Key Points
Question
What is the association between new lesions detected on a first follow-up bone scan and outcomes in enzalutamide-treated men with metastatic castration-resistant prostate cancer with a stable or decreasing prostate-specific antigen level and regressing soft-tissue disease?
Findings
This secondary analysis of the PREVAIL and AFFIRM randomized clinical trials found that chemotherapy-naive men with new early bone lesions whose condition was stable or responding to enzalutamide had similar progression-free and overall survival times and a quality of life similar to that of men without new lesions whose condition was responding to enzalutamide; however, overall survival after chemotherapy may have a negative association with new bone lesions.
Meaning
These findings reinforce the importance of avoiding premature discontinuation of treatment based on new unconfirmed lesions detected on a follow-up bone scan in men with metastatic castration-resistant prostate cancer whose condition is stable or responding to enzalutamide, and the importance of functional imaging for diagnosing bone metastases.
Abstract
Importance
For men with metastatic castration-resistant prostate cancer (mCRPC) whose condition is responding to enzalutamide, new unconfirmed bone lesions detected at posttreatment scinitigraphy may reflect an osteoblastic reaction that represents healing, known as pseudoprogression, which can lead to premature discontinuation of therapy.
Objective
To determine the association between new unconfirmed lesions detected on a follow-up bone scintigram (bone scan) and outcomes in enzalutamide-treated men with mCRPC.
Design, Setting, and Participants
This post hoc, retrospective secondary analysis of 1672 enzalutamide-treated men from 2 phase 3, randomized mCRPC studies (PREVAIL and AFFIRM) before or after treatment with docetaxel was conducted from April 12, 2018, to July 25, 2019. Participants were men from the enzalutamide groups of the 2 studies with a decrease in prostate-specific antigen level at any time or with stable disease or soft-tissue disease responding to treatment based onradiologic findings.
Intervention
Enzalutamide, 160 mg once daily.
Main Outcomes and Measures
The clinical significance of new lesions detected on the first (early) or second (late) posttreatment bone scan, without an unfavorable change in prostate-specific antigen level or soft-tissue progression, was investigated. Associations of new unconfirmed lesions with radiographic progression-free survival, overall survival, decrease in prostate-specific antigen level, objective response in soft tissue, and quality of life were evaluated.
Results
Among the 643 men (median age, 72 years [range, 43-93 years]) in PREVAIL, early and late unconfirmed lesions were observed in 177 men (27.5%) with stable disease or disease responding to enzalutamide. Among the 404 men (median age, 70 years [range, 41-88 years]) in AFFIRM, early and late unconfirmed lesions were observed in 73 men (18.1%) with stable disease or disease responding to enzalutamide. In PREVAIL, men with new unconfirmed lesions had median radiographic progression-free survival (hazard ratio [HR], 1.37 [95% CI, 0.81-2.30]; P = .23) and median overall survival (HR, 1.25 [95% CI, 0.85-1.83]) in the chemotherapy-naive setting similar to men those of men without such new lesions. In AFFIRM, the median overall survival (HR, 1.94 [95% CI, 1.10-3.44]) was reduced among men with unconfirmed bone lesions, but the median radiographic progression-free survival was not reduced (HR, 1.21 [95% CI, 0.83-1.75]; P = .32). Quality of life over time was similar regardless of the presence of new unconfirmed lesions detected on a follow-up bone scan in either setting.
Conclusions and Relevance
These results suggest that new unconfirmed lesions detected on follow-up bone scans may represent pseudoprogression in men with mCRPC and are indicative of a favorable treatment response to enzalutamide. The detection of new unconfirmed bone lesions in men with mCRPC that responded to treatment with enzalutamide after docetaxel appears to be associated with worse overall survival and may represent true progression, thus highlighting the need for improved functional bone metastasis imaging.
Trial Registration
ClinicalTrials.gov Identifiers: NCT01212991 and NCT00974311
This secondary analysis of the PREVAIL and AFFIRM randomized clinical trials examines the association between the detection of new unconfirmed lesions detected on follow-up bone scans and outcomes in enzalutamide-treated men with metastatic castration-resistant prostate cancer (mCRPC).
Introduction
Technetium (Tc) 99m–labeled methylene diphosphonate bone scans are commonly used to both assess and monitor disease progression in bone for men with metastatic castration-resistant prostate cancer (mCRPC), indirectly inferring disease activity based on osteoblastic uptake. It has long been appreciated that bone scans can be misleading in determining whether a patient with bone metastases is benefiting from a treatment, and, in particular, from hormones.1,2 Per the Prostate Cancer Working Group 2 (PCWG2) recommendations, as retained in the PCWG3 guidelines, the assessment of disease progression in bone in the absence of other signs of progression requires that new lesions detected on the first posttreatment scan be confirmed with the documentation of additional new lesions on the next follow-up scan in the absence of other signs of progression.3,4 New unconfirmed bone lesions detected on the first follow-up scan after treatment may either reflect true progression before overall treatment outcomes can be assessed reliably or be the result of a healing response known as pseudoprogression (also known as bone scan flare) that can be misinterpreted as treatment failure and lead to the premature discontinuation of therapy.5,6,7
Pseudoprogression has been described in men with noncastrate prostate cancer7 and in men with chemotherapy-naive mCRPC treated with abiraterone acetate, an androgen biosynthesis inhibitor,5 but it has not been formally associated with clinical outcomes in large prospective studies or been examined in men with mCRPC treated with enzalutamide, an androgen receptor inhibitor. We hypothesized that early unconfirmed lesions detected on follow-up bone scans of men with mCRPC during treatment response to enzalutamide would commonly represent pseudoprogression, irrespective of prior exposure to chemotherapy, and would be associated with outcomes similar to those in patients who are responding to treatment as assessed by other means. We also assessed whether new lesions detected on the second posttreatment scan could also reflect a delayed form of pseudoprogression.
