This cross-sectional study evaluates the association of major adverse cardiac events (MACE) integrated with fractional flow reserve as the management strategy for diabetes with outcomes in patients with ambiguous lesions who undergo angiography.
Key Points
Question
What are the usefulness, rate of major adverse cardiovascular events (MACE), and clinical outcomes of routinely integrating fractional flow reserve in the management strategy for patients with diabetes who undergo coronary angiography?
Findings
In this cross-sectional study of 1983 patients, overall reclassification by fractional flow rate was high and similar in patients with diabetes (41.2%) and patients without diabetes (37.5%); however, reclassification from medical treatment to revascularization was more frequent among patients with diabetes. The rate of 1-year MACE was similar in reclassified (9.7%) and nonreclassified (12.0%) patients with diabetes, and the rate of MACE of patients deferred based on fractional flow reserve was similar among those with and without diabetes.
Meaning
The findings suggest that management strategies guided by fractional flow reserve, including revascularization deferral, may be useful for patients with diabetes.
Abstract
Importance
Approximately one-third of patients considered for coronary revascularization have diabetes, which is a major determinant of clinical outcomes, often influencing the choice of the revascularization strategy. The usefulness of fractional flow reserve (FFR) to guide treatment in this population is understudied and has been questioned.
Objective
To evaluate the usefulness and rate of major adverse cardiovascular events (MACE) of integrating FFR in management decisions for patients with diabetes who undergo coronary angiography.
Design, Setting, and Participants
This cross-sectional study used data from the PRIME-FFR study derived from the merger of the POST-IT study (Portuguese Study on the Evaluation of FFR-Guided Treatment of Coronary Disease [March 2012-November 2013]) and R3F study (French Study of FFR Integrated Multicenter Registries Implementation of FFR in Routine Practice [October 2008-June 2010]), 2 prospective multicenter registries that shared a common design. A population of all-comers for whom angiography disclosed ambiguous lesions was analyzed for rates, patterns, and outcomes associated with management reclassification, including revascularization deferral, in patients with vs without diabetes. Data analysis was performed from June to August 2018.
Main Outcomes and Measures
Death from any cause, myocardial infarction, or unplanned revascularization (MACE) at 1 year.
Results
Among 1983 patients (1503 [77%] male; mean [SD] age, 65 [10] years), 701 had diabetes, and FFR was performed for 1.4 lesions per patient (58.2% of lesions in the left anterior descending artery; mean [SD] stenosis, 56% [11%]; mean [SD] FFR, 0.81 [0.01]). Reclassification by FFR was high and similar in patients with and without diabetes (41.2% vs 37.5%, P = .13), but reclassification from medical treatment to revascularization was more frequent in the former (142 of 342 [41.5%] vs 230 of 730 [31.5%], P = .001). There was no statistical difference between the 1-year rates of MACE in reclassified (9.7%) and nonreclassified patients (12.0%) (P = .37). Among patients with diabetes, FFR-based deferral identified patients with a lower risk of MACE at 12 months (25 of 296 [8.4%]) compared with those undergoing revascularization (47 of 257 [13.1%]) (P = .04), and the rate was of the same magnitude of the observed rate among deferred patients without diabetes (7.9%, P = .87). Status of insulin treatment had no association with outcomes. Patients (6.6% of the population) in whom FFR was disregarded had the highest MACE rates regardless of diabetes status.
Conclusions and Relevance
Routine integration of FFR for the management of coronary artery disease in patients with diabetes may be associated with a high rate of treatment reclassification. Management strategies guided by FFR, including revascularization deferral, may be useful for patients with diabetes.
Introduction
Fractional flow reserve (FFR)–guided revascularization has been shown to be superior to angiography-guided revascularization in reducing both short- and long-term major adverse cardiovascular events (MACE),1,2 and deferral of nonischemic lesions has been associated with improved outcomes.3 In addition, several studies4,5,6,7,8,9 have suggested that routine use of FFR is associated with a high rate of change in treatment decisions (up to 44%) and that reclassification (against angiography) is safe.
Patients with diabetes represent one-third of the population considered for coronary revascularization. Also, diabetes is often associated with a more deleterious clinical outcome, which in turn may be affected by the choice of revascularization method.10 Although there is a need for optimizing clinical decisions, concerns about microcirculatory responsiveness and the potential for accelerated atherosclerosis have cast doubts on the usefulness of FFR among patients with diabetes.11 Data on this subject are scarce, derived from a limited number of retrospective small cohorts, and findings are conflicting.12,13,14,15,16
With use of data from the large, multicenter PRIME-FFR (POST-IT [Portuguese Study on the Evaluation of FFR-Guided Treatment of Coronary Disease] and R3F [French Study of FFR Integrated Multicenter Registries–Implementation of FFR in Routine Practice]) joint international prospective study,5,7 we aimed to describe and evaluate the routine use of FFR among patients with diabetes for whom diagnostic coronary angiography disclosed at least 1 intermediate stenosis. In particular, we sought to describe the rate of reclassification of the patient management strategy, evaluate such change among patients with diabetes compared with those without diabetes, describe the rate of revascularization deferral, and compare the rates of MACE at 1 year in deferred patients with diabetes vs deferred patients without diabetes.
Methods
Patient Population
This cross-sectional study used data from the PRIME-FFR population (n = 1983), which resulted from the merger of the R3F (October 2008-June 2010) and POST-IT cohorts (March 2012-November 2013).5,7,8 These nationwide prospective studies shared a common design and objective dedicated to investigating the routine use of FFR at the time of diagnostic angiography and its association with patient management decisions and 1-year clinical outcomes. A total of 1983 patients were referred for coronary angiography in 40 European centers and prospectively included in the registries. The R3F and POST-IT studies were approved by the relevant institutional review boards or ethics committees. The R3F study was also approved by the Commission National Informatique et Liberté. All patients provided written informed consent to clinical follow-up and to storage and use of their clinical data. All data had been deidentified before storage.
