This survey study describes the testing methods and practices used by a sample of US audiologists when evaluating adults with severe hearing loss who are being considered for cochlear implant.
Key Points
Question
What are the preoperative assessment methods used by audiologists to identify adult candidates for cochlear implants?
Findings
This survey study with 99 respondents found that practices in the evaluation and management of adult cochlear implant candidates vary widely across audiologists in the United States.
Meaning
Variability in clinical testing methods for cochlear implant candidacy may highlight a potential increased risk for health care inequities among adults with clinically significant hearing loss.
Abstract
Importance
Currently, no clear guidelines exist regarding clinical testing methods for identifying adult cochlear implant (CI) candidates. Indications provided by the US Food and Drug Administration, Medicare, and private insurers are ambiguous concerning test materials and the level and mode of test presentation. This could lead to wide variability in clinical assessment and, potentially, unequal access to CIs for individuals with clinically significant hearing loss.
Objective
To examine the preoperative testing methods used by audiologists in evaluating adult CI candidates across the United States.
Design, Setting, and Participants
A survey assessing audiology practice patterns was created using a Research Electronic Data Capture system hosted at the University of Miami. A link to a survey (65 questions in multiple-choice or rank-order format was distributed electronically along with a request for completion to members of the American Cochlear Implant Alliance and to the Institute for Cochlear Implant Training forum. Responses were collected from January 17 to June 4, 2018. Participation was limited to audiologists who evaluate adult CI candidates, and respondents who do not provide adult CI care were excluded. Collected demographic information included work setting, years of experience, and highest level of education attained.
Main Outcomes and Measures
Percentages, medians, and interquartile ranges were from aggregated responses concerning hearing aid verification methods; testing methods, materials, and practices; nonauditory factors that might affect CI candidacy; audiology practice patterns; and expanded indications for CIs.
Results
Anonymized surveys were returned by 99 respondents; because surveys were available electronically, the number of audiologists who viewed the survey but did not respond was not available. Seven respondents identified themselves as pediatric specialists and were excluded, resulting in a total of 92 surveys available for analysis (denominators vary because respondents could complete the survey without answering all questions). Seventy percent of respondents (51 of 72) were doctors of audiology, and nearly 50% (33 of 74) were employed at universities and academic centers performing more than 50 CIs per year. When assessing adult candidacy for implant, most respondents reported using test materials from the Minimum Speech Test Battery: 96% (51 of 53), using AzBio sentences in quiet; 89% (47 of 53), AzBio sentences in noise; and 100% (53 of 53), the consonant–vowel nucleus–consonant, monosyllabic words test. However, these tests were applied inconsistently, with 39 of 53 respondents (74%) reporting use of a sound pressure level scale and the other 14 (26%) a hearing level scale at various decibel levels, and with some using a single signal-to-noise ratio and others using multiple ratios for sound-in-noise tests. Respondents’ definitions of the best aided listening condition for assessing implant candidates also varied widely. Among the nonauditory factors ranked most important for assessing CI candidacy were patient’s level of cognition and expectations of CI; yet, few respondents reported including cognitive or psychological tests in the assessment protocol.
Conclusions and Relevance
Findings of this study reveal considerable variability in preoperative testing methods and practices across health care professionals assessing adult candidates for CI. This lack of standardization in the delivery of care may increase the risk for health care inequities, specifically in access to care for adults with clinically significant hearing loss.
Introduction
The number of individuals with disabling hearing loss continues to increase globally.1,2 Management and treatment options for hearing loss are expanding. Despite these trends, access to hearing health care remains disproportionately low.1 Cochlear implants (CIs) are a widely accepted treatment option for patients with bilateral deafness. Advancements in surgical technique and electrode design have contributed to improved outcomes in patients with CIs, leading to a rapid push to expand indications set by the US Food and Drug Administration (FDA)3 to allow increased access to CI technology. Along these lines, FDA criteria for CIs recently were expanded to include individuals with lesser degrees of hearing loss.3 Evidence now exists to support the success of CIs in those with residual hearing who cannot benefit from traditional amplification.4,5,6,7,8 In accordance with the expanded FDA criteria, testing measures have evolved to more appropriately assess candidacy and avoid potential ceiling effects.9,10
Although CI is a long-standing primary treatment solution for those with irreparable hearing loss, no national or international consensus or clinical practice guidance statement exists, to our knowledge, on the delivery of care specific to preoperative and postoperative audiologic evaluation for CI.
