Abstract
This study uses pharmacoepidemiology data from Danish national health registries to examine the association between first-trimester exposure to modafinil and risk of major congenital malformations.
Modafinil is a stimulant drug used in the treatment of excessive daytime sleepiness associated with narcolepsy.1 In Denmark, it is also sometimes used off-label for fatigue in multiple sclerosis. Modafinil has been discouraged during pregnancy because of a lack of safety data. In June 2019, the manufacturer alerted health care professionals that an interim analysis of postmarketing surveillance data detected major malformations among 15% of children exposed to modafinil during pregnancy compared with 3% in the background population.2
We examined the association between first-trimester exposure to modafinil and risk of major congenital malformations.
Methods
We identified all pregnancies from 2004 to 2017 using the Danish national health registries. Modafinil exposure was defined as any prescription overlapping with the first trimester (first 91 days) of pregnancy. Duration of treatment was calculated by dividing amount prescribed with the standard daily defined dose of 200 mg. The primary comparison was with pregnancies exposed to an active comparator, methylphenidate, which is not associated with congenital malformations and is used for similar indications.3 It is recommended for treatment of attention-deficit/hyperactivity disorder (ADHD) during pregnancy. A second comparator group was pregnancies unexposed to modafinil and methylphenidate 1 year prior to and during pregnancy. Major malformations were identified during pregnancy, at delivery, or during the first year of life using the EUROCAT classification of malformations.
We excluded women not residing in Denmark for at least 2 years before delivery; pregnancies shorter than 18 weeks or missing information on gestational age; pregnancies exposed to known teratogens (retinoids, angiotensin-converting enzyme inhibitors, vitamin K antagonists, valproic acid, lithium, carbamazepine, oxcarbazepine, phenytoin, phenobarbital); and infants/fetuses with chromosomal malformations. Recording of gestational age in the Danish registries is 99% complete.
Logistic regression was used to calculate odds ratios (ORs), adjusting for diagnosis of diabetes and hypertension, concomitant use of psychotropic drugs (listed in the Table), maternal age, year of delivery, smoking status, and body mass index. Stata release 15.1 (StataCorp) was used for analysis, and a 2-sided 95% CI was used to define a significant association.
Table. Participant Characteristics.
| Characteristic | Modafinil | Unexposed | Methylphenidate |
|---|---|---|---|
| No. of pregnancies | 49 | 828 644 | 963 |
| Age, mean (SD), y | 30.6 (5.1) | 30.3 (4.9) | 27.4 (5.5) |
| Smoking status, No. (%) | |||
| Heavy smoker (≥11 cigarettes/d) | (n < 5)a | 23 924 (2.9) | 112 (12) |
| Light smoker (1-10 cigarettes/d) | 6 (12) | 81 222 (9.8) | 307 (32) |
| Nonsmoker | 39 (80) | 703 020 (85) | 513 (53) |
| No information | (n < 5)a | 20 478 (2.5) | 31 (3.2) |
| Body mass index, No. (%)b | |||
| Underweight (<18) | (n < 5)a | 11 621 (1.4) | 29 (3.0) |
| Normal weight (18-24) | 26 (53) | 481 686 (58) | 485 (50) |
| Overweight (25-29) | 12 (25) | 188 254 (23) | 216 (22) |
| Obese class I (30-34) | 7 (14) | 68 061 (8.2) | 118 (12) |
| Obese class II and III (≥35) | (n < 5)a | 41 387 (5.0) | 79 (8.2) |
| No information | (n < 5)a | 37 635 (4.5) | 36 (3.7) |
| Major malformations, No. (%) | 6 (12) | 32 466 (3.9) | 43 (4.5) |
| Termination due to malformation | 0 | 2.553 (0.3) | (n < 5)a |
| Hospitalized during pregnancy, No. (%) | 8 (16) | 118 190 (14) | 238 (25) |
| Maternal comorbidities, No. (%) | |||
| Diabetes | (n < 5)a | 7509 (0.9) | 14 (1.5) |
| Hypertension | (n < 5)a | 10 601 (1.3) | 18 (1.9) |
| Multiple sclerosis | 13 (27) | 1961 (0.2) | (n < 5)a |
| Sleep disorders (ICD-10 G47) | 19 (39) | 2145 (0.3) | 43 (4.5) |
| Narcolepsy (ICD-10 G47.4) | 15 (31) | 274 (0) | 27 (2.8) |
| Sleep-wake schedule disorder (ICD-10 G47.2) | 0 | 74 (0) | (n < 5)a |
| Sleep apnea (ICD-10 G47.3) | 0 | 1201 (0) | 13 (1.3) |
| Mental and behavioral disorders | |||
| Kinetic disorders (ICD-10 F90) | 0 | 370 (0) | 121 (13) |
| ADHD (ICD-10 F90.0) | 0 | 289 (0) | 102 (11) |
| Mood disorders | 6 (12) | 9322 (1.1) | 92 (9.6) |
| Other mental and behavioral disorders | 9 (18) | 48 782 (5.9) | 352 (37) |
| Concomitant drug exposure, No. (%) | |||
| Other ATC groups (not nervous system) | 44 (90) | 487 050 (59) | 694 (72) |
| Nervous system (ATC group) | 30 (61) | 70 852 (8.6) | 488 (51) |
| N02 Analgesics | 9 (18) | 35 531 (4.3) | 137 (14) |
| N03 Antiepileptics | (n < 5)a | 4208 (0.5) | 61 (6.3) |
| N05 Psycholeptics | 15 (31) | 11 285 (1.4) | 189 (20) |
| N06 Psychoanalepticsc | 20 (41) | 28 976 (3.5) | 293 (30) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; ATC, Anatomical Therapeutic Chemical Classification System; ICD-10, International Classification of Diseases and Related Health Problems, Tenth Revision.
