This cohort study examines the association between recurrence score and locoregional recurrence in postmenopausal women who received treatment for node-positive, estrogen receptor– or progesterone receptor–positive breast cancer.
Key Points
Question
Does an association exist between the 21-gene expression assay recurrence score and locoregional recurrence in postmenopausal women with a node-positive, estrogen receptor– or progesterone receptor–positive breast cancer treated with either adjuvant chemotherapy followed by tamoxifen or tamoxifen alone?
Findings
In this cohort study of 316 women with breast cancer who were participants in the Southwest Oncology Group S8814 randomized clinical trial, an intermediate or high recurrence score was associated with a significantly increased risk of locoregional recurrence; the same results were found in a subset of this cohort who underwent a mastectomy without radiotherapy.
Meaning
These findings suggest that the recurrence score may provide valuable prognostic information on locoregional recurrence that may aid in decision-making about radiotherapy for postmenopausal women with breast cancer.
Abstract
Importance
The 21-gene assay recurrence score is increasingly used to personalize treatment recommendations for systemic therapy in postmenopausal women with estrogen receptor (ER)– or progesterone receptor (PR)–positive, node-positive breast cancer; however, the relevance of the 21-gene assay to radiotherapy decisions remains uncertain.
Objective
To examine the association between recurrence score and locoregional recurrence (LRR) in a postmenopausal patient population treated with adjuvant chemotherapy followed by tamoxifen or tamoxifen alone.
Design, Setting, and Participants
This cohort study was a retrospective analysis of the Southwest Oncology Group S8814, a phase 3 randomized clinical trial of postmenopausal women with ER/PR-positive, node-positive breast cancer treated with tamoxifen alone, chemotherapy followed by tamoxifen, or concurrent tamoxifen and chemotherapy. Patients at North American clinical centers were enrolled from June 1989 to July 1995. Medical records from patients with recurrence score information were reviewed for LRR and radiotherapy use. Primary analysis included 316 patients and excluded 37 who received both mastectomy and radiotherapy, 9 who received breast-conserving surgery without documented radiotherapy, and 5 with unknown surgical type. All analyses were performed from January 22, 2016, to August 9, 2019.
Main Outcomes and Measures
The LRR was defined as a recurrence in the breast; chest wall; or axillary, infraclavicular, supraclavicular, or internal mammary lymph nodes. Time to LRR was tested with log-rank tests and Cox proportional hazards regression for multivariate models.
Results
The final cohort of this study comprised 316 women with a mean (range) age of 60.4 (44-81) years. Median (interquartile range) follow-up for those without LRR was 8.7 (7.0-10.2) years. Seven LRR events (5.8%) among 121 patients with low recurrence score and 27 LRR events (13.8%) among 195 patients with intermediate or high recurrence score occurred. The estimated 10-year cumulative incidence rates were 9.7% for those with a low recurrence score and 16.5% for the group with intermediate or high recurrence score (P = .02). Among patients who had a mastectomy without radiotherapy (n = 252), the differences in the 10-year actuarial LRR rates remained significant: 7.7 % for the low recurrence score group vs 16.8% for the intermediate or high recurrence score group (P = .03). A multivariable model controlling for randomized treatment, number of positive nodes, and surgical type showed that a higher recurrence score was prognostic for LRR (hazard ratio [HR], 2.36; 95% CI, 1.02-5.45; P = .04). In a subset analysis of patients with a mastectomy and 1 to 3 involved nodes who did not receive radiation therapy, the group with a low recurrence score had a 1.5% rate of LRR, whereas the group with an intermediate or high recurrence score had a 11.1% LRR (P = .051).
Conclusions and Relevance
This study found that higher recurrence scores were associated with increased LRR after adjustment for treatment, type of surgical procedure, and number of positive nodes. This finding suggests that the recurrence score may be used, along with accepted clinical variables, to assess the risk of LRR during radiotherapy decision-making.