In both studies, baseline clinical and angiographic measures were prospectively recorded in dedicated electronic case report forms. Inclusion and exclusion criteria for each of the original registries and definition of clinical characteristics (including diabetes) are provided in the eMethods in the Supplement.
Angiography and FFR Procedure
Angiography and qualitative and quantitative lesion characterization were performed according to standard practice and accepted criteria. Fractional flow reserve was performed through diagnostic or interventional catheters and after injection of intracoronary nitrate. Hyperemia was achieved using adenosine administered through intracoronary bolus (≥100 μg) or intravenous infusion (>140 μg/kg/min) according to local practice. Extensive FFR evaluation was not mandated, and the operators decided which lesions or vessels to investigate. Analyses were performed according to the validated FFR threshold (0.80).
Management Strategy and Definition of Reclassification and Deferral
Investigators were required to prospectively define a baseline management strategy for each patient based on coronary angiography findings and all available clinical information before FFR measurement was performed. After FFR was evaluated, a final management strategy was defined and recorded. Fractional flow reserve was considered to have been disregarded whenever treatment was performed contrary to the FFR result (eg, revascularization when FFR was >0.80 or the opposite).
Options included medical therapy (with or without additional stress test), percutaneous coronary intervention (PCI), or coronary artery bypass surgery (CABG). Patients for whom a hybrid approach was chosen were classified in the CABG group. When a final decision of revascularization was decided, it was performed immediately or at a later stage. Reclassification of patient management strategy was defined as discordance between the baseline and final strategies. Revascularization deferral was identified when the final strategy was medical treatment for all lesions after FFR and no revascularization procedure was either performed or planned for that patient.
Detailed Objectives
The objective was to describe and evaluate the usefulness and rate of MACE of routine use of FFR among patients with diabetes for whom diagnostic coronary angiography disclosed at least 1 intermediate stenosis. In particular, we aimed to describe the rate of reclassification of patient management strategy and to evaluate such change in patients with diabetes compared with those without diabetes. We compared the rate of 1-year MACE according to the agreement or divergence of the FFR-guided final decision vs the a priori strategy suggested based on angiography findings. In addition, we aimed to describe the rate of revascularization deferral and to compare rate of 1-year MACE in deferred patients with diabetes vs deferred patients without diabetes.
As secondary analyses, we evaluated the outcome of patients in whom the results of FFR measurement were disregarded by the investigators when deciding the final strategy, as well as the potential association of insulin treatment with FFR-based management.
Clinical Follow-up and End Points
The study primary end point (MACE) was a composite of all-cause death, myocardial infarction, or unplanned revascularization at 12 months. Each individual end point was reviewed and adjudicated by an independent clinical event committee, and external monitoring was performed in both registries. Angina status was also obtained at 1-year follow-up. Myocardial infarction was defined according to the third 2012 European Society of Cardiology/American College of Cardiology Foundation/American Heart Association/World Heart Federation universal definition of myocardial infarction.8,17 Revascularization was considered to be unplanned when it was not performed or planned at the time of the index procedure. Thus, both staged PCI and elective CABG resulting from the index FFR evaluation were not considered as events.
Statistical Analysis
Before merging R3F and POST-IT into the PRIME-FFR data set, interim comparisons were made to verify that no major heterogeneity existed in main baseline characteristics of study patients (including epidemiologic, clinical, and angiographic characteristics and FFR) or the 1-year clinical outcome of the 2 study cohorts overall and within subgroups (patients with diabetes and patients without diabetes). To check for the consistency of the results, we replicated the POST-IT analysis on the 3RF database and vice versa.
Continuous variables are presented as means (SDs). Discrete variables are presented as quantities and percentages. For patient-related characteristics, differences among groups were evaluated using a t test or χ2 test. The initial and final management strategy (medical therapy, PCI, and CABG) and overall decision to reclassify were evaluated using a Fisher exact test. Fractional flow reserve was compared between patients with and without diabetes according to lesion stenosis severity and lesion complexity.
Cumulative rates of MACE were estimated using the Kaplan-Meier method. Differences in outcomes were tested using multivariable Cox proportional hazards models adjusted for relevant baseline characteristics. Multiple imputation was used to account for missing covariate data in the Cox proportional hazards models. Analyses were conducted using SAS, version 9.4 (SAS Institute Inc) and the level of statistical significance was set at 2-sided P < .05. Data analysis was performed from June to August 2018.
Results
Baseline Clinical Characteristics
The main clinical characteristics of patients are summarized in Table 1. Of the 1983 patients (1503 [77%] male; mean [SD] age, 65 [10] years) included in this study, 701 (35.3%) were identified as having diabetes based on study criteria, including 175 (8.8%) requiring insulin treatment. Patients with diabetes had a typical clinical profile of such a population; compared with patients without diabetes, they were older (mean [SD] age, 65.5 [9.6] years vs 64.6 [10.9] years, P = .05) and more likely to be women (206 of 701 [29.4%] vs 274 of 1282 [21.4%], P < .001), to be hypertensive (590 of 701 [84.2%] vs 848 of 1282 [68.7%], P < .001), and to have hypercholesterolemia (544 [77.7%] vs 835 [67.9%], P < .001) but were less likely to be smokers (228 [32.5%] vs 564 [44.0%], P < .001). Patients with diabetes were also more likely to have a left ventricular ejection fraction less than or equal to 50% (151 [21.5%] vs 182 [14.2%], P < .001) and to receive secondary prevention medications (any antiplatelet: 601 [87.1%] vs 1048 [82.5%], P = .01; statin: 563 [81.7%] vs 954 [75.3%], P = .001; angiotensin-converting enzyme inhibitor/angiotensin receptor blocker: 470 [68.6%] vs 688 [55.0%], P < .001; β-blockers: 445 [65.0%] vs 753 [59.8%], P = .03) (Table 1).
