This comparative effectiveness study assesses whether proton therapy in the setting of concurrent chemoradiotherapy is associated with fewer 90-day unplanned hospitalizations or other adverse events and similar disease-free and overall survival compared with photon chemoradiotherapy among patients with nonmetastatic cancer.
Key Points
Question
Can proton therapy reduce the risk of severe adverse events associated with unplanned hospitalizations compared with photon therapy for patients undergoing concurrent chemoradiotherapy?
Findings
In this comparative effectiveness study of 1483 adults with nonmetastatic cancer and treated with curative intent, proton therapy was associated with a two-thirds reduction in adverse events associated with unplanned hospitalizations, with no difference in disease-free or overall survival.
Meaning
These findings suggest that, in adults with locally advanced cancer, proton therapy with concurrent chemoradiotherapy may significantly reduce severe adverse events compared with photon therapy, with comparable oncologic outcomes.
Abstract
Importance
Concurrent chemoradiotherapy is the standard-of-care curative treatment for many cancers but is associated with substantial morbidity. Concurrent chemoradiotherapy administered with proton therapy might reduce toxicity and achieve comparable cancer control outcomes compared with conventional photon radiotherapy by reducing the radiation dose to normal tissues.
Objective
To assess whether proton therapy in the setting of concurrent chemoradiotherapy is associated with fewer 90-day unplanned hospitalizations (Common Terminology Criteria for Adverse Events, version 4 [CTCAEv4], grade ≥3) or other adverse events and similar disease-free and overall survival compared with concurrent photon therapy and chemoradiotherapy.
Design, Setting, and Participants
This retrospective, nonrandomized comparative effectiveness study included 1483 adult patients with nonmetastatic, locally advanced cancer treated with concurrent chemoradiotherapy with curative intent from January 1, 2011, through December 31, 2016, at a large academic health system. Three hundred ninety-one patients received proton therapy and 1092, photon therapy. Data were analyzed from October 15, 2018, through February 1, 2019.
Interventions
Proton vs photon chemoradiotherapy.
Main Outcomes and Measures
The primary end point was 90-day adverse events associated with unplanned hospitalizations (CTCAEv4 grade ≥3). Secondary end points included Eastern Cooperative Oncology Group (ECOG) performance status decline during treatment, 90-day adverse events of at least CTCAEv4 grade 2 that limit instrumental activities of daily living, and disease-free and overall survival. Data on adverse events and survival were gathered prospectively. Modified Poisson regression models with inverse propensity score weighting were used to model adverse event outcomes, and Cox proportional hazards regression models with weighting were used for survival outcomes. Propensity scores were estimated using an ensemble machine-learning approach.
Results
Among the 1483 patients included in the analysis (935 men [63.0%]; median age, 62 [range, 18-93] years), those receiving proton therapy were significantly older (median age, 66 [range, 18-93] vs 61 [range, 19-91] years; P < .01), had less favorable Charlson-Deyo comorbidity scores (median, 3.0 vs 2.0; P < .01), and had lower integral radiation dose to tissues outside the target (mean [SD] volume, 14.1 [6.4] vs 19.1 [10.6] cGy/cc × 107; P < .01). Baseline grade ≥2 toxicity (22% vs 24%; P = .37) and ECOG performance status (mean [SD], 0.62 [0.74] vs 0.68 [0.80]; P = .16) were similar between the 2 cohorts. In propensity score weighted–analyses, proton chemoradiotherapy was associated with a significantly lower relative risk of 90-day adverse events of at least grade 3 (0.31; 95% CI, 0.15-0.66; P = .002), 90-day adverse events of at least grade 2 (0.78; 95% CI, 0.65-0.93; P = .006), and decline in performance status during treatment (0.51; 95% CI, 0.37-0.71; P < .001). There was no difference in disease-free or overall survival.
Conclusions and Relevance
In this analysis, proton chemoradiotherapy was associated with significantly reduced acute adverse events that caused unplanned hospitalizations, with similar disease-free and overall survival. Prospective trials are warranted to validate these results.
Introduction
Concurrent chemoradiotherapy is a standard-of-care curative therapy for many locally advanced cancers, including lung cancer,1 glioma,2 head and neck cancer,3 and esophageal cancer.4,5 However, concurrent chemoradiotherapy is associated with substantial morbidity,1,6 including oral mucositis, esophagitis, nausea, vomiting, significant weight loss, and radiation-induced lung injury that can result in unplanned hospitalizations, emergency department visits, treatment interruptions that can diminish the effectiveness of treatment, and decreased patient performance status.1,2,7
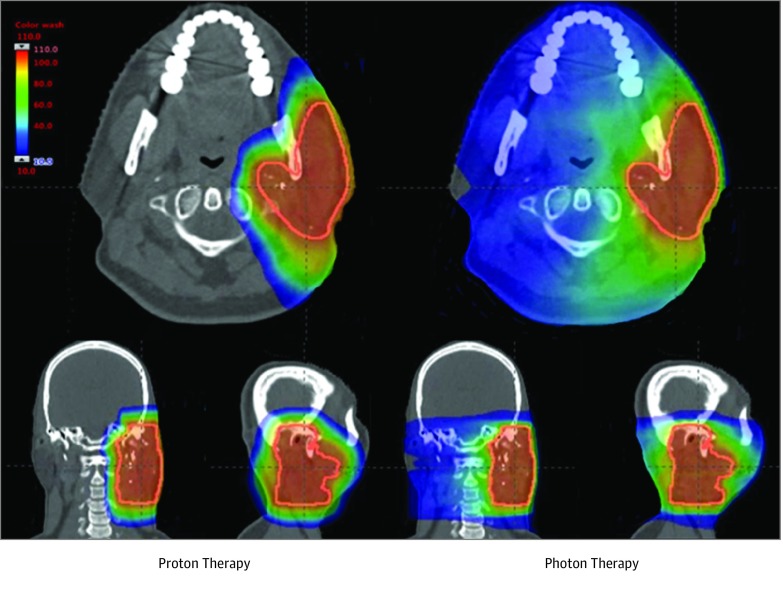
For decades, concurrent chemoradiotherapy has been administered using photon (ie, x-ray) radiation. Photon therapy, delivered as intensity-modulated radiotherapy or 3-dimensional conformal radiotherapy, uses multiple x-ray beams to irradiate a tumor target but unavoidably deposits radiation in normal tissues beyond the target. Proton radiation therapy is a US Food and Drug Administration–approved treatment that has emerged as an alternative radiation treatment modality that directs protons at the tumor target, where they deposit the bulk of their radiation dose to a finite depth in tissue with minimal residual radiation beyond the target (Figure 1 and eFigure in the Supplement).8 Although proton therapy has been in limited clinical use since the 1950s, clinical implementation has been slow owing to the high capital expenditure and maintenance costs required and challenges in dose computational modeling.9 Advances in proton technology and decreasing equipment costs have increased patient access to proton therapy, and it is now often the preferred radiation modality for many pediatric,10 base of skull,11,12 and primary liver13 cancers. Proton therapy as part of concurrent chemoradiotherapy may be able to reduce treatment toxicity, but limited data are available comparing results of proton chemoradiotherapy with chemoradiotherapy delivered with photon therapy, and proton therapy remains unproven in this setting.9,14,15,16,17,18
Figure 1. Representative Proton and Photon Treatment Plan for a Patient With Head and Neck Cancer.
