Key Points
Question
Is there a survival benefit when radial access is used instead of femoral access among patients with ST-segment elevation myocardial infarction referred for primary percutaneous coronary intervention?
Findings
In this multicenter randomized clinical trial that enrolled 2292 patients referred for primary percutaneous coronary intervention and that was stopped prematurely following a futility analysis, no significant difference in 30-day all-cause mortality (17 [1.5%] vs 15 [1.3%] patients) was found between the use of radial access and femoral access.
Meaning
These findings indicate that adequately trained operators may be able to achieve similar results when using either radial access or femoral access for primary percutaneous coronary intervention.
Abstract
Importance
Among patients with ST-segment elevation myocardial infarction (STEMI) referred for primary percutaneous coronary intervention (PCI), a survival benefit associated with radial access compared with femoral access remains controversial.
Objective
To assess whether there is a survival benefit when radial access is used instead of femoral access among patients with STEMI referred for primary PCI.
Design, Setting, and Participants
This multicenter, open-label, randomized clinical trial was conducted at 5 PCI centers in Canada. In total, 2292 patients with STEMI referred for primary PCI were enrolled between July 2011 and December 2018, with a 30-day follow-up. The primary analyses were conducted based on the intention-to-treat population.
Interventions
Patients were randomized to radial access (n = 1136) or to femoral access (n = 1156) for PCI.
Main Outcomes and Measures
Initially, the primary outcome was bleeding, but this outcome was modified to 30-day all-cause mortality following the recommendation of the granting agency. Secondary outcomes included recurrent myocardial infarction, stroke, and Thrombolysis in Myocardial Infarction–defined major or minor bleeding.
Results
Among the 2292 patients enrolled, the mean (SD) age of the patients randomized to radial access was 61.6 (12.3) years and to femoral access was 62.0 (12.1) years, with 883 male patients in the radial access and 901 male patients in the femoral access group. The trial was stopped early following a futility analysis. Primary PCI was performed in 1082 of 1136 patients (95.2%) in the radial access group and 1109 of 1156 patients (95.9%) in the femoral access group. Bivalirudin was administered to 1001 patients (88.1%) in the radial access group and to 1068 patients (92.4%) in the femoral access group, whereas glycoprotein IIb/IIIa inhibitors were administered in only 69 patients (6.1%) in the radial access group and 68 patients (5.9%) in the femoral access group. A vascular closure device was used in 789 patients (68.3%) in the femoral group. The primary outcome, 30-day all-cause mortality, occurred in 17 patients (1.5%) assigned to radial access and in 15 patients (1.3%) assigned to femoral access (relative risk [RR], 1.15; 95% CI, 0.58-2.30; P = .69). There were no significant differences between patients assigned to radial and femoral access in the rates of reinfarction (1.8% vs 1.6%; RR, 1.07; 95% CI, 0.57-2.00; P = .83), stroke (1.0% vs 0.4%; RR, 2.24; 95% CI, 0.78-6.42; P = .12), and bleeding (1.4% vs 2.0%; RR, 0.71; 95% CI, 0.38-1.33; P = .28).
Conclusions and Relevance
No significant differences were found for survival or other clinical end points at 30 days after the use of radial access vs femoral access in patients with STEMI referred for primary PCI. However, small absolute differences in end points cannot be definitively refuted given the premature termination of the trial.
Trial Registration
ClinicalTrials.gov identifier: NCT01398254
This multicenter randomized clinical trial conducted in Canada assesses whether 30-day all-cause mortality differs with the use of radial access or femoral access for primary percutaneous coronary intervention among referred patients with ST-segment elevation myocardial infarction.
Introduction
Primary percutaneous coronary intervention (PCI) is the most effective method of achieving reperfusion in patients with ST-segment elevation myocardial infarction (STEMI). However, patients referred for primary PCI are at risk for periprocedural complications because of exposure to potent antiplatelet and anticoagulant therapy, and the procedure needs to be completed quickly. Access for primary PCI is typically through either the radial or femoral vasculature. Radial access has been endorsed because bleeding is reported to be less frequent than that with femoral access,1 and bleeding associated with PCI is linked to mortality.2
Two recent trials have suggested that radial access is associated with lower mortality in patients with STEMI.3,4 The Radial vs Femoral (RIVAL) trial found no difference in mortality between radial and femoral access in patients with an acute coronary syndrome.3 However, in the STEMI subgroup, 30-day mortality was lower with radial access, which cannot be explained by the very low rates of bleeding in either access group.5 The Radial vs Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome (RIFLE-STEACS) trial also reported a lower mortality favoring radial access.4 As compared with the RIVAL trial, bleeding events in the RIFLE trial were noticeably higher in both access groups, and more so with femoral access.
Without a clear causal mechanism, the claim of a mortality advantage for radial access over femoral access in patients undergoing primary PCI remains a topic of debate.6 The present Safety and Efficacy of Femoral Access vs Radial Access in ST-Elevation Myocardial Infarction (SAFARI-STEMI) trial aimed to determine if radial access improves survival when compared with femoral access in patients referred for primary PCI.