Table 1. Baseline Characteristics According to FFR Use and Patient Reclassification of the Management Strategy.
| Characteristic | Patients With Diabetes (n = 701) | Patients Without Diabetes (n = 1282) | P Valuea | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | FFR Disregarded (n = 48) | FFR Used (n = 653) | P Valueb | All | FFR Disregarded (n = 83) | FFR Used (n = 1199) | P Valueb | ||||||
| Reclassified (n = 269) | Not Reclassified (n = 384) | P Valuec | Reclassified (n = 450) | Not Reclassified (n = 749) | P Valuec | ||||||||
| Age, mean (SD), y | 65.5 (9.6) | 67.1 (8.7) | 64.3 (9.8) | 66.2 (9.4) | .01 | .21 | 64.6 (10.9) | 63.8 (10.0) | 63.9 (10.9) | 65.1 (11.0) | .056 | .48 | .05 |
| Male, No. (%) | 495 (70.6) | 36 (75.0) | 191 (71.0) | 268 (69.8) | .74 | .49 | 1008 (78.6) | 64 (77.1) | 370 (82.2) | 574 (76.6) | .02 | .73 | <.001 |
| Insulin use, No. (%) | 175 (25.0) | 12 (25.0) | 69 (25.6) | 94 (24.5) | .73 | .99 | NA | NA | NA | NA | NA | NA | NA |
| Cardiovascular risk factors, No. (%) | |||||||||||||
| Hypertension | 590 (84.2) | 37 (77.1) | 229 (85.1) | 324 (84.4) | .79 | .16 | 848 (68.7) | 54 (66.7) | 284 (65.6) | 510 (70.7) | .07 | .69 | <.001 |
| Smoker | 228 (32.5) | 14 (29.2) | 91 (33.8) | 123 (32.0) | .45 | .17 | 564 (44.0) | 31 (37.3) | 225 (50.0) | 308 (41.1) | .01 | .38 | <.001 |
| High cholesterol | 544 (77.7) | 37 (77.1) | 216 (80.6) | 291 (75.8) | .15 | .91 | 835 (67.9) | 58 (71.6) | 290 (67.3) | 487 (67.8) | .85 | .46 | <.001 |
| Cardiovascular medication, No. (%) | |||||||||||||
| Any antiplatelet | 601 (87.1) | 41 (87.2) | 231 (87.8) | 329 (86.6) | .64 | .98 | 1048 (82.5) | 72 (86.7) | 377 (84.3) | 599 (80.9) | .14 | .29 | .01 |
| Dual antiplatelet | 388 (56.2) | 23 (48.9) | 164 (62.4) | 201 (52.9) | .02 | .30 | 668 (52.6) | 47 (56.6) | 256 (57.3) | 365 (49.4) | .01 | .45 | .13 |
| Statin | 563 (81.7) | 40 (85.1) | 221 (84.4) | 302 (79.5) | .12 | .53 | 954 (75.3) | 67 (80.7) | 345 (77.5) | 542 (73.3) | .11 | .24 | .001 |
| ACEI/ARB | 470 (68.6) | 32 (69.6) | 181 (69.1) | 257 (68.2) | .81 | .88 | 688 (55.0) | 44 (53.7) | 255 (58.1) | 389 (53.2) | .11 | .81 | <.001 |
| β-Blocker | 445 (65.0) | 30 (65.2) | 175 (67.0) | 240 (63.5) | .35 | .97 | 753 (59.8) | 59 (71.1) | 278 (62.8) | 416 (56.8) | .04 | .03 | .03 |
| Clinical history, No. (%) | |||||||||||||
| Myocardial infarction | 174 (30.8) | 14 (31.1) | 72 (33.2) | 88 (29.0) | .31 | .96 | 373 (36.6) | 27 (40.3) | 134 (38.2) | 212 (35.2) | .36 | .51 | .02 |
| PCI | 249 (44.0) | 26 (57.8) | 99 (45.4) | 124 (40.9) | .31 | .05 | 488 (47.7) | 34 (49.3) | 175 (49.9) | 279 (46.3) | .30 | .793 | .15 |
| CABG | 23 (4.1) | 2 (4.4) | 16 (7.3) | 5 (1.7) | .001 | .89 | 44 (4.3) | 3 (4.3) | 11 (3.1) | 30 (5.0) | .18 | .99 | .82 |
| LVEF ≤50% | 151 (21.5) | 12 (25.0) | 58 (21.6) | 81 (21.1) | .79 | .75 | 182 (14.2) | 11 (13.3) | 76 (16.9) | 95 (12.7) | .05 | .91 | <.001 |
| Clinical status, No. (%) | |||||||||||||
| Typical angina | 193 (27.5) | 12 (25.0) | 78 (29.0) | 103 (26.8) | .43 | .96 | 369 (28.8) | 32 (38.6) | 120 (26.7) | 217 (29.0) | <.001 | .10 | <.001 |
| Atypical angina or nonanginal chest pain | 67 (9.6) | 5 (10.4) | 20 (7.4) | 42 (10.9) | 176 (13.7) | 10 (12.0) | 42 (9.3) | 124 (16.6) | |||||
| Other symptoms or asymptomatic | 281 (40.1) | 19 (39.6) | 115 (42.8) | 147 (38.3) | 364 (28.4) | 19 (22.9) | 156 (34.7) | 189 (25.2) | |||||
| Ongoing NSTEMI or unstable angina | 72 (10.3) | 4 (8.3) | 24 (8.9) | 44 (11.5) | 157 (12.2) | 11 (13.3) | 51 (11.3) | 95 (12.7) | |||||
| Recent NSTEMI or unstable angina | 67 (9.6) | 6 (12.5) | 26 (9.7) | 35 (9.1) | 146 (11.4) | 11 (13.3) | 57 (12.7) | 78 (10.4) | |||||
| Recent STEMI | 21 (3.0) | 2 (4.2) | 6 (2.2) | 13 (3.4) | 70 (5.5) | 0 | 24 (5.3) | 46 (6.1) | |||||
| Initial revascularization strategy, No. (%) | |||||||||||||
| CABG | 63 (9.0) | 4 (8.3) | 22 (8.2) | 37 (9.6) | .91 | .70 | 54 (4.2) | 3 (3.6) | 29 (6.4) | 22 (3.0) | <.001 | .28 | <.001 |
| PCI | 272 (38.8) | 20 (41.7) | 105 (39.0) | 147 (38.3) | NA | NA | 454 (35.4) | 36 (44.4) | 191 (42.4) | 227 (30.3) | NA | NA | NA |
| Medical therapy | 366 (52.2) | 24 (50.0) | 142 (52.8) | 200 (52.1) | NA | NA | 774 (60.4) | 44 (51.0) | 230 (51.2) | 500 (66.7) | NA | NA | NA |
| Final revascularization strategy, No. (%) | |||||||||||||
| CABG | 85 (12.1) | 4 (8.4) | 44 (16.4) | 37 (9.6) | .003 | .32 | 105 (8.2) | 7 (8.4) | 76 (16.9) | 22 (2.9) | <.001 | .12 | <.001 |
| PCI | 279 (39.8) | 16 (33.3) | 116 (43.1) | 147 (38.3) | NA | NA | 462 (36.0) | 38 (45.8) | 197 (43.8) | 227 (30.3) | NA | NA | NA |
| Medical therapy | 337 (48.1) | 28 (58.3) | 109 (40.5) | 200 (52.1) | NA | NA | 715 (55.8) | 38 (45.8) | 177 (39.3) | 500 (66.8) | NA | NA | NA |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass graft; FFR, fractional flow reserve; LVEF, left ventricular ejection fraction; NA, not applicable; NSTEMI, non–ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
P values comparing populations with diabetes vs without diabetes.
