This cross-sectional study evaluates the variation in clinician use and payments for audiograms and vestibular tests across all regions of the United States and by specialty practice.
Key Points
Question
How do audiovestibular test rates vary among Medicare beneficiaries across US regions and by specialty of the billing clinician?
Findings
In this cross-sectional study of 1 307 887 audiovestibular test claims from Medicare beneficiaries in 2014, utilization rates per 100 000 beneficiaries ranged from 166 to 12 021 for audiograms, 15 to 4271 for caloric tests, and 13 to 3556 for rotary chair tests between the lowest- and highest-use regions. Although most audiograms and caloric tests were attributed to audiologists and otolaryngologists, rotary chair tests were more commonly acquired by primary care physicians and neurologists.
Meaning
The findings suggest that efforts to address variation in audiovestibular test use should engage a range of stakeholders, including primary care physicians.
Abstract
Importance
Variations in diagnostic test use may indicate that there are opportunities for quality improvement in vestibular health care. To date, the extent to which clinician acquisition of tests varies nationwide by region and specialty of the clinician is unknown.
Objective
To quantify variation in clinician use and payments for audiograms and vestibular tests across all geographic regions of the United States and by specialty of practice.
Design, Setting, and Participants
This cross-sectional study used a population-based sample of 1 307 887 audiovestibular test claims from fee-for-service Medicare beneficiaries aged 65 years or older in the Medicare Provider Utilization and Payment Public Use File from January 1 through December 31, 2014. The analysis was completed from January 2 through June 1, 2019.
Exposures
Diagnostic audiograms, caloric testing, and rotary chair testing.
Main Outcomes and Measures
Test utilization was analyzed by hospital referral region, medical specialty, and total payments.
Results
In 2014, clinicians performed 1 213 328 audiograms, 317 880 caloric tests (ie, single caloric irrigations), and 62 779 rotary chair tests, for a total of $38 647 350.21 in Medicare payments from the Centers for Medicare & Medicaid Services. No patient or clinician demographic characteristics were available. Across health care referral regions, rates of testing per 100 000 beneficiaries varied from 166 to 12 021 for audiograms, 15 to 4271 for caloric tests, and 13 to 3556 for rotary chair tests between the lowest-use and highest-use regions. Most audiograms and caloric tests were billed by audiologists (797 957 audiograms [65.8%]; 112 485 caloric tests [35.4%]) and otolaryngologists (376 728 audiograms [31.0%]; 70 567 caloric tests [22.2%]). In contrast, primary care physicians (18 933 [30.2%]) and neurologists (15 254 [24.3%]) billed the largest proportion of rotary chair tests compared with other specialists, including audiologists (7253 [11.6%]) and otolaryngologists (6464 [10.3%]).
Conclusions and Relevance
Substantial geographic and clinician-level variation may have been observed in use of audiovestibular tests. Quality improvement efforts in vestibular health care may need to target a range of clinicians, including primary care physicians to be successful.
Introduction
Dizziness and vertigo are highly prevalent vestibular symptoms that are adversely associated with the physical, cognitive, and social functioning of older adults.1,2,3 Half of the US adults older than 65 years who have dizziness seek medical evaluation.4,5 Nevertheless, many affected individuals do not receive prompt and accurate diagnoses needed to drive clinical management.6,7,8,9,10 To facilitate diagnosis, clinicians may acquire audiovestibular tests. The videonystagmography test protocol, which includes caloric testing, assists in lesion localization and assessment of lesion severity and compensation status.11 Some clinicians supplement or replace videonystagmography with rotary chair testing, arguing that rotary chair testing is more sensitive for detection of vestibulopathies,12 whereas others refrain because the nonindependent stimulation of ears during rotation limits specificity and lesion localization.11,12
In a previous analysis of Medicare beneficiaries who did not have cancer and were residing in Surveillance, Epidemiology, and End Results (SEER) areas, approximately 10% of individuals with dizziness underwent vestibular testing.13 Of note, use of caloric testing varied 3-fold and use of rotary chair testing varied 5-fold across registry regions; variation persisted after controlling for patient characteristics.13 Such variation prompts concerns about potential underutilization and quality of diagnostic care in lower-use regions and overutilization and spending in higher-use regions.14
To promote consistent quality and cost-efficiency, modifiable factors contributing to variation in diagnostic testing for vestibular symptoms need to be identified. The previous analysis13 was limited to SEER registry regions and considered variation from the perspective of the beneficiaries and their places of residence. However, the clinician providing the service is often the key locus of decision-making for diagnostic test acquisition. In the present study, we aimed to quantify variation in clinician use and payments for audiovestibular testing by specialty of practice across all geographic regions of the United States. We linked publicly available data from the Centers for Medicare & Medicaid Services to geographic boundary files to obtain nationwide physician utilization and payment data. In addition to caloric and rotary chair tests, we analyzed audiograms as a benchmark comparator test expected to reflect the availability of audiologic services to Medicare beneficiaries.