Radiation dose is represented as a color wash, with blue indicating the region receiving the lowest radiation dose and red indicating the region receiving the highest radiation dose.
In this comparative effectiveness cohort study, we compared the rate of severe 90-day adverse events associated with unplanned hospitalizations (defined as Common Terminology Criteria for Adverse Events, version 4 [CTCAEv4], grade ≥3) ([range, 0 [no toxicity] to 5 [death]) for patients treated with proton vs photon chemoradiotherapy. Secondary end points included a decline in the Eastern Cooperative Oncology Group (ECOG) performance status scores from the start to the completion of radiotherapy for patients treated with proton vs photon chemoradiotherapy and the rate of acute 90-day adverse events that limit the patient’s instrumental activities of daily living (CTCAEv4 grade ≥2).19,20 Decline in ECOG performance status is associated with worse outcomes, including inability to undergo additional planned therapies, decreased survival, decreased quality of life, and inability to perform work activities.21 We hypothesized that, in the setting of chemoradiotherapy, proton therapy compared with photon therapy is associated with reduced 90-day severe adverse events that result in unplanned hospitalizations and less decline in ECOG performance status during chemoradiotherapy, without a reduction in cancer control outcomes.
Methods
Cohort
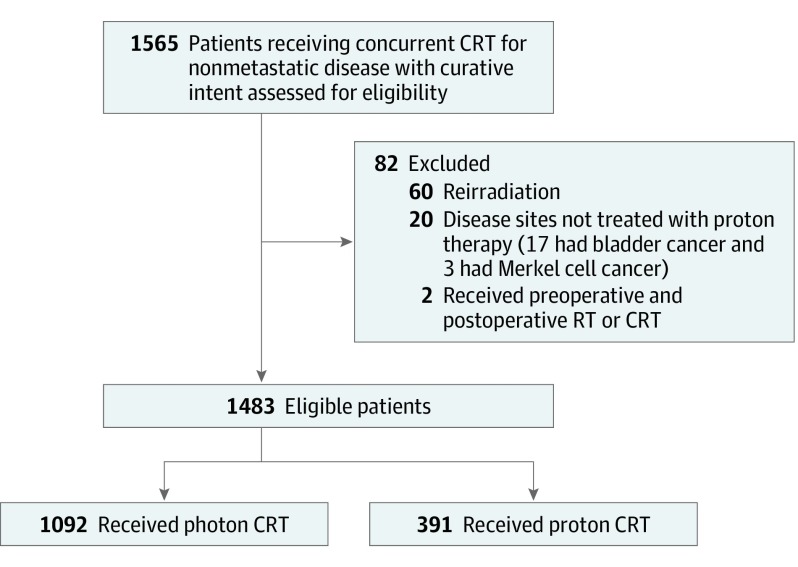
This retrospective, nonrandomized comparative effectiveness study was approved by the institutional review board of the University of Pennsylvania, which waived the need for informed consent for the use of medical records. Adult patients (aged ≥18 years) treated with concurrent chemoradiotherapy with curative intent for nonmetastatic cancer using proton or photon radiotherapy in the University of Pennsylvania Health System from January 1, 2011, through December 31, 2016, were included. Patients treated with salvage reirradiation overlapping with prior radiotherapy portals or those treated with stereotactic body radiotherapy were excluded. Diseases treated at our institution with only photon and not proton chemoradiotherapy (bladder and Merkel cell cancer) were excluded (Figure 2). We classified patients as having received proton therapy if any part of their treatment consisted of proton therapy; otherwise, patients were classified as having been treated with photon therapy. All patients were treated using the same treatment planning system, standard procedures, and radiation dose constraints; all radiation treatment plans were centrally reviewed at a weekly patient management conference.
Figure 2. Consort Diagram.
CRT indicates chemoradiotherapy; RT, radiotherapy.
Variables
The research question, study design, and plan for the analysis followed a prospectively defined protocol (eMethods 1 in the Supplement). Detailed demographic, clinical, pathologic, and treatment-related factors and oncologic outcomes were extracted from Penn’s Oncology Research and Quality Improvement Datamart, a clinical data repository that sources data from multiple databases, including the Epic electronic medical record, the Penn Medicine Cancer Registry, and the ARIA radiation oncology information and treatment planning system (Varian Medical Systems).22 Manual medical record review was used when needed to fill in absent values. Demographic variables included age, race, sex, ECOG performance status (range, 0-5, with 0 meaning no functional impairment and 5 meaning death), body mass index, and Charlson-Deyo comorbidity score (for patients with solid tumor, scores range from 2-33, with higher scores indicating greater comorbidities)23; socioeconomic variables included insurance status, insurance provider, and treatment location (main site vs satellite facility). Complete clinical, pathologic, and surgical variables were obtained, and information on chemotherapeutic agents were also collected. Radiotherapy-specific variables included treatment modality, delivered dose, fractionation, elapsed days for radiotherapy, treatment site, the integral dose to the planning target volume, the integral dose delivered outside of the target volume, and the treating physician. The integral dose delivered outside the target volume is a measure of the total amount of radiation absorbed by normal tissues that are outside of the radiation target volume. We used 131 clinically relevant variables in the development and construction of the model (eMethods 2 in the Supplement).
The only missing covariate data included integral dose delivered outside the target volume (57 [3.8%] missing), starting ECOG score (91 [6.1%] missing), ending ECOG score (68 [4.6%] missing), and body mass index (15 [1.0%] missing). All other variables were complete for all patients.