P values comparing FFR used vs FFR disregarded populations.
P values comparing FFR used, reclassified vs nonreclassified populations.
FFR and Angiographic Characteristics
The main FFR and angiographic characteristics are reported in eTable 1 in the Supplement. Patients with diabetes vs those without diabetes were more likely to have multivessel coronary artery disease (CAD) (347 of 701 [49.5%] vs 506 of 1282 [39.4%], P < .001) and to have FFR evaluation more extensively performed (1.4 vs 1.3 lesions/patient, P = .007). Among patients with diabetes, the investigated lesion was located in the left anterior descending artery in 582 of 1000 cases (58.2%) and was proximal in 333 of 1000 cases (33.3%). Lesions were in the 30% to 70% stenosis range in 782 of 1000 (78.2%) of cases. Despite mean (SD) percentage of angiographic stenosis being similar, patients with diabetes had more complex B2/C lesions (422 of 1000 [42.2%] vs 645 of 1698 [38.1%], P = .03) and FFR was lower (mean [SD]: 0.81 [0.10] vs 0.82 [0.10], P < .001). Ischemic lesions (FFR ≤0.80) were more frequent in patients with diabetes (458 of 1000 [45.8%] vs 616 of 1698 [36.3%], P < .001). In both groups, FFR had a normal distribution (eFigure 1 in the Supplement), and within each stratum of stenosis severity, FFR was significantly lower in patients with diabetes (eFigure 2 in the Supplement). Multivariable analyses of clinical, angiographic, and procedural characteristics identified the same variables associated with a lower FFR value in both groups: age, left anterior descending artery location, American College of Cardiology/American Heart Association lesion type, stenosis percentage, lesion length, and number of diseased vessels. Specific differences in FFR assessment methods, including route of adenosine injection and catheter size, were not associated with FFR.
Diabetes and Clinical Outcome
Regardless of FFR and FFR-based decision, patients with diabetes had a higher rate of MACE (79 of 701 [11.3%] vs 116 of 1282 [9.0%]) and of death or myocardial infarction (37 of 701 [5.3%] vs 46 of 1282 [3.6%]) (eTable 2 in the Supplement). The proportion of patients free from angina at 1 year was similar (643 of 701 [91.7%] vs 1179 of 1282 [92.0%], P = .92).
FFR-Based Reclassification of Treatment Strategy
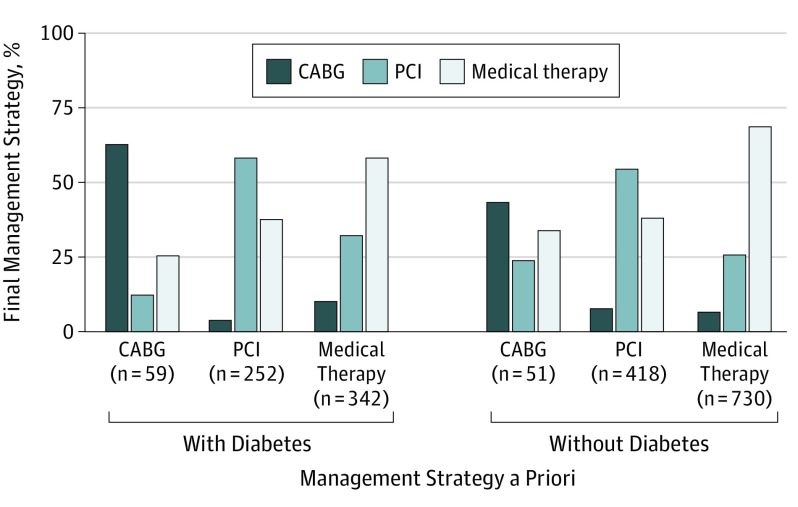
In most procedures, physicians used the information provided by FFR to drive their final management decision in patients with diabetes (653 of 701 [93.2%]) and patients without diabetes (1199 of 1282 [93.5%]) (Figure 1). The overall rate of FFR-based treatment change was numerically higher in patients with diabetes (269 of 653 [41.2%] vs 450 of 1199 [37.5%], P = .13). However, as shown in Figure 2, the reclassification pattern diverged significantly. Specifically, patients with diabetes initially considered for medical treatment were more likely to be reclassified to a revascularization strategy (142 of 342 [41.5%] vs 230 of 730 [31.5%], P = .001) (eFigure 3 in the Supplement). Patients with diabetes considered a priori for CABG were less likely reclassified to PCI or medical treatment (22 of 59 [37.3%] vs 29 of 51 [56.8%], P = .03) (Figure 2). The proportion of patients finally undergoing revascularization (PCI or CABG) after FFR was higher among those with diabetes (344 of 653 [52.7%] vs 522 of 1199 [43.5%], P = .001). Baseline clinical and angiographic characteristics of patients for whom the use of FFR was associated with reclassification of management strategy are presented in Table 1 and eTable 1 in the Supplement. In both patient groups, those reclassified were more likely to have multivessel CAD, a left anterior descending artery lesion interrogated, and lower and more frequent ischemic FFR.
