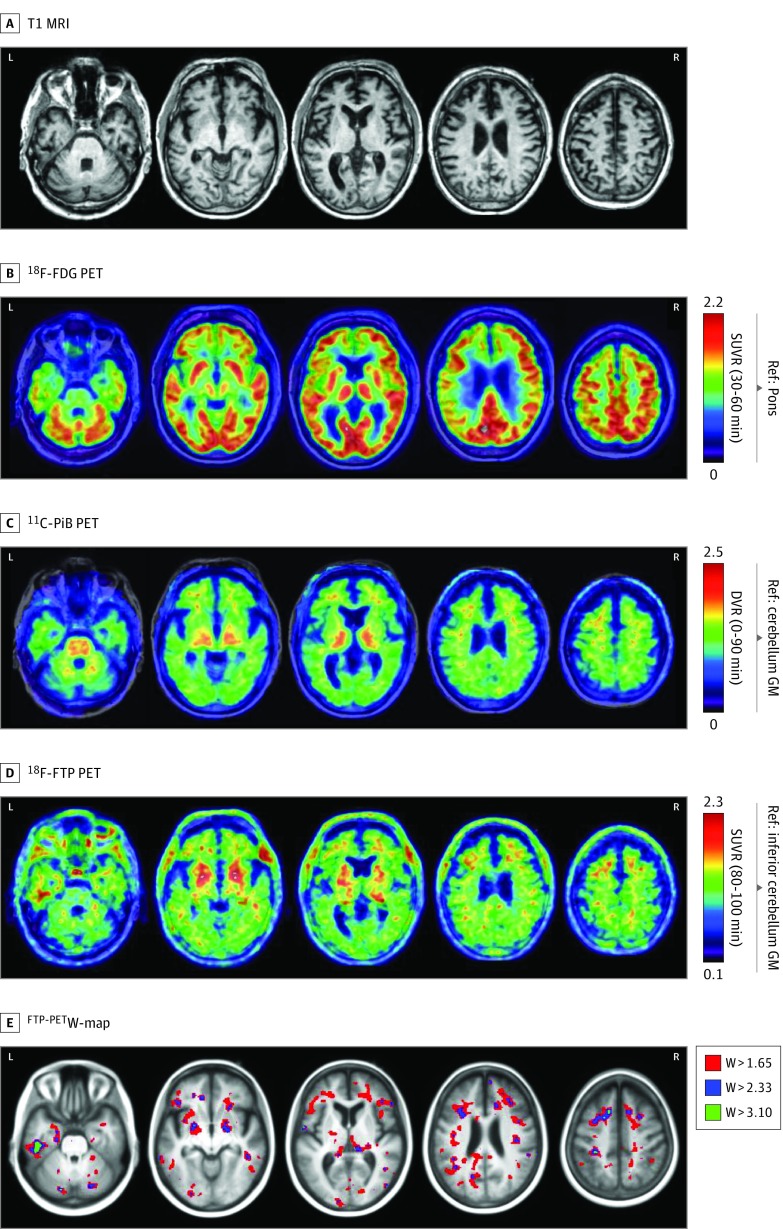
Neuroimaging performed at age 68 years is shown in Figure 1. On visual assessment, T1-weighted MRI revealed a partial cavum septum pellucidum and mild atrophy, predominantly in left greater than right medial temporal and frontal lobes (Figure 1). Findings from FDG PET demonstrated mild hypometabolism in the same regions. Images from PiB PET showed nonspecific white matter binding consistent with absent to sparse neuritic plaques. Flortaucipir SUVR images revealed most intense, confluent uptake in the left inferior temporal and fusiform gyri, with lower signal in the bilateral medial temporal lobes, dorsolateral prefrontal cortex (particularly at the juxtacortical white matter), and parietal regions. Retention was also observed in basal ganglia and thalami, although uptake in these regions is known to be nonspecific.7 W-maps from FTP PET (threshold = 1.65; 95th percentile of age-adjusted controls) showed predominant uptake in frontal subcortical white matter and left posterior medial temporal lobe.
Figure 1. Neuroimaging in Pathologically Proven Chronic Traumatic Encephalopathy.
T1-weighted magnetic resonance (MRI) (top row) demonstrated mild medial temporal and frontal atrophy. Fluorine F 18–labeled (18F)–fludeoxyglucose (FDG) on positron emission tomography (PET) images (second row) demonstrated mild hypometabolism in the medial temporal, medial and dorsolateral frontal, and peri-insular regions, whereas carbon 11–labeled Pittsburgh compound B (PiB) PET scan (third row) was visually rated as negative for cortical retention. On 18F-flortaucipir PET imaging (fourth row), there was widespread patchy binding predominantly in the left greater than right frontotemporal areas. The 18F-flortaucipir PET W-map (bottom row) (thresholded at 3 different levels) showed that these focal increases were higher than what would be expected considering the patient’s age. DVR indicates distribution volume ratio; GM, gray matter; Ref, reference region; and SUVR, standardized uptake value ratio with acquisition windows in parentheses.