Outcomes
Adverse events were defined using the National Cancer Institute’s CTCAEv4 grading system, the criterion standard for assessing adverse events in oncology trials. Patient functional status was defined using the ECOG performance status score.24 We scored CTCAEv4 adverse events and ECOG performance status prospectively using standardized templates at each weekly visit during treatment and all subsequent follow-ups. End points were chosen a priori. Assessment and documentation of adverse events was the same regardless of treatment modality. Hematologic adverse events were not scored prospectively and were not considered in the analysis. A CTCAEv4 grade of at least 3 within the first 90 days of treatment was the primary end point, which is defined as severe adverse events for which unplanned hospitalization is indicated. These adverse events of at least grade 3 are disabling and limit the patient’s ability to perform basic self-care activities. We chose 90-day adverse events of at least grade 3 because (1) most early adverse events for chemoradiotherapy occur within the first 90 days, with many of these events resolved or improved later25,26; (2) acute events after radiotherapy are commonly defined as occurring during this period25,27; and (3) the 90-day interval is an accepted interval for assessing early morbidity and mortality after oncologic surgery.28,29 Patients with any adverse events of grade 3 or greater that were not present at baseline or that had increased in severity from baseline were scored as having the outcome of interest. Patients who did not have the event of interest and had less than 90 days of follow-up were excluded. Change in ECOG status from the start to the completion of radiotherapy was assessed as a binary outcome, with deterioration in ECOG status as the outcome of interest. Less severe acute adverse events that cause significant impairment of the patient’s ability to perform their instrumental activities of daily living (eg, shopping, cleaning) were also assessed (90-day CTCAEv4 grade ≥2). We also assessed disease-free survival (DFS) and overall survival (OS). Events of interest for DFS included death due to any cause or relapse, whichever occurred first. Oncologic outcomes data were obtained from the Penn Medicine Cancer Registry, which is prospectively maintained. All oncologic outcomes data were reviewed, confirmed, and updated by a radiation oncologist (B.C.B.). Survival was measured from the start of treatment to events of interest; otherwise, patients were censored.
Statistical Analysis
Data were analyzed from October 15, 2018, through February 1, 2019. Confounding is an important concern in any observational study but particularly in this study, given the heterogeneity of the patient population and the multiple factors that could affect patient selection for proton vs photon therapy. We used propensity score weighting to account for bias due to measured confounding and conducted an extensive sensitivity analysis to assess the effects of unmeasured confounders. For example, although Medicare generally covers proton therapy for most malignant neoplasms, most commercial payers and Medicaid do not.30 Thus, in general, we expected to find that patients 65 years or older were more likely to receive proton therapy than photon therapy.
We first estimated propensity scores using an ensemble machine-learning algorithm called Super Learner.31 The algorithm combines weighted estimates across several parametric and nonparametric approaches based on the accuracy of the estimations from the models to create an overall propensity score estimate, which increases the robustness of the analysis. All covariates that were deemed clinically relevant a priori were included in the propensity score model, and no further filtering or selection was conducted. These covariates included age, sex, race, comorbidity score, cancer site, clinical tumor stage, clinical nodal stage, chemoradiotherapy timing, delivered radiation dose, and ECOG score before starting radiotherapy. We used standardized mean differences to assess the covariate balance before and after propensity score weighting.32 We used these estimated propensity scores to calculate each patient’s inverse probability of being treated with protons.33 The inverse probability treatment weights were the inverse of the propensity score for the patients in the proton cohort and were the inverse of 1 minus the propensity score for the patients in the photon cohort. These weights were then included in a modified Poisson regression model with robust standard errors to estimate relative risks and corresponding 95% CIs for each of the binary outcomes.34 Overlap of propensity score distributions between treatment groups was assessed graphically, and balance on all confounders was assessed using balance diagnostics.35 Using a more traditional approach, we also estimated propensity scores using logistic regression and found nearly identical results; however, Super Learner results demonstrated efficiency gains. Missing covariate data were imputed using multiple imputation before propensity score estimation (6.1% missing starting ECOG score and 3.8% missing integral dose). We assessed DFS and OS using a propensity score–weighted Cox proportional hazards regression model. The proportional hazards regression assumption was evaluated using Schoenfeld residuals.
To assess the potential effect of unmeasured confounding, we conducted a regression-based sensitivity analysis in which we evaluated the sensitivity of our relative risk (RR) estimates to the presence of a binary unmeasured confounder (such as physical frailty, which was not collected in our data). The presence of physical frailty could increase the risk of severe adverse events and may also be independently associated with worse survival.36 We varied the prevalence and strength of the unmeasured confounder to assess whether our primary findings would have been altered if we had been able to adjust for the unmeasured confounder.37
All analyses were conducted in R, version 3.5 (R Foundation for Statistical Computing). All tests were 2 sided. We used a Bonferroni correction given the 5 analytic approaches used, such that P < .01 (0.05/5.0) was considered statistically significant. Analysis was based on intention to treat; all patients who started radiotherapy with either modality were included.
Results
We identified 1483 consecutive adult patients undergoing chemoradiotherapy (935 men [63.0%] and 548 women [37.0%]; median age, 62 [range, 18-93] years), including 391 in the proton cohort and 1092 in the photon cohort. Common tumor sites included head and neck, lung, brain, esophagus/gastric tract, rectum, and pancreas (Table). Patients treated with protons were significantly older (median age, 66 [range, 18-93] vs 61 [range, 19-91] years; mean [SD] age, 63.3 [14.9] vs 60.2 [11.1] years; P < .01) and had higher age-unadjusted Charlson-Deyo comorbidity index scores (median, 3.0 [range, 2-16] vs 2.0 [range, 2-13]; mean [SD], 3.2 [1.5] vs 3.0 [1.6]; P < .01). The proton therapy group had a lower integral radiation dose to tissues outside the target (mean [SD], 14.1 [6.4] vs 19.1 [10.6] cGy/cc × 106; P < .01). There was no statistically significant difference in the integral dose to the planning target volume, which is a measure of the mean dose delivered to the treatment volume (mean [SD], 32.9 [25.6] vs 33.5 [31.1] cGy/cc × 107; P = .72). One thousand sixteen patients (93.0%) in the photon therapy group were treated with intensity-modulated radiotherapy. Baseline toxicity of at least grade 2 (24% vs 22%; P = .37) and baseline ECOG performance status (median, 0 [range, 0-3] vs 1 [range, 0-3]; mean [SD], 0.62 [0.74] vs 0.68 [0.80]; P = .16) were similar between the 2 cohorts.