Methods
Study Design and Conduct
From April 12, 2018, to July 25, 2019, we conducted a post hoc retrospective analysis of the PREVAIL (A Safety and Efficacy Study of Oral MDV3100 in Chemotherapy-Naive Patients With Progressive Metastatic Prostate Cancer; NCT01212991) and AFFIRM (Safety and Efficacy Study of MDV3100 in Patients With Castration-Resistant Prostate Cancer Who Have Been Previously Treated With Docetaxel-based Chemotherapy; NCT00974311) phase 3 prospective randomized clinical trial data sets (trial protocols in Supplement 1). The study designs of PREVAIL8 and AFFIRM9 have previously been described. Participants in PREVAIL were men with mCRPC who were asymptomatic or minimally symptomatic and who had not received prior chemotherapy. Participants in AFFIRM were men with mCRPC who had received prior treatment with docetaxel. The coprimary end points of PREVAIL were overall survival (OS) and radiographic progression-free survival (rPFS). The primary end point of AFFIRM was OS. These studies were conducted in accordance with the Declaration of Helsinki,10 and the Duke University Institutional Review Board approved the PREVAIL and AFFIRM protocols, which specifically covered the objectives of the present analysis to examine the association of radiographic progression with overall survival, including bone scan progression. The PREVAIL and AFFIRM protocols were approved by the institutional review boards at all participating sites. All participants provided written informed consent before enrollment.
Analysis of Pseudoprogression
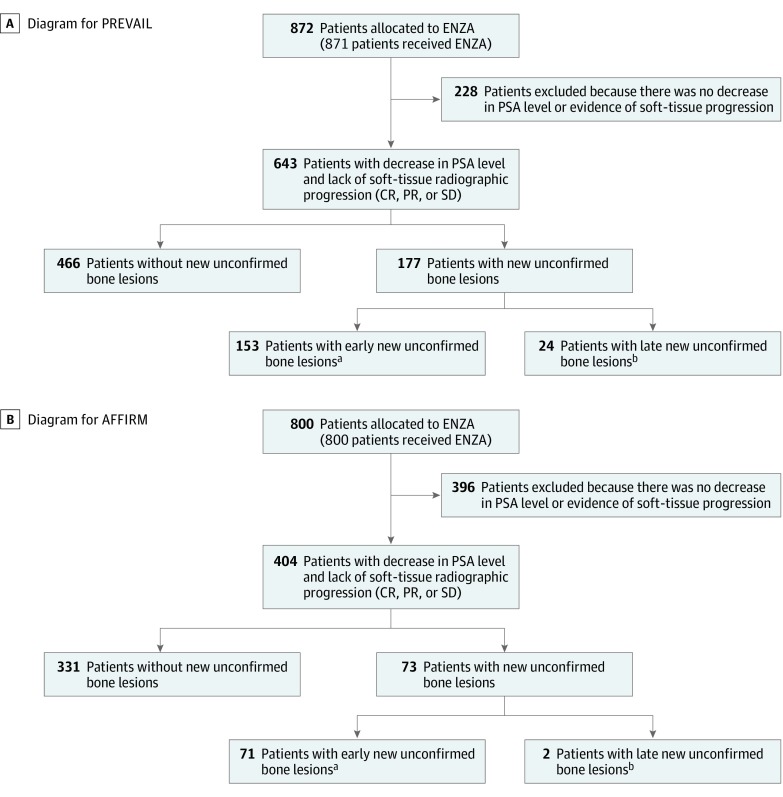
For the present analysis, only enzalutamide-treated men with mCRPC were evaluated, and the focus was on men with stable disease or disease responding to therapy according to nonbone disease manifestations, including prostate-specific antigen (PSA) level and soft-tissue criteria (Figure 1). Thus, men with no decrease in PSA level or with soft-tissue progression confirmed by radiography were excluded from the bone-scan pseudoprogression analysis. Tc 99m–labeled methylene diphosphonate bone scans were performed and interpreted locally at each center, but they were also analyzed centrally for each study. Scans were performed at weeks 9, 17, and 25 and every 12 weeks thereafter in PREVAIL and at weeks 13 and 25 and, subsequently, every 12 weeks thereafter in AFFIRM. Pseudoprogression was defined as the detection of 1 or more new lesions on a first or second postbaseline bone scan, without subsequent new lesions detected on later scans for men with any decrease in PSA level from baseline or those with a complete response, partial response, or stable disease in soft tissue based on the Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1).11 The definition was further refined to differentiate between early and late pseudoprogression. Early pseudoprogression was defined as new lesions detected on the first posttreatment scan in men with disease otherwise responding to treatment (week 9 in PREVAIL and week 13 in AFFIRM), without subsequent new lesions detected at later assessments (week 17 or later in PREVAIL and week 25 or later in AFFIRM). Late pseudoprogression was defined as new lesions detected on the second posttreatment scan in men with disease otherwise responding to treatment (week 17 in PREVAIL and week 25 in AFFIRM), without subsequent new lesions detected at later assessments.