Figure 1. Flowchart.
FFR indicates fractional flow reserve.
Figure 2. Rate of Fractional Flow Reserve–Based Reclassification of the Management Strategy Among Patients With and Without Diabetes According to the Management Strategy a Priori.
CABG indicates coronary artery bypass graft; PCI, percutaneous coronary intervention.
Clinical Outcome in Patients With Diabetes and FFR Reclassification
Among the patients with diabetes in whom FFR was used for decision reclassification of the management strategy (ie, FFR-based decision discordant with angiography findings), the 1-year clinical outcome was as good for those for whom the decision was not reclassified by FFR (ie, FFR-based decision concordant with angiography findings). One-year MACE rates were 9.7% vs 12.0% (log-rank P = .37), respectively (Figure 1 and Figure 3A and eTable 3 in the Supplement). Similarly, no increase in clinical events was observed in reclassified vs nonreclassified patients with diabetes, including death or myocardial infarction (10 of 269 [3.7%] vs 22 of 384 [5.7%], log-rank P = .25), myocardial infarction alone (3 of 269 [1.1%] vs 10 of 384 [2.6%], log-rank P = .18), or unplanned revascularization (16 of 269 [5.9%] vs 31 of 384 [8.1%], log-rank P = .30). These findings were not modified after multivariable adjustment (eTables 3 and 4 in the Supplement). As previously reported, reclassification of the management strategy was also associated with a low rate of MACE for patients without diabetes (Figure 1 and Figure 3A) (eTable 3 in the Supplement).
Figure 3. Fractional Flow Reserve (FFR)–Based Decision and Clinical Outcome in the Overall Population of Patients With Diabetes Compared With Patients Without Diabetes.
A, One-year outcome of FFR-based reclassification of the treatment strategy was associated with a low rate of major adverse cardiovascular events (all-cause death, myocardial infarction, and revascularization) among patients with diabetes. B, One-year outcome of FFR-based deferral to medical treatment was associated with a low rate of major adverse cardiovascular events among patients with diabetes.
FFR-Based Deferral of Revascularization in Patients With Diabetes
Deferral of all lesions was less frequent in patients with diabetes (296 of 653 [45.3%]) compared with those without diabetes (662 of 1199 [55.2%]) (P < .001) (Table 2). The main clinical, angiographic, and FFR characteristics of deferred patients are reported in eTable 5 in the Supplement. Deferred patients had significantly less extensive and less complex CAD, and FFR was higher than in those deemed to need revascularization. Among patients with diabetes, FFR-based deferral identified a group of patients with a lower risk of MACE at 12 months (25 of 296 [8.4%]) compared with those undergoing revascularization (47 of 257 [13.1%]) (P = .04), and the rate was of the same magnitude of the observed rate among deferred patients without diabetes (52 of 662 [7.9%], P = .87) (Table 2 and Figure 3B). The occurrence of the individual clinical end points was consistently lower in deferred vs nondeferred patients with diabetes (Table 2) (eTable 4 in the Supplement).
Table 2. Clinical Outcomes at 12 Months by Management Strategy in Deferred vs Nondeferred Patients.
| Analysis Group | Fractional Flow Reserve Used, No. (%) | ||||||
|---|---|---|---|---|---|---|---|
| Patients With Diabetes (n = 653) | Patients Without Diabetes (n = 1199) | ||||||
| Deferred (n = 296)a | Nondeferred (n = 357)b | P Value, Log Rank | Deferred (n = 662)a | Nondeferred (n = 537)b | P Value, Log Rank | P Value, Log Rankc | |
| Major adverse cardiovascular event | 25 (8.4) | 47 (13.1) | .04 | 52 (7.9) | 48 (9.0) | .50 | .87 |
| Death or myocardial infarction | 11 (3.7) | 21 (5.9) | .18 | 24 (3.6) | 18 (3.4) | .78 | .94 |
| Total deaths | 9 (3.0) | 12 (3.4) | .81 | 17 (2.6) | 12 (2.2) | .70 | .67 |
| Myocardial infarction | 2 (0.7) | 11 (3.1) | .02 | 8 (1.2) | 8 (1.5) | .67 | .44 |
| Unplanned coronary revascularization | 15 (5.1) | 33 (9.2) | .03 | 34 (5.2) | 34 (6.4) | .33 | .96 |
Deferred patients: all lesions deferred by fractional flow reserve.
Nondeferred patients: at least 1 lesion revascularized by percutaneous coronary intervention or coronary artery bypass graft surgery.
Deferred diabetes vs deferred no diabetes.
Interaction of Insulin Treatment With Reclassification and Outcome
Compared with patients with noninsulin-dependent diabetes, those with insulin-dependent diabetes had more angiographically defined multivessel CAD (103 of 175 [68.8%] vs 244 of 526 [46.5%], P = .01) (eTable 6 in the Supplement) and higher 1-year rates of MACE (24 of 175 [13.8%] vs 55 of 526 [10.4%], P = .21) and death or myocardial infarction (12 of 175 [6.9%] vs 25 of 526 [4.7%], P = .18). Although overall reclassification rate was similar between patients with insulin-dependent vs noninsulin-dependent diabetes (69 of 163 [42.3%] vs 200 of 490 [40.8%] patients, P = .73), less deferral was observed (68 of 163 [41.7%] vs 228 of 490 [46.5%] patients, P = .22), and the proportion of patients referred for CABG surgical procedure was higher in the insulin-dependent diabetes group (29 of 163 [17.8%] vs 53 of 490 [10.8%], P = .02). Furthermore, in patients with insulin-dependent diabetes, 1-year MACE rate was not associated with treatment reclassification (reclassified vs nonreclassified: 10 of 69 [14.4%] vs 13 of 94 [14.0%], P = .92), and deferral of revascularization was not associated with an increased risk of 1-year MACE (deferred vs nondeferred: 7 of 68 [10.3%] vs 16 of 95 [16.8%], P = .20) (eFigure 4 in the Supplement).
