Key Points
Question
Are procedural blood pressure parameters, including specific blood pressure thresholds, associated with neurologic outcomes after endovascular treatment for acute ischemic stroke?
Findings
In this cohort study of 365 patients in 3 randomized clinical trials, critical mean arterial blood pressure thresholds and durations for poor outcome were found to be less than 70 mm Hg for more than 10 minutes and greater than 90 mm Hg for more than 45 minutes. Both durations had a number needed to harm of 10 patients.
Meaning
Mean arterial blood pressure is a modifiable therapeutic target to prevent or reduce poor functional outcome after endovascular therapy and should possibly be maintained within narrow limits.
Abstract
Importance
The optimal blood pressure targets during endovascular therapy (EVT) for acute ischemic stroke (AIS) are unknown.
Objective
To study whether procedural blood pressure parameters, including specific blood pressure thresholds, are associated with neurologic outcomes after EVT.
Design, Setting, and Participants
This retrospective cohort study included adults with anterior-circulation AIS who were enrolled in randomized clinical trials assessing anesthetic strategy for EVT between February 2014 and February 2017. The trials had comparable blood pressure protocols, and patients were followed up for 90 days. A total of 3630 patients were initially approached, and 3265 patients were excluded.
Exposure
Endovascular therapy.
Main Outcomes and Measures
The primary efficacy variable was functional outcome as defined by the modified Rankin Scale (mRS) score at 90 days. Associations of blood pressure parameters and time less than and greater than mean arterial blood pressure (MABP) thresholds with outcome were analyzed.
Results
Of the 365 patients included in the analysis, the mean (SD) age was 71.4 (13.0) years, 163 were women (44.6%), and the median National Institutes of Health Stroke Scale score was 17 (interquartile range [IQR], 14-21). For the entire cohort, 182 (49.9%) received general anesthesia and 183 (50.1%) received procedural sedation. A cumulated period of minimum 10 minutes with less than 70 mm Hg MABP (adjusted OR, 1.51; 95% CI, 1.02-2.22) and a continuous episode of minimum 20 minutes with less than 70 mm Hg MABP (adjusted OR, 2.30; 95% CI, 1.11-4.75) were associated with a shift toward higher 90-day mRS scores, corresponding to a number needed to harm of 10 and 4, respectively. A cumulated period of minimum 45 minutes with greater than 90 mm Hg MABP (adjusted OR, 1.49; 95% CI, 1.11-2.02) and a continuous episode of minimum 115 minutes with greater than 90 mm Hg MABP (adjusted OR, 1.89; 95% CI, 1.01-3.54) were associated with a shift toward higher 90-day mRS scores, corresponding to a number needed to harm of 10 and 6, respectively.
Conclusions and Relevance
Critical MABP thresholds and durations for poor outcome were found to be MABP less than 70 mm Hg for more than 10 minutes and MABP greater than 90 mm Hg for more than 45 minutes, both durations with a number needed to harm of 10 patients. Mean arterial blood pressure may be a modifiable therapeutic target to prevent or reduce poor functional outcome after EVT.
This cohort study investigates whether procedural blood pressure parameters, including specific blood pressure thresholds, are associated with neurological outcomes after endovascular therapy.
Introduction
Endovascular therapy (EVT) improves neurologic outcome in patients with acute ischemic stroke (AIS) owing to large vessel occlusion.1,2,3 The optimal blood pressure targets during EVT for AIS have not been defined.4,5 Blood pressure is often lower in patients who receive general anesthesia (GA) for the EVT procedure than those who receive procedural sedation (PS), a factor that is considered a contributor to the worse outcomes associated with EVT under GA.6,7,8,9,10,11 It is conceivable that hypotensive episodes less than critical thresholds and their duration may influence collateral circulation, final infarct volume, and hence functional outcome.12,13,14 Since 2016, 3 randomized clinical trials, SIESTA (Sedation vs Intubation for Endovascular Stroke Treatment), ANSTROKE (Anesthesia During Stroke), and GOLIATH (General or Local Anesthesia in Intra-Arterial Therapy), demonstrated similar neurologic outcomes after EVT with GA and PS.15,16,17 Strict hemodynamic management according to specific blood pressure protocols may be an important part of the explanation for the equivalent outcomes in the randomized trials.15,16,17
The association of blood pressure parameters and blood pressure thresholds with outcome warrants further investigation. In this study, individual blood pressure and outcome data from the 3 randomized clinical trials (SIESTA, ANSTROKE, and GOLIATH) with comparable anesthetic and hemodynamic protocols were aggregated and analyzed. The aim was to study whether procedural blood pressure parameters, including specific blood pressure thresholds, were associated with neurologic outcome after EVT.
Methods
Patients
This analysis included patients with AIS in the anterior circulation who were enrolled in the SIESTA, ANSTROKE, and GOLIATH trials between April 2014 and February 2017. The 3 trials were single-center, randomized trials that randomized patients with AIS to either GA or PS for the EVT procedure. Primary outcomes were functional outcome (SIESTA and ANSTROKE) and infarct growth (GOLIATH).15,16,17 Study designs, inclusion and exclusion criteria, and randomization have been described in detail previously (eTable 1 in the Supplement).15,16,17,18 The trials had comparable anesthetic and hemodynamic protocols (eTable 1 in the Supplement). Access to original study data resulted from a cross-institutional SIESTA, ANSTROKE, and GOLIATH Association collaboration.18 All recruited patients or their legal representatives had provided written informed consent according to each trial protocol, and all trials had been approved by their respective local ethics committee. The decision for EVT was based on local treatment protocols. Trial registrations are provided in eTable 2 in the Supplement
Blood Pressure Measurements
In the SIESTA and ANSTROKE trials, noninvasive blood pressure measurements were performed every 5 minutes during the EVT procedure.16,19 In the GOLIATH trial, invasive blood pressure variables were recorded every minute during the first 5 minutes, followed by recording of measurements for every 5 minutes throughout the procedure.20 Baseline blood pressure was defined as the last blood pressure measured before induction of GA or PS.