In 2011, Cochlear Americas, Med-El Corporation, and Advanced Bionics LLC, leading global manufacturers of CIs, recommended a revised Minimum Speech Test Battery (MSTB)11,12 for assessment of CI candidacy. The revised MSTB consists of the AzBio12 sentences presented in quiet and in noise; an adaptive speech-in-noise (SIN) test, the Bamford-Kowal-Bench SIN13; and a monosyllabic word test that uses consonant–vowel nucleus–consonant stimuli. Although the revised MSTB provides audiologists with recommended testing materials, thereby encouraging greater consistency in testing protocols and methods, the revised recommendations are not supported by evidence and do not represent a consensus of commissioned experts. Moreover, the MSTB recommendations remain ambiguous, allowing for considerable variation in how the tests are administered. This situation is further complicated by the fact that indications for CI are often vague.14 Specifically, testing conditions (ie, quiet vs noise), testing material (ie, sentence characteristics), and definitions of “best aided” (monaural vs binaural) listening conditions are poorly defined. Although leaving room for clinical judgment in such decisions is appropriate, the ambiguity that currently exists may lead to inconsistency across clinics in regard to important life-changing decisions regarding CI candidacy. Similarly, criteria for Medicare candidates15 tend to be more stringent than the FDA criteria despite increasing criticism of their appropriateness for selecting adult candidates for CI.6,9,16
The primary objective of this study was to investigate preoperative assessment methods used by audiologists to evaluate adult candidates for CI in the United States. Secondarily, we investigated clinical practice variables that might contribute to decision-making regarding assessment methods.
Methods
Survey Methods
A survey investigating practice patterns of CI audiologists in the United States was developed using the Research Electronic Data Capture system hosted at the University of Miami, Miami, Florida.17 The survey was developed using existing literature and information obtained from a focus group with recognized experts in audiologic evaluation and management of CIs from leading institutions around the United States (University of Miami; University of Michigan, Ann Arbor; University of Iowa, Iowa City; Vanderbilt University, Nashville, Tennessee; University of North Carolina, Charlotte; Johns Hopkins University, Baltimore, Maryland; and Medical University of South Carolina, Charleston). This focus group convened at the 2017 American Cochlear Implant Alliance meeting in San Francisco, California. Content was recorded with participant agreement, and key concepts used in the survey were established based on identified discrepancies, stated topics of importance, and high-volume discussion points. In total, 8 audiologists and researchers from 7 academic institutions participated.
The survey was distributed electronically by the American Cochlear Implant Alliance to its members, and to the Institute for Cochlear Implant Training forum. Responses were anonymously recorded between January 17, 2018, and June 4, 2018, and data were password protected. The study was approved as exempt research by the University of Miami Institutional Review Board, which also waived the need for informed participant consent because responses were anonymized.
Survey Design
The survey consisted of 65 questions presented in multiple-choice or rank-order format (eAppendix in the Supplement). With the knowledge that clinical practice patterns may be highly variable, a branched conditional structure was used to direct respondents to questions that were relevant to their individual practice pattern.
The survey was limited to audiologists evaluating adults for CI candidacy. Respondents who did not provide adult CI care were excluded. Demographic information collected included work setting, years of experience, and highest level of education attained. Major topics covered in the survey included nonauditory factors considered in preoperative evaluation of adults for CI, hearing aid (HA) verification methods, auditory evaluation criteria, expanded indications for CI, and audiology practice patterns.
Statistical Analysis
Aggregated data were summarized using descriptive statistics. Where appropriate, percentages, medians, and interquartile ranges were calculated using spreadsheet software (Excel, version 2016; Microsoft Corporation).
Results
Descriptive Data
Ninety-nine anonymized surveys were returned; because surveys were available electronically, the number of audiologists who viewed the survey but did not respond was not available. Seven respondents who identified themselves as pediatric specialists were excluded, resulting in a total of 92 surveys available for analysis (denominators vary because respondents could complete the survey without answering all questions). Seventy-one percent of respondents (51 of 72) had a doctorate of audiology. Most respondents were employed at university or other academic centers (33 of 74 [45%]) or hospitals (29 of 74 [39%]), and 58% (41 of 71) worked at large CI centers, defined as performing more than 50 CIs per year (Table 1).