The Danish Data Health Authority does not allow specified numbers below 5.
Calculated as weight in kilograms divided by height in meters squared.
Excluding methylphenidate and modafinil.
Registry-based studies are exempt from informed consent and ethical review in Denmark. The study was approved by the Danish Data Health Authority.
Results
There were 49 pregnancies (0.006%) exposed to modafinil, 963 (0.12%) exposed to methylphenidate, and 828 644 unexposed pregnancies. Among modafinil-exposed women, 27% had a diagnosis of multiple sclerosis, and of methylphenidate exposed women, 11% had a diagnosis of ADHD. Sleep disorders occurred in 39% of modafinil users and 4.5% of methylphenidate users, and use of other psychoanalytics in the modafinil- and methylphenidate-exposed groups was reported to be 41% and 30%, respectively (Table).
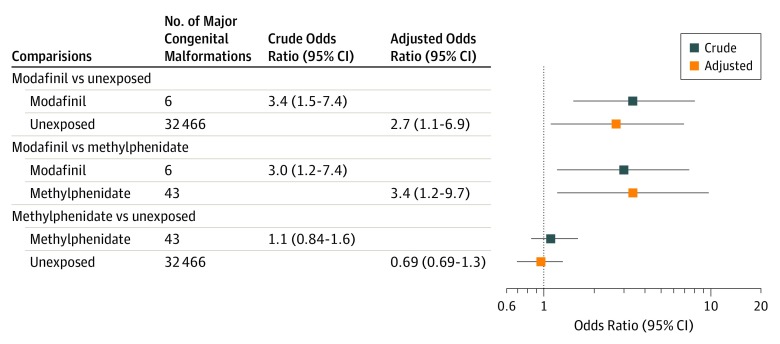
There were 6 major congenital malformations in the modafinil-exposed group (no patient with multiple sclerosis received teriflunomide, a suspected teratogen), 43 in the methylphenidate-exposed group, and 32 466 in the unexposed pregnancies group, for absolute risks of 12% for modafinil, 4.5% for methylphenidate, and 3.9% for unexposed. The adjusted ORs were 3.4 (95% CI, 1.2-9.7) comparing modafinil and methylphenidate and 2.7 (95% CI, 1.1-6.9) comparing modafinil with unexposed (Figure).
Figure. Risk of Congenital Malformations From First-Trimester Modafinil Exposure.
Odds ratios and 95% CIs comparing first-trimester exposure to modafinil with those who were unexposed and with those who were exposed to methylphenidate. Those who were methylphenidate exposed are also compared with those who were unexposed.
Discussion
In this nationwide study, first-trimester in utero exposure to modafinil compared with methylphenidate or no medication was significantly associated with an increased risk of congenital malformations.
In 2 case series totaling 274 patients with narcolepsy, no malformations among 13 modafinil-exposed pregnancies were reported.4,5 Narcolepsy itself does not appear to increase the risk of congenital malformations.6
There are several limitations. First, the potential for residual confounding exists. For example, baseline differences in psychiatric medications were adjusted for, but adjusting for psychiatric diagnoses was not feasible. Although methylphenidate was used as an active comparator to address confounding by indication, methylphenidate is indicated for ADHD as well as narcolepsy. Second, prescription redemptions were a proxy for actual use. Third, the small numbers of exposed women and events reflect the rarity of the exposure and decrease the precision of the point estimates. Fourth, specific malformations were not identifiable.
Although further research is needed, women contemplating pregnancy should currently avoid or discontinue modafinil.
Section Editor: Jody W. Zylke, MD, Deputy Editor.
References
- 1.Electronic Medicines Compendium Modafinil 100 mg tablets. https://www.medicines.org.uk/emc/product/4319/smpc. Accessed June 26, 2019.
- 2.Teva Pharmaceuticals Ireland Modafinil: potential risk of congenital malformations when administered during pregnancy. http://www.hpra.ie/docs/default-source/default-document-library/important-safety-information---modafinil99170c2697826eee9b55ff00008c97d0.pdf. Accessed December 14, 2019.
- 3.Huybrechts KF, Bröms G, Christensen LB, et al. Association between methylphenidate and amphetamine use in pregnancy and risk of congenital malformations: a cohort study from the International Pregnancy Safety Study Consortium. JAMA Psychiatry. 2018;75(2):167-175. doi: 10.1001/jamapsychiatry.2017.3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calvo-Ferrandiz E, Peraita-Adrados R. Narcolepsy with cataplexy and pregnancy: a case-control study. J Sleep Res. 2018;27(2):268-272. doi: 10.1111/jsr.12567 [DOI] [PubMed] [Google Scholar]
- 5.Maurovich-Horvat E, Kemlink D, Högl B, et al. ; European Narcolepsy Network . Narcolepsy and pregnancy: a retrospective European evaluation of 249 pregnancies. J Sleep Res. 2013;22(5):496-512. doi: 10.1111/jsr.12047 [DOI] [PubMed] [Google Scholar]
- 6.Maurovich-Horvat E, Tormášiová M, Slonková J, et al. Assessment of pregnancy outcomes in Czech and Slovak women with narcolepsy. Med Sci Monit. 2010;16(12):SR35-SR40. [PubMed] [Google Scholar]