Figure 1. CONSORT Diagrams for PREVAIL and AFFIRM.
CR indicates complete response; ENZA, enzalutamide; PR, partial response; PSA, prostate-specific antigen; and SD, stable disease.
aEarly was defined as new lesions detected on the first posttreatment scan (week 9 in PREVAIL, week 13 in AFFIRM).
bLate was defined as new lesions detected on the second posttreatment scan (week 17 or later in PREVAIL, week 25 or later in AFFIRM).
Statistical Analysis
The primary objective of the present analysis was to determine whether new lesions detected on bone scans were associated with enzalutamide treatment efficacy based on OS, rPFS, confirmed decreases in PSA level and time to PSA progression, changes in serum alkaline phosphatase levels over time, objective response rates by soft-tissue imaging, and quality of life over time.8,9 Estimates of the median and 95% CIs for the time-to-event analyses were determined using the Kaplan-Meier method. The hazard ratio (HR) was determined using an unstratified Cox proportional hazards regression model and was relative to men without new unconfirmed lesions detected on bone scans. An unstratified Cochran-Mantel-Haenszel mean test score was used for comparisons of PSA response rates and best overall soft-tissue response rates.
For PREVAIL, the data cutoff date was September 16, 2013, for OS, time to PSA progression, Functional Assessment of Cancer Therapy–Prostate Cancer (FACT-P) degradation, and best overall soft-tissue response (per RECIST v1.1) and was May 6, 2012, for rPFS. The data on FACT-P were collected at day 1, weeks 5 and 13, and every 12 weeks thereafter. For AFFIRM, the data cutoff date was September 25, 2011, for all end points. The data on FACT-P were collected at baseline and then at every regular study visit beginning at week 13. Analyses were performed with SAS Enterprise Guide, version 7.1 (SAS Institute Inc). All P values were from 2-sided tests, and results were deemed statistically significant at P < .05 for the associations of new unconfirmed bone scan lesions with each individual efficacy outcome.
Results
Patient Disposition
In PREVAIL, 872 men were assigned to receive enzalutamide; 643 had a decrease in PSA level at any time or had soft-tissue responses or stable disease. Of these 643 men, 177 (27.5%) had new unconfirmed bone lesions detected on the first or second posttreatment scan, which led to treatment discontinuation for 13 men based on the first posttreatment scan and for 3 mean based on the second posttreatment scan (Figure 1A). In AFFIRM, 800 men were assigned to receive enzalutamide; 404 had a decreased PSA level at any time or had soft-tissue responses or stable disease, and 73 of these 404 men (18.1%) had new unconfirmed bone lesions, none of whom discontinued treatment owing to unconfirmed bone lesions (Figure 1B). Most of the new unconfirmed bone lesions were detected on the first posttreatment scan and were considered to be associated with pseudoprogression until proven otherwise. New unconfirmed bone lesions detected on the second posttreatment scan were seen in 24 of 643 men (3.7%) with disease otherwise responding to treatment in PREVAIL and in 2 of 404 men (0.5%) with disease otherwise responding to treatment in AFFIRM.
Data on demographic characteristics and baseline disease characteristics were generally similar between men with and men without new unconfirmed bone lesions in both PREVAIL (median age, 72 years [range, 43-93 years]) and AFFIRM (median age, 70 years [range, 41-88 years]) (eTables 1 and 2 in Supplement 2). No pretreatment characteristics were associated with the detection or nondetection of new unconfirmed lesions on follow-up bone scans in patients considered to be responding to treatment based on PSA or soft-tissue criteria, including the following: age, race/ethnicity, Gleason score, PSA levels, the burden of bone metastases, the number of prior hormonal therapies, or the use of bone antiresorptive therapies. The prevalence of new unconfirmed bone lesions ranged from 1 of 8 (12.5%) to 37 of 101 (36.6%) in PREVAIL and from 1 of 14 (7.1%) to 7 of 26 (26.9%) in AFFIRM, based on these various subgroups (eTables 1 and 2 in Supplement 2).