Methods
Data Source
For this cross-sectional study, we used the 2014 Medicare Provider Utilization and Payment Data Physician and Other Supplier Public Use File.15 The Public Use File includes information on services and procedures, including counts and average Medicare paid amount, provided to Medicare beneficiaries by physicians and other health care professionals. All beneficiaries enrolled in a traditional fee-for-service Medicare plan were included in the database. Detailed beneficiary utilization information was extracted using Healthcare Common Procedure Coding System (HCPCS) codes. We augmented the Public Use File data with a linkage to the Dartmouth Atlas of Health Care file of geographic boundary lines and hospital referral regions (HRR).16 The HRRs were established as a framework to examine geographic variation in health care practices and were developed by dividing the country into 306 regional market areas based on the hospitals in each area.16 The institutional review board at the University of Minnesota in Minneapolis, Minnesota, declared this project exempt from review because all data were deidentified; a waiver of informed consent was not necessary.
Variables and Outcome Measures
Audiovestibular test services were selected using HCPCS codes for audiograms (92552, 92553, and 92557), caloric tests (92543), and rotary chair tests (92546). In 2014, the caloric test HCPCS code (92543) covered 1 irrigation, equivalent to a unilateral monothermal caloric test. Thus, bilateral bithermal irrigations yielded 4 billable units. In the presentation of results, caloric test refers to a unilateral monothermal caloric test. Audiogram and rotary chair HCPCS codes could be billed only once per day per Medicare guidelines.
The zip code for the physician practice address (ie, the billing entity for the claim) was used to map unique national provider identification numbers to HRRs. Clinicians who billed fewer than 11 procedures were suppressed by Medicare. We divided clinician types into 11 specialty groups: (1) audiology; (2) otolaryngology; (3) neurology and neuropsychiatry; (4) primary care incorporating family medicine, internal medicine, and emergency medicine; (5) internal medicine subspecialties; (6) nonotolaryngology surgical specialties; (7) anesthesia and interventional pain clinics; (8) allied health clinicians including nurse practitioners and physician assistants; (9) physical medicine incorporating physical medicine and rehabilitation, and osteopathic manipulative medicine; (10) radiology; and (11) independent testing facilities.
We identified total Medicare payment amounts using the average Medicare standardized amount variable. This variable is a Medicare Public Use File–defined variable that reports the average amount that Medicare paid after beneficiary insurance deductible and coinsurance amounts were deducted for the service and after Medicare payment standardization. The payment standardization that Medicare uses removes geographic differences in payment rates for individual services that result from factors such as local clinician wages (wage index for the clinician or facility area) or input prices.17 The standardization used by Medicare to make payments across geographic areas comparable allows differences across regions to reflect variation in factors, such as physicians’ practice patterns and Medicare beneficiaries’ ability and willingness to obtain care.18
Statistical Analyses