Table 1. Survey Respondents’ Practice-Related Characteristics .
| Characteristic | Respondents, No./Total No. (%)a |
|---|---|
| Clinical setting | |
| Academic university | 33/74 (45) |
| Hospital | 29/74 (39) |
| Private practice | 11/74 (14) |
| Other | 4/74 (5) |
| Government/Veterans Affairs | 2/74 (3) |
| Level of education | |
| Doctorate of audiology | 51/72 (70) |
| Master’s degree | 14/72 (20) |
| Doctorate of philosophy | 6/72 (9) |
| Audiology assistant | 1/72 (1) |
| Audiology experience, y | |
| 0-5 | 25/73 (35) |
| 6-10 | 14/73 (19) |
| 11-15 | 20/73 (27) |
| ≥16 | 14/73 (19) |
Denominators vary because respondents could complete the survey without answering all questions.
Nonauditory Factors
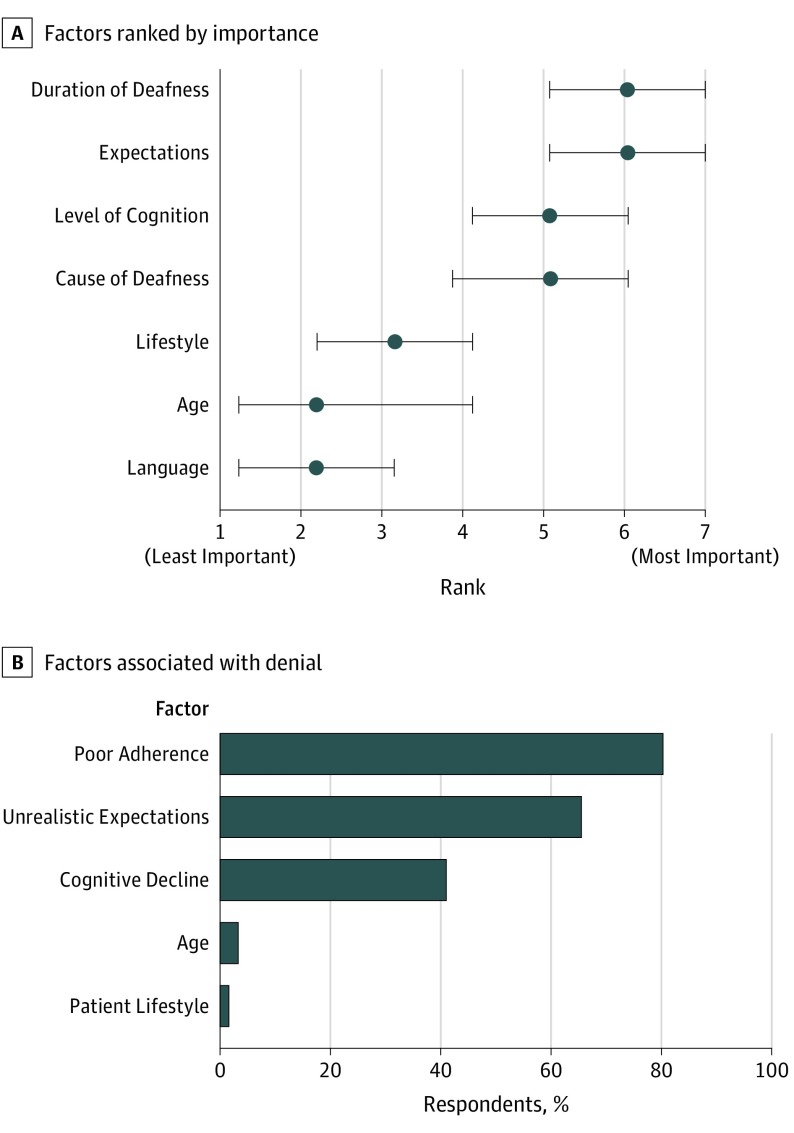
Respondents were asked to report how often psychological testing and cognitive screening were included as part of the standard pre-CI workup. Most respondents (53 of 65 [82%]) reported seldom or never including a psychological evaluation. Similarly, cognitive screening tests (ie, Mini-Mental State Examination or Montreal Cognitive Assessment) were seldom or never included (43 of 65 [74%]). Of the 40 respondents who reported administering cognitive screening, 18 (45%) reported modifying their testing protocol based on the results of cognitive screening. Modifications included changing testing material to make the tasks easier (eg, using a Hearing-in-Noise Test instead of an AzBio test) and reducing the amount of testing (ie, number of test items). Only 3 (8%) of the 40 respondents reported that an abnormal result in cognitive screening would warrant additional referral for psychological, neurological, or speech-language evaluation. Although fewer than half of respondents reported administering cognitive testing as part of their preoperative protocol, cognition was considered a highly important factor in clinical decision-making regarding CI candidacy, with 57% (35 of 61) of respondents ranking cognition as moderately important to extremely important (Figure 1A).