Association of Bone Scan Pseudoprogression With Efficacy
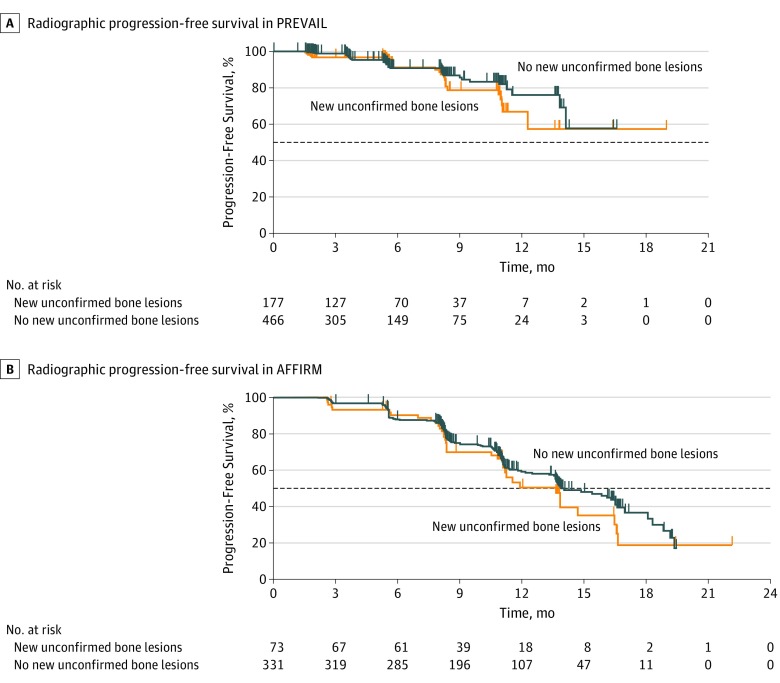
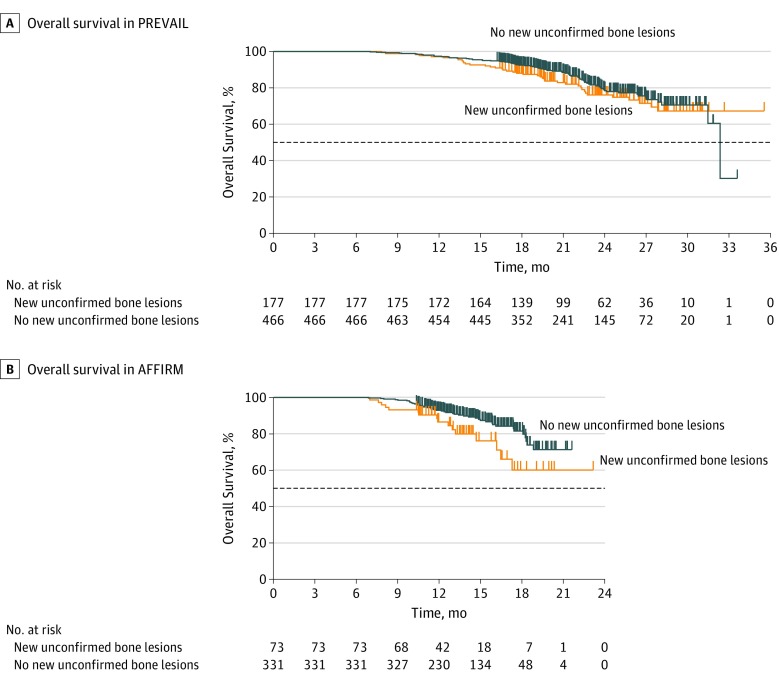
In PREVAIL, chemotherapy-naive men with stable disease or disease responding to enzalutamide who developed new unconfirmed lesions detected on follow-up bone scans (n = 177) had outcomes of rPFS (HR, 1.37; 95% CI, 0.81-2.30; P = .23) (Table and Figure 2A), OS (HR, 1.25; 95% CI, 0.85-1.83) (Table and Figure 3A), and time to PSA progression (HR, 1.16; 95% CI, 0.93-1.45) similar to those in men treated with enzalutamide whose disease was responding to treatment by PSA or soft-tissue standards with no new unconfirmed lesions detected on follow-up bone scans (Table; eFigure 1A in Supplement 2). Soft-tissue responses and decreases in PSA level did not significantly differ between men with and men without new unconfirmed lesions detected on follow-up bone scans (Table; eFigure 2A in Supplement 2).
Table. Summary of Trial Outcomes.