For each test, we identified the total claims and the total standardized payment amounts (in US dollars) in each HRR and by clinician specialty. Utilization intensity was estimated as test claims per 100 000 Medicare beneficiaries in each HRR. We divided HRRs into quartiles of utilization intensity to examine geographic variation. The interquartile ratio of utilization intensity was defined as the 75th percentile divided by the 25th percentile and the extremal ratio as the highest observed utilization intensity divided by the lowest. In addition, provider types associated with claims were identified and compared between high and low utilization quartiles. Variance was mapped across geographic regions. Analyses were completed using SAS, version 9.3 (SAS Institute).
Results
Test Utilization Intensity
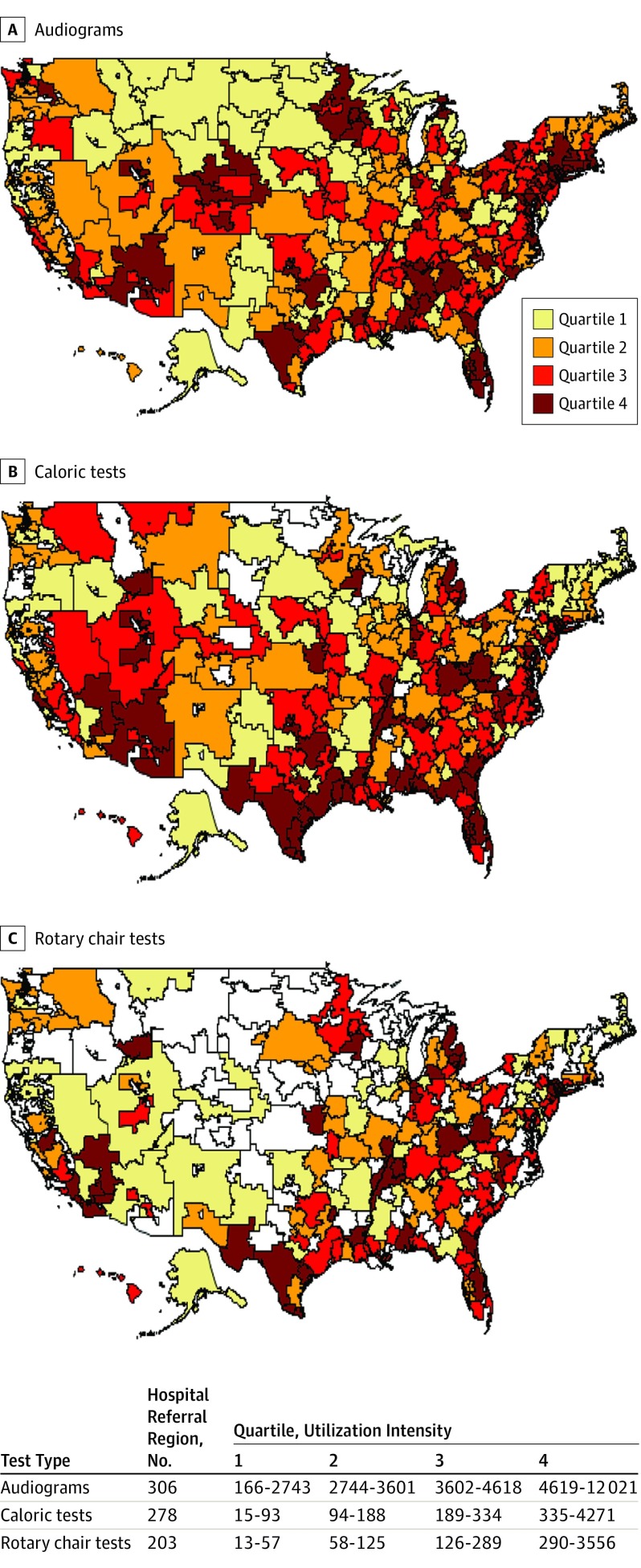
In 2014, a total of 1 213 328 diagnostic audiograms, 317 880 caloric tests (ie, unilateral monothermal caloric tests), and 62 779 rotary chair tests were performed for fee-for-service Medicare beneficiaries across 306 HRRs, for a total of $38 647 350.21 in Medicare payments. No patient characteristics were available. There was greater geographic variation in the use of vestibular tests compared with audiograms (Figure). For audiograms, the interquartile ratio for utilization intensity was 1.68 and the extremal ratio was 72.42 (range, 166-12 021 per 100 000 Medicare beneficiaries). For caloric testing, the interquartile ratio was 3.60 and the extremal ratio was 284.73 (range, 15-4271 per 100 000 Medicare beneficiaries). For rotary chair testing, the interquartile ratio was 5.09 and the extremal ratio was 273.54 (range, 13-3556 per 100 000 Medicare beneficiaries).
Figure. Utilization Intensity of Audiograms and Vestibular Tests Across Health Care Referral Regions.
Utilization intensity is defined as tests per 100 000 Medicare beneficiaries within each hospital referral region. Quartiles indicate lowest (1) to highest (4) utilization intensity. Hospital referral regions (white) indicate that no clinician billed Medicare for more than 10 tests.
High utilization of all 3 tests occurred in overlapping regions of the Southern coast and Eastern coast, but there was less overlap in utilization intensity among the 3 test categories in other regions. We identified 9 HRRs (Palm Springs, California; Idaho Falls, Idaho; Gary, Indiana; Detroit, Michigan; Saginaw, Michigan; Harlingen, Texas; Longview, Texas; Odessa, Texas; and Charleston, West Virginia) that fell in the upper quartile of use for rotary chair and caloric testing while also falling in the lower quartile of use for audiograms. Conversely, we identified 1 HRR (Wilmington, North Carolina) in the lower quartile for rotary chair and caloric testing that tested in the upper quartile for audiograms.
Test Utilization by Clinician Specialty Type
Test utilization by clinician type is presented in Table 1. Audiogram claims were submitted predominantly by audiologists and otolaryngologists (1 174 685 of 1 212 328; 97%). In contrast, vestibular test claims were more highly distributed across specialty types, including primary care, neurology, and independent testing facilities. Audiology and otolaryngology submitted claims for 57.6% (183 052 of 317 880) of caloric tests and 22.9% (13 717 of 62 779) of rotary chair tests billed. Primary care, neurology, and independent testing facilities submitted claims for 36.1% (114 615 of 317 880) of caloric tests and 63.2% (39 717 of 62 779) of rotary chair tests. The ratio of the number of claims for caloric tests to rotary chair tests by selected clinician specialties was as follows: audiology (16:1), otolaryngology (11:1), neurology (4:1), and primary care (2:1).
Table 1. Nationwide Use of Audiogram and Vestibular Test Services for Fee-for-Service Medicare Beneficiaries by Clinician Specialty.