Figure 1. Factors Considered When Determining Cochlear Implant (CI) Candidacy.
A, Respondents (n = 59) ranked 7 patient-specific factors in order of their importance in assessing adult candidates for CIs: duration of deafness, realistic expectations of CI, level of cognition, cause of deafness, lifestyle, age, and native language. On a scale of 1-7 (with 1 indicating the least important and 7 the most important factor), cognition was ranked the third most important nonauditory factor when considering candidacy for a CI. Data markers indicate the median; error bars, the interquartile range. B, Percentage of 61 respondents indicating that the factors listed might contribute to denial of adult patients’ candidacy for a CI. Four of 61 respondents (7%) reported that they would not deny CI to any patient who meets criteria.
The respondents were asked to identify any of 5 patient-related factors (ie, unrealistic expectations, poor compliance [eg, high no-show rate, lack of interest in aural rehabilitation], cognitive decline, lifestyle, or age) that might contribute to clinician denial of CI. The factor most frequently identified was poor compliance (49 of 61 [80%]), followed by unrealistic expectations (40 of 61 [66%]) (Figure 1B). Cognitive impairment was considered a contraindication by 41% of respondents (25 of 61). Patient age and lifestyle were reported to have little influence on denial, and 7% of respondents (4 of 61) indicated that no patient meeting candidacy criteria would be denied CI.
Preimplantation Verification Methods
The fundamental criterion for CI candidacy of adults is lack of HA benefit.3,15 However, lack of HA benefit is highly subjective and is rarely defined. Respondents were queried regarding their HA fitting and verification practices to investigate their definitions of best fit and “HA benefit.” When respondents were asked to select all criteria that apply to evaluating HA fit and benefit, their responses were highly consistent, with the majority (41 of 65 [63%]) identifying functional gain (assessment of aided sound-field thresholds as decibels in hearing level [HL]), in combination with on-ear and/or simulated real-ear measurements. Only 1 respondent solely relied on the patient’s subjective report. Of note, respondents indicated that when HAs are verified, they meet prescriptive targets less than 50% of the time. When HAs do not meet prescriptive targets and the patient’s current HA cannot be reprogrammed, 94% of respondents (60 of 64) reported that they program loaner HAs to use in testing for CI candidacy.
Evaluation Criteria
Of the respondents who reported using speech-recognition test materials from the MSTB, a majority reported using the AzBio sentences in quiet (51 of 53 [96%]) and/or in noise (47 of 53 [89%]), and all reported using the consonant–vowel nucleus–consonant, monosyllabic words test. Only 17 of 53 (32%) reported using the Bamford-Kowal-Bench SIN test. A few respondents incorporated test materials from outside the MSTB, including Hearing-in-Noise Test sentences in quiet (18 of 53 [35%]) and Hearing-in-Noise Test sentences in noise (8 of 53 [15%]).
Although the most commonly used test battery included consonant–vowel nucleus–consonant, AzBio in quiet, and AzBio in noise, inconsistencies emerged in the administration of these tests. Of 53 respondents, 39 (74%) presented speech stimuli using a sound pressure level (SPL) scale, while the remaining 14 (26%) used HL. The vast majority using SPL (37 of 39) presented stimuli at 60 dB, although some used more than 1 sound level ranging from 50 to 70 dB SPL. Of the 14 using HL, 7 (50%) presented stimuli at 60 dB, whereas the other 7 presented stimuli ranging from 50 to greater than 70 dB.
Response data revealed that SIN measures are conditionally applied on the basis of speech-in-quiet (SIQ) scores by 38 of 51 respondents (74%). The reported SIQ scores used to indicate a need for SIN testing varied from 20% to 70%, suggesting little consistency in application or in methods.
When using SIN as a candidacy measure, 37 of 52 respondents (71%) reported using a single signal to noise ratio (SNR), whereas 15 of 52 (29%) use multiple SNRs. Among the 37 respondents testing at a single SNR, 21 (58%) reported using +10-dB SNR; 13 (36%), +5-dB SNR; and 2 (6%), +8-dB SNR. Of 15 respondents who reported testing at multiple SNRs, most reported using +10-dB SNR (13 [87%]) and +5-dB SNR (14 [93%]), with a small percentage incorporating +8-dB SNR (2 [13%]).