Introduction
Several genomic tools are available for making prognostic estimates and evaluating possible chemotherapy outcomes for patients with early-stage breast cancer.1,2,3,4,5,6 However, few of these tools have been incorporated into routine use explicitly to estimate local recurrence risk and to personalize local treatments. The 21-gene expression assay (OncotypeDX; GenomicHealth Inc) provides a recurrence score, which is frequently used to estimate the 10-year distant recurrence risk and the likely advantages of adjuvant chemotherapy for patients with estrogen receptor (ER)– or progesterone receptor (PR)–positive, node-negative, tamoxifen citrate–treated breast cancer.2,7,8 The recurrence score has also been correlated with locoregional recurrence (LRR) rates in the same setting for patients who have undergone either mastectomy or breast-conserving surgery (BCS).9 Clinical trials are ongoing that incorporate genomic-based prognostic information into locoregional treatment decisions for patients with low-risk, largely node-negative breast cancer. These trials include the PRECISION Trial (Profiling Early Breast Cancer for Radiotherapy Omission), the IDEA Study (Individualized Decisions for Endocrine Therapy Alone), and the LUMINA Trial (A Prospective Cohort Study Evaluating Risk of Local Recurrence Following Breast Conserving Surgery and Endocrine Therapy in Low Risk Luminal A Breast Cancer).
The recurrence score has been demonstrated to be prognostic for breast cancer–specific survival and for chemotherapy outcomes in postmenopausal patients with ER/PR-positive, node-positive disease.5 However, limited information exists on the ability of genomic expression assays to identify the risk of LRR in patients with ER/PR-positive, node-positive breast cancer. Mamounas et al10 examined LRR rates and recurrence scores among patients with node-positive breast cancer treated with both chemotherapy and endocrine therapy in the National Surgical Adjuvant Breast and Bowel Project (NSABP). The authors reported that high recurrence scores were statistically significantly correlated with greater LRR and low recurrence scores with decreased LRR among patients with 1 to 3 positive nodes.10 The TailorRT (Regional Radiotherapy in Biomarker Low Risk Node Positive Breast Cancer) study has recently begun accrual and will examine the safety of omitting radiotherapy among patients with low recurrence scores and 1 to 3 positive nodes.11 Until the TailorRT results are reported, the controversy will continue in the setting of patients with low nodal tumor burden and less aggressive tumor features.
Therefore, in the present study, we sought to analyze the association of recurrence score and LRR in patients in the Southwest Oncology Group (SWOG) Intergroup Trial S8814 (INT-0100).12 We hypothesized that, regardless of therapy, the recurrence score would be correlated with LRR and therefore would represent a clinical tool that, in conjunction with standard clinical risk factors, could inform borderline local therapy decisions for patients who were unable or unwilling to participate in the ongoing SWOG S8814 trial.
Methods
Translational Study Design
This present cohort study has a translational design and is an extension of a previously published study,5 which showed the prognostic utility of the recurrence score in the tamoxifen and CAF-T groups of the SWOG S8814 trial. A higher recurrence score was associated with chemotherapy advantages, but associated disease-free survival (DFS) or breast cancer–specific survival advantages were not found in those patients with tumors with low recurrence scores.5 SWOG S8814 participants signed a separate informed consent document for optional tumor banking for assessing biomarkers in tumor tissue (SWOG S9445 protocol). This study was reviewed and approved by each site’s institutional review board committee and was reported according to the Reporting Recommendations for Tumour Marker Prognostic Studies (REMARK) guidelines.13
The recurrence score values for the 367 patients in the initial translational study were determined and described in a previous publication.5 For the present study, all records were reviewed for the use of radiotherapy and the LRR end point, defined as a documented recurrence in the breast, the chest wall, or the axillary, infraclavicular, supraclavicular, or internal mammary lymph nodes. Patients who underwent mastectomy and radiotherapy (n = 37), BCS without radiotherapy (n = 9), and unknown surgical type (n = 5) were excluded from this analysis, for a final cohort of 316 patients (eFigure in the Supplement).
Parent Trial Summary
The parent trial for this analysis was SWOG S8814, a phase 3 study of postmenopausal women with ER/PR-positive, node-positive breast cancer treated with tamoxifen alone, CAF (cyclophosphamide, doxorubicin, and fluorouracil) chemotherapy followed by tamoxifen, or concurrent tamoxifen and CAF.12 The trial enrolled patients at North American clinical centers from June 1989 to July 1995. Postmenopausal women with axillary node–positive breast cancer were eligible for inclusion if they had ER/PR-positive tumors based on the standards of the treating institution. Patients were randomized in a 2:3:3 ratio to 1 of 3 drug regimens: (1) tamoxifen alone (20 mg per day orally) for 5 years; (2) 6 cycles of CAF (cyclophosphamide 100 mg/m2 orally on days 1-14, doxorubicin 30 mg/m2 intravenously on days 1 and 8, and fluorouracil 500 mg/m2 intravenously on days 1 and 8) followed by tamoxifen (CAF-T); or (3) CAF with concurrent tamoxifen. The CAF cycles were repeated every 28 days.