Methods
Study Design
The SAFARI-STEMI trial was a multicenter, open-label, randomized clinical trial with blinded end-point adjudication. The details of the trial design can be found in the trial protocol in Supplement 1. The University of Ottawa Heart Institute Cardiovascular Research Methods Centre coordinated the trial and was responsible for all data collection and analysis. The trial was an investigator-initiated study, and funding was provided by the University of Ottawa Heart Institute STEMI program and the Canadian Institutes of Health Research. All authors vouch for the accuracy and completeness of the data and all analyses and for the fidelity of this report to the trial protocol. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. The study was approved by the institutional review board at each participating center. All patients provided written informed consent obtained in a manner consistent with the Tri-Council Policy Statement requirements prior to intervention. No one received compensation or was offered any incentive for participating in this study.
Patient Population
Patients presenting with STEMI who were referred for primary PCI within 12 hours after symptom onset were eligible to participate in the trial. Patients who had received fibrinolytic therapy, had been prescribed oral anticoagulant therapy, or had undergone previous coronary artery bypass graft (CABG) surgery were excluded. The detailed inclusion and exclusion criteria are provided in the trial protocol in Supplement 1.
Randomization
Patients were randomized to radial or femoral access in a 1:1 ratio. Randomization was performed with the use of computer-generated permuted blocks and stratified by study center. Access assignment was provided in sealed, opaque, serially numbered envelopes to ensure allocation concealment and to avoid selection bias. The envelopes were allocated sequentially in the order in which the participants were enrolled.
Intervention
Catheterization was performed via the allocated access site. Among patients allocated to radial access, verapamil, 2 mg, or nitroglycerin, 200 μg, was given through the access sheath prior to catheter insertion to eliminate radial artery spasm. At the end of the procedure, the sheath was removed using a radial band. Among patients allocated to femoral access, the puncture site was facilitated by fluoroscopic landmark identification. The use of vascular closing devices was strongly encouraged, and deployment was carried out according to the manufacturer’s recommendation. If a vascular closing device was not used, then the femoral arterial sheath was removed 4 hours after completing the procedure. Diagnostic and interventional catheters used in the 2 access groups were 5 or 6 French size at the discretion of the operator.
Concomitant Study Treatment
Before cardiac catheterization, patients were prescribed chewable aspirin, 160 mg, and an oral P2Y12 inhibitor per guidelines.7 Patients also received unfractionated heparin, 60 U/kg (maximum, 4000 U), given as an intravenous bolus.
Intravenous unfractionated heparin or bivalirudin was administered during the PCI. The choice of the anticoagulant was decided by the center, and each center was to use only the site-chosen anticoagulant during the PCI. An activated clotting time (ACT) was measured once arterial access had been obtained. When heparin was used, the operator targeted an ACT of 250 seconds or more. If the ACT was below target, boluses of heparin, 2000 to 3000 U, were given to achieve and maintain the ACT at target during the procedure. When bivalirudin was used, an initial intravenous bolus of 0.75 mg/kg was given, followed by an infusion of 1.75 mg/kg per hour. An additional bolus of 0.3 mg/kg was given to maintain the ACT at 250 seconds or more during the PCI. On completion of the PCI, the infusion was adjusted to 0.25 mg/kg per hour for 2 hours and then stopped.
Glycoprotein IIb/IIIa inhibitors (GPIs) were not prescribed except in the following situations: the presence of a large, residual intracoronary thrombus mass; the inability to achieve or maintain Thrombolysis in Myocardial Infarction (TIMI) grade 2 or 3 flow; or an abrupt vessel closure.
Study Outcomes
The study was initially designed with bleeding as the primary outcome. However, the peer-review granting agency (ie, the Canadian Institutes of Health Research) subsequently recommended modifying the study to use mortality as the primary outcome. The primary outcome was then revised to all-cause mortality measured at 30 days. This metric was chosen because mortality at 30 days is likely to be related to a cardiovascular cause, and mortality at 30 days is standard for STEMI studies.3,4 Key secondary outcomes included stroke, reinfarction, stent thrombosis, and bleeding within 30 days. Stent thrombosis was defined according to the Academic Research Consortium definition.8 Bleeding was defined as major or minor using the TIMI definition.9 The Bleeding Academic Research Consortium (BARC) bleeding definition was used for comparison.10 A central adjudication committee whose members were blinded to the study group assignments reviewed all deaths, strokes, reinfarction, stent thrombosis, and bleeding events. An independent data and safety monitoring board (DSMB) oversaw the safety and scientific validity of the trial.
Statistical Analysis
The sample size was calculated with an expected mortality at 30 days of 4.0% in the femoral access group based on the experience of previous trials.3,4,11 A difference in mortality of 1.5% between femoral access and radial access was considered the minimal clinically important difference that would change practice based on a Delphi process involving cardiologists. We anticipated a crossover rate in the radial access group of 5% and in the femoral access group of 1%. Taking the more conservative 5% for both groups plus an anticipated minimal loss to follow-up of 0.5%, we determined that a sample size of 2442 patients per group (a total of 4884 patients) was required to identify this difference with a level of statistical significance of 0.05 and 80% power.
Two interim analyses were planned after recruiting 33% and 66% of patients. A group sequential procedure using O’Brien-Fleming boundaries was followed. The first interim analysis was reviewed by the DSMB in January 2018. Subsequently, the DSMB requested a futility analysis because of a lower-than-expected rate of the primary outcome. On the basis of this analysis, the DSMB recommended terminating the trial because it was unlikely that the trial would show a minimal clinically important difference between the access site strategies using the planned sample size. The steering committee met to discuss the recommendation, and enrollment was terminated in December 2018.