| Characteristic | Men in the PREVAIL Trial | Men in the AFFIRM Trial | ||
|---|---|---|---|---|
| Responding to Enzalutamide With New Unconfirmed Bone Lesions (n = 177) | Responding to Enzalutamide Without New Unconfirmed Bone Lesions (n = 466) | Responding to Enzalutamide With New Unconfirmed Bone Lesions (n = 73) | Responding to Enzalutamide Without New Unconfirmed Bone Lesions (n = 331) | |
| Median rPFS (95% CI), mo | NR (12.3 to NR) | NR (14.1 to NR) | 13.6 (11.1 to 16.5) | 13.9 (13.6 to 16.5) |
| HR (95% CI) | 1.37 (0.81 to 2.30) | 1.21 (0.83 to 1.75) | ||
| P value | .23 | .32 | ||
| Median OS (95% CI), mo | NR (NR to NR) | 32.4 (31.5 to NR) | NR (16.5 to NR) | NR (NR to NR) |
| HR (95% CI) | 1.25 (0.85 to 1.83) | 1.94 (1.10 to 3.44) | ||
| Median time to PSA progression (95% CI), mo | 12.0 (11.1 to 13.9) | 13.9 (13.7 to 16.6) | 8.4 (8.3 to 11.0) | 11.0 (8.5 to 11.1) |
| HR (95% CI) | 1.16 (0.93 to 1.45) | 1.29 (0.93 to 1.81) | ||
| Decrease in PSA level ≥30% from baseline (95% CI), %a | 98.9 (96.0 to 99.9) | 98.1 (96.4 to 99.1) | 98.6 (92.6 to 100.0) | 97.3 (94.9 to 98.7) |
| Difference (95% CI), % | 0.8 (−1.2 to 2.8) | 1.4 (−1.8 to −4.5) | ||
| P value | .48 | .50 | ||
| Decrease in PSA level ≥50% from baseline (95% CI), %a | 93.8 (89.2 to 96.9) | 93.6 (90.9 to 95.6) | 94.5 (86.6 to 98.5) | 89.1 (85.3 to 92.3) |
| Difference (95% CI), % | 0.2 (−4.0 to 4.4) | 5.4 (−0.8 to 11.6) | ||
| P value | .92 | .16 | ||
| Decrease in PSA level ≥90% from baseline (95% CI), %a | 59.9 (52.3 to 67.2) | 60.9 (56.3 to 65.4) | 37.0 (26.0 to 49.1) | 49.8 (44.3 to 55.4) |
| Difference (95% CI), % | −1.1 (−9.5 to 7.4) | −12.9 (−25.2 to −0.6) | ||
| P value | .81 | .05 | ||
| Objective response rate (95% CI), %b | 67.5 (58.1 to 76.0) | 60.1 (54.2 to 65.9) | 50.0 (36.1 to 63.9) | 44.4 (37.7 to 51.3) |
| Difference (95% CI), % | 7.4 (−2.9 to 17.7) | 5.6 (−9.3 to 20.5) | ||
| P value | .17 | .46 | ||
Abbreviations: AFFIRM, Safety and Efficacy Study of MDV3100 in Patients With Castration-Resistant Prostate Cancer Who Have Been Previously Treated With Docetaxel-based Chemotherapy; HR, hazard ratio; NR, not reached; OS, overall survival; PREVAIL, A Safety and Efficacy Study of Oral MDV3100 in Chemotherapy-Naive Patients With Progressive Metastatic Prostate Cancer; PSA, prostate-specific antigen; rPFS, radiographic progression-free survival.
Includes confirmed and unconfirmed response.
Calculated by excluding nonevaluable patients (PREVAIL, n = 63 with new bone lesions and n = 180 without new bone lesions; and AFFIRM, n = 19 with new bone lesions and n = 115 without new bone lesions).
Figure 2. Radiographic Progression-Free Survival (rPFS) in PREVAIL and AFFIRM Among Men Treated With Enzalutamide Who Had a Decrease in Prostate-Specific Antigen Level or an Objective Soft-Tissue Response, With or Without New Unconfirmed Lesions Detected on Follow-up Bone Scans Over Time.
A, Median rPFS in PREVAIL among men with new unconfirmed bone lesions (n = 177), not reached (NR [95% CI, 12.3 months to NR]); and median rPFS in PREVAIL among men with no new unconfirmed bone lesions (n = 466), NR (95% CI, 14.1 months to NR); hazard ratio, 1.37 (95% CI, 0.81-2.30); P = .23. B, Median rPFS in AFFIRM among men with new unconfirmed bone lesions (n = 73), 13.6 months (95% CI, 11.1-16.5 months); and median rPFS in AFFIRM among men with no new unconfirmed bone lesions (n = 331), 13.9 months (95% CI, 13.6-16.5 months); hazard ratio, 1.21 (95% CI, 0.83-1.75); P = .32. Horizontal dashed lines indicate the median.
Figure 3. Overall Survival (OS) in PREVAIL and AFFIRM Among Men Treated With Enzalutamide Who Had a Decrease in Prostate-Specific Antigen Level or an Objective Soft-Tissue Response, With or Without New Unconfirmed Lesions Detected on Follow-up Bone Scans Over Time.
A, Median OS in PREVAIL among men with new unconfirmed bone lesions (n = 177), not reached (NR [95% CI, NR to NR]); and median OS in PREVAIL among men with no new unconfirmed bone lesions (n = 466), 32.4 months (95% CI, 31.5 months to NR); hazard ratio, 1.25 (95% CI, 0.85-1.83). B, Median OS in AFFIRM among men with new unconfirmed bone lesions (n = 73), NR (95% CI, 16.5 months to NR); and median OS in AFFIRM among men with no new unconfirmed bone lesions (n = 331), NR; hazard ratio, 1.94 (95% CI, 1.10-3.44). Horizontal dashed lines indicate the median.