FFR-Disregarded Group: Profile and Clinical Outcome
The baseline clinical and angiographic characteristics of the 131 patients (6.6% of the total population; 48 with diabetes and 83 without diabetes) for whom FFR was disregarded for the final decision are presented in Table 1 and eTable 1 in the Supplement. Although clinical characteristics were similar between the FFR-disregarded vs FFR groups, patients for whom FFR was disregarded had more extensive disease, longer lesions, and lower FFR both among patients with diabetes (0.77 vs 0.81) and patients without diabetes (0.79 vs 0.82). In most cases (80% among patients with diabetes), lesions with an FFR <0.80 were left untreated rather than treated (eTable 1 in the Supplement). In this subgroup, the rate of MACE at 1 year was twice as high compared with patients for whom the FFR was included in the management strategy (17.5% vs 9.2%, P = .002) (eTables 2 and 4 in the Supplement) (Figure 1). Similarly, patients for whom FFR was disregarded had more angina at 1 year.
Discussion
To our knowledge, this was the largest prospective study to report the use of FFR among patients with diabetes who undergo angiography and the association of FFR with treatment decisions and clinical outcomes. The key findings can be summarized as follows: (1) diabetes was not associated with higher FFR values for any given stenosis severity; (2) treatment reclassification was high among patients with diabetes (41.2%) and comparable with that observed among patients without diabetes, although the pattern of reclassification was different; (3) integrating FFR information into patient management was associated with a low rate of MACE among patients with diabetes, and FFR-based deferral to medical treatment was associated with a low rate of MACE among patients without diabetes; and (4) disregarding the information derived from FFR was associated with a worse outcome regardless of diabetes status.
Reliability of FFR Measurements Among Patients With Diabetes
Diabetes has the potential to impair microvascular responsiveness to hyperemia. As such, FFR could theoretically underestimate lesion severity. However, in our study within each stratum of stenosis severity, FFR was lower in patients with diabetes. The evidence was not associated with higher lesion complexity, which in turn was associated with a lower FFR. This observation was consistent with most previous reports (eTable 7 in the Supplement).12,16,18,19,20,21 Altogether, the findings suggest that microvascular disease, when present, may have a limited association with FFR reliability and its performance as a risk stratification tool in this population.
Management Strategy for Patients With Diabetes and CAD
We showed that patients with and without diabetes had an overall high and similar reclassification rate. However, the pattern of change was different. Patients with diabetes were more likely to be reclassified into a revascularization strategy (PCI or CABG) and less likely from revascularization to medical therapy. This finding may be the consequence of the more complex disease profile, which as previously reported, was associated with lower FFR regardless of diameter stenosis severity,5 and was further supported by the observation that within each category of stenosis severity, FFR was lower in patients with more complex lesions (B2/C vs A/B1).
The present study also extended to a population with diabetes the previous observation from both the R3F5 and the POST-IT7 studies that the routine integration of FFR in management decisions was not associated with a reduced overall percentage of patients undergoing revascularization (eTable 7 in the Supplement), contrary to common belief.
Rate of MACE Associated With FFR in Patients With Diabetes
Our study showed that pursuing a treatment strategy based on FFR that was different from that suggested by angiography findings was not associated with worse clinical outcomes despite patients with diabetes having a higher CAD burden and complexity. Of importance, not integrating FFR in the management strategy was associated with increases in the rates of both MACE and death or myocardial infarction in patients with or without diabetes. Although bias (associated with unmeasured comorbidity, such as patient frailty and lesion complexity) cannot be excluded and causality could not be determined, the findings support the importance of fully integrating FFR findings into treatment decisions. This is in line with the previous observations from Fractional Flow Reserve Versus Angiography for Multivessel Evaluation 2 (FAME 2), R3F, and POST-IT.2,5,7,22
Deferring Lesions Based on FFR and Clinical Outcome
To our knowledge, no dedicated randomized clinical trial or subgroup analysis has specifically addressed the outcomes of lesions adequately deferred in the context of diabetes. A single-center retrospective study including 250 patients recruited during a 4-year period and representing less than 7% of the population treated during the same period (n = 3379) observed a 10% increase in the risk of target vessel failure among 122 deferred patients with diabetes and FFR more than 0.80 compared with 128 deferred patients without diabetes.13,14 In the present study, the incidence of the composite primary end point at 12 months was similar among patients in whom all lesions were deferred based on an FFR more than 0.80, and no revascularization was undertaken regardless of diabetes status (Figure 3B and Table 2 and eFigure 4 and eTable 5 in the Supplement).
Overall, our results support the potential role of FFR in this context and showed that among patients diagnosed with diabetes for whom clinicians felt that FFR was appropriate for decision-making, an FFR value more than 0.80 may identify a group of patients in which revascularization may be deferred without an increase in the rate of MACE compared with patients without diabetes.
Patients Requiring Insulin
Insulin treatment has been consistently associated with the highest risk of cardiovascular events among patients with diabetes.10 Although hampered by the small number of patients (rendering statistical comparisons underpowered), the pattern of clinical outcomes was consistent with the overall population in that despite their intrinsic higher risk, insulin-treated patients for whom revascularization was deferred based on FFR had a numerically lower rate of MACE compared with those warranting PCI or CABG (eFigure 4 in the Supplement). Also, there was no association of use of FFR (vs angiography findings) and changes in management strategy according to the FFR values vs angiography were not associated with worse clinical outcomes.
Limitations
This study has limitations. The present study was the combination of 2 nationwide prospective FFR studies, and we cannot exclude that unaccounted differences between the 2 cohorts might have influenced our findings. However, the previous published results of R3F5 and POST-IT7 as well as the outcome analyses performed separately in each data set showed consistency between the 2 studies.