| Specialty | No. (%) | |||
|---|---|---|---|---|
| Audiograms (n = 1 213 328) | Caloric Tests (n = 317 880) | Rotary Chair Tests (n = 62 779) | ||
| Audiology | 797 957 (65.8) | 112 485 (35.4) | 7253 (11.6) | |
| Otolaryngology | 376 728 (31.0) | 70 567 (22.2) | 6464 (10.3) | |
| Neurology | 1389 (0.1) | 58 398 (18.4) | 15 254 (24.3) | |
| Primary care | 28 578 (2.4) | 33 728 (10.6) | 18 933 (30.2) | |
| Independent testing facility | 149 (<0.1) | 21 489 (6.8) | 5527 (8.8) | |
| Anesthesia, pain specialists | 185 (<0.1) | 5297 (1.7) | 2186 (3.5) | |
| Physical medicine | 4556 (0.4) | 5224 (1.5) | 2241 (3.6) | |
| Internal medicine subspecialties | 1203 (0.1) | 4731 (1.5) | 3198 (5.1) | |
| Surgical subspecialties | 677 (0.1) | 2154 (0.7) | 761 (1.2) | |
| Allied health providers | 1906 (0.2) | 2572 (0.8) | 674 (1.1) | |
| Radiology | 0 | 1235 (0.4) | 288 (0.5) | |
The clinician specialty types that billed Medicare for testing services are compared between the HRRs in the lowest and highest utilization quartiles in Table 2. There was no difference between quartiles in clinician type predominance for audiograms, with audiologists billing most of the testing services. As shown in Table 2, 66.4% of audiograms (54 945 of 82 723) were billed by audiologists in lowest quartile regions vs 62.7% (378 919 of 604 342) of audiograms billed by audiologists in upper quartile regions. For caloric tests, higher use regions had a greater proportion of tests billed by neurologists (23.2%), primary care physicians (12.7%), and independent testing facilities (10.7%) and a smaller proportion billed by otolaryngologists (18.9%) and audiologists (28.6%). Rotary chair tests followed a similar pattern except that primary care physicians had the highest use (30.2%) and billed 30.9% in low-use and 30.1% in high-use quartiles.
Table 2. Total Audiograms and Vestibular Tests Billed in Health Care Referral Regions With Lowest and Highest Utilization Intensity by Clinician Specialtya.
| Specialty | No. (%) | |||||
|---|---|---|---|---|---|---|
| Audiograms | Caloric Tests | Rotary Chair Tests | ||||
| Lower Quartile (n = 82 723) | Upper Quartile (n = 604 342) | Lower Quartile (n = 9875) | Upper Quartile (n = 192 180) | Lower Quartile (n = 1608) | Upper Quartile (n = 42 788) | |
| Audiology | 54 945 (66.4) | 378 919 (62.7) | 4200 (42.5) | 54 948 (28.6) | 260 (16.2) | 3337 (7.8) |
| Otolaryngology | 25 156 (30.4) | 199 467 (33.0) | 3192 (32.3) | 36 397 (18.9) | 226 (14.1) | 3612 (8.4) |
| Primary care | 2191 (2.6) | 19 240 (3.2) | 666 (6.7) | 24 400 (12.7) | 497 (30.9) | 12 876 (30.1) |
| Neurology | NA | 2000 (0.3) | 1444 (14.6) | 44 506 (23.2) | 230 (14.3) | 11 622 (27.2) |
| Independent testing facility | NA | NA | NA | 20 547 (10.7) | 34 (2.1) | 5345 (12.5) |
| Anesthesia pain specialists | NA | NA | 161 (1.6) | 4043 (2.1) | 52 (3.2) | 1344 (3.1) |
| Physical medicine | 262 (0.3) | 2721 (0.4) | NA | 3236 (1.7) | 96 (6.0) | NA |
| Internal medicine specialties | NA | 1046 (0.2) | 63 (0.6) | 1788 (0.9) | 23 (1.4) | 2212(5.2) |
| Surgical subspecialties | NA | NA | NA | 1144 (0.6) | 52 (3.2) | 417 (1.0) |
| Allied health clinicians | 169 (0.3) | 949 (0.2) | 149 (1.5) | NA | 138 (8.6) | 1735 (4.1) |
| Radiology | NA | NA | NA | 1171 (0.6) | NA | 288 (0.7) |
Abbreviation: NA, not applicable (no tests were ascribed to clinicians of this medical specialty in the quartile).
Utilization intensity was measured as tests per 100 000 Medicare beneficiaries.
Medicare Total Average Standardized Payment Amount by Clinician Specialty Type
The total average Medicare standardized payment amount by clinician type is presented in Table 3. The national median payment amounts for audiograms were $27.23, for caloric tests (ie, a unilateral monothermal caloric test) were $3.19, and for rotary chair testing were $81.16. Total payments varied by clinician type. Audiology and otolaryngology received the largest Medicare standardized amount for audiometric testing ($27.02) and caloric testing ($3.41). However, primary care, neurology, and independent testing facilities received the largest percentage amount (62.1%; $2 835 641.56 of $4 562 722.77) for rotary chair testing. In contrast, audiologists and otolaryngologists received a Medicare standardized amount of $976 986.04 for rotary chair testing (22.4%). We identified 92 HRRs (eg, Seattle, Washington; Orange County, California; Palm Springs, California; Mesa, Arizona; Phoenix, Arizona; Little Rock, Arkansas; Birmingham, Alabama; Mobile, Alabama; and East Long Island, New York) that were in the upper quartile of cost for rotary chair and caloric testing while also in the lower quartile of cost for audiograms. Conversely, we identified 93 HRRs in the lower quartile of cost for rotary chair and caloric testing that tested in the upper quartile of cost for audiograms (eg, Minneapolis, Minnesota; Tucson, Arizona; San Diego California; Fort Worth, Texas; Milwaukee, Wisconsin; Roanoke, Virginia; and Syracuse, New York).