The primary analysis was performed on an intention-to-treat basis. Descriptive statistics were used to compare patients randomized to radial or femoral access with respect to baseline variables. For the analysis of the primary outcome, the 2-tailed χ2 test was used to compare radial access and femoral access, and relative risk (RR) and 95% CI were calculated. Discrete variables were compared using χ2 tests. In addition, the cumulative incidence of mortality and bleeding during the 30-day follow-up was estimated with the Kaplan-Meier method. The 2 access groups were compared with the log-rank test, and the hazard ratio and 95% CI were calculated. Continuous variables were compared using t tests; however, if the parametric assumption was not met, then the Wilcoxon rank sum test was used. Subgroups of interest were identified, such as age, sex, body mass index, renal function, and use of antiplatelet and anticoagulant therapy. The primary outcome was compared within each subgroup, and the interaction P value was also calculated. A 2-sided P < .05 was considered statistically significant. Analyses were conducted using SAS, version 9.4 (SAS Institute Inc).
Results
On December 7, 2018, recruitment was stopped early as recommended by the DSMB as a result of a futility analysis. Based on data available, a futility index of 0.83 for the primary outcome was calculated using the PASS software (version 13), indicating that the SAFARI-STEMI trial would fail to demonstrate superiority given the results observed, with a conditional power of only 17%.
Patient Characteristics
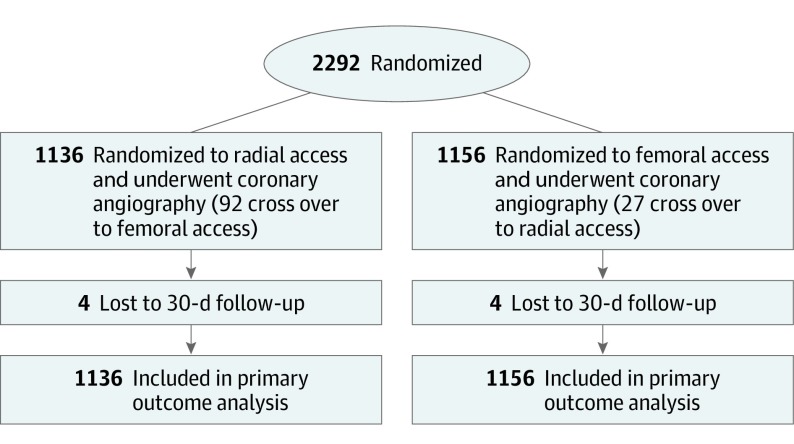
Between July 2011 and December 2018, 2292 patients were enrolled in the study at 5 PCI centers; 1136 patients were randomized to radial access, and 1156 patients were randomized to femoral access (Figure 1). The baseline characteristics of the patients were similar in the 2 groups (Table 1). The mean (SD) age of the patients assigned to radial access was 61.6 (12.3) years and assigned to femoral access was 62.0 (12.1) years, with 883 male patients (77.7%) in the radial access group and 901 male patients (77.9%) in the femoral access group. Killip class II to IV at presentation was evident in 78 patients (6.9%) assigned to radial access and in 77 patients (6.7%) assigned to femoral access.
Figure 1. CONSORT Flow Diagram for the Safety and Efficacy of Femoral Access vs Radial Access in ST-Elevation Myocardial Infarction Randomized Clinical Trial.
Table 1. Baseline Characteristics of the Patientsa.
| Characteristic | Radial Access (n = 1136) | Femoral Access (n = 1156) |
|---|---|---|
| Age, mean (SD), y | 61.6 (12.3) | 62.0 (12.1) |
| Age ≥75, No. (%) | 186 (16.4) | 186 (16.1) |
| Male sex, No. (%) | 883 (77.7) | 901 (77.9) |
| Medical history, No. (%) | ||
| Hypertension | 558 (49.1) | 541 (46.8) |
| Diabetes | 190 (16.7) | 212 (18.3) |
| Current smoker | 450 (39.6) | 442 (38.2) |
| Dyslipidemia | 423 (37.2) | 431 (37.3) |
| Previous myocardial infarction | 125 (11.0) | 116 (10.0) |
| Previous percutaneous coronary intervention | 105 (9.2) | 104 (9.0) |
| Previous stroke or TIA | 41 (3.6) | 40 (3.5) |
| Anterior myocardial infarction, No. (%) | 428 (37.7) | 396 (34.3) |
| Heart rate, mean (SD), beats/min | 76.7 (30.3) | 77.5 (25.5) |
| Systolic blood pressure, mean (SD), mm Hg | 141.0 (28.5) | 142.4 (27.7) |
| Killip class, No. (%) | 79 (7.0) | 77 (6.7) |
| I | 1055 (93.1) | 1071 (93.2) |
| II | 69 (6.1) | 62 (5.4) |
| III | 6 (0.5) | 12 (1.0) |
| IV | 3 (0.3) | 3 (0.3) |
| Weight, mean (SD), kg | 84.1 (17.5) | 84.5 (17.9) |
| BMI, mean (SD) | 28.2 (4.9) | 28.2 (4.9) |
| Creatinine clearance, mean (SD), mL/minb | 101.7 (4.5) | 103.8 (5.3) |
| Peripheral vascular disease, No. (%) | 8 (0.7) | 15 (1.3) |
| Presentation by ambulance, No. (%) | 685 (60.3) | 682 (59.0) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); TIA, transient ischemic attack.