Randomization was stratified by the number of positive nodes (1-3 vs ≥4), PR status (positive vs negative), and interval from surgical procedure (≤6 weeks vs >6 weeks). Use of radiotherapy was in accordance with standard practice: expected after BCS and at the discretion of the treating physician after mastectomy if the stage was T3, if 4 or more nodes were present, or if there was extranodal extension. The primary end point of the SWOG S8814 trial was DFS. The combined chemotherapy groups (CAF-T and concurrent tamoxifen and CAF) showed superior DFS and overall survival for 10 years, compared with the tamoxifen group.12 The sequential addition of chemotherapy (CAF-T) was better than concurrent tamoxifen and CAF, and, as such, the former group was not included in the initial translational study5 or the present study.
Statistical Analysis
The primary prespecified outcome was LRR. Time from randomization to LRR was analyzed with censoring at distant recurrence, death, or last follow-up in the absence of LRR. We used 2-sided α = .05 as the level of statistical significance. The primary analysis specified modeling continuous recurrence score as a linear term in a Cox proportional hazards regression model. Although the primary analysis used recurrence score as a continuous variable, the secondary analysis used the clinical recurrence score categories of low score (<18) and combined intermediate and high scores (≥18), owing to event numbers. Time to LRR was tested with log-rank tests and Cox proportional hazards regression for multivariate models. Independent variables included in the multivariable analysis were recurrence score (low vs intermediate and high), randomized treatment (chemotherapy or not), number of positive nodes (1-3 vs ≥4), and surgical type. Post hoc exploratory analysis examined alternative choices for the cut point and treated recurrence score as a continuous variable. Subset analyses were exploratory and thus did not adjust for multiple comparisons.
Statistical analyses were done with Stata, version 10.1 (StataCorp LLC). All analyses were performed from January 22, 2016, to August 9, 2019.
Results
Comparing the final cohort of this study (n = 316 women) with the cohort in the parent trial (n = 927 women) revealed similar age (mean [range] age, 60.4 [44-81] years vs 61.1 [37-81] years), node involvement (with 1-3 positive nodes: 210 [66.5%] vs 541 [58.4%]), and representation of the treatment group (tamoxifen only: 124 [39.2%] vs 361 [38.9%]). The DFS events were similar between the 2 cohorts (117 [37.0%] vs 395 [42.6%]) (Table 1). In the present study, the mean (SD) LRR follow-up time was 8.2 (2.66) years. The median (interquartile range) follow-up for those without LRR was 8.7 (7.0-10.2) years.
Table 1. Patient and Tumor Characteristics in the Present Study vs the Parent Trial.
| Variable | No. (%) | |||
|---|---|---|---|---|
| Present Study | Parent Trial: Tamoxifen Only and CAF-T Groups (n = 927) | |||
| Low RS Group (n = 121) | Intermediate or High RS Group (n = 195) | Overall (n = 316) | ||
| Age | ||||
| Mean (range), y | 61.0 (44-81) | 60.1 (45-79) | 60.4 (44-81) | 61.1 (37-81) |
| 30-54 | 27 (22.3) | 51 (26.2) | 78 (24.7) | 205 (22.1) |
| 55-64 | 55 (45.5) | 90 (46.1) | 145 (45.9) | 443 (47.8) |
| ≥65 | 39 (32.2) | 54 (27.7) | 933 (29.4) | 279 (30.1) |
| Positive nodes, No. | ||||
| 1-3 | 87 (71.9) | 123 (63.1) | 210 (66.5) | 541 (58.4) |
| ≥4 | 34 (28.1) | 72 (36.9) | 106 (33.5) | 386 (41.6) |
| ER/PR+ by RT-PCR assaya | 121 (100) | 184 (94.4) | 305 (96.5) | NA |
| ERBB2 by RT-PCR | NA | |||
| Negative | 120 (99.2) | 158 (81.0) | 278 (88.0) | |
| Positive | 1 (0.8) | 37 (19.0) | 38 (12.0) | |
| Tumor grade (by central review) | NA | |||
| 1 | 14 (11.6) | 100 (51.3) | 114 (36.1) | |
| 2 | 86 (71.1) | 81 (41.5) | 167 (52.8) | |
| 3 | 21 (17.4) | 14 (7.2) | 35 (11.1) | |
| Treatment | ||||
| Tamoxifen only | 44 (36.4) | 80 (41.0) | 124 (39.2) | 361 (38.9) |
| Chemotherapy then tamoxifen | 77 (63.6) | 115 (59.0) | 192 (60.8) | 566 (61.1) |
| Mean (SD) follow-up for LRR (censored only), y | 8.4 (2.39); median [IQR]: 8.6 [7.1-10.1] | 8.1 (2.83); median [IQR]: 8.8 [6.5-10.3] | 8.2 (2.66); median [IQR]: 8.7 [7.0-10.2] | NA |
| With LRR | 7 (5.8) | 27 (13.8) | 34 (10.8) | NA |
| With DFS event | 33 (27.3) | 84 (43.1) | 117 (37.0) | 395 (42.6) |
| Deaths | 23 (19.0) | 60 (30.8) | 83 (26.3) | 321 (34.6) |
Abbreviations: CAF, cyclophosphamide, doxorubicin, fluorouracil; CAF-T, CAF then tamoxifen; DFS, disease-free survival; ER/PR+, estrogen receptor– or progesterone receptor–positive; ERBB2, human epidermal growth factor receptor 2; IQR, interquartile range; LRR, locoregional recurrence; NA, not applicable; RS, recurrence score; RT-PCR, reverse-transcriptase polymerase chain reaction.