Neuropathological Analysis
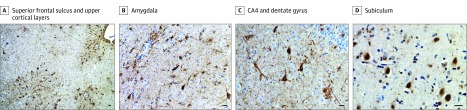
Tau immunohistochemistry identified frequent clusters of tau-immunoreactive astrocytes and neurofibrillary tangles, predominantly in perivascular regions or at the depths of cortical sulci (Figure 2A and B). Perivascular clusters of tau-immunoreactive astrocytes were observed in the spinal cord. Findings were consistent with stage 4 CTE.1
Figure 2. Neuropathologic Findings.
Photomicrographs from immunohistochemical analyses with CP13 antibody for detection of phosphorylated tau (A-C) and TDP-43 antibody (D). Bars indicate 25 μm. A, Frequent perivascular and depth-of-sulci clusters of tau-immunoreactive astrocytes and neurons are seen in the superior frontal sulcus, together with frequent pretangles, fuzzy astrocytes, and dystrophic neurites in upper cortical layers. B, Numerous neurofibrillary tangles, dystrophic neurites, and perivascular astrocytic and neuronal clusters are present in the amygdala. C, Abundant perinuclear ringlike inclusions in the CA4 region of the hippocampus and frequent pretangles in the dentate gyrus are consistent with argyrophilic grain disease. D, Neuronal cytoplasmic inclusions of transactive response DNA-binding protein of 43 kDa (TDP-43) are seen in neurons lacking nuclear TDP-43 staining, and dystrophic neurites are seen in the subiculum.
In addition, moderate flame-shaped neurofibrillary tangles typical of Alzheimer disease (AD) (Braak stage 3), and frequent tau-immunoreactive pretangles and grains consistent with argyrophilic grain disease, were observed in the hippocampus, entorhinal cortex, and fusiform gyrus (Figure 2C). Transactive response DNA-binding protein of 43 kDa (TDP-43) inclusions were seen in the hippocampus and entorhinal cortex (limbic-predominant, age-related TDP-43 encephalopathy, stage 2), but not in sulcal depths or perivascular regions typical of CTE-related TDP-43 (Figure 2D). Results of immunohistochemical analyses for α-synuclein and β-amyloid were negative.
Regions with high tau burden and elevated SUVRs and W-scores at FTP PET included the hippocampus, entorhinal cortex, amygdala, and fusiform gyrus. Regions with high FTP W-scores but low tau burden included the motor cortex and the calcarine cortex. Basal ganglia and thalamus PET signal was associated with low tau-immunohistochemistry burden, consistent with reported off-target FTP binding in these regions.7 High W-scores in left medial temporal regions corresponded to regions that showed additional copathologies at autopsy, including AD-type neurofibrillary tangles, argyrophilic grain disease, and TDP-43 inclusions. No regions with high tau burden corresponded to low FTP signal.
Correlation of Regional FTP Uptake With Tau Area Fraction
A modest correlation (ρ = 0.35) was observed between regional FTP SUVRs and tau-positive area fraction, which did not reach statistical significance (P = .17) (Figure 3). Regions with the highest pathological tau burden (as measured by area fraction), such as the hippocampus, entorhinal cortex, amygdala, and fusiform gyrus, had the highest SUVR values, although similar average SUVR values were also found in a few regions with low pathological tau area fraction.
Figure 3. Comparison Between Regional Flortaucipir Standardized Uptake Value Ratio (SUVR) and CP13-Labeled Tau Area Fraction.
A modest correlation that did not reach statistical significance was found between regional fluorine F 18–labeled (18F)–flortaucipir SUVR and tau-positive area fraction at postmortem pathologic analysis. aMCC indicates anterior cingulate cortex; AMY, amygdala; ANG, angular gyrus; AOG, anterior orbital gyrus; CA, hippocampus; CAL, calcarine cortex; ENT, entorhinal cortex; FP, frontal pole; FUS, fusiform gyrus; IFG, inferior frontal gyrus; ITG, inferior temporal gyrus; MFG, middle frontal gyrus; MOT, primary motor cortex; PCC, posterior cingulate cortex; SEN, primary sensory cortex; SFS, superior frontal sulcus; and STG, superior/middle temporal gyrus.