Table. Baseline Characteristics of the Entire Cohort.
| Characteristic | CRT Cohorta | P Value | |
|---|---|---|---|
| Proton (n = 391) | Photon (n = 1092) | ||
| Age, mean (SD), y | 63.3 (14.9) | 60.2 (11.1) | <.01 |
| Sex | |||
| Male | 230 (58.8) | 705 (64.6) | .05 |
| Female | 161 (41.2) | 387 (35.4) | |
| Race | |||
| White | 327 (83.6) | 842 (77.1) | <.01 |
| Black | 46 (11.8) | 206 (18.9) | |
| Asian or other | 18 (4.6) | 44 (4.0) | |
| Insurance status | |||
| Medicare | 207 (52.9) | 305 (27.9) | <.01 |
| Private insurance | 178 (45.5) | 742 (67.9) | |
| Medicaid | 2 (0.5) | 40 (3.7) | |
| Other | 4 (1.0) | 5 (0.5) | |
| BMI, mean (SD) | 27.3 (5.5)b | 27.4 (5.6)c | .89 |
| Charlson-Deyo comorbidity score, mean (SD)d | 3.2 (1.5) | 3.0 (1.6) | <.01 |
| Starting ECOG performance status score, mean (SD)e | 0.62 (0.74) | 0.68 (0.80) | .16 |
| Treatment facility | |||
| Main site | 391 (100) | 874 (80.0) | <.01 |
| Satellite facility | 0 | 218 (20.0) | |
| Year of treatment | |||
| 2011-2012 | 53 (13.6) | 483 (44.2) | <.01 |
| 2013-2014 | 195 (49.9) | 364 (33.3) | |
| 2015-2016 | 143 (36.6) | 245 (22.4) | |
| Disease site | |||
| Head and neck | 40 (10.2) | 397 (36.4) | <.01 |
| Lung | 132 (33.8) | 195 (17.9) | |
| Brain | 57 (14.6) | 183 (16.8) | |
| Esophagus/gastric tract | 55 (14.1) | 95 (8.7) | |
| Pancreas, duodenum, or hepatobiliary | 44 (11.3) | 47 (4.3) | |
| Rectal | 41 (10.5) | 83 (7.6) | |
| Anal | 18 (4.6) | 62 (5.7) | |
| Gynecologic | 4 (1.0) | 30 (2.7) | |
| Clinical tumor stage | |||
| T1 | 58 (14.8) | 153 (14.0) | <.01 |
| T2 | 97 (24.8) | 281 (25.7) | |
| T3 | 130 (33.2) | 275 (25.2) | |
| T4 | 49 (12.5) | 200 (18.3) | |
| NAf | 57 (14.6) | 183 (16.8) | |
| Clinical nodal stage | |||
| N0 | 110 (28.1) | 230 (21.1) | <.01 |
| N1 | 87 (22.3) | 229 (21.0) | |
| N2 | 112 (28.6) | 397 (36.4) | |
| N3 | 25 (6.4) | 53 (4.9) | |
| NAf | 57 (14.6) | 183 (16.8) | |
| Timing of CRT | |||
| Preoperative | 86 (22.0) | 135 (12.4) | <.01 |
| Definitive | 103 (26.3) | 372 (34.1) | |
| Postoperative | 202 (51.7) | 585 (53.6) | |
| Delivered radiation dose, mean (SD), Gy | 58.5 (7.6) | 60.3 (10.5) | <.01 |
| Integrated radiation dose delivered to the target volume, mean (SD), cGy/cc × 106 | 32.9 (25.6)g | 33.5 (31.1)h | .72 |
| Integral radiation dose outside of the target volume, mean (SD), cGy/cc × 107 | 14.1 (6.4)g | 19.1 (10.6)h | <.01 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by square of height in meters); CRT, concurrent chemoradiotherapy; ECOG, Eastern Cooperative Oncology Group; NA, not applicable.
Unless otherwise indicated, data are expressed as number (percentage) of patients. Percentages have been rounded and may not total 100.
Values available for 387 of 391 patients.
Values available for 1081 of 1092 patients.
Patient scores ranged from 2 to 16, with higher scores indicating higher burden of comorbid disease.
Patient scores ranged from 0 to 3, with higher scores indicating worse performance status.
Denotes central nervous system cancers without TNM staging.
Values available for 364 of 391 patients.
Values available for 1062 of 1092 patients.
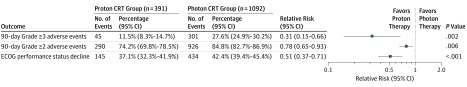
Forty-five 90-day adverse events of at least grade 3 occurred in the proton cohort (11.5%; 95% CI, 8.3%-14.7%) vs 301 in the photon cohort (27.6%; 95% CI, 24.9%-30.2%; P < .001). Adverse event outcomes are summarized in Figure 3.
Figure 3. Adverse Events and Decline in Eastern Cooperative Oncology Group (ECOG) Performance Status for Proton vs Photon Chemoradiotherapy (CRT) and Propensity Analysis Results.
Ninety-day adverse events are measured using Common Terminology Criteria for Adverse Events, version 4 (CTCAEv4). Patients were identified with CTCAEv4 grades of at least 3 and at least 2. ECOG performance status scores range from 0 to 5, with higher scores indicating worse performance status.
In propensity score–weighted analyses, proton chemoradiotherapy was associated with statistically significantly lower acute 90-day adverse events of at least grade 3 compared with photon chemoradiotherapy (RR, 0.31; 95% CI, 0.15-0.66; P = .002) (Figure 3). Proton chemoradiotherapy was associated with significantly less decline in ECOG performance status scores during chemoradiotherapy (RR, 0.51; 95% CI, 0.37-0.71; P < .001). Proton chemoradiotherapy was also associated with a significantly lower risk of acute 90-day adverse events of at least grade 2 (RR, 0.78; 95% CI, 0.65-0.93; P = .006).