More than half of those using SIN measures (28 of 51) modify their standard test battery by adjusting the SNR to alter the difficulty of the test condition. Of those 28, a total of 23 (82%) reported they would not increase the SNR to make the test condition easier; however, 21 of 28 (75%) reported that they would increase the difficulty of the test condition by decreasing the SNR. Response data did not reveal trends or consistency in threshold criteria used to decide when to decrease the SNR but do suggest that this protocol modification is used when the SIN score exceeds 40% to 80%.
Audiologic Testing Criteria
The respondents were queried on SNR and overall percentage correct criteria used when determining CI candidacy (Table 2). Results showed considerable differences in both criteria among respondents.
Table 2. Statements Reflecting Respondents’ Sound-in-Noise (SIN) Criteria for Qualifying a Patient for CI.
| Statement | Respondents, No. (%) (n = 49)a |
|---|---|
| If scores are <50% at +5-dB SNR, the patient is eligible for CI | 28 (57) |
| If scores are >60% at +5-dB SNR, the patient is not eligible for CI | 34 (69) |
| If scores are >60% at a +10-dB SNR, the patient is not eligible for CI | 27 (55) |
Abbreviations: dB, decibels; CI, cochlear implant; SNR, signal to noise ratio.
Data are the number and percentage of respondents who indicated that the statement is true. The variability in SIN criteria among respondents suggests that CI may be denied to adults who could benefit from it.
Cochlear implant candidacy criteria as defined by the Centers for Medicare & Medicaid Services and the FDA are based on hearing outcomes in the best aided listening condition. Figure 2 presents data demonstrating the ambiguity in the definition of best aided performance for both unilateral CI (Figure 2A) and sequential, second CI (Figure 2B) in adults with bilateral hearing impairment. Selection of adult candidates for a second CI was based solely on the scores obtained while using a HA alone in the ear to be implanted, according to 30 of 49 respondents (61%). The remaining 19 respondents (39%) based adult CI candidacy on the bimodal (CI + HA) scores.
Figure 2. Interpretation of “Best Aided” Condition for Unilateral and Sequential Bilateral CIs.
A, Respondents’ definition of “best aided” listening condition when evaluating adult candidacy for initial, unilateral cochlear implant (CI). The majority (21 of 51 [42%]) reported testing 3 hearing conditions—right aided, left aided, and bilaterally aided —and selecting the highest scoring condition as the best aided. B, Respondents’ definition of “best aided” listening condition when evaluating adult candidacy for second, sequential CI. The majority (30 of 49 [61%]) reported that the best aided condition was use of a hearing aid (HA) in the unimplanted ear. WRS indicates word recognition score.
Expanding Indications
To explore factors related to expanding indications for CI, respondents were queried on consideration of residual hearing during preoperative assessments. For the purpose of this survey, residual hearing was defined as aidable hearing up to and including 1000 Hz. Most respondents (44 of 51 [85%]) reported that they do not modify their preoperative assessment protocol based on the patient’s residual hearing.
Medicare Criteria
Eligibility for CI among Medicare beneficiaries is limited to those with bilateral, moderate-to-profound sensorinueral hearing loss. Interpretation of moderate-to-profound hearing loss varied, with 21 of 48 respondents (44%) reporting that some audiometric thresholds must fall in the severe-to-profound range (ie, not purely moderate in degree), 12 (25%) indicated that thresholds must fit within the moderate-to-profound range, and 4 (8%) required thresholds to be both moderate and profound. The remaining 11 (23%) reported that they do not consider unaided thresholds when assessing CI candidacy of Medicare beneficiaries. Only 14 (29%) reported testing Medicare recipients in noise consistently, with considerable variability in both the application of such measures and the resulting interpretation. As in the assessment of non-Medicare candidates, SIQ was often used to decide whether to test adult Medicare beneficiaries in noise, with 34 of 48 respondents (71%) using SIN if SIQ scores were 40% or higher. In regard to provision of a second, bilateral CI to Medicare recipients, 27 of the 48 respondents (56%) recommend bilateral CI. Of those 27 respondents, 12 (44%) recommend bilateral CI when performance is less than 40% in the ear without CI; 10 (37%), when scores are less than 40% in the bimodal test condition (CI + HA); 1 (3.7%), for reasons of medical necessity (eg, blindness); and 4 (15%), when the patient is self-paying.
Discussion
In a diverse sample of US audiologists, our study results show considerable variability in preoperative assessment practices for the evaluation and management of adult CI candidates. Test administration, both in terms of presentation level and SNR, can influence outcomes. Although most audiologists report presenting stimuli at a level of 60 dB, the scale used varies, suggesting a lack of clarity with regard to the use of HL vs SPL in testing methods.