There were no significant differences between groups in the listed characteristics.
To convert creatinine clearance to milliliters per second, multiply by 0.0167.
Medications and Procedures
Procedural characteristics are shown in Table 2. All patients were administered a loading dose of the P2Y12 platelet inhibitor before cardiac catheterization, and more than 90% of patients received ticagrelor (1040 patients in the radial access group and 1058 patients in the femoral access group). All patients underwent immediate cardiac catheterization: in the radial access group, 1082 patients (95.2%) underwent PCI, and in the femoral access group, 1109 patients (95.9%) underwent PCI. The rate of access crossover was 8.1% in patients assigned to radial access and 2.3% in patients assigned to femoral access (P < .001). Four centers chose bivalirudin for anticoagulation treatment during PCI, and 1 center chose heparin. As such, bivalirudin was prescribed in 1001 (88.1%) of the patients assigned to radial access and in 1068 (92.4%) of the patients assigned to femoral access. By contrast, GPIs were used in only 69 patients (6.1%) in the radial access group and 68 patients (5.9%) in the femoral access group. The median interval between administration of the local anesthetic lidocaine and balloon inflation/device was 2 minutes longer in the radial access group (radial access median, 13 minutes; interquartile range [IQR], 10-17 minutes vs femoral access median, 11 minutes; IQR, 9-14 minutes; P < .001). Median (IQR) fluoroscopy time was also significantly longer in the radial access group (9.4 [6.5-13.5] min vs 8.2 [6.0-12.5] min; P < .001).
Table 2. Medications and Procedural Results.
| Variable | Radial Access (n = 1136) | Femoral Access (n = 1156) |
|---|---|---|
| Medications administered before procedure, No. (%) | ||
| Aspirin | 1135 (99.9) | 1151 (99.6) |
| P2Y12 inhibitora | ||
| Clopidogrel | 211 (18.6) | 236 (20.4) |
| Prasugrel | 1 (0.1) | 1 (0.1) |
| Ticagrelor | 1040 (91.5) | 1058 (91.5) |
| Antithrombin | ||
| Intravenous unfractionated heparin | 1116 (98.2) | 1127 (97.5) |
| Medications administered during procedure, No. (%) | ||
| Antithrombinb | ||
| Bivalirudin | 1001 (88.1) | 1068 (92.4) |
| Unfractionated heparin | 135 (11.9) | 88 (7.6) |
| Glycoprotein IIb/IIIa inhibitor | 69 (6.1) | 68 (5.9) |
| Cardiac catheterization procedure | ||
| Coronary angiography, No. (%) | 1136 (100) | 1156 (100) |
| PCI performed, No. (%) | 1082 (95.2) | 1109 (95.9) |
| Stenting performed, No. (%) | 1038 (91.3) | 1070 (92.6) |
| Stents per patient, mean (SD) | 1.5 (1.2) | 1.5 (1.0) |
| Type of stent, No./total No. (%) | ||
| Drug-eluting | 906/1038 (87.3) | 946/1070 (88.4) |
| Bare metal | 123/1038 (11.8) | 113/1070 (10.6) |
| Both | 9/1038 (0.9) | 11/1070 (1.0) |
| Use of manual aspiration thrombectomy, No./total No. (%) | 420/1082 (38.8) | 476/1109 (42.9) |
| No. of diagnostic and guiding catheters per patient, mean (SD) | 3.2 (1.4) | 3.1 (1.0) |
| Use of mechanical support, No. (%) | ||
| Intra-aortic balloon pump | 20 (1.8) | 29 (2.5) |
| Impella device or extracorporeal membrane oxygenation | 3 (0.26) | 4 (0.35) |
| Use of vascular closing device, No. (%)b | 63 (5.5) | 789 (68.3) |
| Contrast volume, mean (SD), mL | 227.4 (3.4) | 232.3 (73.4) |
| Fluoroscopy time, median (IQR), minb | 9.4 (6.5-13.5) | 8.2 (6.0-12.5) |
| Peak activated clotting time, mean (SD), s | 395 (130) | 389 (116) |
| Crossover, No. (%)b | 92 (8.1) | 27 (2.3) |
| Critical time intervals, median (IQR), min | ||
| Symptom onset to first balloon inflation/device | 166 (111-247) | 161 (109-239) |
| Arrival at PCI center to first balloon inflation/devicec | 47 (35-63) | 44 (33-60) |
| Arrival at catheterization laboratory to first balloon inflation/deviceb | 20 (16-25) | 18 (14-22) |
| Lidocaine administration to first balloon inflation/deviceb | 13 (10-17) | 11 (9-14) |
| Angiographic results | ||
| Multivessel disease, No. (%) | 648 (57.0) | 674 (58.3) |
| Infarct-related coronary artery, No. (%) | ||
| Left main | 6 (0.5) | 10 (0.9) |
| Left anterior descending | 456 (40.1) | 427 (36.9) |
| Left circumflex | 158 (13.9) | 176 (15.2) |
| Right | 500 (44.0) | 524 (45.3) |
| Unknown | 17 (1. 5) | 19 (1.6) |
| Coronary flow TIMI grade at baseline, No./total No. (%)d | ||
| 0 | 701/1126 (58.8) | 636/1147 (55.4) |
| 1 | 72/1126 (7.6) | 100/1147 (8.7) |
| 2 | 148/1126 (13.1) | 155/1147 (13.5) |
| 3 | 205/1126 (18.2) | 256/1147 (22.3) |
| Coronary flow TIMI grade after procedure, No./total No. (%) | ||
| 0 | 57/1131 (5.0) | 38/1150 (3.3) |
| 1 | 12/1131 (1.1) | 8/1150 (0.7) |
| 2 | 43/1131 (3.8) | 34/1150 (3.0) |
| 3 | 1019/1131 (90.1) | 1070/1150 (93.0) |
| Contrast volume, mean (SD), mL | 227.4 (3.4) | 232.3 (73.4) |
Abbreviations: IQR, interquartile range; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction, flow graded from 0 to 3, with higher grade indicating better flow.