In AFFIRM, we also observed that men with stable disease or disease responding to treatment after docetaxel who were treated with enzalutamide and developed new unconfirmed lesions detected on follow-up bone scans (n = 73) had rPFS (HR, 1.21; 95% CI, 0.83-1.75; P = .32) (Table and Figure 2B) and time to PSA progression (HR, 1.29; 95% CI, 0.93-1.81) outcomes similar to men with disease responding to enzalutamide who had no new unconfirmed lesions detected on follow-up bone scans (Table; eFigure 1B in Supplement 2). In addition, soft-tissue responses and decreases in PSA level did not significantly differ between men with and men without new unconfirmed lesions (Table; eFigure 2B in Supplement 2). However, OS was significantly worse in men with new unconfirmed lesions detected on follow-up bone scans (HR, 1.94; 95% CI, 1.10-3.44; Table and Figure 3B).
A higher proportion of men with no decrease in PSA level or with bone or soft-tissue disease progression with enzalutamide treatment had an increase in serum alkaline phosphatase level over time compared with patients whose disease was responding to enzalutamide with or without new unconfirmed bone lesions, in both PREVAIL and AFFIRM (eFigure 3 in Supplement 2). A higher proportion of men whose disease was responding to enzalutamide with or without new unconfirmed bone lesions had an increase in alkaline phosphatase level at week 13 and had a subsequent decrease compared with men with no decrease in PSA level or with bone scan or soft-tissue progression in PREVAIL and AFFIRM (eFigure 4 in Supplement 2).
Association of Pseudoprogression With Quality of Life
In PREVAIL, men with new unconfirmed lesions detected on follow-up bone scans had a quality of life over time similar to that of men whose disease was responding to enzalutamide without new lesions detected on follow-up bone scans. We found no association of these newly observed unconfirmed bone metastases with time to degradation of FACT-P global score (HR, 1.03; 95% CI, 0.81-1.32; P = .79) or any subdomain thereof, or with time to pain progression (HR, 1.11; 95% CI, 0.80-1.53; P = .54) (eTable 3 in Supplement 2).
In AFFIRM, new unconfirmed bone lesions also had no association with time to degradation of FACT-P global score (HR, 0.95; 95% CI, 0.64-1.41; P = .81) or any subdomain (eTable 3 in Supplement 2). Time to pain progression was not associated with these newly observed unconfirmed bone lesions (HR, 1.04; 95% CI, 0.61-1.76; P = .89) (eTable 3 in Supplement 2).
Discussion
Whether new lesions detected on posttreatment bone scans, in the setting of treatment response as defined by nonbone disease outcomes, represent a favorable treatment response or disease progression can be challenging to determine in men with metastatic prostate cancer, in part because Tc 99m–labeled methylene diphosphonate imaging reveals osteoblastic activity and does not directly depict the cancer. The PCWG2 guidelines3 prioritize maximizing the opportunity to benefit from treatment by discouraging the premature discontinuation of therapy based on the detection of new unconfirmed bone lesions on a first follow-up scan without confirming that additional new lesions were detected on a second posttreatment scan. The occurrence of pseudoprogression is well documented; however, the association with disease outcomes has not been systematically evaluated, to our knowledge.5,7 Here we studied the survival and key secondary outcomes among men in whom new unconfirmed lesions detected on follow-up bone scans were or were not observed using cohorts of men enrolled in large-scale randomized clinical trials. The PREVAIL and AFFIRM trials were designed in accordance with the PCWG2 guidelines.3 Only 16 men in PREVAIL and no men in AFFIRM discontinued treatment because of unconfirmed lesions detected on follow-up bone scans, which demonstrates the successful implementation of the guidelines and may have resulted in more men maintaining clinical benefit from enzalutamide.
In our analysis, the following findings are important to highlight. First, new unconfirmed lesions detected on the first posttreatment bone scan in 2 large phase 3 trials were observed in 18.1% to 27.5% of men with mCRPC whose disease was otherwise responding to enzalutamide. Second, these new unconfirmed lesions were associated with similar clinical outcomes in chemotherapy-naive men with mCRPC and, thus, likely represent a healing response and pseudoprogression. However, such new unconfirmed bone lesions in the postdocetaxel mCRPC setting were associated with a decrease in OS, although no differences were seen in the secondary end points of rPFS and time to PSA progression. These results suggest that newly observed lesions in some men with mCRPC who had received prior docetaxel may more likely reflect the first evidence of true progression. In addition, differences in pseudoprogression in earlier settings may reflect the higher probability and quality and durability of responses earlier in the course of mCRPC, when disease is less heterogeneous. Given these findings, we recommend a patient-level decision around the clinical benefits of continuing therapy, based not solely on bone scan findings but also on other disease manifestations, including pain, toxic effects, serum markers such as PSA and alkaline phosphatase levels, and soft-tissue disease as well as patient preference and informed decision-making.
There was no pretreatment factor associated with the development of new unconfirmed lesions among men with mCRPC responding to enzalutamide in either setting. Although we originally hypothesized that men with more androgen-receptor–dependent prostate cancer, such as those with low Gleason scores, younger age, or African ancestry,12,13,14 and those with fewer prior hormonal therapies would have a greater probability of pseudoprogression, baseline characteristics were similar in men with or without such new unconfirmed lesions detected on follow-up bone scans. Therefore, men who are likely to have pseudoprogression cannot presently be identified prospectively, and data suggest that all men be carefully observed over time for this phenomenon.