Neurologic Outcome and Hemodynamic Exposure Variables
Neurologic outcome as defined by the modified Rankin Score (mRS) after 90 days was assessed by an independent certified assessor blinded to randomization. The selection of hemodynamic exposure variables was mainly based on previous retrospective studies where 1 or more of the selected variables were suggested to be associated with poor outcome after EVT for AIS.8,9,10,11,21,22 The following hemodynamic parameters were defined as exposure variables:
Baseline mean arterial blood pressure (MABP) and systolic blood pressure (SBP) (millimeters of mercury).
Procedure MABP and SBP (millimeters of mercury).
A 20% drop in MABP and SBP during the EVT procedure (yes/no).
Lowest and highest MABP and SBP during the EVT procedure (mm Hg).
Any recording of SBP less than 140 mm Hg during the procedure (yes/no).
Vasopressor treatment during procedure (yes/no).
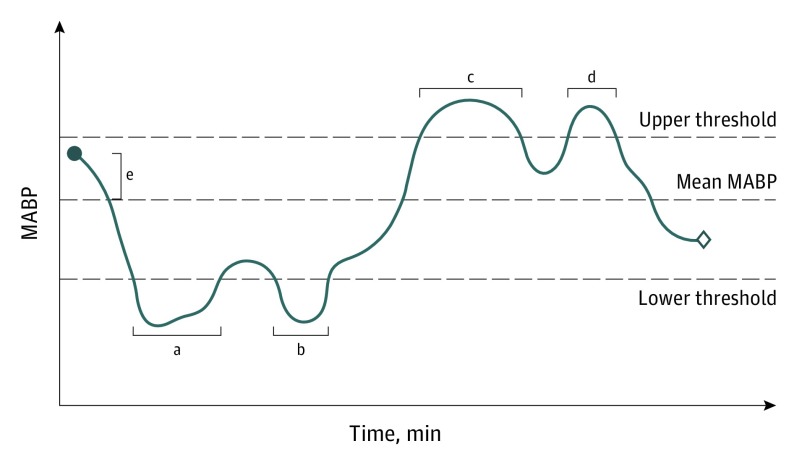
Blood pressure variability (change in MABP) calculated as the magnitude of the difference between baseline MABP and the mean of all MABP values measured during the procedure11,20 (Figure 1).
Figure 1. Schematic Presentation of Fluctuations in Mean Arterial Blood Pressure (MABP) Around Upper and Lower Thresholds.
Cumulated time (minutes) and longest continuous episode (minutes) less than and greater than specific thresholds are defined. Change in MABP (Δ MABP) is calculated as the magnitude of the difference between baseline MABP and the average of all MABP values measured during the procedure.11,19 The blue dot indicates baseline and the diamond indicates end of endovascular therapy.
aLongest continuous episode with MABP less than lower threshold.
bCombined with period A for cumulated time with MABP less than lower threshold.
cLongest continuous episode with MABP greater than upper threshold.
dCombined with period C for cumulated time with MABP greater than upper threshold.
eChange in MABP.
Definitions of Blood Pressure Thresholds
The following definitions (thresholds) were used a priori to define intraprocedural hypotension and hypertension: any recording of MABP less than 70 mm Hg, less than 80 mm Hg, or less than 90 mm Hg and greater than 90 mm Hg, greater than 100 mm Hg, or greater than 110 mm Hg, respectively (yes/no). Cumulated time (in minutes) and longest continuous episode (in minutes) with MABP less than 70 mm Hg, less than 80 mm Hg, or less than 90 mm Hg and greater than 90 mm Hg, greater than 100 mm Hg, or greater than 110 mm Hg during the procedure were calculated (Figure 1).
Statistical Analysis
Missing blood pressure measurements during the procedure were imputed using single-imputation linear regression on the first subsequent and the last preceding nonmissing measurements following the course of time. Hence, if a single measurement was missing between 2 nonmissing blood pressure measurements, then the missing measurement was imputed as the mean of the 2 nonmissing values. For multiple consecutive missing measurements, we assigned values such that all imputed values were on a straight line between the 2 nonmissing values.
Initially, we compared the original randomized groups on patient covariates and greater than defined blood pressure variables with t test for continuous variables and Fisher exact test for factor variables. For the primary analysis, we used a mixed-effect ordered logistic regression model with a random intercept on a group variable indicating center. The entire distribution of the mRS (0-6 points) was considered as outcome. To avoid overfitting, we put a random intercept on type of anesthesia as well. Further adjustment was performed for the following patient covariates: age, sex, neurologic deficit measured by National Institutes of Health Stroke Scale, prestroke disability (mRS score), early brain ischemic change extent (Alberta Stroke Program Early Computed Tomography Score [ASPECTS]), location of occlusion, and treatment with intravenous alteplase (yes or no). An adjusted odds ratio (OR) greater than 1 constituted a change in a blood pressure variable associated with a shift on the mRS toward a worse 90-day outcome. The analysis was conducted separately for each of these defined exposures. In addition, we fitted a similar model implementing restricted cubic splines for each of the variables describing maximum and cumulative time less than or greater than a given MABP threshold, using patients with zero minutes less than or greater than the blood pressure threshold as the reference group. To further quantify the potential association of the BP thresholds with outcome, we estimated the number needed to harm (NNH) for each of the points in this analysis, using the proportions of patients in the reference group with an mRS greater than 2 at 90 days’ follow-up. The NNH analysis indicates the number of patients with blood pressure less than/greater than the stated blood pressure threshold for the given continuous and cumulative time for 1 patient to have a poor clinical outcome. Because reperfusion is a main predictor of functional outcome, a sensitivity analysis was performed on the subgroup of reperfused patients. Blood pressure data obtained in the period from arrival at the neurointerventional suite to reperfusion were used for this analysis. Baseline blood pressure was defined as the first blood pressure measurement obtained in the neurointerventional suite. An additional sensitivity analysis was performed on patients stratified according to randomization (GA vs PS). Again, the analyses were conducted for each of the defined exposures separately and adjusted for the same covariates as in the primary analyses. Because of the exploratory nature of the study as well as the high association between the exposure variables, it was decided not to conduct multiple comparison analyses. All analyses were conducted in Stata, version 15 (StataCorp LLC). A P value less than .05 was considered statistically significant, and all P values were 2-sided.