Patients given a loading dose of clopidogrel at the sending hospital were allowed a loading dose of ticagrelor on arrival at the PCI center.
P < .001.
P = .007.
P = .004.
Enrolling Centers
All 5 participating centers were high-volume PCI academic centers. Operators typically performed more than 250 PCI procedures annually. At least 95% of the PCIs were performed by operators who performed more than 142 PCIs per year via the radial approach, a benchmark that has been established by the RIVAL investigators as a high-volume operator of radial PCI.3 In SAFARI-STEMI, none of the PCIs were performed by low-volume radial operators (ie, ≤70 PCIs per year). In addition, all of the operators were highly trained at using femoral access, with more than 95% of the PCIs performed by operators with a mean of more than 100 PCIs per year via this approach.
Primary Outcome
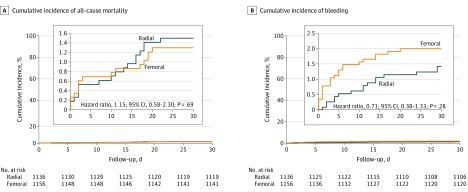
Follow-up information on all-cause 30-day mortality was available for 2283 of 2292 patients (99.6%). This outcome occurred in 17 of 1136 patients (1.5%) assigned to radial access and in 15 of 1156 patients (1.3%) assigned to femoral access, yielding an absolute risk difference favoring femoral access of −0.002 (95% CI, −0.012 to 0.008; P = .69) and a corresponding RR of 1.15 (95% CI, 0.58-2.30; P = .69) (Table 3). As illustrated in eFigure 1 in Supplement 2, the results were consistent across each of the subgroups. The findings on mortality are further supported by Figure 2A, which depicts the cumulative rates during the 30-day follow-up.
Table 3. Primary and Secondary Outcomes at 30 Days.
| Outcome | No. (%) of Patients | Relative Risk (95% CI) | P Value | |
|---|---|---|---|---|
| Radial Access (n = 1136) | Femoral Access (n = 1156) | |||
| Primary outcome | ||||
| All-cause mortality | 17 (1.5) | 15 (1.3) | 1.15 (0.58-2.30) | .69 |
| Secondary outcome | ||||
| Reinfarction | 20 (1.8) | 19 (1.6) | 1.07 (0.57-2.00) | .83 |
| Stroke | 11 (1.0) | 5 (0.4) | 2.24 (0.78-6.42) | .12 |
| Death or stroke | 25 (2.2) | 20 (1.7) | 1.27 (0.71-2.28) | .42 |
| Death, reinfarction, or stroke | 45 (4.0) | 39 (3.4) | 1.17 (0.77-1.79) | .45 |
| Stent thrombosisa | 17 (1.5) | 13 (1.1) | 1.33 (0.65-2.73) | .43 |
| Acute (≤24 h) | 10 (0.9) | 6 (0.5) | 1.70 (0.62-4.65) | .32 |
| Subacute (>24 h to 30 d) | 7 (0.6) | 7 (0.6) | 1.02 (0.36-2.89) | >.99 |
| Repeat angiogram, No. (%) | 150 (13.2) | 157 (13.6) | 0.97 (0.76-1.23) | .79 |
| Noninfarct-related artery PCI | 101(8.9) | 111(9.6) | 0.92 (0.69-1.21) | .56 |
| CABG | 43 (3.8) | 40 (3.5) | 1.10 (0.71-1.70) | .68 |
| Length of stay, median (IQR), d | 3.0 (2.0-5.0) | 3.0 (3.0-5.0) | .37 | |
| Bleeding | ||||
| TIMI classification | ||||
| Non-CABG | ||||
| Major | 10 (0.9) | 15 (1.3) | 0.68 (0.31-1.50) | .34 |
| Minor | 6 (0.5) | 8 (0.7) | 0.76 (0.27-2.19) | .61 |
| Major or minor | 16 (1.4) | 23 (2.0) | 0.71 (0.38-1.33) | .28 |
| CABG major | 2 (0.2) | 5 (0.4) | 0.41 (0.08-2.09) | .45 |
| Any major or minor bleed | 18 (1.6) | 28 (2.4) | 0.65 (0.36-1.18) | .15 |
| BARC classification | ||||
| BARC (type 3-5) | 19 (1.7) | 27 (2.3) | 0.72 (0.40-1.28) | .26 |
| Blood transfusion | 36 (3.2) | 41 (3.5) | 0.89 (0.58-1.39) | .62 |
| Intracranial | 1 (0.1) | 0 | ND | .32 |
| Retroperitoneal | 0 | 5 (0.4) | ND | .06 |
Abbreviations: BARC, Bleeding Academic Research Consortium; CABG, coronary artery bypass graft; IQR, interquartile range; PCI, percutaneous coronary intervention; ND, not determined; TIMI, Thrombolysis in Myocardial Infarction.