Trial inclusion was based on protein level from trial entry, not the RT-PCR assay.
Among patients who underwent BCS (excision and breast radiation) and mastectomy without radiation, 34 LRR events (10.8%) occurred, including 27 (13.8%) from the intermediate or high recurrence score group and 7 (5.8%) from the low recurrence score group.
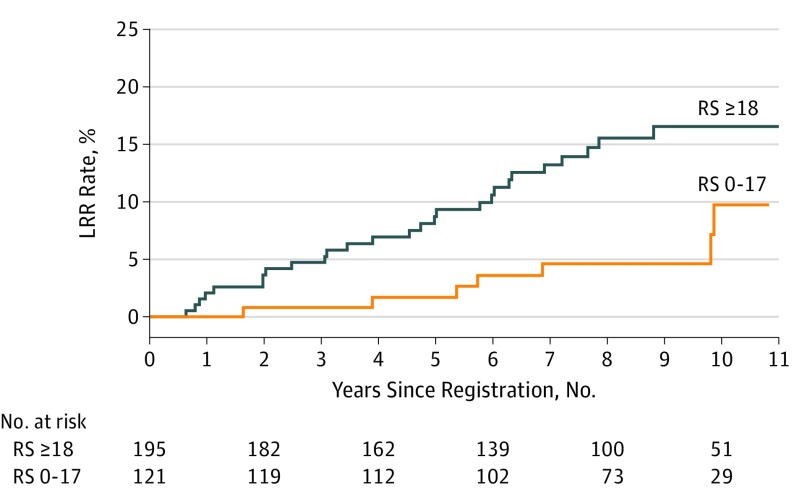
Separate log-rank tests for individual characteristics were conducted in univariate analyses on the total cohort. Both recurrence score and number of nodes were important factors associated with LRR. The 10-year LRR rates were 9.7% for those with a low recurrence score vs 16.5% for the group with an intermediate or high recurrence score (P = .02; Figure 1) and 9.0% for those with 1 to 3 positive nodes vs 24.4% for those with 4 or more involved nodes (P = .002). However, associations between LRR and age, HER2 (now ERBB2) status, tumor grade, and treatment groups were not statistically significant.
Figure 1. Kaplan-Meier Curves for Locoregional Recurrence (LRR) by Recurrence Score (RS).
The 10-year actuarial LRR rates for all patients were 9.7% for those with low RS and 16.5% for those with intermediate or high RS (P = .02).
The multivariable analysis of the entire cohort for LRR included the recurrence score, randomized treatment (chemotherapy or not), number of positive nodes (1-3 vs ≥4), and surgical type variables (Table 2). The statistically significant independent variables were intermediate to high recurrence scores (hazard ratio [HR], 2.36; 95% CI, 1.02-5.45; P = .04) and 4 or more involved nodes (HR, 3.37; 95% CI, 1.69-6.72; P = .001). The post hoc exploratory analysis confirmed that a recurrence score of 18 was the optimal cut point for the association of recurrence score with LRR. Modeling recurrence score as a linear continuous variable in the multivariate analysis was not statistically significant (10-point increase in recurrence score; HR, 1.14; 95% CI, 0.97-1.35; P = .11), but other models for continuous recurrence score were not explored.