We also determined that, although the change in serum alkaline phosphatase level at week 13 is unlikely to identify or be associated with subsequent progression in bone, a subsequent decrease in serum alkaline phosphatase level after an initial increase at week 13 may be an indicator of response to therapy and bone pseudoprogression. These findings mirror those of Huggins and Hodges15 in 1941 in their initial description of the changes in alkaline phosphatase level after orchiectomy and likely reflect osteoblastic bone remodeling. Finally, pseudoprogression at the second posttreatment scan was uncommon (3.7% of men in PREVAIL and 0.5% of men in AFFIRM), suggesting that this phenomenon is restricted largely to the first 4 months of treatment.
To clarify and validate these outcomes in each setting, improved functional imaging of the actual tumor in bone is needed. Combined positron emission tomography and computed tomography performed with fluorine F 18–labeled sodium fluoride does not allow direct visualization of all tumors, and, to our knowledge, objective standards do not yet exist for acquiring and interpreting images with this method. Uptake of F18-labeled fluordeoxyglucose allows more direct visualization of the tumor, but interpretation may be confounded by the presence of osteoblastic remodeling within the responding tumor microenvironment.16 Additional tumor-specific positron emission tomography probes, such as prostate-specific membrane antigen, choline, fluciclovine, or dihydrotestosterone,17,18 may provide useful discrimination in this clinical setting, where bone imaging results are disconnected from PSA, soft-tissue imaging, and patient symptoms, provided that proper analytic and clinical validation studies are performed in this mCRPC setting. Our work highlights this unmet need for functional bone imaging over time to more fully assess patient benefits.
Limitations
This study has some limitations, including the lack of functional imaging of bone metastases, which limits the ability to differentiate cases of pseudoprogression from true progression. Although most patients did not stop enzalutamide therapy owing to pseudoprogression in this study, our results may inform clinical practice by raising awareness of these unconfirmed bone lesions and the need for subsequent confirmation and attention to other patient and disease manifestations. A second limitation is the retrospective nature of the analysis. However, patients were managed according to PCWG2 guidelines, which anticipated this issue of bone scan pseudoprogression and thus permitted the present analysis of 2 prospective randomized phase 3 trials.
Conclusions
Newly observed but unconfirmed lesions detected on follow-up bone scans are common in patients with mCRPC who have been treated with enzalutamide and should not trigger premature discontinuation of treatment if they are detected within the first 4 months of treatment initiation, particularly in men with chemotherapy-naive mCRPC whose disease is otherwise responding to enzalutamide. However, new unconfirmed bone lesions in men with mCRPC who were previously treated with docetaxel may reflect disease heterogeneity and true progression in some men. In these men, treatment discontinuation can be considered, but ideally in the context of other disease manifestations such as changes in PSA level, soft-tissue imaging, symptoms, and patient preferences. Most importantly, quality-of-life outcomes did not differ based on the presence of new unconfirmed bone lesions in either trial. These results illustrate the need for close follow-up assessments for these patients. Improvements in imaging assessments of metastatic bone disease are needed.
Trial Protocols
eTable 1. Demographics and Baseline Disease Characteristics in PREVAIL
eTable 2. Demographics and Baseline Disease Characteristics in AFFIRM
eTable 3. Quality of Life Summary
eFigure 1. Kaplan-Meier Plot of Time to PSA Progression in PREVAIL and AFFIRM in Men Treated With Enzalutamide Who Had a Decline in PSA or Objective Soft-Tissue Response, With or Without New Unconfirmed Bone Scan Lesions Over Time
eFigure 2. Best Overall Soft-Tissue Response in PREVAIL and AFFIRM in Men Treated With Enzalutamide Who Had a Decline in PSA or Objective Soft-Tissue Response, With or Without New Unconfirmed Bone Scan Lesions Over Time
eFigure 3. Best Overall Change in Alkaline Phosphatase Over Time in PREVAIL and AFFIRM in Men Treated With Enzalutamide With No PSA Decline or With Soft-Tissue Radiographic Progression, or Who Had a Decline in PSA or Objective Soft-Tissue Response, With or Without New Unconfirmed Bone Scan Lesions
eFigure 4. Change in Alkaline Phosphatase at Week 13 in PREVAIL and AFFIRM in Men Treated With Enzalutamide With No PSA Decline or With Soft-Tissue Radiographic Progression, or Who Had a Decline in PSA or Objective Soft-Tissue Response, With or Without New Unconfirmed Bone Scan Lesions
Data Sharing Statement
References