Presentation at 60 dB HL results in an SPL of approximately 72 dB, which has been shown to be vocally unsustainable18 and, it is important to note, may falsely inflate sentence and word discrimination scores.19 According to Skinner et al,18 presenting test materials at a normal conversational level results in effective everyday communication and understanding of vowels, consonants, and sentences in CI recipients. Their findings, along with those of Alkaf and Firszt,19 showed that presentation at 70 dB SPL resulted in inflated scores compared with the scores achieved during presentation at a normal conversational level of 60 dB SPL, at which CI candidates had a (statistically) significantly poorer performance. This practice may exclude potential candidates that could benefit from a CI. Despite the evidence that 70 dB SPL is not representative of real-world situations and the fact that 60 dB SPL is the recommended level in the MSTB instructions, some clinics continue to present test materials at inappropriate levels that may hinder access to CI for viable candidates.
In the present study, SIN was performed by less than 30% of the survey population despite evidence supporting the importance of testing in noise.20 Speech-in-noise measures more appropriately reflect real-world listening performance and appear to have a higher sensitivity for selecting appropriate CI candidates than SIQ.6,10,16 When SIN testing was used, it was conditionally based on scores obtained in quiet; yet no clear performance marker for when to include SIN was identified. These results differ from those reported by Carlson et al,21 wherein 68% of respondents reported that SIN testing is performed routinely. This divergence likely results from the differences between the survey populations; Carlson et al21 included only a small sample of neurotologists, which did not allow for representation of the diversity of CI audiologists and surgeons. Furthermore, our data were derived primarily from audiologists who actually perform the candidacy testing.
Of the respondents who do consistently rely on SIN testing in assessing candidacy, marked variability in the application and the resulting interpretation of these measures was observed. Risks of selecting noise levels that degrade speech recognition more than would be expected in everyday situations could falsely deflate patients’ scores and qualify those who may not benefit. Our data show inconsistencies in the SNR applied to SIN measures. The differences in performance for a given patient at +10 SNR vs +5 SNR could be clinically significant and could be the deciding factor for candidacy, although to date no clear guidelines exist on appropriate SNRs for CI candidates. Based on survey responses, the same individual may not have access to a CI if tested at a center using +10 SNR to decide candidacy, but may have access if tested at a center using a +5 SNR (Table 2). Furthermore, discrepancies in the interpretation of best aided performance can lead to varying recommendations.
The results of this survey identified some important trends. Two primary factors affecting candidacy decisions rely predominantly on clinical observation, including cognition and the patient’s possession of realistic expectations. Most respondents placed value on the importance of cognition, yet greater than 80% did not include cognitive assessments or screening in their evaluation. Mild cognitive impairment increases as a function of age and is highly prevalent in adults older than 65 years.2 Some studies have failed to show a correlation between cognition and overall variability in word recognition outcomes in CI recipients,22,23,24 while others have shown that older CI recipients tend to score poorer on tasks of listening in noise than their younger cohorts.25,26 Age was not considered by the respondents and was not identified as an influential factor in denial of a CI in this study, suggesting that the correlation between age and cognition was not readily considered during preoperative assessment of adult CI candidates.
It was also revealed that less than half of the patients referred for a CI evaluation are wearing appropriately fit HAs. This finding is consistent with those of Kochkin27 in a survey of 533 HA users, which showed that only 36% participated in verification and validation measures during their HA fitting appointments. Similar findings were reported by Holder et al,28 whereby only 29.1% of patients presenting to the clinic for a CI evaluation were fitted with HAs achieving the targets established in the National Acoustic Laboratories nonlinear fitting procedure, version 2.29 Conversely, our survey results suggest that 1 area where respondents were in agreement was in the area of HA verification. In our survey, more than 90% of CI audiologists reported that they use verification methods prior to candidacy testing; when HAs are below target, they program loaner HAs to meet prescriptive targets for the purposes of candidacy testing. The practice of fitting HAs appropriately for CI candidacy testing is essential to obtaining accurate SIN measures and understanding the candidates’ full potential, both of which play important roles in the surgical decision-making process.