Table 2. Locoregional Recurrence Multivariable Model.
| Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Intermediate or high recurrence score | 2.36 (1.02-5.45) | .04 |
| Mastectomy | 1.11 (0.45-2.71) | .82 |
| ≥4 Nodes | 3.37 (1.69-6.72) | .001 |
| Chemotherapy | 0.58 (0.29-1.15) | .12 |
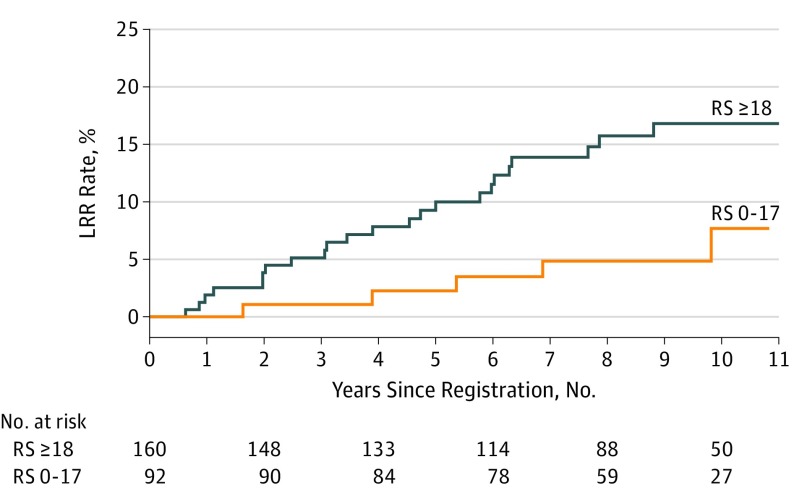
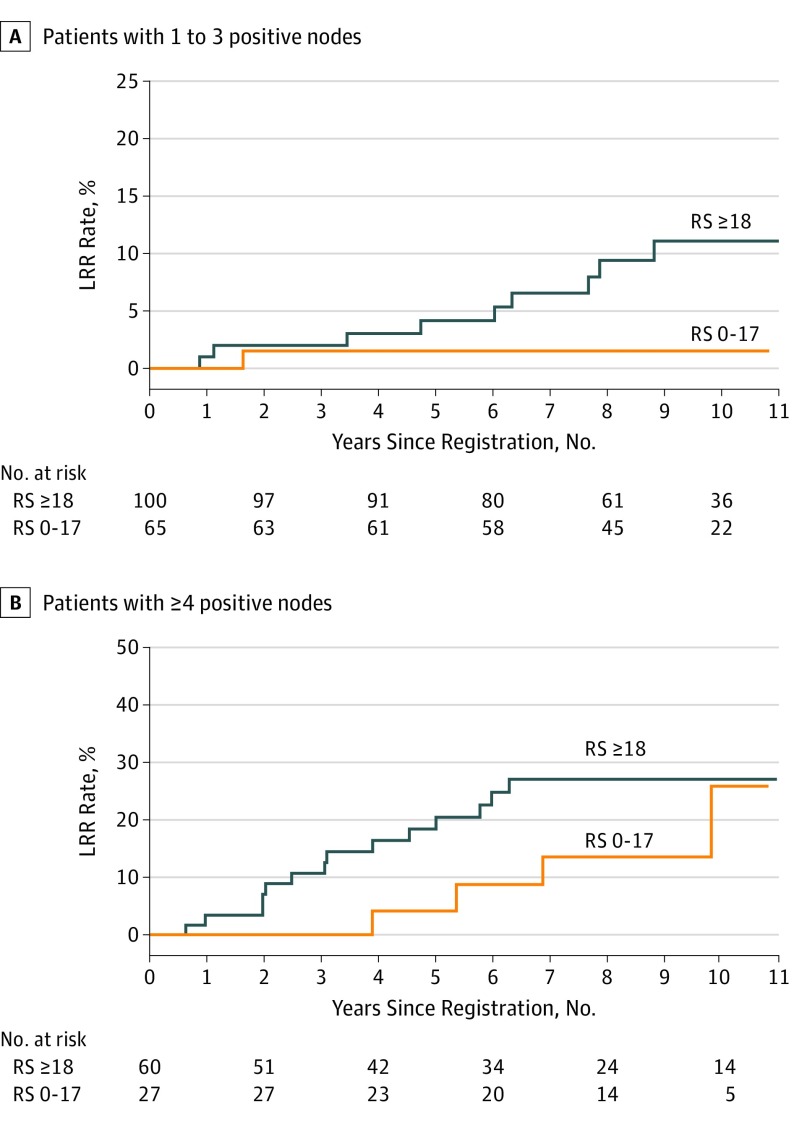
Restricting the analysis to those patients who underwent a mastectomy without radiotherapy (n = 252) demonstrated that intermediate or high recurrence score was associated with increased LRR. The 10-year actuarial LRR rates for all patients treated with a mastectomy without radiotherapy were 7.7% for low recurrence score vs 16.8% for intermediate or high recurrence score (P = .03; Figure 2). In the subset of patients with 1 to 3 positive nodes (n = 165), a trend of increased LRR was observed among those with intermediate or high recurrence score: the 10-year actuarial LRR rates in the subset of patients with 1 to 3 nodes who underwent a mastectomy without radiotherapy were 1.5% for those with low recurrence score vs 11.1% for those with intermediate or high recurrence score (P = .051; Figure 3A). No difference by recurrence score was found in the 10-year rates of LRR among those with 4 or more positive nodes who received a mastectomy without radiotherapy (25.9% vs 27.0%; P = .27; Figure 3B). Only 6 events occurred among patients who underwent BCS, all of whom received radiotherapy, on the basis of the inclusion criteria for this analysis (2 of 29 among those with a low recurrence score vs 4 of 35 among those with an intermediate or high recurrence score). As such, insufficient power existed for comparison by recurrence score in this group.
Figure 2. Kaplan-Meier Curves for Locoregional Recurrence (LRR) by Recurrence Score (RS) for Patients With Mastectomy Only.
The 10-year actuarial LRR rates for all patients treated with mastectomy without adjuvant radiotherapy were 7.7% for those with low RS and 16.8% for those with intermediate or high RS (P = .03).
Figure 3. Kaplan-Meier Curves for Locoregional Recurrence (LRR) by Recurrence Score (RS) for Patients With Mastectomy Only, by Number of Positive Nodes.