Table 3. Total Average Standardized Medicare Payment Amount for Audiometric and Vestibular Test Services by Clinician Specialty.
| Specialty | No. (%), US$ | ||
|---|---|---|---|
| Audiograms | Caloric Tests | Rotary Chair Tests | |
| All clinicians | 32 956 511.59 (100) | 1 128 115.85 (100) | 4 562 722.77 (100) |
| Audiology | 21 565 650.16 (65) | 383 906.11 (34.0) | 540 019.33 (11.8) |
| Otolaryngology | 10 463 711.91 (31.8) | 247 070.11 (21.9) | 436 966.71 (9.6) |
| Neurology | 38 642.24 (0.1) | 172 145.31 (15.3) | 927 718.74 (20.3) |
| Primary care | 654 671.65 (2.0) | 116 460.03 (10.3) | 1 504 625.96 (33.0) |
| Independent testing facility | 4097.04 (<0.1) | 137 774.58 (12.2) | 403 296.86 (8.8) |
| Anesthesia, pain specialists | 4582.04 (<0.1) | 18 255.15 (1.6) | 176 440.21 (3.9) |
| Physical medicine | 125 287.67 (0.4) | 17 923.48 (1.6) | 177 117.30 (3.9) |
| Internal medicine subspecialties | 20 975.70 (0.1) | 15 375.09 (1.4) | 258 991.65 (5.7) |
| Surgical subspecialties | 17 604.11 (0.1) | 6991.73 (0.6) | 60 143.29 (1.3) |
| Allied health clinicians | 51 542.06 (0.2) | 8248.16 (0.7) | 54 012.98 (1.2) |
| Radiology | 0 | 3966.1 (0.3) | 23 389.73 (0.5) |
Discussion
This study examined nationwide utilization of audiovestibular testing among Medicare beneficiaries from the perspective of the billing clinician. There was substantial variation in clinician use and payments for audiovestibular testing across health care referral regions, with the least variation in audiograms and the greatest variation in rotary chair testing. Audiologists and otolaryngologists submitted 97% of the audiogram claims. In contrast, vestibular test claims originated from a broader range of specialists, including primary care physicians and neurologists. In both high- and low-use quartiles, primary care physicians had the greatest use of rotary chair tests, submitting 30.2% of the claims. Audiologists and otolaryngologists received the largest total Medicare payments for audiograms and caloric testing, whereas primary care, neurology, and independent testing facilities received 62.1% of the total payment amount for rotary chair testing.
By examining test use by service provider rather than by beneficiary, the present analysis complements previous observations from the United States and elsewhere. The UK National Health Service evaluated rates of diagnostic audiology assessments per 1000 weighted population across regions defined by clinical commissioning groups in 2016.19 Clinical commissioning groups are comprised of all general practitioner practices in defined contiguous geographic regions across England and are responsible for buying and ensuring provision of local National Health Service services.20 After adjusting for age, sex, and medical need, there remained a 123-fold difference in audiologic testing rates between clinical commissioning groups.19 Similarly, in work among Medicare beneficiaries in 15 SEER registries, regional variation in vestibular test utilization persisted after controlling for patient age, sex, race/ethnicity, rurality, and Medicaid participation status.13 The variation also could not be accounted for by geographic differences in disease prevalence.13 In both the SEER regional and present nationwide analyses, there was variation in use of vestibular testing, but the nationwide examination revealed the extremes of small-area variation. We are not aware of another cross-specialty analysis of audiovestibular test claims for comparison, but there is previous evidence of within-specialty practice variation. Among otolaryngology practices in the Creating Healthcare Excellence Through Education and Research network, 21% of patients presenting with dizziness in 1 year had at least 1 vestibular test; rates varied by clinic (3%-72%) and practice type (18% for community vs 31% for academic).21
The purpose of examining clinical variation was to improve the quality and value of health care for patients with dizziness and balance disorders. The integrity of the vestibulo-ocular reflex, in particular the horizontal vestibulo-ocular reflex, may be assessed across its frequency range using caloric testing (low frequencies), rotary chair testing (middle frequencies), and head impulse testing (high frequencies). Among adults, the traditional entry point for objective vestibular assessment is the videonystagmography test protocol, including caloric testing. The caloric weakness is a readily interpretable marker for distinguishing peripheral from nonperipheral vestibular disease and defining lesion laterality.11 Nevertheless, clinicians need to remain mindful of prevalent variations in laboratory protocols (eg, medium and volume of irrigations) and normative values.22,23 Rotary chair testing is the consensus standard test for identifying and quantifying bilateral vestibular hypofunction,24,25,26 an uncommon condition with a prevalence of 28 cases per 100 000, or approximately 64 000 individuals from the United States.27 Rotary chair testing may also be used for vestibular deficit detection and assessment of compensation status. However, there is less clinical consensus on the value of routinely using rotary chair testing for these broader applications, in part because its mid-frequency stimulation paradigm has limited ability to detect low- and high-frequency vestibulo-ocular reflex deficits.11,12 Furthermore, in comparison with videonystagmography