Results
Missing Data, Demographics, Clinical Characteristics, and Time Metrics
A total of 368 patients were enrolled in the 3 trials. All blood pressure measurements were missing for a total of 3 patients. These patients were excluded entirely from all analyses, leaving 365 patients for further investigation. Of these, 182 patients (49.9%) were randomized to GA and 183 patients (50.1%) to PS. For the entire cohort, the mean (SD) age was 71.4 (13.0) years, 163 were women (44.6%), and the median National Institutes of Health Stroke Scale score was 17 (interquartile range [IQR], 14-21).
The study population included 8065 blood pressure measurements at 5-minute intervals. Of these, 70 measurements (0.9%) were imputed. The distribution of demographic and clinical characteristics was balanced between the 2 groups (Table 1). The median time intervals from symptom onset to groin puncture and from arrival at angiosuite to groin puncture were significantly shorter in the PS group (Table 1).
Table 1. Baseline Demographic, Clinical Characteristics, and Time Metrics Stratified According to Randomization (General Anesthesia vs Procedural Sedation).
| Characteristic | No. (%) | |
|---|---|---|
| General Anesthesia (n = 182) | Procedural Sedation (n = 183) | |
| Demographic characteristics | ||
| Age, mean (SD), y | 71.4 (12.1) | 71.4 (13.8) |
| Sex | ||
| Female | 73 (40.1) | 90 (49.2) |
| Male | 109 (59.9) | 93 (50.8) |
| Patients included from each study | ||
| SIESTA | 73 (40.1) | 77 (42.1) |
| ANSTROKE | 44 (24.2) | 43 (23.5) |
| GOLIATH | 65 (35.7) | 63 (34.4) |
| Vascular risk factors | ||
| Hypertension | 118 (64.8) | 107 (58.8) |
| Atrial fibrillation | 77 (42.3) | 80 (44.0) |
| Hyperlipidemia | 72 (39.8) | 74 (40.4) |
| Diabetes mellitus | 35 (19.2) | 32 (17.6) |
| Smoking | 33 (18.1) | 41 (22.8) |
| Pretreatment imaging | ||
| ASPECTSa | ||
| 6-10 | 162 (89.0) | 157 (87.2) |
| <6 | 20 (10.9) | 23 (12.8) |
| Median (IQR) | 8 (7-10) | 8 (6-10) |
| Scores on admission | ||
| Premorbid mRSb | ||
| 0 | 132 (72.5) | 130 (71.0) |
| 1 | 23 (12.6) | 30 (16.4) |
| 2 | 15 (8.2) | 16 (8.7) |
| >2 | 12 (6.6) | 7 (3.8) |
| NIHSS on admission, median (IQR)c | 18 (14-21) | 17 (14-21) |
| Occlusion | ||
| Localization of occlusion | ||
| Single ICA | 8 (4.4) | 12 (6.6) |
| Single ICA-T4 | 27 (14.8) | 32 (17.4) |
| Single MCA | 101 (55.4) | 113 (61.7) |
| M1 | 80 (44.3) | 95 (51.9) |
| M2 | 21 (11.5) | 18 (9.8) |
| Tandem | 46 (25.3) | 26 (14.2) |
| ICA + ICA-Td | 15 (8.2) | 7 (3.8) |
| ICA + M1 | 29 (15.9) | 13 (7.1) |
| ICA + M2 | 2 (1.1) | 6 (3.3) |
| Occlusion side right | 73 (40.1) | 92 (50.3) |
| Reperfusion treatments (%) | ||
| Premechanical thrombectomy IV tPA | 128 (70.3) | 130 (71.0) |
| Successful reperfusion (mTICI 2b-3) | 155 (85.2) | 138 (75.4) |
| Time-related parameters, median (IQR), min | ||
| Symptom onset-to-groin puncture | 180 (136-255) | 170 (133-240) |
| Symptom onset-to-reperfusion | 237 (199-347) | 261 (203-325) |
| Door-to-reperfusion | 150 (113-200) | 166 (120-208) |
| Door-to-groin puncture | 75 (60.0-90.0) | 70 (54.0-90.0)e |
| Groin puncture-to-reperfusion | 51 (31-90) | 72 (34-105)e |
| Angio suite arrival-to-groin puncture | 23 (14-29) | 15 (10-25)f |
| Duration of intervention | 158 (82-232) | 160 (97-225) |
Abbreviations: ANSTROKE, Anesthesia During Stroke; ASPECTS, Alberta Stroke Program Early Computed Tomography Score; GOLIATH, General or Local Anesthesia in Intra-Arterial Therapy; ICA, internal carotid artery; IQR, interquartile range; IV tPA, intravenous thrombolysis; MCA, middle cerebral artery; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; SIESTA, Sedation vs Intubation for Endovascular Stroke Treatment.
The Alberta Stroke Program Early Computed Tomography Score is a measure of the extension of stroke. Score ranges from 0 to 10, higher scores indicating fewer early ischemic changes.
The mRS scores run between 0 and 6; 0 means no symptoms, 1, no clinically relevant disability; 2, slight disability (able to look after own affairs without assistance, but not to full extent); 3, moderate disability (requires some help, but able to walk unassisted); 4, moderately severe disability (requires assistance, and unable to walk unassisted); 5, severe disability (requires constant nursing care); 6, dead.
The NIHSS classifies neurologic deficit from 0 (no deficit) to 42 (most severe deficit).
ICA-T was defined as occlusion of the internal carotid artery less than the bifurcation of the middle and the anterior cerebral artery.
P < .05.
P < .001.
Comparison of Stratified Hemodynamic Variables
Procedural MABP values in the GA and PS groups for the combined population and for each trial are shown in the eFigure in the Supplement. Baseline MABP and SBP were similar between the groups (Table 2). Procedure MABP was lower in the GA group (Table 2; eFigure in the Supplement). Variables associated with hypotension were more pronounced and frequent in the GA group and included minimum MABP during procedure, number of patients with a 20% drop in MABP, number of patients with a 20% drop in SBP, number of patients with any recording of SBP less than 140 mm Hg, MABP less than 90 mm Hg, MABP less than 80 mm Hg, MABP less than 70 mm Hg, duration of longest continuous episode with MABP less than 70 mm Hg, and use of vasopressors (Table 2). The number of patients with MABP greater than 90 mm Hg was higher in the GA group, and longest continuous episode with MABP greater than 110 mm Hg was longer in the PS group.