(A) The 10-year actuarial LRR rates in the subset of patients with 1 to 3 nodes who underwent mastectomy without adjuvant radiotherapy (RT) were 1.5% for those with low RS and 11.1% for those with intermediate or high RS (P = .05). The 10-year actuarial LRR rates in the subset of patients with ≥4 or more positive nodes who underwent mastectomy without RT were 25.9% for those with low RS and 27.0% for those with intermediate or high RS (P = .27).
Discussion
To our knowledge, genomic data have not been routinely incorporated into local therapy decisions for patients with breast cancer and positive lymph nodes. This study demonstrated that recurrence score appeared to be an independent prognostic factor for LRR in the SWOG S8814 trial for postmenopausal patients with node-positive, ER/PR-positive, nonmetastatic breast cancer. In the NSABP B-28 trial, Mamounas et al10 found a correlation between the recurrence score and LRR among women treated with chemotherapy. We analyzed node-positive patients treated with CAF-T as well as those treated with tamoxifen alone. The women in the mastectomy cohort in the SWOG S8814 trial represented patients for whom radiotherapy was not recommended, a subset for whom additional prognostic information would be clinically useful. We believe that the recurrence score adds independent prognostic information that could be used with standard clinical factors for identifying LRR risk and making radiotherapy decisions.
These findings suggest that radiotherapy should remain as a standard treatment in patients with any recurrence score and 4 or more positive nodes. The findings also suggest the possibility of omitting radiotherapy in patients with low recurrence score and 1 to 3 positive nodes. Thus, these results support the conduct of randomized clinical trials, such as the Canadian Cancer Trials Group (CCTG) MA39 (TailorRT), to investigate whether radiotherapy may safely be omitted in patients with N1 disease and a low recurrence score. The findings that patients with N1 disease but a higher recurrence score had a nontrivial risk (11.1%) of LRR after mastectomy alone may also be helpful in informing treatment decisions in patients not eligible to participate in the TailorRT trial.