equipment, rotational chairs are expensive and require additional expertise for performance and interpretation. Video head impulse testing has emerged as a relatively affordable means of assessing high-frequency vestibulo-ocular reflex function in multiple planes and, as such, has proved to be a useful complement to the traditional test battery.28,29 Evidence-based guidelines directing the use of specific audiovestibular tests for patients with dizziness are lacking,30 leaving the optimal rate of testing undefined for clinicians. Some guidance may be derived from the Canadian Choosing Wisely campaign,31 a program that encourages physicians and patients to avoid unnecessary testing and treatment. The campaign encourages clinicians to abstain from ordering audiovestibular tests to screen for peripheral vestibular disease and to consider clinical indications based on specific diagnoses supported by the history and physical examination.31 As such, published standardized diagnostic criteria for vestibular disorders32,33,34,35,36,37 may be particularly useful as clinicians seek to optimize test utilization. The Canadian Choosing Wisely campaign also states that, “[i]n general, advanced balance tests should be ordered and interpreted by otolaryngologists with specialized training in the diagnosis and treatment of vestibular disorders.”31
The observed distribution of test claims among medical specialties revealed unexpected practice patterns. Most audiogram and caloric test claims were attributable to audiologists and otolaryngologists. In contrast, most rotary chair test claims were billed by other clinicians, notably neurologists and primary care physicians. Among neurologists, acquired caloric (ie, unilateral monothermal caloric) tests to rotatory chair tests ratio was 4:1 and, among primary care physicians, acquired caloric tests to rotary chair tests ratio was 2:1, which diverged from the 16:1 ratio among audiologists and 11:1 ratio among otolaryngologists. Some neurologists may use rotational chair testing preferentially for the evaluation of central vestibulopathies, whereas otolaryngologists and audiologists may favor caloric tests to evaluate suspected peripheral vestibulopathies. Primary care physicians are expected to be involved in vestibular health care because most patients who seek care for dizziness do so initially with their primary care physician.38,39 However, given the aforementioned indications, expense, and required expertise, why was utilization of rotary chair tests by primary care physicians so high in comparison with other specialists? Possible explanations include differences in clinician education, payment incentives for fee-for-service Medicare clinicians, complexity in patient case mix, and potential differences in what clinicians constituted as a billable test. These findings showed that any clinician-level interventions aimed at reducing unnecessary variation should consider a broad range of stakeholders, including primary care physicians, who are responsible for a substantial proportion of vestibular test use.
Differences in resource supply (eg, availability of clinicians, testing facilities) may also contribute to the observed geographic and clinician-level variation.40,41 Low-use regions may lack clinicians or testing facilities, whereas those with sufficient access may be at greater risk of overutilization, thus requiring other approaches to promote appropriate use.42 A study by Planey43 recently showed the markedly uneven supply and availability of audiologists across the United States. Approximately 57% of US counties do not have audiologists, and those that do are more urban and have fewer older individuals. A regional absence of audiologists may lay the groundwork for the participation of specialists not typically engaged in vestibular testing. However, other local factors or incentives may also contribute because a similar specialty distribution for audiogram claims was not observed.
Limitations
Claims-based analyses do not permit confirmation of test indications, results, or actual performance of the test billed. Similarly, tests lacking billing codes (vestibular-evoked myogenic potentials, video head impulse tests) cannot be assessed. Cell-size suppression requirements in the Medicare Public Use File excluded clinicians with lower volume who submitted claims for fewer than 11 tests. Claims from clinicians who are not individually registered in the Medicare database were also not represented. Claims were limited to fee-for-service Medicare beneficiaries aged 65 years or older, and no patient characteristics were available; thus, the findings may not be generalizable to a younger population, Medicare Advantage participants, or particular subgroups.
Conclusions
This study identified variation in vestibular and audiometric test utilization by different clinician specialties across the United States. Audiologists and otolaryngologists billed most of the audiograms and caloric tests, whereas primary care physicians billed more rotary chair tests than other specialists. Additional work should investigate the degree to which the observed variation is warranted or unwarranted and whether it may be improved with establishing and implementing evidence-based guidelines. To be successful, quality improvement efforts in vestibular health care may need to target a range of clinicians, including primary care physicians.