Discussion
We compared FTP PET and postmortem tau pathological burden in a professional US football player with clinical traumatic encephalopathy syndrome and pathologically confirmed CTE. Flortaucipir PET showed a patchy, frontotemporal-predominant uptake pattern that overlapped with regions showing neurodegeneration/hypometabolism on MRI and FDG PET, similar to the pattern previously reported in symptomatic retired professional football players.2,3 Flortaucipir SUVR values were elevated compared with those for age-adjusted controls but lower than those reported in patients with AD.8 Regions of FTP retention generally corresponded to regions that showed CTE-related tau pathology at autopsy, although their correspondence was modest and not as strong as that previously reported in a patient with AD.9
The binding of FTP to pathological tau inclusions in other, non-AD tauopathies is characterized by lower signal intensity than is seen in AD,10 with similar binding also reported in some non-tau neurodegenerative disorders, especially in degenerating brain regions.10 Our findings in CTE parallel these previous observations in that, although the FTP uptake was observed in some tau-positive areas, the signal intensity was low and could be explained by local neurodegeneration-related changes that are independent of tau aggregation. Flortaucipir was developed as a radiotracer based on its ability to selectively bind to tau in AD postmortem tissue.11 Although tau aggregates in AD and CTE are both composed of 6-isoform tau filaments, differences in filament tertiary structure between the 2 diseases may be relevant to FTP binding.12 Autoradiography has shown relatively low FTP binding to postmortem CTE tissue.13 A focused effort may be needed to develop novel radiotracers that reliably detect CTE tau aggregates.
Limitations
Our study has limitations. The results should be interpreted in the context of a single case report. It is conceivable that interindividual variations in the expression of CTE, neurodegeneration, or copathologies may affect FTP binding. The interval between FTP PET and autopsy in this case was more than 4 years, and pathology may have progressed in this time frame. The quantitative correlation between PET and tau immunohistochemistry may have been affected by sampling error, low spatial resolution of PET, and differences in target/epitope affinity when comparing radiotracer and antibody binding.
Conclusions
We detected elevated FTP binding during life in a pattern that generally corresponded to CTE pathology post mortem. However, the relatively low signal intensity and only modest correlation between PET and autopsy findings suggest that FTP may have limited utility as a biomarker of tau pathology in CTE. Additional PET-to-autopsy reports with FTP and alternative tau radiotracers are needed to validate and extend our findings.
eMethods. Imaging and Neuropathological Analyses
eReferences
eTable. Neuropsychological Testing Results at Age 68
eFigure. Timeline
References
- 1.McKee AC, Cairns NJ, Dickson DW, et al. ; TBI/CTE group . The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016;131(1):75-86. doi: 10.1007/s00401-015-1515-z. doi: 10.1007/s00401-015-1515-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stern RA, Adler CH, Chen K, et al. Tau positron-emission tomography in former national football league players. N Engl J Med. 2019;380(18):1716-1725. doi: 10.1056/NEJMoa1900757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lesman-Segev OH, La Joie R, Stephens ML, et al. Tau PET and multimodal brain imaging in patients at risk for chronic traumatic encephalopathy. Neuroimage Clin. 2019;24:102025. doi: 10.1016/j.nicl.2019.102025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kramer JH, Jurik J, Sha SJ, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16(4):211-218. doi: 10.1097/00146965-200312000-00002 [DOI] [PubMed] [Google Scholar]
- 5.Spina S, Brown JA, Deng J, et al. Neuropathological correlates of structural and functional imaging biomarkers in 4-repeat tauopathies. Brain. 2019;142(7):2068-2081. doi: 10.1093/brain/awz122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montenigro PH, Baugh CM, Daneshvar DH, et al. Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res Ther. 2014;6(5):68. doi: 10.1186/s13195-014-0068-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker SL, Harrison TM, Maass A, La Joie R, Jagust WJ. Effect of off-target binding on 18F-Flortaucipir variability in healthy controls across the life span. J Nucl Med. 2019;60(10):1444-1451. doi: 10.2967/jnumed.118.224113. doi: 10.2967/jnumed.118.224113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ossenkoppele R, Schonhaut DR, Schöll M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. 2016;139(pt 5):1551-1567. doi: 10.1093/brain/aww027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith R, Wibom M, Pawlik D, Englund E, Hansson O. Correlation of in vivo [18F]flortaucipir with postmortem Alzheimer disease tau pathology. JAMA Neurol. 2019;76(3):310-317. doi: 10.1001/jamaneurol.2018.3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai RM, Bejanin A, Lesman-Segev O, et al. 18F-flortaucipir (AV-1451) tau PET in frontotemporal dementia syndromes. Alzheimers Res Ther. 2019;11(1):13. doi: 10.1186/s13195-019-0470-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marquié M, Normandin MD, Vanderburg CR, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol. 2015;78(5):787-800. doi: 10.1002/ana.24517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falcon B, Zivanov J, Zhang W, et al. Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature. 2019;568(7752):420-423. doi: 10.1038/s41586-019-1026-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Y-G, Liang Q, Gomez F, McKee A, Mintun MA, Attardo G [18F]Flortaucipir, aka [18F]AV-1451, autoradiography matches immunofluorescent staining from AT8 tau antibody in Chronic Traumatic Encephalopathy (CTE) post-mortem brain tissue sections. Poster presented at the 11th Human Amyloid Imaging conference; January 11-13, 2017; Miami, FL. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Imaging and Neuropathological Analyses
eReferences
eTable. Neuropsychological Testing Results at Age 68
eFigure. Timeline