The present study included mostly patients with intermediate lesions for whom the likelihood of reclassification by FFR was expected to be high. Therefore, our results may not extend to patients with angiographically severe multivessel disease and tight lesions. In addition, our data cannot speak to the relative safety of a strict FFR vs angiography-based approach because there was no formal control group in which patients were managed according to angiography findings alone. However, in the FFR-disregarded group, information from angiography (and maybe clinical characteristics) prevailed compared with information derived from the FFR, and these patients had a higher rate of MACE.
Conclusions
Among patients with diabetes and mostly intermediate coronary lesions, routine use of FFR was associated with reclassification of clinical management in a significant proportion of cases. A treatment plan guided by FFR that is divergent from that suggested by angiography findings (including revascularization deferral) was associated with good clinical outcome in these patients. Dedicated randomized clinical trials powered for clinical outcomes are needed to further refine the role of FFR-based strategies (as well of other physiologic indexes) for the clinical management of this important subgroup of patients.23,24
eMethods. Inclusion Criteria in POST-IT and R3F Studies and Definition of Diabetes and Other Baseline Characteristics
eTable 1. Baseline Angiographic Characteristics According to Patient and Lesion Reclassification of the Management Strategy and FFR
eTable 2. Clinical Outcomes at 12 Months by Management Strategy: FFR Used vs FFR Disregarded
eTable 3. Clinical Outcomes at 12 Months: Diabetes Group and FFR Reclassification Status
eTable 4. Results of Cox Model Analysis by Diabetes and Reclassification Status (A), by Diabetes and Deferral Status (B) and by Diabetes and FFR Usage (C)
eTable 5. Patient’s Baseline Characteristics by “Deferral” and Diabetes Status (in Patients in Which FFR Was Used)
eTable 6. Patient’s Baseline Characteristics in Diabetic Patients According to Insulin Treatment
eTable 7. Studies Dedicated to the Use of FFR in Diabetic Patients for Treatment Decision and Clinical Outcome
eFigure 1. A) FFR Value Distribution in the DM Population. B) FFR Value Distribution in the non-DM Population
eFigure 2. FFR Value Stratified by Stenosis Severity Group in Diabetic and Nondiabetic Patients
eFigure 3. Rate of FFR-based reclassification of the management strategy in DM and non-DM Patients According to the Management Strategy A “Priori”
eFigure 4. One-year MACE According to Subgroup Diabetic Status and Revascularization Deferral (All Lesions Deferred)
References
- 1.Tonino PA, De Bruyne B, Pijls NH, et al. ; FAME Study Investigators . Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213-224. doi: 10.1056/NEJMoa0807611 [DOI] [PubMed] [Google Scholar]
- 2.De Bruyne B, Pijls NH, Kalesan B, et al. ; FAME 2 Trial Investigators . Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease [published correction appears in N Engl J Med. 2012;367(18):1768]. N Engl J Med. 2012;367(11):991-1001. doi: 10.1056/NEJMoa1205361 [DOI] [PubMed] [Google Scholar]
- 3.Zimmermann FM, Ferrara A, Johnson NP, et al. Deferral vs performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur Heart J. 2015;36(45):3182-3188. doi: 10.1093/eurheartj/ehv452 [DOI] [PubMed] [Google Scholar]
- 4.Sant’Anna FM, Silva EE, Batista LA, Ventura FM, Barrozo CA, Pijls NH. Influence of routine assessment of fractional flow reserve on decision making during coronary interventions. Am J Cardiol. 2007;99(4):504-508. doi: 10.1016/j.amjcard.2006.09.097 [DOI] [PubMed] [Google Scholar]
- 5.Van Belle E, Rioufol G, Pouillot C, et al. ; Investigators of the Registre Français de la FFR–R3F . Outcome impact of coronary revascularization strategy reclassification with fractional flow reserve at time of diagnostic angiography: insights from a large French multicenter fractional flow reserve registry. Circulation. 2014;129(2):173-185. doi: 10.1161/CIRCULATIONAHA.113.006646 [DOI] [PubMed] [Google Scholar]
- 6.Curzen N, Rana O, Nicholas Z, et al. Does routine pressure wire assessment influence management strategy at coronary angiography for diagnosis of chest pain? the RIPCORD study. Circ Cardiovasc Interv. 2014;7(2):248-255. doi: 10.1161/CIRCINTERVENTIONS.113.000978 [DOI] [PubMed] [Google Scholar]
- 7.Baptista SB, Raposo L, Santos L, et al. Impact of routine fractional flow reserve evaluation during coronary angiography on management strategy and clinical outcome: one-year results of the POST-IT. Circ Cardiovasc Interv. 2016;9(7):e003288. doi: 10.1161/CIRCINTERVENTIONS.115.003288 [DOI] [PubMed] [Google Scholar]
- 8.Van Belle E, Baptista SB, Raposo L, et al. ; PRIME-FFR Study Group . Impact of routine fractional flow reserve on management decision and 1-year clinical outcome of patients with acute coronary syndromes: PRIME-FFR (insights from the POST-IT [Portuguese Study on the Evaluation of FFR-Guided Treatment of Coronary Disease] and R3F [French FFR Registry] Integrated Multicenter Registries–Implementation of FFR [Fractional Flow Reserve] in routine practice). Circ Cardiovasc Interv. 2017;10(6):e004296. doi: 10.1161/CIRCINTERVENTIONS.116.004296 [DOI] [PubMed] [Google Scholar]
- 9.Van Belle E, Gil R, Klauss V, et al. Impact of routine invasive physiology at time of angiography in patients with multivessel coronary artery disease on reclassification of revascularization strategy: results from the DEFINE REAL study. JACC Cardiovasc Interv. 2018;11(4):354-365. doi: 10.1016/j.jcin.2017.11.030 [DOI] [PubMed] [Google Scholar]