References
- 1.Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001-2004. Arch Intern Med. 2009;169(10):938-944. doi: 10.1001/archinternmed.2009.66 [DOI] [PubMed] [Google Scholar]
- 2.Semenov YR, Bigelow RT, Xue QL, du Lac S, Agrawal Y. Association between vestibular and cognitive function in US Adults: data from the National Health and Nutrition Examination Survey. J Gerontol A Biol Sci Med Sci. 2016;71(2):243-250. doi: 10.1093/gerona/glv069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bisdorff A, Von Brevern M, Lempert T, Newman-Toker DE. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res. 2009;19(1-2):1-13. [DOI] [PubMed] [Google Scholar]
- 4.Roberts DS, Lin HW, Bhattacharyya N. Health care practice patterns for balance disorders in the elderly. Laryngoscope. 2013;123(10):2539-2543. doi: 10.1002/lary.24087 [DOI] [PubMed] [Google Scholar]
- 5.Sloane PD. Dizziness in primary care: results from the National Ambulatory Medical Care Survey. J Fam Pract. 1989;29(1):33-38. [PubMed] [Google Scholar]
- 6.To-Alemanji J, Ryan C, Schubert MC. Experiences engaging healthcare when dizzy. Otol Neurotol. 2016;37(8):1122-1127. doi: 10.1097/MAO.0000000000001145 [DOI] [PubMed] [Google Scholar]
- 7.Fife D, FitzGerald JE. Do patients with benign paroxysmal positional vertigo receive prompt treatment? Analysis of waiting times and human and financial costs associated with current practice. Int J Audiol. 2005;44(1):50-57. doi: 10.1080/14992020400022629 [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharyya N, Gubbels SP, Schwartz SR, et al. Clinical practice guideline: benign paroxysmal positional vertigo (update). Otolaryngol Head Neck Surg. 2017;156(3_suppl)(suppl):S1-S47. doi: 10.1177/0194599816689667 [DOI] [PubMed] [Google Scholar]
- 9.Yardley L, Owen N, Nazareth I, Luxon L. Prevalence and presentation of dizziness in a general practice community sample of working age people. Br J Gen Pract. 1998;48(429):1131-1135. [PMC free article] [PubMed] [Google Scholar]
- 10.Kroenke K, Lucas CA, Rosenberg ML, et al. Causes of persistent dizziness: a prospective study of 100 patients in ambulatory care. Ann Intern Med. 1992;117(11):898-904. doi: 10.7326/0003-4819-117-11-898 [DOI] [PubMed] [Google Scholar]
- 11.Ahmed MF, Goebel JA, Sinks BC. Caloric test versus rotational sinusoidal harmonic acceleration and step-velocity tests in patients with and without suspected peripheral vestibulopathy. Otol Neurotol. 2009;30(6):800-805. doi: 10.1097/MAO.0b013e3181b0d02d [DOI] [PubMed] [Google Scholar]
- 12.Arriaga MA, Chen DA, Cenci KA. Rotational chair (ROTO) instead of electronystagmography (ENG) as the primary vestibular test. Otolaryngol Head Neck Surg. 2005;133(3):329-333. doi: 10.1016/j.otohns.2005.05.002 [DOI] [PubMed] [Google Scholar]
- 13.Adams ME, Marmor S, Yueh B, Kane RL. Geographic variation in use of vestibular testing among Medicare beneficiaries. Otolaryngol Head Neck Surg. 2017;156(2):312-320. doi: 10.1177/0194599816676450 [DOI] [PubMed] [Google Scholar]
- 14.Parker L, Levin DC, Frangos A, Rao VM. Geographic variation in the utilization of noninvasive diagnostic imaging: national Medicare data, 1998-2007. AJR Am J Roentgenol. 2010;194(4):1034-1039. doi: 10.2214/AJR.09.3528 [DOI] [PubMed] [Google Scholar]
- 15.The Centers for Medicare & Medicaid Services Medicare Provider Utilization and Payment Data. Physician and other supplier data CY 2014. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/Physician-and-Other-Supplier2014.html. Accessed March 1, 2018.
- 16.The Dartmouth Atlas of Health Care https://atlasdata.dartmouth.edu/static/supp_research_data#crosswalks. Accessed November 11, 2019.
- 17.O’Donnell BE, Schneider KM, Brooks JM, et al. Standardizing Medicare payment information to support examining geographic variation in costs. Medicare Medicaid Res Rev. 2013;3(3):mmrr.003.03.a06. doi: 10.5600/mmrr.003.03.a06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Centers for Medicare & Medicaid Services Medicare Provider Utilization and Payment Data: Physician and Other Supplier. 2017. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/Physician-and-Other-Supplier.html. Accessed June 30, 2018.
- 19.Public Health England The 2nd atlas of variation in NHS diagnostic services in England: reducing unwarranted variation to improve health outcomes and value. https://fingertips.phe.org.uk>documents>Diagnostic Atlas_FINAL Accessed November 8, 2019.
- 20.The King’s Fund The new NHS: clinical commissioning groups. https://www.kingsfund.org.uk/publications/what-commissioning-and-how-it-changing#what-is-commissioning. Accessed November 11, 2019.