Table 2. Comparison of Hemodynamic Variables Stratified According to Randomization.
| Hemodynamic Variables | Mean (SD) | P Value | |
|---|---|---|---|
| General Anesthesia (n = 182) | Procedural Sedation (n = 183) | ||
| Baseline values, mm Hg | |||
| SBP | 162 (25) | 165 (27) | .31 |
| MABP | 111 (16) | 113 (18) | .49 |
| Periprocedural variables, mm Hg | |||
| MABP during procedure | 96 (10) | 100 (11) | .01 |
| SBP during procedure | 143 (15) | 149 (17) | .15 |
| Minimum MABP during procedure | 76 (14) | 86 (15) | .04 |
| Maximum MABP during procedure | 120 (15) | 119 (16) | .83 |
| Minimum SBP during procedure | 111 (21) | 126 (23) | .10 |
| Maximum SBP during procedure | 178 (21) | 176 (23) | .38 |
| No. of patients and time less than specified MABP and SBP thresholds during procedure | |||
| 20% Drop in MABP during procedure, No. (%) | 143 (79) | 93 (51) | <.001 |
| 20% Drop in SBP during procedure, No. (%) | 140 (77) | 90 (49) | <.001 |
| Patients with systolic blood pressure less than 140 mm Hg, No. (%) | 167 (92) | 132 (72) | <.001 |
| Patients with MABP <90 mm Hg, No. (%) | 148 (81) | 106 (58) | <.001 |
| Cumulated time with MABP <90 mm Hg, min | 35 (37) | 24 (32) | .11 |
| Longest continuous episode with MABP <90 mm Hg, min | 24 (27) | 17 (24) | .05 |
| Patients with MABP <80 mm Hg, No. (%) | 109 (60) | 56 (31) | <.001 |
| Cumulated time with MABP <80 mm Hg, min | 15 (20) | 9 (18) | .12 |
| Longest continuous episode with MABP 80 mm Hg, min | 11 (13) | 6 (13) | .06 |
| Patients with MABP <70 mm Hg, No. (%) | 58 (32) | 26 (14) | <.001 |
| Cumulated time with MABP <70 mm Hg, min | 5 (10) | 2 (8) | .07 |
| Longest continuous episode with MABP <70 mm Hg, min | 4 (6) | 2 (6) | .046 |
| No. of patients and time greater than specified MABP thresholds during procedure | |||
| Patients with MABP >90 mm Hg, No. (%) | 182 (100) | 177 (97) | .030 |
| Cumulated time with MABP >90 mm Hg, min | 75 (51) | 87 (62) | .33 |
| Longest continuous episode with MABP >90 mm Hg, min | 58 (47) | 71 (57) | .21 |
| Patients with MABP >100 mm Hg, No. (%) | 169 (93) | 165 (90) | .45 |
| Cumulated time with MABP >100 mm Hg, min | 43 (46) | 58 (56) | .14 |
| Longest continuous episode with MABP >100 mm Hg, min | 29 (38) | 40 (45) | .12 |
| Patients with MABP >110 mm Hg | 124 (68) | 129 (70) | .65 |
| Cumulated time with MABP >110 mm Hg, min | 18 (28) | 27 (38) | .06 |
| Longest continuous episode with MABP >110 mm Hg, min | 11 (19) | 17 (25) | .005 |
| Blood pressure variability and use of vasopressors | |||
| Δ MABP, mm Hga | 17.4 (12.1) | 14.8 (12.9) | .39 |
| Use of vasopressors, No. (%) | 180 (99) | 108 (59) | <.001 |
Abbreviations: EVT, endovascular therapy; Δ MABP, change in MABP; MABP, mean arterial blood pressure; SBP, systolic blood pressure.
Δ MABP was calculated as the difference between baseline MABP and the mean of all MABP values measured during the procedure. Cumulated time less than or greater than a given threshold is defined as the total cumulative duration of minutes less than or greater than the threshold during the EVT procedure. Longest continuous episode is defined as the longest continuous period in minutes less than or greater than a given threshold during the EVT procedure.
Hemodynamic Parameters and Neurologic Outcomes
Univariate hemodynamic parameters associated with an adjusted OR for a shift toward higher 90-day mRS scores were minimum MABP during procedure (adjusted OR, 0.83; 95% CI, 0.72-0.96; P = .01) and minimum SBP during procedure (adjusted OR, 0.88; 95% CI, 0.80-0.96; P = .004) (the OR less than 1 in these cases indicates that the higher minimum MABP/SBP, the more favorable outcomes and vice versa), maximum MABP during procedure (adjusted OR, 1.20; 95% CI, 1.05-1.36; P = .006), greater than 20% reduction in MABP during procedure (adjusted OR, 1.65; 95% CI, 1.08-2.5; P = .02), greater than 20% reduction in SBP during procedure (adjusted OR, 1.80; 95% CI, 1.17-2.75; P = .007), and change in MABP (adjusted OR, 1.23; 95% CI, 1.06-1.43; P = .005) (Table 3).
Table 3. Association Between Hemodynamic Parameters and Neurologic Outcome in the Patients With Acute Ischemic Stroke Undergoing Endovascular Therapya.