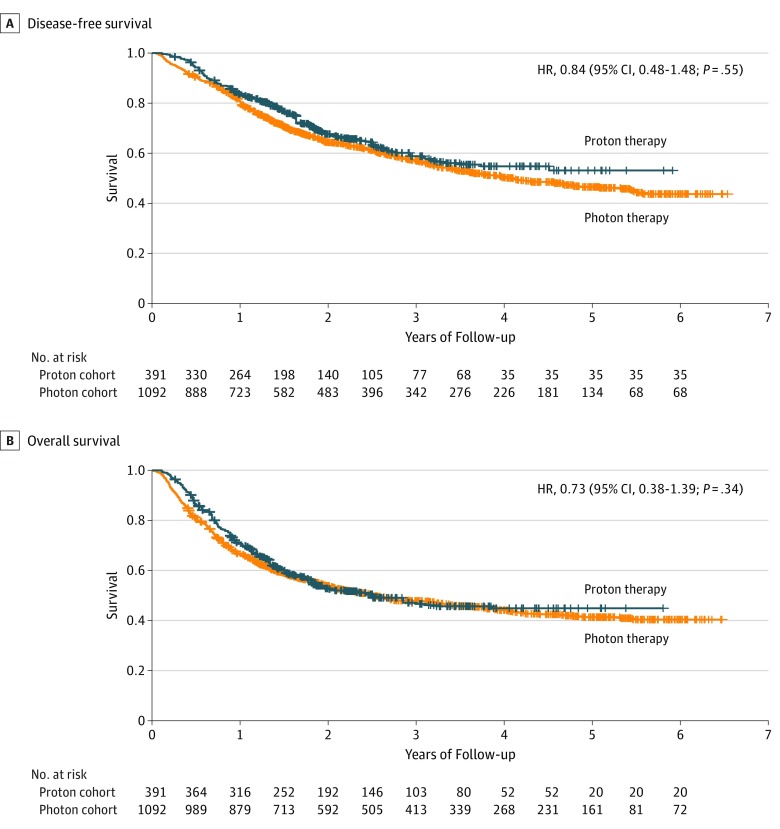
Median follow-up for the proton cohort was 3.7 (range, 0.1-6.5) years; for the photon cohort, 4.2 (range, 0.1-5.9) years. One- and 3-year adjusted DFS for the proton cohort was 70.0% (95% CI, 65.5%-74.7%) and 45.9% (95% CI, 40.8%-51.8%), respectively; for the photon cohort, 67.3% (95% CI, 64.6%-79.2%) and 48.5% (95% CI, 45.5%-51.7%), respectively. We found no significant difference in DFS for proton chemoradiotherapy compared with photon chemoradiotherapy in our propensity score–weighted Cox proportional hazards regression model (hazard ratio, 0.84; 95% CI, 0.48-1.48; P = .55). One- and 3-year adjusted OS for the proton cohort was 83.0% (95% CI, 79.3%-86.8%) and 56.2% (95% CI, 50.7%-62.2%), respectively; for the photon cohort, 81.1% (95% CI, 78.8%-83.4%) and 57.9% (95% CI, 54.8%-61.1%), respectively. There was no significant difference in OS (hazard ratio, 0.73; 95% CI, 0.38-1.39; P = .34) (Figure 4).
Figure 4. Adjusted Disease-Free and Overall Survival for the Proton vs Photon Chemoradiotherapy Cohorts.
HR indicates hazard ratio.
Sensitivity Analysis
We performed an extensive sensitivity analysis to assess the potential effect of an unmeasured confounder, such as physical frailty, on the primary outcome of 90-day toxicity of at least grade 3. We found that a substantial imbalance in physical frailty would be needed to significantly alter the overall results of the study (eTable in the Supplement). For instance, assuming an RR of 2.00 for unplanned hospitalizations for physical frailty, the prevalence of the unmeasured confounder would need to be 200% higher in the photon group than the proton group for our RR estimates to no longer be statistically significant.
Discussion
We conducted a study of 1483 adult patients with locally advanced cancer treated with chemoradiotherapy to assess the comparative effectiveness of photon vs proton therapy using prospectively gathered data on adverse events and oncologic outcomes. We found that, compared with photon therapy, proton therapy was associated with a nearly two-thirds reduction in 90-day severe adverse events associated with unplanned hospitalizations. Proton therapy was also associated with significantly lower risk of a decline in ECOG performance status and significantly less risk of adverse events causing impairment in patient’s instrumental activities of daily living. There were no statistically significant differences in DFS or OS between treatment groups.
We observed substantial morbidity associated with conventional photon chemoradiotherapy; 27.6% of patients developed severe 90-day adverse events associated with unplanned hospitalizations (CTCAEv4 grade ≥3), whereas in the proton group, 11.5% of patients had severe 90-day adverse events. Previous research has demonstrated that proton chemoradiotherapy is associated with significant morbidity.38 One study39 found that one-third of patients undergoing definitive radiotherapy with or without chemotherapy had an unplanned emergency department visit or hospitalization within 30 days. In a pooled analysis, patients receiving chemoradiotherapy accounted for 6% of all intensive care unit admissions and 26% of all cancer-related intensive care unit admissions.40
Our results demonstrating significant reduction in toxicity with the use of proton chemoradiotherapy are consistent with and extend previous research,11,41,42,43,44,45,46,47,48 although this outcome remains an area of controversy. Before this study, data on the toxicity differences between proton vs photon chemoradiotherapy have been limited, with relatively small patient numbers, although most studies11,41,42,43,44,45,46,47,48 have found a toxicity advantage and/or dosimetric advantage in favor of proton chemoradiotherapy. To our knowledge, only 1 randomized trial of chemoradiotherapy with proton vs photon therapy16 has been reported in locally advanced non–small cell lung cancer. There was no difference in the primary end point (grade ≥3 radiation pneumonitis). Other toxicity end points have not yet been reported. Exploratory subset analysis of the patients with non–small cell lung cancer in our cohort revealed a reduction in overall toxicity of at least grade 3 with proton therapy that was comparable to the reduction seen in the entire cohort. Although several cooperative group trials randomizing patients to proton vs photon chemoradiotherapy are in progress, including NRG BN-005 in glioma and RTOG 1308 in lung cancer,14,49 results will not be available for years, and accrual is challenging.30
The significant reduction in adverse events that we observed with proton therapy was not accompanied by a reduction in treatment efficacy. We compared DFS and OS between the 2 cohorts and found that survival outcomes were comparable. This finding is noteworthy because it suggests that, with careful delineation of the target volume, proton therapy is not associated with worse cancer control outcomes that could occur due to a marginal miss if the proton beam stops short of covering the full extent of the subclinical disease.11
Our finding that proton therapy is not associated with worse cancer control outcomes is also consistent with and extends the existing literature. Preliminary results from the lung cancer trial found no difference in local control at 12 months.16 Similarly, retrospective studies have not shown worse oncologic outcomes with proton chemoradiotherapy, with some studies showing improvement in survival with proton therapy.17,42 These studies were smaller and/or relied on the National Cancer Database, which is missing key variables and has no cancer-specific outcomes data.50
Limitations
This study has several limitations. First, this observational study cannot draw causal inferences. However, we used several approaches to adjust for measured confounding and consider unmeasured confounding. The database included 131 relevant demographic, socioeconomic, clinical, pathologic, and treatment variables. We used robust statistical approaches to try to account for measured confounding, including a modern ensemble machine-learning approach for propensity score estimation that may better reduce residual bias.33,34,35 This analysis helps us to account for the heterogeneity of the proton and photon cohorts, although we acknowledge that these statistical techniques to reduce residual bias have limitations. At our institution, 80% of patients receiving photon therapy were treated at the same hospital by the same physicians as those receiving proton therapy; radiation target volumes were not significantly different; and the choice of proton therapy was usually determined not by clinical or disease-specific factors but by whether the patient’s insurance approves proton therapy.51 In addition, the sensitivity analysis suggests—although it does not confirm—that a very large imbalance in an unmeasured confounder, such as physical frailty or socioeconomic status, with effects on unplanned hospitalizations would be required to alter the findings of the study.