- 10.Farkouh ME, Domanski M, Sleeper LA, et al. ; FREEDOM Trial Investigators . Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367(25):2375-2384. doi: 10.1056/NEJMoa1211585 [DOI] [PubMed] [Google Scholar]
- 11.Seto AH, Kern MJ. Deferred lesion failure in diabetes: a truly bad actor. Catheter Cardiovasc Interv. 2017;90(7):1084-1085. doi: 10.1002/ccd.27419 [DOI] [PubMed] [Google Scholar]
- 12.Domínguez-Franco AJ, Jiménez-Navarro MF, Muñoz-García AJ, Alonso-Briales JH, Hernández-García JM, de Teresa Galván E. Long-term prognosis in diabetic patients in whom revascularization is deferred following fractional flow reserve assessment [in Spanish]. Rev Esp Cardiol. 2008;61(4):352-359. doi: 10.1016/S1885-5857(08)60144-9 [DOI] [PubMed] [Google Scholar]
- 13.Kennedy MW, Hermanides RS, Kaplan E, et al. Fractional flow reserve–guided deferred versus complete revascularization in patients with diabetes mellitus. Am J Cardiol. 2016;118(9):1293-1299. doi: 10.1016/j.amjcard.2016.07.059 [DOI] [PubMed] [Google Scholar]
- 14.Kennedy MW, Kaplan E, Hermanides RS, et al. Clinical outcomes of deferred revascularisation using fractional flow reserve in patients with and without diabetes mellitus. Cardiovasc Diabetol. 2016;15:100. doi: 10.1186/s12933-016-0417-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z, Matsuzawa Y, Herrmann J, et al. Relation between fractional flow reserve value of coronary lesions with deferred revascularization and cardiovascular outcomes in non-diabetic and diabetic patients. Int J Cardiol. 2016;219:56-62. doi: 10.1016/j.ijcard.2016.05.032 [DOI] [PubMed] [Google Scholar]
- 16.Gargiulo G, Stabile E, Ferrone M, et al. ; CONTRST Study Investigators . Diabetes does not impact the diagnostic performance of contrast-based fractional flow reserve: insights from the CONTRAST study. Cardiovasc Diabetol. 2017;16(1):7. doi: 10.1186/s12933-016-0494-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thygesen K, Alpert JS, Jaffe AS, et al. ; Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction . Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020-2035. doi: 10.1161/CIR.0b013e31826e1058 [DOI] [PubMed] [Google Scholar]
- 18.Adjedj J, Xaplanteris P, Toth G, et al. Visual and quantitative assessment of coronary stenoses at angiography versus fractional flow reserve: the impact of risk factors. Circ Cardiovasc Imaging. 2017;10(7):e006243. doi: 10.1161/CIRCIMAGING.117.006243 [DOI] [PubMed] [Google Scholar]
- 19.Yanagisawa H, Chikamori T, Tanaka N, Usui Y, Takazawa K, Yamashina A. Application of pressure-derived myocardial fractional flow reserve in assessing the functional severity of coronary artery stenosis in patients with diabetes mellitus. Circ J. 2004;68(11):993-998. doi: 10.1253/circj.68.993 [DOI] [PubMed] [Google Scholar]
- 20.Sahinarslan A, Kocaman SA, Olgun H, et al. The reliability of fractional flow reserve measurement in patients with diabetes mellitus. Coron Artery Dis. 2009;20(5):317-321. doi: 10.1097/MCA.0b013e32832c8ca3 [DOI] [PubMed] [Google Scholar]
- 21.Reith S, Battermann S, Hellmich M, Marx N, Burgmaier M. Impact of type 2 diabetes mellitus and glucose control on fractional flow reserve measurements in intermediate grade coronary lesions. Clin Res Cardiol. 2014;103(3):191-201. doi: 10.1007/s00392-013-0633-7 [DOI] [PubMed] [Google Scholar]
- 22.De Bruyne B, Fearon WF, Pijls NH, et al. ; FAME 2 Trial Investigators . Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med. 2014;371(13):1208-1217. doi: 10.1056/NEJMoa1408758 [DOI] [PubMed] [Google Scholar]
- 23.Davies JE, Sen S, Dehbi HM, et al. Use of the instantaneous wave-free ratio or fractional flow reserve in PCI. N Engl J Med. 2017;376(19):1824-1834. doi: 10.1056/NEJMoa1700445 [DOI] [PubMed] [Google Scholar]
- 24.Götberg M, Christiansen EH, Gudmundsdottir IJ, et al. ; iFR-SWEDEHEART Investigators . Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N Engl J Med. 2017;376(19):1813-1823. doi: 10.1056/NEJMoa1616540 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Inclusion Criteria in POST-IT and R3F Studies and Definition of Diabetes and Other Baseline Characteristics
eTable 1. Baseline Angiographic Characteristics According to Patient and Lesion Reclassification of the Management Strategy and FFR
eTable 2. Clinical Outcomes at 12 Months by Management Strategy: FFR Used vs FFR Disregarded
eTable 3. Clinical Outcomes at 12 Months: Diabetes Group and FFR Reclassification Status
eTable 4. Results of Cox Model Analysis by Diabetes and Reclassification Status (A), by Diabetes and Deferral Status (B) and by Diabetes and FFR Usage (C)
eTable 5. Patient’s Baseline Characteristics by “Deferral” and Diabetes Status (in Patients in Which FFR Was Used)
eTable 6. Patient’s Baseline Characteristics in Diabetic Patients According to Insulin Treatment
eTable 7. Studies Dedicated to the Use of FFR in Diabetic Patients for Treatment Decision and Clinical Outcome
eFigure 1. A) FFR Value Distribution in the DM Population. B) FFR Value Distribution in the non-DM Population
eFigure 2. FFR Value Stratified by Stenosis Severity Group in Diabetic and Nondiabetic Patients
eFigure 3. Rate of FFR-based reclassification of the management strategy in DM and non-DM Patients According to the Management Strategy A “Priori”
eFigure 4. One-year MACE According to Subgroup Diabetic Status and Revascularization Deferral (All Lesions Deferred)