All stent thrombosis adjudicated as definite.
Figure 2. Cumulative Incidence of the Primary Outcome and of Bleeding Within 30 Days.
Kaplan-Meier estimates of the cumulative incidence of the primary outcome of all-cause mortality (A) and noncoronary artery bypass graft Thrombolysis in Myocardial Infarction–defined major or minor bleeding (B). Insets show detailed views of the same data.
Secondary Outcomes
Secondary outcomes are shown in Table 3. At 30 days, there were no significant differences between patients assigned to radial or femoral access in the rates of reinfarction (1.8% vs 1.6%; RR, 1.07; 95% CI, 0.57-2.00; P = .83), stroke (1.0% vs 0.4%; RR, 2.24; 95% CI, 0.78-6.42; P = .12), and stent thrombosis (1.5% vs 1.1%; RR, 1.33; 95% CI, 0.65-2.73; P = .43). There were also no significant differences in the rates of bleeding defined as non-CABG TIMI major or minor (1.4% vs 2.0%; RR, 0.71; 95% CI, 0.38-1.33; P = .28) or defined as BARC type 3 to 5 (1.7% vs 2.3%; RR, 0.72; 95% CI, 0.40-1.28; P = .26). Figure 2B illustrates the cumulative rates of non-CABG TIMI major or minor bleeding during the 30-day follow-up. Blood transfusion was required in 36 patients (3.2%) assigned to radial access and in 41 patients (3.5%) assigned to femoral access (RR, 0.89; 95% CI, 0.58-1.39; P = .62). One patient in the radial group had an intracranial hemorrhage, and 5 patients in the femoral group had a retroperitoneal bleed. A vascular closure device was used in 789 patients (68.3%) assigned to femoral access; among the patients in the femoral access group, non-CABG TIMI major or minor bleeding occurred in 9 patients (1.1%) in whom a closure device was used and in 14 patients (3.8%) in whom the device was not used (P = .005).
Discussion
In this multicenter randomized clinical trial, the use of radial access compared with femoral access was not associated with lower mortality among patients with STEMI referred for primary PCI. This observation was consistent across all patient subgroups. In addition, we found no differences in the rates of reinfarction, stroke, stent thrombosis, or major bleeding between access sites, although a small absolute reduction in bleeding associated with radial access cannot be definitively refuted given the premature termination of the trial.
The SAFARI-STEMI trial was specifically designed to assess whether there is a survival benefit when radial access is used instead of femoral access in patients referred for primary PCI. It is the largest primary PCI trial dedicated to evaluate the merits of radial access compared with femoral access. The trial design was straightforward, focused on a homogeneous patient population, and used standardized, contemporary pharmacological and procedural strategies intended to optimize outcomes in both study groups.
Two trials,3,4 RIVAL and RIFLE-STEACS, have suggested that radial access is associated with lower mortality compared with femoral access in patients with STEMI. The authors of both of these trials have indicated that future randomized clinical trials would be useful to confirm these findings. The subgroup of patients with STEMI in the RIVAL trial3 consisted of 1471 patients (74%) referred for primary PCI and 507 patients (26%) for secondary PCI. The STEMI cohort was reported as a subgroup analysis5 of the larger parent acute coronary syndrome trial that failed to demonstrate a difference in major bleeding or ischemic events between femoral access and radial access. However, in the STEMI subgroup, 30-day mortality was significantly lower with radial access (1.3% vs 3.2%), which cannot be explained by the very low rates of bleeding at 0.84% (radial access) vs 0.91% (femoral access). The majority of deaths occurred in patients who had neither a major bleed nor an access site complication.5 Because randomization did not stratify patients by STEMI and non-STEMI, any comparison in the patients with STEMI is a subgroup analysis and prone to potential differences between access groups that may confound the relationship.