| Hemodynamic Variable | Adjusted OR (95% CI) for Higher mRS Score (0-6 Points) | P Value |
|---|---|---|
| Baseline variables (per 10 mm Hg) | ||
| SBP | 1.03 (0.97-1.12) | .29 |
| MABP | 1.10 (0.99-1.23) | .07 |
| Periprocedural variables (per 10 mm Hg) | ||
| MABP during procedure | 1.0 (0.83-1.20) | .99 |
| SBP during procedure | 0.97 (0.86-1.09) | .59 |
| Minimum MABP during procedure | 0.83 (0.72-0.96) | .01 |
| Maximum MABP during procedure | 1.20 (1.05-1.36) | .006 |
| Minimum SBP during procedure | 0.88 (0.80-0.96) | .004 |
| Maximum SBP during procedure | 1.07 (0.98-1.17) | .13 |
| Lower MABP and SBP threshold variables | ||
| >20% Reduction in MABP during procedure | 1.65 (1.08-2.5) | .02 |
| >20% Drop in SBP during procedure | 1.80 (1.17-2.75) | .007 |
| SBP <140 mm Hg vs no | 1.66 (0.99-2.79) | .05 |
| MABP <90 mm Hg vs no | 1.26 (0.82-1.94) | .29 |
| Longest continuous episode with MABP <90 mm Hg (per 10 min change) | 1.04 (0.96-1.12) | .37 |
| Cumulated time with MABP <90 mm Hg (per 10 min change) | 1.05 (0.99-1.12) | .09 |
| MABP <80 mm Hg vs no | 1.52 (1.01-2.28) | .04 |
| Longest continuous episode with MABP <80 mm Hg (per 10 min change) | 1.01 (0.87-1.17) | .90 |
| Cumulated time with MABP <80 mm Hg (per 10 min change) | 1.05 (0.94-1.16) | .39 |
| MABP <70 mm Hg vs no | 1.81 (1.12-2.90) | .02 |
| Longest continuous episode with MABP <70 mm Hg (per 10 min change) | 1.62 (1.15-2.27) | .005 |
| Cumulated time with MABP <70 mm Hg (per 10 min change) | 1.30 (1.03-1.65) | .03 |
| Upper MABP threshold variables | ||
| MABP >90 mm Hg vs no | 1.41 (0.31-6.54) | .66 |
| Longest continuous episode with MABP >90 mm Hg (per 10 min change) | 1.05 (1.01-1.09) | .007 |
| Cumulated time with MABP >90 mm Hg (per 10 min change) | 1.08 (1.04-1.11) | <.001 |
| MABP >100 mm Hg vs no | 0.92 (0.48-1.79) | .82 |
| Longest continuous episode with MABP >100 mm Hg (per 10 min change) | 1.05 (1.00-1.10) | .03 |
| Cumulated time with MABP >100 mm Hg (per 10 min change) | 1.06 (1.02-1.11) | .002 |
| MABP >110 mm Hg vs no | 1.20 (0.78-1.82) | .41 |
| Longest continuous episode with MABP >110 mm Hg (per 10 min change) | 1.08 (0.99-1.18) | .09 |
| Cumulated time with MABP >110 mm Hg (per 10 min change) | 1.06 (0.99-1.12) | .05 |
| Blood pressure variability variables and vasopressors | ||
| Δ MABP per 10 mm Hgb | 1.23 (1.06-1.43) | .005 |
| Use of vasopressors vs no | 1.55 (0.91-2.65) | .11 |
Abbreviations: EVT, endovascular therapy; Δ MABP, change in MABP; MABP, mean arterial blood pressure; mRS, modified Rankin Scale; OR, odds ratio; SBP, systolic blood pressure.
Results presented for the entire study population. Ordered logistic regression was performed across the entire ordinal mRS score (0-6) to compute adjusted ORs for any increase in mRS.
Δ MABP was calculated as the difference between baseline MABP and the mean of all MABP values measured during the procedure. Cumulated time less than or greater than a given threshold is defined as the total cumulative duration of minutes less than or greater than the threshold during the EVT procedure. Longest continuous episode is defined as the longest continuous period in minutes less than or greater than a given threshold during the EVT procedure.
Lower Thresholds
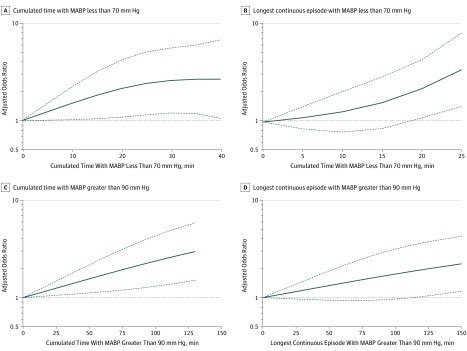
Blood pressure thresholds associated with a shift toward higher 90-day mRS scores were MABP less than 80 mm Hg (adjusted OR, 1.52; 95% CI, 1.01-2.28; P = .04) and MABP less than 70 mm Hg (adjusted OR, 1.81; 95% CI, 1.12-2.90; P = .02). For every 10 minutes of cumulated time with MABP less than 70 mm Hg, there was a 30% increase in odds (adjusted OR, 1.30; 95% CI, 1.03-1.65; P = .03) of shifting toward worse outcomes on the mRS at 90 days. A cumulated time of minimum 5 minutes less than 70 mm Hg was the shortest period associated with a statistically significant shift toward higher 90-day mRS scores (adjusted OR, 1.23; 95% CI, 1.01-1.49), which corresponds to an NNH of 19 (ie, to harm 1 patient, 19 patients are needed with procedural MABP less than 70 mm Hg for a cumulative time of minimum 5 minutes). At cumulated periods of minimum 10, 15, and 20 minutes less than 70 mm Hg MABP, the adjusted ORs increased to 1.51 (95% CI, 1.02-2.22), 1.82 (95% CI, 1.04-3.17), and 2.14 (95% CI, 1.08-4.22), corresponding to an NNH of 10, 7, and 6, respectively (Figure 2A).
Figure 2. Spline Plots of Cumulated Time (Minutes) and Longest Continuous Episode (Minutes) Less Than Lower and Greater Than Upper Mean Arterial Blood Pressure (MABP) Thresholds vs Adjusted Odds Ratio (OR).
A, Cumulated time with MABP less than 70 mm Hg vs adjusted OR (95% CI) for shift toward worse 90-day outcome. B, Longest continuous episode with MABP less than 70 mm Hg vs adjusted OR (95% CI) for a shift toward worse 90-day outcome. C, Cumulated time with MABP greater than 90 mm Hg vs adjusted OR (95% CI) for a shift toward worse 90-day outcome. D, Longest continuous episode with MABP over 90 mm Hg vs adjusted OR (95% CI) toward worse 90-day outcome.