The recurrence score was an independent prognostic factor for LRR in the analysis from 2 prospective randomized clinical trials of ER/PR-positive, node-negative breast cancer (NSABP B-14 and B-20)9 and 2 independent randomized clinical trials of ER/PR-positive, node-positive disease (SWOG S8814 and NSABP B-28).9,10 Several differences were observed in the patients in the present study and the NSABP B-28 trial.10 No postmastectomy radiotherapy was permitted in the NSABP B-28 trial, although no studies have assessed adherence to these guidelines. This exclusion meant there were higher-risk mastectomy patients in the NSABP B-28 cohort compared to the SWOG S8814 cohort, in which postmastectomy radiotherapy was permitted for those with higher-risk disease (and these patients were excluded from the present analysis). In addition, all patients in the NSABP B-28 trial received systemic chemotherapy, unlike in the SWOG S8814 trial, in which some patients were randomized to receive tamoxifen. In both studies, recurrence score was independently associated with LRR. Among all patients with 1 to 3 positive nodes in the NSABP B-28 trial, LRR rates were low regardless of recurrence score (10-year cumulative incidence rate for low recurrence score was 2.4%; for intermediate, 4.1%; and for high, 6.0%).10 Among patients who underwent a mastectomy and had 1 to 3 positive nodes in the SWOG S8814 trial, the higher rate of 11.1% among those with a recurrence score of 18 or higher highlighted that caution must be taken in eliminating radiation in these patients. In the TailorRT trial, which recently enrolled patients with ER/PR-positive disease and 1 to 3 nodes, only those with a low recurrence score will be randomized, an approach supported by the current study findings. Considering the subset with 4 or more nodes who underwent a mastectomy in the NSABP B-28 study,10 recurrence score was significantly associated with LRR (10-year cumulative incidence rate for low recurrence was 5.5%; for intermediate, 9.6%; and for high, 23.5%). Among patients with 4 or more nodes and a low recurrence score treated with mastectomy in the SWOG S8814 trial, low LRR rates were not identified (10-year LRR rate among those with a low recurrence score was 27%), highlighting the need for caution and further study before considering omission of radiotherapy in this higher-risk cohort.
Omitting radiotherapy from patient treatment because of the estimated low risk for LRR has potential risks. The CCTG MA-20 trial was a randomized clinical trial of patients with node-positive and high-risk node-negative breast cancer who were randomized to radiotherapy of the breast alone (50 Gy) or radiotherapy of breast and regional nodes (45 Gy).14 The LRR rates in that trial were low, at 4.8% for the radiotherapy of the breast group and 7.9% for the radiotherapy of breast and regional nodes groups at 10 years (HR, 0.59; 95% CI, 0.39-0.88; P = .009). However, despite this small difference in LRR rates and low failure rates overall, significant DFS outcomes of 5.0% (HR, 0.76; 95% CI, 0.61-0.94; P = .01) in the overall cohort and 14.6% (HR, 0.56; 95% CI, 0.39-0.81; P = .04) in a preplanned subset analysis were found among patients with ER-negative breast cancer. No difference was found among patients with node-positive tumors and the small cohort of patients with node-negative disease.14 Results from the CCTG MA-20 trial supported the consideration of radiotherapy for patients with trial-eligible early-stage breast cancer, despite of their low LRR rates. This conclusion was bolstered by the results of the European Organisation for Research and Treatment of Cancer 22922 study, which randomized more than 4000 women with node-positive or medial/central node–negative tumors either to breast or chest wall radiation therapy or to the same with regional nodal irradiation.15 At 10 years, DFS was improved by regional nodal irradiation with an absolute benefit of 3.0% (HR, 0.89; 95% CI, 0.80-1.00; P = .04). Although the LRR rates were not explicitly provided, the difference between the distant DFS and DFS was 5.9 in both groups.15 Additional tools for selecting patients who are truly at risk for LRR and greater understanding of the association between radiotherapy and DFS are needed.
Overall, subgroup analyses demonstrated that recurrence score was associated with LRR, but the absolute LRR rates varied. We recommend considering the recurrence score, when available, as one of the factors in selecting patients for postmastectomy radiotherapy. All available trial results enhanced by the present study supported the continued use of radiotherapy in any patient with pN2a disease and N1 disease with an intermediate or high recurrence score. Until the results of ongoing prospective trials, such as TailorRT, are known, enrollment of patients with low recurrence scores should be encouraged.