The RIFLE-STEACS trial enrolled 1001 patients with STEMI and also reported a significantly lower 30-day mortality favoring radial access (5.2% vs 9.2%).4 In contrast to the RIVAL trial, bleeding events were significantly higher in the femoral access group (7.8% vs 12.2%). However, the RIFLE-STEACS trial4 used GPIs in more than two-thirds of patients, drugs shown to increase bleeding and mortality.12,13
The Minimizing Adverse Hemorrhagic Events by Transradial Access Site and Systemic Implementation of AngioX (MATRIX) trial concluded that radial access reduces net adverse clinical events in patients presenting with an acute coronary syndrome through a reduction in major bleeding and all-cause mortality.14 However, the benefit was confined to patients randomized in centers where the proportion of radial PCI was very high (ie, 80%-98%), with no difference between radial access and femoral access even when the proportion of the radial procedures at a center was as high as 79%. Furthermore, in the STEMI cohort, there was no difference in mortality between radial access vs femoral access at 2.4% vs 2.7%, respectively.15
In a meta-analysis that included patients with STEMI from 9 randomized trials comparing radial access with femoral access, mortality at 30 days was significantly lower among patients assigned to radial access.15 We performed an updated meta-analysis of these trials, including SAFARI-STEMI (eFigure 2 in Supplement 2). The overall survival benefit was only slightly reduced and still favored radial access. However, eFigure 2 in Supplement 2 shows that only 2 trials, RIVAL STEMI and RIFLE-STEACS,3,4 achieved statistical significance and essentially drive the overall reduction in mortality. The sample size of both of these trials was smaller than that of SAFARI-STEMI, and the shortcomings of these 2 trials are discussed above. An influence analysis identified RIVAL as having the largest contribution to the overall result, and on removing RIVAL from the meta-analysis, the upper 95% CI nears unity (0.61-0.99). Moreover, the 30-day mortality results of the SAFARI-STEMI trial align with the results shown for the patients with STEMI in the larger MATRIX trial.
The mortality rates in SAFARI-STEMI were lower than anticipated. Yet, low-risk patients were not targeted. The eligibility criteria and the baseline characteristics for the patients with STEMI were similar to those in the RIVAL trial.3,5 A number of contributing factors may explain the relatively low mortality reported in our trial. First, the study was performed in a contemporary setting in which well-designed systems of care allowed rapid reperfusion of the infarct-related artery. Second, the interventions were performed by experienced teams, with the PCI volume per operator set high and with each operator highly qualified in performing the procedure via either access site. Third, contemporary pharmacological strategies were used in the majority of patients. Ticagrelor, an orally administered P2Y12 receptor inhibitor associated with survival benefits,16 was prescribed to more than 90% of the patients. By contrast, the use of GPIs was curtailed to single-digit numbers. Bivalirudin was used for anticoagulation in the majority of the patients in our study. In the MATRIX trial, the rates of bleeding and all-cause mortality were lower with the use of bivalirudin compared with the use of unfractionated heparin.17 Some of these strategies may have contributed to the relatively low rates of bleeding in our study and may have attenuated the likelihood of finding any potential difference between access groups. Fourth, vascular closing devices were used in more than two-thirds of patients assigned to femoral access. Although the data remain limited, 1 study using a propensity score–matching analysis involving 271 845 PCI procedures reported a mortality benefit favoring the use of a vascular closing device over a clamp or manual compression.18
Although the SAFARI-STEMI trial was terminated early, our results suggested that experienced operators practicing in high-volume PCI centers may achieve equally good survival outcomes using either access site for primary PCI. The 2017 European Society of Cardiology STEMI Guidelines give a class IA recommendation for radial access over femoral access.19 Given the shortcomings of previous trials, some authors have questioned this recommendation.6 Furthermore, this recommendation may not be applicable for all patients, institutions, and cardiologists. Proficiency at performing PCI via either access would provide flexibility and help ensure better outcomes. Therefore, it is important that educational programs for trainees aim to ensure competency using the 2 approaches.
Limitations
This study has some limitations. The results may not apply to all patients with STEMI. For example, we excluded patients treated with fibrinolytic therapy and patients prescribed oral anticoagulant therapy because bleeding at the femoral access site could be more difficult to control. A second limitation is the rate of crossovers at 8.1% with radial access vs 2.3% with femoral access; yet, these rates are similar to those reported in the RIVAL study (7.6% vs 2.0%, respectively)3 and in the RIFLE study (9.4% vs 2.8%, respectively).4 A third limitation is the small number of participating centers, which could imply that the results are not generalizable. In a randomized clinical trial, internal validity (comparability) is often of greater importance than external validity (generalizability). As such, in SAFARI-STEMI, the method was highly standardized and the trial conducted in highly experienced centers, which had the effect of minimizing differences among the operators while assessing the value of the operation itself. Finally, it could be argued that the study was terminated early for futility and that a larger sample size was needed for adequate precision to justify our conclusions. However, the precision surrounding the primary outcome is similar to that found for mortality in patients with STEMI enrolled in the RIVAL and RIFLE-STEACS trials.15 In addition, our results align with the absence of mortality benefit in the patients with STEMI reported in the MATRIX study.15
Conclusions
When comparing the use of radial and femoral access for patients with STEMI referred for primary PCI, we detected no difference in survival or other clinical end points at 30 days, although small, absolute differences in end points cannot be definitively refuted given the premature termination of the trial.