Limitations
One limitation of this survey is that the behaviors of respondents could not be fully assessed because the survey inherently limited respondents to a forced-choice option. It is possible that some alternatives were overlooked or misrepresented. In addition, academic institutions were overrepresented in the survey population, which may not accurately represent the diversity of practices among audiologists performing CI candidacy assessments. It is reasonable to assume that those who are active members of the American Cochlear Implant Alliance and/or graduates of the Institute for Cochlear Implant Training program may have increased awareness and advanced training in assessing CI candidacy. Similarly, an individual respondent’s implementation of candidacy criteria may reflect the skill level at that particular clinic. Specifically, large academic institutions with extensive experience and access to large databases may implement less stringent CI candidacy criteria, whereas a smaller clinic with less experience may implement strict criteria owing to a lack of necessary skills (ie, inability to monitor residual hearing or to manage the care of patients with bimodal [HA and electroacoustic stimulation] devices) to optimize postoperative outcomes. As such, the findings here are of even greater importance because those with less access to continuing education and on-the-job training may further represent greater deviations from the reported results.
Conclusions
Current indications for obtaining a CI, as provided by the FDA, Medicare, and private insurers, are ambiguous in regard to test materials, presentation level, and mode of presentation. There are advantages as well as disadvantages to this ambiguity. On a positive note, audiologists have the opportunity to use their professional judgment when making decisions about CI candidacy and may base those decisions on careful consideration of additional patient-specific factors beyond audiometric findings and aided speech recognition scores. Conversely, this same ambiguity has led to wide variability in reported practice methods by audiologists across the United States, which could foster confusion among referring professionals regarding CI candidacy and potentially result in fewer referrals, thus limiting access to CIs. To adequately evaluate candidacy for CI and accurately measure benefit gained from CI, preoperative and postoperative test batteries should be better streamlined, with improved consistency in methods of application across CI centers nationwide.
eAppendix. Survey Questions
References
- 1.World Health Organization Global costs of unaddressed hearing loss and cost-effectiveness of interventions: a WHO report, 2017. https://apps.who.int/iris/bitstream/handle/10665/254659/9789241512046-eng.pdf;jsessionid=EE8DD97DAB4158B6476C620671F9492D?sequence=1. Accessed October 24, 2019.
- 2.Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med. 2013;29(4):753-772. doi: 10.1016/j.cger.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration Premarket Approval (PMA) [online database]. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm. Updated February 25, 2019. Accessed October 24, 2019.
- 4.Gantz BJ, Dunn C, Oleson J, Hansen M, Parkinson A, Turner C. Multicenter clinical trial of the Nucleus Hybrid S8 cochlear implant: final outcomes. Laryngoscope. 2016;126(4):962-973. doi: 10.1002/lary.25572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hornsby BW, Ricketts TA. The effects of hearing loss on the contribution of high- and low-frequency speech information to speech understanding, II: sloping hearing loss. J Acoust Soc Am. 2006;119(3):1752-1763. doi: 10.1121/1.2161432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes ML, Neff DL, Simmons JL, Moeller MP. Performance outcomes for borderline cochlear implant recipients with substantial preoperative residual hearing. Otol Neurotol. 2014;35(8):1373-1384. doi: 10.1097/MAO.0000000000000367 [DOI] [PubMed] [Google Scholar]
- 7.Pillsbury HC III, Dillon MT, Buchman CA, et al. Multicenter US clinical trial with an electric-acoustic stimulation (EAS) system in adults: final outcomes. Otol Neurotol. 2018;39(3):299-305. doi: 10.1097/MAO.0000000000001691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheffield SW, Jahn K, Gifford RH. Preserved acoustic hearing in cochlear implantation improves speech perception. J Am Acad Audiol. 2015;26(2):145-154. doi: 10.3766/jaaa.26.2.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gifford RH, Dorman MF, Shallop JK, Sydlowski SA. Evidence for the expansion of adult cochlear implant candidacy. Ear Hear. 2010;31(2):186-194. doi: 10.1097/AUD.0b013e3181c6b831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gifford RH, Shallop JK, Peterson AM. Speech recognition materials and ceiling effects: considerations for cochlear implant programs. Audiol Neurootol. 2008;13(3):193-205. doi: 10.1159/000113510 [DOI] [PubMed] [Google Scholar]
- 11.Minimum Speech Test Battery (MSTB) for Adult Cochlear Implant Users 2011 http://www.auditorypotential.com/MSTBfiles/MSTBManual2011-06-20%20.pdf. Published June 2011. Accessed October 24, 2019.