For every continuous 10 minutes with less than an MABP of 70 mm Hg, there was a 62% increase in odds (adjusted OR, 1.62; 95% CI, 1.15-2.27; P = .005) of shifting toward worse outcomes on the mRS at 90 days. A continuous episode of minimum 20 minutes with less than 70 mm Hg MABP was associated with a shift toward higher 90-day mRS scores (adjusted OR, 2.30; 95% CI, 1.11-4.75) (Figure 2B), which corresponds to an NNH of 4 patients. However, at 15 minutes, the OR was reduced to a statistically insignificant value of 1.62 (95% CI, 0.85-3.10) (Figure 2B).
Upper Thresholds
For every 10 minutes of cumulated time with MABP greater than 90 mm Hg, there was an 8% increase in odds (adjusted OR, 1.08; 95% CI, 1.04-1.11; P <.001) of shifting toward worse outcomes on the mRS at 90 days. Figure 2C shows statistically significant harm for all cumulative times greater than an MABP of 90 mm Hg. The NNH increased as the cumulative time approached zero, with an NNH of 82 and 135 patients for cumulative times of 10 and 5 minutes greater than 90 mm Hg, respectively. At cumulated time of minimum 80 minutes greater than 90 mm Hg, MABP was associated with a shift to higher mRS scores (adjusted OR, 2.02; 95% CI, 1.22-3.59), which corresponds to an NNH of 6 patients (Figure 2C). At a cumulated time of minimum 45 minutes, the adjusted OR was reduced to a statistically significant value of 1.49 (95% CI, 1.11-2.02) corresponding to an NNH of 10 (Figure 2C).
For every continuous 10 minutes greater than an MABP of 90 mm Hg, there was an 8% increase in odds of shifting toward worse outcomes on the mRS at 90 days. A continuous episode of minimum 115 minutes greater than 90 mm Hg MABP was associated with a shift toward higher 90-day mRS scores (adjusted OR, 1.89; 95% CI, 1.01-3.54), which corresponds to an NNH of 6 patients (Figure 2D).
Sensitivity Analyses
Hemodynamic parameters associated with a shift toward higher 90-day mRS scores in the subgroup of reperfused patients and not identified in the combined analysis included SBP less than 140 mm Hg and use of vasopressors (eTable 3 in the Supplement). In the reperfused patients, durations for worse outcome less than or greater than the prespecified thresholds had similar or less statistical significance (eResults in the Supplement). There was no significant association between hemodynamic variables and neurologic outcomes when patients were stratified according to randomization (GA vs PS) (eTable 4 in the Supplement).
Discussion
Both variables indicating procedural hypotension and hypertension were associated with worse 90-day functional outcome. Critical MABP thresholds and durations for poor outcome were found to be MABP less than 70 mm Hg for more than 10 minutes and MABP greater than 90 mm Hg for more than 45 minutes. Both durations were associated with an NNH of 10 patients. During EVT, hypotension may occur as a result of administration of anesthetic drugs, which typically exhibits a dose-dependent effect on vascular tone and depression of cardiovascular function.19 Infarct progression and negative effect on collateral circulation have been suggested as potential mechanisms of hypotension, leading to worse outcomes.12,13
As expected, MABP was lower in the GA group. This finding is in agreement with studies that have suggested that low blood pressure and blood pressure variability during the EVT procedure are responsible for the negative outcomes reported with GA.8,9,10,11,12 However, these risks are not associated with GA alone. During PS for EVT, hemodynamic intervention was reported to be required in more than one-third of patients and a more than 10% reduction in blood pressure prior to recanalization was predictive of poor outcome.22,23 Collectively, these findings suggest that worse outcome after EVT is associated with changes in blood pressure rather than with the anesthetic procedure itself. This observation may also partly explain the neutral findings of GA vs PS in the randomized clinical trials, which all had rigorous blood pressure protocols.15,16,17 Isolated analyses of hemodynamic data from the GOLIATH and SIESTA trials could not demonstrate an association between blood pressure variables and neurologic outcome.19,20 This discrepancy is possibly explained by the difference in sample sizes between the aggregated cohort in this study and the small randomized trials. The lack of significant associations in the stratified analysis according to randomization (GA vs PS) further supports the suggestion of low sample size as the main explanation.
We identified maximum procedure MABP, but not maximum procedure SBP, to be associated with poor outcome. To our knowledge, this is the first report that has suggested that high blood pressure during EVT is potentially harmful. This finding is supported by a stroke registry study24 where high poststroke blood pressure was associated with unfavorable clinical outcomes and studies24,25,26 and where SBP greater than thresholds of approximately 120 mm Hg and 130 mm Hg at the time of stroke onset was associated with worse outcomes. Further, a U-shaped association has been shown to exist between blood pressure and outcome in patients with AIS.24,27
Our findings further suggest that MABP is more sensitive than SBP in the assessment of hypertension and hypotension. Cerebral perfusion pressure, defined as the difference between MABP and intracranial pressure, is considered the physiologic driving force behind cerebral blood flow.28 Furthermore, MABP is a combination of systolic and diastolic blood pressures and is considered a more valid index of tissue perfusion.29 We hypothesize that an MABP threshold is a more appropriate indicator of hypertension and hypotension during EVT for AIS.
Despite the possible importance of maintaining SBP within narrow limits, guidelines do not provide specific procedural blood pressure targets or define the duration of hypotension or hypertension that is potentially harmful.5,30 The guidelines only recommend maintaining SBP at less than 180 mm Hg.5,30 This is similar to an expert consensus recommending a target SBP between 140 and 180 mm Hg, which is based on weak evidence.31 However, these suggestions are not based on studies involving patients with AIS undergoing EVT. This study identified MABP less than 70 mm Hg for a cumulated time of more than 10 minutes and MABP greater than 90 mm Hg for a cumulated time more than 45 minutes to be potentially harmful, with an NNH of 10 patients. A longer period outside these thresholds was associated with a lower NNH and suggests a dose-response association between MABP and outcome. We also identified that a single sustained episode of 20 minutes less than 70 mm Hg MABP was associated with poor outcome. The lower tolerance toward cumulated time less than an MABP of 70 mm Hg, compared with a single sustained episode of MABP less than 70 mm Hg, further suggests that fluctuations in MABP less than and greater than a certain threshold is potentially more detrimental than a sustained episode with stable blood pressure less than a similar threshold. This is supported by a study where change in MABP, which is indicative of blood pressure variability, was associated with worse outcome after EVT.12
In the subgroup of reperfused patients, the significant associations between hemodynamic parameters and outcome were very similar to the analysis of the combined population. Further, critical MABP thresholds and durations for poor outcome in patients who achieved full reperfusion were similar or had less statistical significance, which is possibly a result of reduced power in the subgroup analysis. Overall, these findings support the assumption that the associations of blood pressure and durations less than and greater than the thresholds are independent of reperfusion status. This hypothesis is supported by a 2019 retrospective study12 that reported that the association between decreases in blood pressure during EVT and worse functional outcome was independent of reperfusion status.