Second, the study involved nonblinded outcomes assessment and is subject to possible observer bias. However, the National Cancer Institute cooperative group studies of radiotherapy use similar approaches to outcome assessment. Moreover, our primary end point of adverse events associated with unplanned hospitalizations and survival outcomes is not subtle, and the likelihood of systematic bias in the reporting of these events is quite low. Although there was some difference in median follow-up between the 2 cohorts, our primary end point was short term, and patients with insufficient follow-up for the primary end point were excluded.
Last, hematologic adverse events and detailed data on chemotherapy administration, including number of cycles and doses, were not included in the analysis; however, choice of chemotherapy agent(s) is standardized by disease site at our institution and did not vary based on radiation modality. Hematologic adverse events are far more likely to be chemotherapy related and would be unlikely to favor the photon cohort because photon therapy would be expected to expose more bone marrow to clinically meaningful doses of radiation owing to the higher integral dose of photon therapy.
Conclusions
This study has 3 important implications for future research. First, proton therapy’s lower observed toxicity and its more favorable association with performance status at least raises the tantalizing possibility that the higher up-front cost of proton therapy may be offset by cost savings from reduced hospitalizations52 and enhanced productivity from patients and caregivers.53 Second, the lower observed toxicity of proton therapy offers an opportunity to explore clinical trials combining proton therapy with intensified systemic therapy and/or dose-escalated radiotherapy, which may, in turn, improve survival outcomes.54,55,56 Third, in our study, older patients with more comorbid disease were more likely to receive proton therapy and experienced less toxicity; thus, proton therapy may allow older patients with more comorbidities to receive the most effective combined-modality treatments. Inclusion criteria for clinical trials of proton chemoradiotherapy may be liberalized to include older, sicker patients who have been excluded from combined modality trials in the past.57,58,59,60
In adults with locally advanced cancer treated at the University of Pennsylvania, proton chemoradiotherapy compared with photon chemoradiotherapy was associated with a significant reduction in 90-day adverse events associated with unplanned hospitalizations (CTCAEv4 grade ≥3), less decline in ECOG performance status, and fewer 90-day adverse events of at least grade 2. We found no difference in DFS and OS. Reduced adverse events associated with proton therapy could offer an opportunity to intensify chemoradiotherapy treatments and/or broaden inclusion criteria on clinical trials to include older, sicker patients, which may improve oncologic outcomes. Prospective clinical trials of proton vs photon chemoradiotherapy are warranted to validate these results.
eFigure. Representative Proton and Photon Treatment Plans With Radiation Dose Represented as a Color Wash
eMethods 1. Prospective Analysis Plan (2017)
eMethods 2. List of the 131 Variables in the Database
eTable. Unmeasured Confounding Sensitivity Analysis for Acute Toxicity of Grade ≥3
eReferences.
References
- 1.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non–small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187-199. doi: 10.1016/S1470-2045(14)71207-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996. doi: 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 3.Pignon JP, le Maître A, Maillard E, Bourhis J; MACH-NC Collaborative Group . Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17 346 patients. Radiother Oncol. 2009;92(1):4-14. doi: 10.1016/j.radonc.2009.04.014 [DOI] [PubMed] [Google Scholar]
- 4.Cooper JS, Guo MD, Herskovic A, et al. ; Radiation Therapy Oncology Group . Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). JAMA. 1999;281(17):1623-1627. doi: 10.1001/jama.281.17.1623 [DOI] [PubMed] [Google Scholar]
- 5.van Hagen P, Hulshof MC, van Lanschot JJ, et al. ; CROSS Group . Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074-2084. doi: 10.1056/NEJMoa1112088 [DOI] [PubMed] [Google Scholar]
- 6.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567-578. doi: 10.1056/NEJMoa053422 [DOI] [PubMed] [Google Scholar]
- 7.Nguyen-Tan PF, Zhang Q, Ang KK, et al. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: long-term report of efficacy and toxicity. J Clin Oncol. 2014;32(34):3858-3866. doi: 10.1200/JCO.2014.55.3925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ollendorf DA, Colby J Proton beam therapy: ICER final evidence report. Washington State Health Technology Assessment Program. http://icer-review.org/wp-content/uploads/2014/07/pbt_final_report_040114.pdf. Published March 28, 2014. Accessed November 12, 2015.