Trial Protocol
eFigure 1. Subgroup Analysis of the Primary Outcome
eFigure 2. Updated Meta-analysis
Data Sharing Statement
References
- 1.Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J. 2009;157(1):132-140. doi: 10.1016/j.ahj.2008.08.023 [DOI] [PubMed] [Google Scholar]
- 2.Manoukian SV, Voeltz MD, Eikelboom J. Bleeding complications in acute coronary syndromes and percutaneous coronary intervention: predictors, prognostic significance, and paradigms for reducing risk. Clin Cardiol. 2007;30(10)(suppl 2):II24-II34. doi: 10.1002/clc.20238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jolly SS, Yusuf S, Cairns J, et al. ; RIVAL Trial Group . Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377(9775):1409-1420. doi: 10.1016/S0140-6736(11)60404-2 [DOI] [PubMed] [Google Scholar]
- 4.Romagnoli E, Biondi-Zoccai G, Sciahbasi A, et al. Radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome: the RIFLE-STEACS (Radial Versus Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome) study. J Am Coll Cardiol. 2012;60(24):2481-2489. doi: 10.1016/j.jacc.2012.06.017 [DOI] [PubMed] [Google Scholar]
- 5.Mehta SR, Jolly SS, Cairns J, et al. ; RIVAL Investigators . Effects of radial versus femoral artery access in patients with acute coronary syndromes with or without ST-segment elevation. J Am Coll Cardiol. 2012;60(24):2490-2499. doi: 10.1016/j.jacc.2012.07.050 [DOI] [PubMed] [Google Scholar]
- 6.Bates ER. Bleeding avoidance strategies, performance measures, and the emperor’s new clothes. JACC Cardiovasc Interv. 2016;9(8):780-783. doi: 10.1016/j.jcin.2016.02.040 [DOI] [PubMed] [Google Scholar]
- 7.O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(4):e78-e140. doi: 10.1016/j.jacc.2012.11.019 [DOI] [PubMed] [Google Scholar]
- 8.Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356(10):1020-1029. doi: 10.1056/NEJMoa067731 [DOI] [PubMed] [Google Scholar]
- 9.TIMI Study Group The Thrombolysis in Myocardial Infarction (TIMI) trial: phase I findings. N Engl J Med. 1985;312(14):932-936. doi: 10.1056/NEJM198504043121437 [DOI] [PubMed] [Google Scholar]
- 10.Valgimigli M; MATRIX Investigators . Design and rationale for the Minimizing Adverse Haemorrhagic Events by Transradial Access Site and Systemic Implementation of AngioX program. Am Heart J. 2014;168(6):838-845. doi: 10.1016/j.ahj.2014.08.013 [DOI] [PubMed] [Google Scholar]
- 11.Bernat I, Horak D, Stasek J, et al. ST-segment elevation myocardial infarction treated by radial or femoral approach in a multicenter randomized clinical trial: the STEMI-RADIAL trial. J Am Coll Cardiol. 2014;63(10):964-972. doi: 10.1016/j.jacc.2013.08.1651 [DOI] [PubMed] [Google Scholar]
- 12.Stone GW, Witzenbichler B, Guagliumi G, et al. ; HORIZONS-AMI Trial Investigators . Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358(21):2218-2230. doi: 10.1056/NEJMoa0708191 [DOI] [PubMed] [Google Scholar]
- 13.Stone GW, Witzenbichler B, Guagliumi G, et al. ; HORIZONS-AMI Trial Investigators . Heparin plus a glycoprotein IIb/IIIa inhibitor versus bivalirudin monotherapy and paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction (HORIZONS-AMI): final 3-year results from a multicentre, randomised controlled trial. Lancet. 2011;377(9784):2193-2204. doi: 10.1016/S0140-6736(11)60764-2 [DOI] [PubMed] [Google Scholar]
- 14.Valgimigli M, Gagnor A, Calabró P, et al. ; MATRIX Investigators . Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet. 2015;385(9986):2465-2476. doi: 10.1016/S0140-6736(15)60292-6 [DOI] [PubMed] [Google Scholar]
- 15.Singh S, Singh M, Grewal N, Khosla S. Transradial vs transfemoral percutaneous coronary intervention in ST-segment elevation myocardial infarction: a systemic review and meta-analysis. Can J Cardiol. 2016;32(6):777-790. doi: 10.1016/j.cjca.2015.08.019 [DOI] [PubMed] [Google Scholar]
- 16.Steg PG, James S, Harrington RA, et al. ; PLATO Study Group . Ticagrelor versus clopidogrel in patients with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: a Platelet Inhibition and Patient Outcomes (PLATO) trial subgroup analysis. Circulation. 2010;122(21):2131-2141. doi: 10.1161/CIRCULATIONAHA.109.927582 [DOI] [PubMed] [Google Scholar]
- 17.Valgimigli M, Frigoli E, Leonardi S, et al. ; MATRIX Investigators . Bivalirudin or unfractionated heparin in acute coronary syndromes. N Engl J Med. 2015;373(11):997-1009. doi: 10.1056/NEJMoa1507854 [DOI] [PubMed] [Google Scholar]
- 18.Farooq V, Goedhart D, Ludman P, de Belder MA, Harcombe A, El-Omar M; British Cardiovascular Intervention Society and the National Institute for Cardiovascular Outcomes Research . Relationship between femoral vascular closure devices and short-term mortality from 271 845 percutaneous coronary intervention procedures performed in the United Kingdom between 2006 and 2011: a propensity score-corrected analysis from the British Cardiovascular Intervention Society. Circ Cardiovasc Interv. 2016;9(6):e003560. doi: 10.1161/CIRCINTERVENTIONS.116.003560 [DOI] [PubMed] [Google Scholar]
- 19.Ibanez B, James S, Agewall S, et al. ; ESC Scientific Document Group . 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119-177. doi: 10.1093/eurheartj/ehx393 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Subgroup Analysis of the Primary Outcome
eFigure 2. Updated Meta-analysis
Data Sharing Statement