This study suggests maintaining MABP within fixed lower and upper MABP thresholds; however, drops in both MABP and SBP greater than 20%, relative to baseline, were associated with worse outcome and suggest that an individualized approach to blood pressure management may be preferable. Randomized studies are needed to determine the optimal blood pressure management strategy during EVT.
Limitations
This study has a number of limitations. First, although the analysis is based on prospectively collected data, it remains a retrospective analysis with its natural inherent shortcomings. A selected group of patients were initially randomized according to anesthesia method and not to hemodynamic management, ie, confounding and selection bias cannot be excluded despite the multivariable analyses. In this study baseline, blood pressure was defined as the last blood pressure before induction of GA or PS. Consequently, hypotensive and hypertensive episodes including blood pressure interventions prior to EVT were not taken into account and may have influenced infarct progression and functional outcome. Blood pressure was mostly measured every 5 minutes during the procedures. It is possible that some patients experienced reductions or increases in blood pressure within the 5-minute intervals, which may have influenced their 90-day outcomes. Finally, blood pressure may have a different effect depending on the phase of procedure (eg, before vs after recanalization) that were not accounted for in this analysis.
Conclusions
Our findings suggest that both hypotension and hypertension during EVT for AIS are associated with poor functional outcome. Critical MABP thresholds and durations for poor outcome were found to be MABP less than 70 mm Hg for more than 10 minutes and MABP greater than 90 mm Hg for more than 45 minutes, both durations with an NNH of 10 patients. These results suggest MABP may be a modifiable therapeutic target to prevent or reduce poor functional outcome in patients undergoing EVT for AIS and that MABP should possibly be maintained within such narrow limits. To determine the optimal blood pressure management strategy during EVT, these results provide a rationale for future randomized studies.
eFigure. Procedural MABP values in the GA and PS groups for the combined population and for each trial
eTable 1. Trial-specific information of the SIESTA, ANSTROKE and GOLIATH trials
eTable 2. Trial registrations
eTable 3. Association of hemodynamic parameters and neurological outcome in the subgroup of reperfused patients
eResults. Association of time below and above blood pressure thresholds with outcome in the subgroup of reperfused patients
eTable 4. Association of hemodynamic parameters and neurological outcome, stratified according to randomization (GA vs PS)
References
- 1.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 2.Albers GW, Marks MP, Kemp S, et al. ; DEFUSE 3 Investigators . Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. doi: 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 4.Maïer B, Fahed R, Khoury N, et al. Association of blood pressure during thrombectomy for acute ischemic stroke with functional outcome: a systematic review. Stroke. 2019;50(10):2805-2812. doi: 10.1161/STROKEAHA.119.024915 [DOI] [PubMed] [Google Scholar]
- 5.Powers WJ, Rabinstein AA, Ackerson T, et al. ; American Heart Association Stroke Council . 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2018;49(3):e46-e110. doi: 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 6.Berkhemer OA, van den Berg LA, Fransen PSS, et al. ; MR CLEAN investigators . The effect of anesthetic management during intra-arterial therapy for acute stroke in MR CLEAN. Neurology. 2016;87(7):656-664. doi: 10.1212/WNL.0000000000002976 [DOI] [PubMed] [Google Scholar]
- 7.Campbell BCV, van Zwam WH, Goyal M, et al. ; HERMES collaborators . Effect of general anaesthesia on functional outcome in patients with anterior circulation ischaemic stroke having endovascular thrombectomy versus standard care: a meta-analysis of individual patient data. Lancet Neurol. 2018;17(1):47-53. doi: 10.1016/S1474-4422(17)30407-6 [DOI] [PubMed] [Google Scholar]
- 8.Davis MJ, Menon BK, Baghirzada LB, et al. ; Calgary Stroke Program . Anesthetic management and outcome in patients during endovascular therapy for acute stroke. Anesthesiology. 2012;116(2):396-405. doi: 10.1097/ALN.0b013e318242a5d2 [DOI] [PubMed] [Google Scholar]
- 9.Löwhagen Hendén P, Rentzos A, Karlsson J-E, et al. Hypotension during endovascular treatment of ischemic stroke is a risk factor for poor neurological outcome. Stroke. 2015;46(9):2678-2680. doi: 10.1161/STROKEAHA.115.009808 [DOI] [PubMed] [Google Scholar]
- 10.Jagani M, Brinjikji W, Rabinstein AA, Pasternak JJ, Kallmes DF. Hemodynamics during anesthesia for intra-arterial therapy of acute ischemic stroke. J Neurointerv Surg. 2016;8(9):883-888. doi: 10.1136/neurintsurg-2015-011867 [DOI] [PubMed] [Google Scholar]
- 11.Treurniet KM, Berkhemer OA, Immink RV, et al. ; MR CLEAN investigators . A decrease in blood pressure is associated with unfavorable outcome in patients undergoing thrombectomy under general anesthesia. J Neurointerv Surg. 2018;10(2):107-111. doi: 10.1136/neurintsurg-2017-012988 [DOI] [PubMed] [Google Scholar]
- 12.Petersen NH, Ortega-Gutierrez S, Wang A, et al. Decreases in blood pressure during thrombectomy are associated with larger infarct volumes and worse functional outcome. Stroke. 2019;50(7):1797-1804. doi: 10.1161/STROKEAHA.118.024286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raychev R, Liebeskind DS, Yoo AJ, et al. Physiologic predictors of collateral circulation and infarct growth during anesthesia: detailed analyses of the GOLIATH trial. J Cereb Blood Flow Metab. 2019:X19865219. doi: 10.1177/0271678X19865219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vitt JR, Trillanes M, Hemphill JC III. Management of blood pressure during and after recanalization therapy for acute ischemic stroke. Front Neurol. 2019;10:138. doi: 10.3389/fneur.2019.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schönenberger S, Uhlmann L, Hacke W, et al. Effect of conscious sedation vs general anesthesia on early neurological improvement among patients with ischemic stroke undergoing endovascular thrombectomy: a randomized clinical trial. JAMA. 2016;316(19):1986-1996. doi: 10.1001/jama.2016.16623 [DOI] [PubMed] [Google Scholar]