- 21.Piker EG, Schulz K, Parham K, et al. Variation in the use of vestibular diagnostic testing for patients presenting to otolaryngology clinics with dizziness. Otolaryngol Head Neck Surg. 2016;155(1):42-47. doi: 10.1177/0194599816650173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams ME, Telian SA, Kane RL, Butler M. Monothermal caloric screening test accuracy: a systematic review. Otolaryngol Head Neck Surg. 2016;154(6):982-996. doi: 10.1177/0194599816630963 [DOI] [PubMed] [Google Scholar]
- 23.Barin K. Background and technique of caloric testing In: Jacobson GP, Shepard NT, eds. Balance function assessment and management. 2nd ed San Diego: Plural Publishing Inc; 2016:283-317. [Google Scholar]
- 24.Judge PD, Janky KL, Barin K. Can the video head impulse test define severity of bilateral vestibular hypofunction? Otol Neurotol. 2017;38(5):730-736. doi: 10.1097/MAO.0000000000001351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fife TD, Tusa RJ, Furman JM, et al. Assessment: vestibular testing techniques in adults and children: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2000;55(10):1431-1441. doi: 10.1212/WNL.55.10.1431 [DOI] [PubMed] [Google Scholar]
- 26.Hain TC, Cherchi M, Yacovino DA. Bilateral vestibular loss. Semin Neurol. 2013;33(3):195-203. doi: 10.1055/s-0033-1354597 [DOI] [PubMed] [Google Scholar]
- 27.Ward BK, Agrawal Y, Hoffman HJ, Carey JP, Della Santina CC. Prevalence and impact of bilateral vestibular hypofunction: results from the 2008 US National Health Interview Survey. JAMA Otolaryngol Head Neck Surg. 2013;139(8):803-810. doi: 10.1001/jamaoto.2013.3913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahringer A, Rambold HA. Caloric test and video-head-impulse: a study of vertigo/dizziness patients in a community hospital. Eur Arch Otorhinolaryngol. 2014;271(3):463-472. doi: 10.1007/s00405-013-2376-5 [DOI] [PubMed] [Google Scholar]
- 29.Zellhuber S, Mahringer A, Rambold HA. Relation of video-head-impulse test and caloric irrigation: a study on the recovery in unilateral vestibular neuritis. Eur Arch Otorhinolaryngol. 2014;271(9):2375-2383. doi: 10.1007/s00405-013-2723-6 [DOI] [PubMed] [Google Scholar]
- 30.Kerber KA, Fendrick AM. The evidence base for the evaluation and management of dizziness. J Eval Clin Pract. 2010;16(1):186-191. doi: 10.1111/j.1365-2753.2009.01133.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canadian Society of Otolaryngology–Head & Neck Surgery Five things physicians and patients should question in otolaryngology—otology/neurotology. Choosing Wisely Canada. https://choosingwiselycanada.org/otolaryngology/. Updated June 2017. Accessed March 11, 2019.
- 32.Lempert T, Olesen J, Furman J, et al. Vestibular migraine: diagnostic criteria. J Vestib Res. 2012;22(4):167-172. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Escamez JA, Carey J, Chung WH, et al. ; Classification Committee of the Barany Society; Japan Society for Equilibrium Research; European Academy of Otology and Neurotology (EAONO); Equilibrium Committee of the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS); Korean Balance Society . Diagnostic criteria for Menière’s disease. J Vestib Res. 2015;25(1):1-7.25882471 [Google Scholar]
- 34.Strupp M, Kim JS, Murofushi T, et al. Bilateral vestibulopathy: diagnostic criteria consensus document of the Classification Committee of the Bárány Society. J Vestib Res. 2017;27(4):177-189. doi: 10.3233/VES-170619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agrawal Y, Van de Berg R, Wuyts F, et al. Presbyvestibulopathy: diagnostic criteria consensus document of the classification committee of the Bárány Society. J Vestib Res. 2019;29(4):161-170. doi: 10.3233/VES-190672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim HA, Bisdorff A, Bronstein AM, et al. Hemodynamic orthostatic dizziness/vertigo: diagnostic criteria. J Vestib Res. 2019;29(2-3):45-56. doi: 10.3233/VES-190655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Staab JP, Eckhardt-Henn A, Horii A, et al. Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): consensus document of the Committee for the Classification of Vestibular Disorders of the Bárány Society. J Vestib Res. 2017;27(4):191-208. doi: 10.3233/VES-170622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grill E, Penger M, Kentala E. Health care utilization, prognosis and outcomes of vestibular disease in primary care settings: systematic review. J Neurol. 2016;263(suppl 1):S36-S44. doi: 10.1007/s00415-015-7913-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin HW, Bhattacharyya N. Balance disorders in the elderly: epidemiology and functional impact. Laryngoscope. 2012;122(8):1858-1861. doi: 10.1002/lary.23376 [DOI] [PubMed] [Google Scholar]
- 40.Wennberg JE. Unwarranted variations in healthcare delivery: implications for academic medical centres. BMJ. 2002;325(7370):961-964. doi: 10.1136/bmj.325.7370.961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finkelstein A, Gentzkow M, Williams H. Sources of geographic variation in health care: evidence From patient migration. Q J Econ. 2016;131(4):1681-1726. doi: 10.1093/qje/qjw023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colla CH, Mainor AJ, Hargreaves C, Sequist T, Morden N. Interventions aimed at reducing use of low-value health services: a systematic review. Med Care Res Rev. 2017;74(5):507-550. doi: 10.1177/1077558716656970 [DOI] [PubMed] [Google Scholar]
- 43.Planey AM. Audiologist availability and supply in the United States: a multi-scale spatial and political economic analysis. Soc Sci Med. 2019;222:216-224. doi: 10.1016/j.socscimed.2019.01.015 [DOI] [PubMed] [Google Scholar]