- 9.Kong FS. What happens when proton meets randomization: is there a future for proton therapy? J Clin Oncol. 2018;36(18):1777-1779. doi: 10.1200/JCO.2017.76.5479 [DOI] [PubMed] [Google Scholar]
- 10.Indelicato DJ, Merchant T, Laperriere N, et al. Consensus report from the Stockholm Pediatric Proton Therapy Conference. Int J Radiat Oncol Biol Phys. 2016;96(2):387-392. doi: 10.1016/j.ijrobp.2016.06.2446 [DOI] [PubMed] [Google Scholar]
- 11.Leeman JE, Romesser PB, Zhou Y, et al. Proton therapy for head and neck cancer: expanding the therapeutic window. Lancet Oncol. 2017;18(5):e254-e265. doi: 10.1016/S1470-2045(17)30179-1 [DOI] [PubMed] [Google Scholar]
- 12.Baumann BC, Lustig RA, Mazzoni S, et al. A prospective clinical trial of proton therapy for chordoma and chondrosarcoma: feasibility assessment. J Surg Oncol. 2019;120(2):200-205. doi: 10.1002/jso.25502 [DOI] [PubMed] [Google Scholar]
- 13.Hong TS, Wo JY, Yeap BY, et al. Multi-institutional phase II study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2016;34(5):460-468. doi: 10.1200/JCO.2015.64.2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas CR., Jr Potential of prospective particle therapy trials to increase the therapeutic ratio for locally advanced lung cancer. JAMA Oncol. 2017;3(8):e172165. doi: 10.1001/jamaoncol.2017.2165 [DOI] [PubMed] [Google Scholar]
- 15.Pragmatic randomized trial of proton vs photon therapy for patients with non-metastatic breast cancer receiving comprehensive nodal radiation: a Radiotherapy Comparative Effectiveness (RADCOMP) Consortium trial NCT02603341. Clinicaltrials.gov. identifier: NCT02603341. https://clinicaltrials.gov/ct2/show/NCT02603341. Updated July 26, 2019. Accessed July 30, 2019.
- 16.Liao Z, Lee JJ, Komaki R, et al. Bayesian adaptive randomization trial of passive scattering proton therapy and intensity-modulated photon radiotherapy for locally advanced non–small-cell lung cancer. J Clin Oncol. 2018;36(18):1813-1822. doi: 10.1200/JCO.2017.74.0720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins KA, O’Connell K, Liu Y, et al. National Cancer Database analysis of proton versus photon radiation therapy in non–small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;97(1):128-137. doi: 10.1016/j.ijrobp.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 18.Mitin T, Zietman AL. Promise and pitfalls of heavy-particle therapy. J Clin Oncol. 2014;32(26):2855-2863. doi: 10.1200/JCO.2014.55.1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michalski JM, Moughan J, Purdy J, et al. Effect of standard vs dose-escalated radiation therapy for patients with intermediate-risk prostate cancer: the NRG oncology RTOG 0126 randomized clinical trial. JAMA Oncol. 2018;4(6):e180039. doi: 10.1001/jamaoncol.2018.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whelan TJ, Olivotto IA, Parulekar WR, et al. ; MA.20 Study Investigators . Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373(4):307-316. doi: 10.1056/NEJMoa1415340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan CJ, Smith MR, de Bono JS, et al. ; COU-AA-302 Investigators . Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138-148. doi: 10.1056/NEJMoa1209096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matuszak MM, Fuller CD, Yock TI, et al. Performance/outcomes data and physician process challenges for practical big data efforts in radiation oncology. Med Phys. 2018;45(10):e811-e819. doi: 10.1002/mp.13136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 24.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649-655. doi: 10.1097/00000421-198212000-00014 [DOI] [PubMed] [Google Scholar]
- 25.Kachnic LA, Winter K, Myerson RJ, et al. RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2013;86(1):27-33. doi: 10.1016/j.ijrobp.2012.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.James ND, Hussain SA, Hall E, et al. ; BC2001 Investigators . Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012;366(16):1477-1488. doi: 10.1056/NEJMoa1106106 [DOI] [PubMed] [Google Scholar]
- 27.Bernier J, Domenge C, Ozsahin M, et al. ; European Organization for Research and Treatment of Cancer Trial 22931 . Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945-1952. doi: 10.1056/NEJMoa032641 [DOI] [PubMed] [Google Scholar]
- 28.Stitzenberg KB, Chang Y, Smith AB, Nielsen ME. Exploring the burden of inpatient readmissions after major cancer surgery. J Clin Oncol. 2015;33(5):455-464. doi: 10.1200/JCO.2014.55.5938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeong IG, Khandwala YS, Kim JH, et al. Association of robotic-assisted vs laparoscopic radical nephrectomy with perioperative outcomes and health care costs, 2003 to 2015. JAMA. 2017;318(16):1561-1568. doi: 10.1001/jama.2017.14586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bekelman JE, Denicoff A, Buchsbaum J. Randomized trials of proton therapy: why they are at risk, proposed solutions, and implications for evaluating advanced technologies to diagnose and treat cancer. J Clin Oncol. 2018;36(24):2461-2464. doi: 10.1200/JCO.2018.77.7078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pirracchio R, Petersen ML, van der Laan M. Improving propensity score estimators’ robustness to model misspecification using Super Learner. Am J Epidemiol. 2015;181(2):108-119. doi: 10.1093/aje/kwu253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan F, Mitra N. An evaluation of bias in propensity score-adjusted non-linear regression models. Stat Methods Med Res. 2018;27(3):846-862. doi: 10.1177/0962280216643739 [DOI] [PubMed] [Google Scholar]
- 34.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 35.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661-3679. doi: 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalbasi A, Li J, Berman A, et al. Dose-escalated irradiation and overall survival in men with nonmetastatic prostate cancer. JAMA Oncol. 2015;1(7):897-906. doi: 10.1001/jamaoncol.2015.2316 [DOI] [PubMed] [Google Scholar]
- 37.Mitra N, Heitjan DF. Sensitivity of the hazard ratio to nonignorable treatment assignment in an observational study. Stat Med. 2007;26(6):1398-1414. doi: 10.1002/sim.2606 [DOI] [PubMed] [Google Scholar]
- 38.Jairam V, Lee V, Park HS, et al. Treatment-related complications of systemic therapy and radiotherapy. JAMA Oncol. 2019;5(7):1028-1035. doi: 10.1001/jamaoncol.2019.0086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marar M, Gabriel P, Hwang WT, et al. Acute hospital encounters in cancer patients treated with definitive radiation therapy. Int J Radiat Oncol Biol Phys. 2018;101(4):935-944. doi: 10.1016/j.ijrobp.2018.04.025 [DOI] [PubMed] [Google Scholar]