Limitations
This study has some limitations that are inherent in retrospective analyses. First, the use of radiotherapy was extracted retrospectively and may be underreported. Second, lower LRR rates were reported in a series using more modern chemotherapy and increased radiotherapy, so this report may overestimate current risks. Third, too few events were included to address the predictive role of recurrence score for radiotherapy or to distinguish between local and regional recurrences. Fourth, smaller numbers necessitated grouping the intermediate and high scores and precluded secondary analysis as a continuous variable. As such, the risk group cutoffs were not aligned with those in the recently published TailorRx study8 or the ongoing RxPonder trial. TailorRx was a randomized study of 6711 patients with ER-positive, ERBB2-negative, axillary node–negative breast cancer with recurrence scores ranging from 11 to 25, demonstrating that endocrine therapy was noninferior to chemoendocrine therapy for the end point of invasive DFS.8 RxPonder is a randomized phase 3 study that compares endocrine therapy to chemoendocrine therapy in patients with ER-positive, ERBB2-positive, axillary node–positive breast cancer with a recurrence score lower than 25; the trial’s expected primary completion date is February 2022. Nevertheless, the prospectively treated cohort of SWOG 8814 represents a uniformly treated cohort with long-term follow-up and extends in an independent analysis the findings of NSABP trials in patients with node-negative and node-positive tumors.
Conclusions
In this retrospective analysis of data from the SWOG S8814 and other randomized clinical trials including patients with node-positive and node-negative breast cancer, recurrence score appeared to be an independent prognostic factor for LRR among those who received whole-breast radiotherapy and those who underwent a mastectomy without postmastectomy radiotherapy. Subgroup analyses demonstrated that recurrence score was associated with LRR, but the absolute LRR rates varied.
eFigure. Specimen Acquisition, Distribution and Processing for the RT-PCR Analyses, Resulting in the Final Sample Size of 316 Patients
References
- 1.Drukker CA, Elias SG, Nijenhuis MV, et al. Gene expression profiling to predict the risk of locoregional recurrence in breast cancer: a pooled analysis. Breast Cancer Res Treat. 2014;148(3):599-613. doi: 10.1007/s10549-014-3188-z [DOI] [PubMed] [Google Scholar]
- 2.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817-2826. doi: 10.1056/NEJMoa041588 [DOI] [PubMed] [Google Scholar]
- 3.Scott JG, Berglund A, Schell MJ, et al. A genome-based model for adjusting radiotherapy dose (GARD): a retrospective, cohort-based study. Lancet Oncol. 2017;18(2):202-211. doi: 10.1016/S1470-2045(16)30648-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W, Mao JH, Zhu W, et al. Centromere and kinetochore gene misexpression predicts cancer patient survival and response to radiotherapy and chemotherapy. Nat Commun. 2016;7:12619. doi: 10.1038/ncomms12619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albain KS, Barlow WE, Shak S, et al. ; Breast Cancer Intergroup of North America . Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11(1):55-65. doi: 10.1016/S1470-2045(09)70314-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardoso F, van’t Veer LJ, Bogaerts J, et al. ; MINDACT Investigators . 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375(8):717-729. doi: 10.1056/NEJMoa1602253 [DOI] [PubMed] [Google Scholar]
- 7.Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010;28(11):1829-1834. doi: 10.1200/JCO.2009.24.4798 [DOI] [PubMed] [Google Scholar]
- 8.Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111-121. doi: 10.1056/NEJMoa1804710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mamounas EP, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol. 2010;28(10):1677-1683. doi: 10.1200/JCO.2009.23.7610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mamounas EP, Liu Q, Paik S, et al. 21-gene recurrence score and locoregional recurrence in node-positive/ER-positive breast cancer treated with chemo-endocrine therapy. J Natl Cancer Inst. 2017;109(4). doi: 10.1093/jnci/djw259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canadian Cancer Trials Group. Trial activation: MA.39 TAILOR RT. https://www.ctg.queensu.ca/bulletin/trial-activation-ma39-tailor-rt. Published June 6, 2018. Accessed December 5, 2019.
- 12.Albain KS, Barlow WE, Ravdin PM, et al. ; Breast Cancer Intergroup of North America . Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374(9707):2055-2063. doi: 10.1016/S0140-6736(09)61523-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM; Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics . Reporting recommendations for tumor marker prognostic studies. J Clin Oncol. 2005;23(36):9067-9072. doi: 10.1200/JCO.2004.01.0454 [DOI] [PubMed] [Google Scholar]
- 14.Whelan TJ, Olivotto IA, Levine MN. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373(19):1878-1879. doi: 10.1056/NEJMoa1415340 [DOI] [PubMed] [Google Scholar]
- 15.Poortmans PM, Collette S, Kirkove C, et al. ; EORTC Radiation Oncology and Breast Cancer Groups . Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373(4):317-327. doi: 10.1056/NEJMoa1415369 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Specimen Acquisition, Distribution and Processing for the RT-PCR Analyses, Resulting in the Final Sample Size of 316 Patients