- 12.Spahr AJ, Dorman MF, Litvak LM, et al. Development and validation of the AzBio sentence lists. Ear Hear. 2012;33(1):112-117. doi: 10.1097/AUD.0b013e31822c2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.BKB-SIN Speech-in-Noise Test, Version 1.03 [audio CD]. Elk Grove Village, IL: Etymotic Research; 2005. [Google Scholar]
- 14.US Food and Drug Administration Summary and certification: 510(K) summary, No. K021837. https://www.accessdata.fda.gov/cdrh_docs/pdf2/k021837.pdf. Accessed October 28, 2019.
- 15.Centers for Medicare & Medicaid Services Cochlear implantation. https://www.cms.gov/Medicare/Coverage/Coverage-with-Evidence-Development/Cochlear-Implantation-.html. Accessed October 24, 2019. [Google Scholar]
- 16.Mudery JA, Francis R, McCrary H, Jacob A. Older individuals meeting medicare cochlear implant candidacy criteria in noise but not in quiet: are these patients improved by surgery? Otol Neurotol. 2017;38(2):187-191. doi: 10.1097/MAO.0000000000001271 [DOI] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skinner MWHL, Holden LK, Holden TA, Demorest ME, Fourakis MS. Speech recognition at simulated soft, conversational, and raised-to-loud vocal efforts by adults with cochlear implants. J Acoust Soc Am. 1997;101(6):3766-3782. doi: 10.1121/1.418383 [DOI] [PubMed] [Google Scholar]
- 19.Alkaf FM, Firszt JB. Speech recognition in quiet and noise in borderline cochlear implant candidates. J Am Acad Audiol. 2007;18(10):872-882. doi: 10.3766/jaaa.18.10.6 [DOI] [PubMed] [Google Scholar]
- 20.Lindley G. They say “I can’t hear in noise,” we say “say the word base.” Audiology Today July/August 2015:45-49. https://www.audiology.org/sites/default/files/AT274_JulAug_15.pdf. Accessed October 25, 2019. [Google Scholar]
- 21.Carlson ML, Sladen DP, Gurgel RK, Tombers NM, Lohse CM, Driscoll CL. Survey of the American Neurotology Society on cochlear implantation: part 1, candidacy assessment and expanding indications. Otol Neurotol. 2018;39(1):e12-e19. [DOI] [PubMed] [Google Scholar]
- 22.Collison EA, Munson B, Carney AE. Relations among linguistic and cognitive skills and spoken word recognition in adults with cochlear implants. J Speech Lang Hear Res. 2004;47(3):496-508. doi: 10.1044/1092-4388(2004/039) [DOI] [PubMed] [Google Scholar]
- 23.Heydebrand G, Hale S, Potts L, Gotter B, Skinner M. Cognitive predictors of improvements in adults’ spoken word recognition six months after cochlear implant activation. Audiol Neurootol. 2007;12(4):254-264. doi: 10.1159/000101473 [DOI] [PubMed] [Google Scholar]
- 24.Knutson JF, Schartz HA, Gantz BJ, Tyler RS, Hinrichs JV, Woodworth G. Psychological change following 18 months of cochlear implant use. Ann Otol Rhinol Laryngol. 1991;100(11):877-882. doi: 10.1177/000348949110001103 [DOI] [PubMed] [Google Scholar]
- 25.Lenarz M, Sönmez H, Joseph G, Büchner A, Lenarz T. Cochlear implant performance in geriatric patients. Laryngoscope. 2012;122(6):1361-1365. doi: 10.1002/lary.23232 [DOI] [PubMed] [Google Scholar]
- 26.Sladen DP, Zappler A. Older and younger adult cochlear implant users: speech recognition in quiet and noise, quality of life, and music perception. Am J Audiol. 2015;24(1):31-39. doi: 10.1044/2014_AJA-13-0066 [DOI] [PubMed] [Google Scholar]
- 27.Kochkin S. MarkeTrak VIII: reducing patient visits through verification and validation. Hearing Rev. 2011;18(6):10-12. http://www.hearingreview.com/2011/06/marketrak-viii-reducing-patient-visits-through-verification-amp-validation/. Accessed October 29, 2019. [Google Scholar]
- 28.Holder JT, Reynolds SM, Sunderhaus LW, Gifford RH. Current profile of adults presenting for preoperative cochlear implant evaluation. Trends Hear. 2018;22:2331216518755288. doi: 10.1177/2331216518755288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keidser G, Dillon H, Flax M, Ching T, Brewer S. The NAL-NL2 prescription procedure. Audiol Res. 2011;1(1):e24. doi: 10.4081/audiores.2011.e24 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Survey Questions