- 40.Torres VB, Vassalo J, Silva UV, et al. Outcomes in critically ill patients with cancer-related complications. PLoS One. 2016;11(10):e0164537. doi: 10.1371/journal.pone.0164537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romesser PB, Cahlon O, Scher E, et al. Proton beam radiation therapy results in significantly reduced toxicity compared with intensity-modulated radiation therapy for head and neck tumors that require ipsilateral radiation. Radiother Oncol. 2016;118(2):286-292. doi: 10.1016/j.radonc.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xi M, Xu C, Liao Z, et al. Comparative outcomes after definitive chemoradiotherapy using proton beam therapy versus intensity modulated radiation therapy for esophageal cancer: a retrospective, single-institutional analysis. Int J Radiat Oncol Biol Phys. 2017;99(3):667-676. doi: 10.1016/j.ijrobp.2017.06.2450 [DOI] [PubMed] [Google Scholar]
- 43.Warren S, Hurt CN, Crosby T, Partridge M, Hawkins MA. Potential of proton therapy to reduce acute hematologic toxicity in concurrent chemoradiation therapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2017;99(3):729-737. doi: 10.1016/j.ijrobp.2017.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang JY, Zhang X, Wang X, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in stage I or stage III non–small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65(4):1087-1096. doi: 10.1016/j.ijrobp.2006.01.052 [DOI] [PubMed] [Google Scholar]
- 45.Apinorasethkul O, Kirk M, Teo K, Swisher-McClure S, Lukens JN, Lin A. Pencil beam scanning proton therapy vs rotational arc radiation therapy: a treatment planning comparison for postoperative oropharyngeal cancer. Med Dosim. 2017;42(1):7-11. doi: 10.1016/j.meddos.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 46.Blanchard P, Garden AS, Gunn GB, et al. Intensity-modulated proton beam therapy (IMPT) versus intensity-modulated photon therapy (IMRT) for patients with oropharynx cancer—a case matched analysis. Radiother Oncol. 2016;120(1):48-55. doi: 10.1016/j.radonc.2016.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDonald MW, Liu Y, Moore MG, Johnstone PA. Acute toxicity in comprehensive head and neck radiation for nasopharynx and paranasal sinus cancers: cohort comparison of 3D conformal proton therapy and intensity modulated radiation therapy. Radiat Oncol. 2016;11:32. doi: 10.1186/s13014-016-0600-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hirano Y, Onozawa M, Hojo H, et al. Dosimetric comparison between proton beam therapy and photon radiation therapy for locally advanced esophageal squamous cell carcinoma. Radiat Oncol. 2018;13(1):23. doi: 10.1186/s13014-018-0966-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giaddui T, Chen W, Yu J, et al. Establishing the feasibility of the dosimetric compliance criteria of RTOG 1308: phase III randomized trial comparing overall survival after photon versus proton radiochemotherapy for inoperable stage II-IIIB NSCLC. Radiat Oncol. 2016;11:66. doi: 10.1186/s13014-016-0640-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fischer-Valuck BW, Michalski JM, Contreras JA, et al. A propensity analysis comparing definitive chemo-radiotherapy for muscle-invasive squamous cell carcinoma of the bladder vs urothelial carcinoma of the bladder using the National Cancer Database. Clin Transl Radiat Oncol. 2018;15:38-41. doi: 10.1016/j.ctro.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bekelman JE, Asch DA, Tochner Z, et al. Principles and reality of proton therapy treatment allocation. Int J Radiat Oncol Biol Phys. 2014;89(3):499-508. doi: 10.1016/j.ijrobp.2014.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong W, Yim YM, Kim A, et al. Assessment of costs associated with adverse events in patients with cancer. PLoS One. 2018;13(4):e0196007. doi: 10.1371/journal.pone.0196007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bradley CJ, Yabroff KR, Dahman B, Feuer EJ, Mariotto A, Brown ML. Productivity costs of cancer mortality in the United States: 2000-2020. J Natl Cancer Inst. 2008;100(24):1763-1770. doi: 10.1093/jnci/djn384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choy H, Jain AK, Moughan J, et al. RTOG 0017: a phase I trial of concurrent gemcitabine/carboplatin or gemcitabine/paclitaxel and radiation therapy (“Ping-Pong trial”) followed by adjuvant chemotherapy for patients with favorable prognosis inoperable stage IIIA/B non–small cell lung cancer. J Thorac Oncol. 2009;4(1):80-86. doi: 10.1097/JTO.0b013e318191503f [DOI] [PubMed] [Google Scholar]
- 55.Deutsch E, Le Péchoux C, Faivre L, et al. Phase I trial of everolimus in combination with thoracic radiotherapy in non-small-cell lung cancer. Ann Oncol. 2015;26(6):1223-1229. doi: 10.1093/annonc/mdv105 [DOI] [PubMed] [Google Scholar]
- 56.Langer CJ, Gadgeel SM, Borghaei H, et al. ; KEYNOTE-021 Investigators . Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non–small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497-1508. doi: 10.1016/S1470-2045(16)30498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sacher AG, Le LW, Leighl NB, Coate LE. Elderly patients with advanced NSCLC in phase III clinical trials: are the elderly excluded from practice-changing trials in advanced NSCLC? J Thorac Oncol. 2013;8(3):366-368. doi: 10.1097/JTO.0b013e31827e2145 [DOI] [PubMed] [Google Scholar]
- 58.Rutter CE, Park HS, Corso CD, et al. Comparison of survival outcomes among standard radiotherapy regimens in limited-stage small cell lung cancer patients receiving concurrent chemoradiation. Lung Cancer. 2015;90(2):243-248. doi: 10.1016/j.lungcan.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 59.Rusthoven CG, Koshy M, Sher DJ, et al. Combined-modality therapy with radiation and chemotherapy for elderly patients with glioblastoma in the temozolomide era: a National Cancer Database analysis. JAMA Neurol. 2016;73(7):821-828. doi: 10.1001/jamaneurol.2016.0839 [DOI] [PubMed] [Google Scholar]
- 60.Lee SJ, Clark MA, Cox JV, Needles BM, Seigel C, Balasubramanian BA. Achieving coordinated care for patients with complex cases of cancer: a multiteam system approach. J Oncol Pract. 2016;12(11):1029-1038. doi: 10.1200/JOP.2016.013664 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Representative Proton and Photon Treatment Plans With Radiation Dose Represented as a Color Wash
eMethods 1. Prospective Analysis Plan (2017)
eMethods 2. List of the 131 Variables in the Database
eTable. Unmeasured Confounding Sensitivity Analysis for Acute Toxicity of Grade ≥3
eReferences.