- 1.Pollen JJ, Witztum KF, Ashburn WL. The flare phenomenon on radionuclide bone scan in metastatic prostate cancer. AJR Am J Roentgenol. 1984;142(4):773-776. doi: 10.2214/ajr.142.4.773 [DOI] [PubMed] [Google Scholar]
- 2.Johns WD, Garnick MB, Kaplan WD. Leuprolide therapy for prostate cancer: an association with scintigraphic “flare” on bone scan. Clin Nucl Med. 1990;15(7):485-487. doi: 10.1097/00003072-199007000-00006 [DOI] [PubMed] [Google Scholar]
- 3.Scher HI, Halabi S, Tannock I, et al. ; Prostate Cancer Clinical Trials Working Group . Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(7):1148-1159. doi: 10.1200/JCO.2007.12.4487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scher HI, Morris MJ, Stadler WM, et al. ; Prostate Cancer Clinical Trials Working Group 3 . Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34(12):1402-1418. doi: 10.1200/JCO.2015.64.2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan CJ, Shah S, Efstathiou E, et al. Phase II study of abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response. Clin Cancer Res. 2011;17(14):4854-4861. doi: 10.1158/1078-0432.CCR-11-0815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman RE, Mashiter G, Whitaker KB, Moss DW, Rubens RD, Fogelman I. Bone scan flare predicts successful systemic therapy for bone metastases. J Nucl Med. 1988;29(8):1354-1359. [PubMed] [Google Scholar]
- 7.Pollen JJ, Shlaer WJ. Osteoblastic response to successful treatment of metastatic cancer of the prostate. AJR Am J Roentgenol. 1979;132(6):927-931. doi: 10.2214/ajr.132.6.927 [DOI] [PubMed] [Google Scholar]
- 8.Beer TM, Armstrong AJ, Rathkopf DE, et al. ; PREVAIL Investigators . Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424-433. doi: 10.1056/NEJMoa1405095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scher HI, Fizazi K, Saad F, et al. ; AFFIRM Investigators . Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187-1197. doi: 10.1056/NEJMoa1207506 [DOI] [PubMed] [Google Scholar]
- 10.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 12.Weischenfeldt J, Simon R, Feuerbach L, et al. Integrative genomic analyses reveal an androgen-driven somatic alteration landscape in early-onset prostate cancer. Cancer Cell. 2013;23(2):159-170. doi: 10.1016/j.ccr.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 13.Kishan AU, Wang X, Seiferheld W, et al. Association of Gleason grade with androgen deprivation therapy duration and survival outcomes: a systematic review and patient-level meta-analysis. JAMA Oncol. 2019;5(1):91-96. doi: 10.1001/jamaoncol.2018.3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.George DJ, Heath EI, Sartor O, et al. Abi race: a prospective, multicenter study of black (B) and white (W) patients (pts) with metastatic castrate resistant prostate cancer (mCRPC) treated with abiraterone acetate and prednisone (AAP). J Clin Oncol. 2018;36(18_suppl):LBA5009. doi: 10.1200/JCO.2018.36.18_suppl.LBA5009 [DOI] [Google Scholar]
- 15.Huggins C, Hodges CV. Studies on prostatic cancer, I: the effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22(4):232-240. doi: 10.3322/canjclin.22.4.232 [DOI] [PubMed] [Google Scholar]
- 16.Harmon SA, Perk T, Lin C, et al. Quantitative assessment of early [18F]sodium fluoride positron emission tomography/computed tomography response to treatment in men with metastatic prostate cancer to bone. J Clin Oncol. 2017;35(24):2829-2837. doi: 10.1200/JCO.2017.72.2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindenberg L, Choyke P, Dahut W. Prostate cancer imaging with novel PET tracers. Curr Urol Rep. 2016;17(3):18. doi: 10.1007/s11934-016-0575-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith CP, Laucis A, Harmon S, et al. Novel imaging in detection of metastatic prostate cancer. Curr Oncol Rep. 2019;21(4):31. doi: 10.1007/s11912-019-0780-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocols
eTable 1. Demographics and Baseline Disease Characteristics in PREVAIL
eTable 2. Demographics and Baseline Disease Characteristics in AFFIRM
eTable 3. Quality of Life Summary
eFigure 1. Kaplan-Meier Plot of Time to PSA Progression in PREVAIL and AFFIRM in Men Treated With Enzalutamide Who Had a Decline in PSA or Objective Soft-Tissue Response, With or Without New Unconfirmed Bone Scan Lesions Over Time
eFigure 2. Best Overall Soft-Tissue Response in PREVAIL and AFFIRM in Men Treated With Enzalutamide Who Had a Decline in PSA or Objective Soft-Tissue Response, With or Without New Unconfirmed Bone Scan Lesions Over Time
eFigure 3. Best Overall Change in Alkaline Phosphatase Over Time in PREVAIL and AFFIRM in Men Treated With Enzalutamide With No PSA Decline or With Soft-Tissue Radiographic Progression, or Who Had a Decline in PSA or Objective Soft-Tissue Response, With or Without New Unconfirmed Bone Scan Lesions
eFigure 4. Change in Alkaline Phosphatase at Week 13 in PREVAIL and AFFIRM in Men Treated With Enzalutamide With No PSA Decline or With Soft-Tissue Radiographic Progression, or Who Had a Decline in PSA or Objective Soft-Tissue Response, With or Without New Unconfirmed Bone Scan Lesions
Data Sharing Statement