- 16.Löwhagen Hendén P, Rentzos A, Karlsson J-E, et al. General anesthesia versus conscious sedation for endovascular treatment of acute ischemic stroke: the anstroke trial (anesthesia during stroke). Stroke. 2017;48(6):1601-1607. doi: 10.1161/STROKEAHA.117.016554 [DOI] [PubMed] [Google Scholar]
- 17.Simonsen CZ, Yoo AJ, Sørensen LH, et al. Effect of general anesthesia and conscious sedation during endovascular therapy on infarct growth and clinical outcomes in acute ischemic stroke: a randomized clinical trial. JAMA Neurol. 2018;75(4):470-477. doi: 10.1001/jamaneurol.2017.4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schönenberger S, Hendén PL, Simonsen CZ, et al. Association of general anesthesia vs. procedural sedation with functional outcome among patients with acute ischemic stroke undergoing thrombectomy: a systematic review and meta-analysis. JAMA. 2019;322(13):1283-1293. doi: 10.1001/jama.2019.11455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schönenberger S, Uhlmann L, Ungerer M, et al. Association of blood pressure with short- and long-term functional outcome after stroke thrombectomy: post hoc analysis of the SIESTA trial. Stroke. 2018;49(6):1451-1456. doi: 10.1161/STROKEAHA.117.019709 [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen M, Espelund US, Juul N, et al. The influence of blood pressure management on neurological outcome in endovascular therapy for acute ischaemic stroke. Br J Anaesth. 2018;120(6):1287-1294. doi: 10.1016/j.bja.2018.01.039 [DOI] [PubMed] [Google Scholar]
- 21.Whalin MK, Lopian S, Wyatt K, et al. Dexmedetomidine: a safe alternative to general anesthesia for endovascular stroke treatment. J Neurointerv Surg. 2014;6(4):270-275. doi: 10.1136/neurintsurg-2013-010773 [DOI] [PubMed] [Google Scholar]
- 22.Whalin MK, Halenda KM, Haussen DC, et al. Even small decreases in blood pressure during conscious sedation affect clinical outcome after stroke thrombectomy: an analysis of hemodynamic thresholds. AJNR Am J Neuroradiol. 2017;38(2):294-298. doi: 10.3174/ajnr.A4992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alcaraz G, Chui J, Schaafsma J, et al. Hemodynamic management of patients during endovascular treatment of acute ischemic stroke under conscious sedation: a retrospective cohort study. J Neurosurg Anesthesiol. 2019;31(3):299-305. doi: 10.1097/ANA.0000000000000514 [DOI] [PubMed] [Google Scholar]
- 24.Bangalore S, Schwamm L, Smith EE, et al. ; Get With the Guidelines-Stroke Steering Committee and Investigators . Blood pressure and in-hospital outcomes in patients presenting with ischaemic stroke. Eur Heart J. 2017;38(37):2827-2835. doi: 10.1093/eurheartj/ehx330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulder MJHL, Ergezen S, Lingsma HF, et al. ; Multicenter Randomized Clinical Trial of Endovascular Treatment of Acute Ischemic Stroke in the Netherlands (MR CLEAN) Investigators . Baseline blood pressure effect on the benefit and safety of intra-arterial treatment in MR CLEAN (Multicenter Randomized Clinical Trial of Endovascular Treatment of Acute Ischemic Stroke in the Netherlands). Stroke. 2017;48(7):1869-1876. doi: 10.1161/STROKEAHA.116.016225 [DOI] [PubMed] [Google Scholar]
- 26.Ishitsuka K, Kamouchi M, Hata J, et al. ; FSR Investigators . High blood pressure after acute ischemic stroke is associated with poor clinical outcomes: Fukuoka Stroke Registry. Hypertension. 2014;63(1):54-60. doi: 10.1161/HYPERTENSIONAHA.113.02189 [DOI] [PubMed] [Google Scholar]
- 27.Leonardi-Bee J, Bath PMW, Phillips SJ, Sandercock PAG; IST Collaborative Group . Blood pressure and clinical outcomes in the International Stroke Trial. Stroke. 2002;33(5):1315-1320. doi: 10.1161/01.STR.0000014509.11540.66 [DOI] [PubMed] [Google Scholar]
- 28.Markus HS. Cerebral perfusion and stroke. J Neurol Neurosurg Psychiatry. 2004;75(3):353-361. doi: 10.1136/jnnp.2003.025825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henry JB, Miller MC, Kelly KC, Champney D. Mean arterial pressure (MAP): an alternative and preferable measurement to systolic blood pressure (SBP) in patients for hypotension detection during hemapheresis. J Clin Apher. 2002;17(2):55-64. doi: 10.1002/jca.10022 [DOI] [PubMed] [Google Scholar]
- 30.Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO): European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg. 2019;11(6):535-538. doi: 10.1136/neurintsurg-2018-014568 [DOI] [PubMed] [Google Scholar]
- 31.Talke PO, Sharma D, Heyer EJ, Bergese SD, Blackham KA, Stevens RD. Republished: society for neuroscience in anesthesiology and critical care expert consensus statement: anesthetic management of endovascular treatment for acute ischemic stroke. Stroke. 2014;45(8):e138-e150. doi: 10.1161/STROKEAHA.113.003412 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Procedural MABP values in the GA and PS groups for the combined population and for each trial
eTable 1. Trial-specific information of the SIESTA, ANSTROKE and GOLIATH trials
eTable 2. Trial registrations
eTable 3. Association of hemodynamic parameters and neurological outcome in the subgroup of reperfused patients
eResults. Association of time below and above blood pressure thresholds with outcome in the subgroup of reperfused patients
eTable 4. Association of hemodynamic parameters and neurological outcome, stratified according to randomization (GA vs PS)